Abstract
Background:
Aspergillosis infection is common in the patients with insufficient immunity. The role of mammalian target of rapamycin (mTOR), T-box expressed in T-cells (T-bet), and eomesodermin (EOMES) in mediating T lymphocytes differentiation in response to Aspergillus fumigatus infection in immunocompromised rats was investigated in this study.
Methods:
Invasive pulmonary aspergillosis (IPA) of immunosuppressive twenty male rats were established and sacrificed at 24 h (n = 5), 48 h (n = 5), 72 h (n = 5), and 96 h (n = 5) after A. fumigatus infection. In addition, control (n = 5), cyclophosphamide (CTX) (n = 5), and aspergillosis (n = 5) group were also established the tissues and pathology of lung tissue was examined by hematoxylin and eosin staining. CD8+ T-cells was sorted by flow cytometry. Serum mTOR, S6K, T-bet, and EOMES were quantified by enzyme-linked immunosorbent assay.
Results:
Histology of lung tissue indicated severe lung tissue injury including infiltration of inflammatory cells, alveolar wall damage or degradation, blood congestion, and hemorrhage in the CTX, IPA, and CTX + IPA rats. Hyphae were seen in the IPA, and CTX + IPA groups. The proportion of CD8+ T-cells was significantly increased in the animals of CTX + IPA. Memory CD8+ T-cells was significantly increased in early stage (24 h and 48 h, P < 0.001), but decreased in the late phase of fungal infection (72 h and 96 h) in the animals of CTX + IPA. In addition, at early stage of fungal infection (24 h and 48 h), serum mTOR (P < 0.001), S6K (P < 0.001), and T-bet (P < 0.05) was significantly higher, while EOMES was significantly lower (P < 0.001), in CTX + IPA group than that in control, CTX alone or IPA alone group. Conversely, serum mTOR, S6K, T-bet, and EOMES showed opposite changed in the late stage (72 h and 96 h). Pearson's correlation analysis indicated that mTOR and S6K were significantly correlated with T-bet (r = 0.901 and 0.91, respectively, P < 0.001), but negatively and significantly correlated with EOMES (r = −0.758 and −0.751, respectively, P < 0.001).
Conclusions:
mTOR may regulate transcription factors of EOMES and T-bet, and by which mechanism, it may modulate lymphocytes differentiation in animals with immune suppression and fungal infection.
Keywords: Aspergillus fumigatus, Immunocompromised, mTOR, Transcription Factor
INTRODUCTION
The immunocompromised population is increasing worldwide. Aspergillosis is one of the devastating comorbidities in the patients with insufficient immunity. Aspergillus species are ubiquitous, and Aspergillus fumigatus is the primary causative agent of human infections. Aspergilla produce small, hydrophobic conidia that disperse easily into the air and can survive a broad range of environmental conditions.[1] A. fumigatus causes a wide range of human diseases depending on the immune status of the host. For instance, in patients with asthma or cystic fibrosis, aspergilla can cause allergic bronchopulmonary aspergillosis, a hypersensitive response to fungal components. While noninvasive aspergillomas may form following repeated exposure to conidia in some patients such as healed tuberculosis, invasive aspergillosis may be the most devastating of Aspergillus-related diseases, targeting severely immunocompromised patients such as cancer patients, organ transplantation patients, patients on prolonged corticosteroid therapy, or acquired immune deficient disease patients.[1,2]
CD8+ T-cell is a vital component of the adaptive immune system and important for eliminating intracellular pathogens.[3,4] CD8+ T-cells are activated through their T-cell receptor (TCR) followed by secreting cytokines and functioning as effector cells. Following infection or peptide stimulation, small numbers of naïve CD8+ T-cells expand to form a large effector population. When the large population of effector CD8+ T undergoes contraction or apoptosis, a smaller long-lived memory CD8+ T-cells will be responsible to protect the host from subsequent reinfection with the same pathogen by quickly expanding and rapidly expressing lytic activity and effector cytokines.[5] In this regard, we have previously reported that a number of CD8+ T-cells significantly decreased at an early stage of invasive pulmonary aspergillosis (IPA) and that the IPA patients’ prognosis was associated with differentiation of CD8+ T-cells.[6,7] Thus, the current study was designed to further investigate the mechanisms of regulating CD8+ T-cell differentiation in an animal model of cyclophosphamide (CTX)-induced immune suppression and Aspergillus infection.
mTOR is a serine/threonine kinase with the ability to integrate environmental stimuli to regulate cell metabolism, survival, growth, and proliferation. mTor forms two complexes, mTORC1 and mTORC2, with distinct signaling properties and sensitivities to rapamycin. mTORC1 phosphorylates S6K1, a 4EBP-1 to promote protein translation, and is sensitive to rapamycin inhibition.[8] Recent studies have indicated that mTOR may regulate T-cell differentiation and adaptive immunity following the binding of TCR to antigens.[8,9] Transcription factors T-box expressed in T-cells (T-bet) and eomesodermin (EOMES) are important transcription factors which regulate the effector T-cell and participated in the process of the differentiation and development of CD4 effector I type T-cells, helper T-cells, Th1 cells, NK cells, and CD8+ cytotoxic T lymphocyte. Many studies had shown that T-bet and Eomes involved in the regulation of CD8+ T-cells into effector T-cells and memory T-cells.[10,11,12]
Therefore, the role of mTOR and downstream T-bet and EOMES in modulating T-cell differentiation in immunocompromised animal models of IPA was explored in the current study.
METHODS
Pathogen preparation
The strain of A. fumigatus was obtained from a case of pulmonary aspergillosis at the Peking Union Medical College Hospital. Viable conidia (>95%) were obtained by growth on Sabouraud dextrose agar for 5 days at 35°C. Conidia was harvested with 10 ml 0.1% Tween-80 and filtered through five-layer of gauze. The concentration of conidia was adjusted to 1 × 108 CFU/ml by the method of turbidity adjustment.
Animal model preparation
Healthy Wistar rats, male, 7–8 weeks old, and weight of 220 ± 20 g were obtained from the Animal Facility Center, Peking Union Medical College Hospital. All animals were housed in a pathogen-free facility and used according to protocols approved by the IACUC of Peking Union Medical College Hospital. Total 35 rats were randomly divided into the following groups (five animals each group or subgroup): (1) control group (CON): nontreated normal rats. (2) Immune suppression group (CTX): animals were treated with CTX only. (3) IPA group: animals were infected with A. fumigatus. (4) Immune suppression plus aspergillosis group (CTX + IPA): animals were given CTX and infected with A. fumigatus. CTX + IPA group was further subgrouped by the time of posttreatment: CTX + IPA-24 h, -48 h, -72 h, and -96 h subgroups. Regarding to IPA group, the rats were sacrificed after aspergillosis injection 96 h.
CTX (Jiangsu Hengrui Medicine Co., Ltd., China) was injected intraperitoneally at a dose of 200 mg · kg−1· d−1, for 5 consecutive days. Tracheostomy was then performed under systemic anesthesia followed by intratracheal instillation of 0.1 ml conidia solution. Animals were sacrificed at 24 h, 48 h, 72 h, and 96 h after intratracheal instillation of the conidia, respectively. Blood samples were obtained through the heart-punctuation. Part of lung tissue was minced and used for A. fumigatus culture. Part of lung tissue was also fixed with 4% formaldehyde, and sections were stained with hematoxylin and eosin for histology.
CD8+ T-cells and memory CD8+ T-cell count
Mononuclear cells were isolated from blood samples and counted. Cells were then labeled with the following fluorescence-conjugated monoclonal antibodies: anti-Rat CD45 PE, anti-Rat CD8a APC, anti-Rat CD44 PE, and anti-Rat CD62L (eBioscience, San Diego, CA, USA). CD8+ T-cells and memory CD8+ T-cells were sorted by flow cytometry (EPICS-XL, Beckman-Coulter, USA).
Cytokine quantification
Cytokines or proteins of transcription factors were quantified using the enzyme-linked immunosorbent assay kits following the manufacture's instruction. mTOR: cat# ab168538, Abcam, Cambridge, MA, USA. S6K: cat#: ab176665, Abcam, Cambridge, MA, USA. T-bet: cat#: 85-86051, Affymetrix eBioscience, San Diego, CA, USA. EOMES: cat# MBS078844, MyBioSource, San Diego, CA, USA.
Statistical analysis
Data were analyzed by SPSS 18.0 software (SPSS Inc., IBM Corp., Armonk, NY, USA). All the data for the continuous variables in this study were proved to be normal distributions, which are given as mean ± standard deviation (SD). Results for continuous variables that were not normally distributed are given as medians (interquartile ranges) and were compared using non-parametric tests. Student's t-test or analysis of variance (ANOVA) followed by Bonferroni's test was used to determine the statistical significance (P) of differences. Pearson's correlation coefficient was used to analyze the correlation of two parameters. A P < 0.05 was considered as statistically significant.
RESULTS
Laboratory blood test
As shown in Figure 1, total number of white blood cells were significantly decreased in the animals of CTX group [5.8 (5.1–8.2) ×109/L] and CTX + IPA groups [0.18 (0.07–0.24) ×109/L, 0.03 (0.02–0.10) ×109/L, 0.05 (0.03–0.30) ×109/L, 0.06 (0.04–0.13) ×109/L, respectively for 24 h, 48 h, 72 h, 96 h] compared to that of normal control group [5.67 (3.50–9.00) ×109] and Aspergillus fumigatus-infected IPA group [0.1 (0.1–0.4) ×109/L] (P< 0.001). The neutrophils [0.54 (0.26–1.35)×109/L, 3.36 (0.31–3.57) ×109/L, 0.02 (0.01–0.40) ×109/L, 0.04 (0.02–0.06)×109/L, 0.02 (0.01–0.03) ×109/L, 0.02 (0.01–0.08) ×109/L, 0.02 (0.02–0.04) ×109/L, respectively for CON group, CTX group, IPA group, 24 h, 48 h, 72 h, 96 h] and lymphocytes 3.97 (2.02–6.52)×109/L, 2.61 (2.06–3.52) ×109/L, 0.06 (0.05–0.20) ×109/L, 0.06 (0.03–0.08) ×109/L, 0.008 (0.003–0.030)×109/L, 0.006 (0.006–0.014)×109/L, respectively for CON group, CTX group, IPA group, 24 h, 48 h, 72 h, 96 h) were the same as white blood cells. There was no significant difference in blood cell counts among the samples of CTX + IPA collected at 24 h, 48 h, 72 h, or 96 h after infection. Neutrophils were significantly increased while lymphocytes were significantly decreased in IPA group compared to that in normal control group (P < 0.01).
Figure 1.
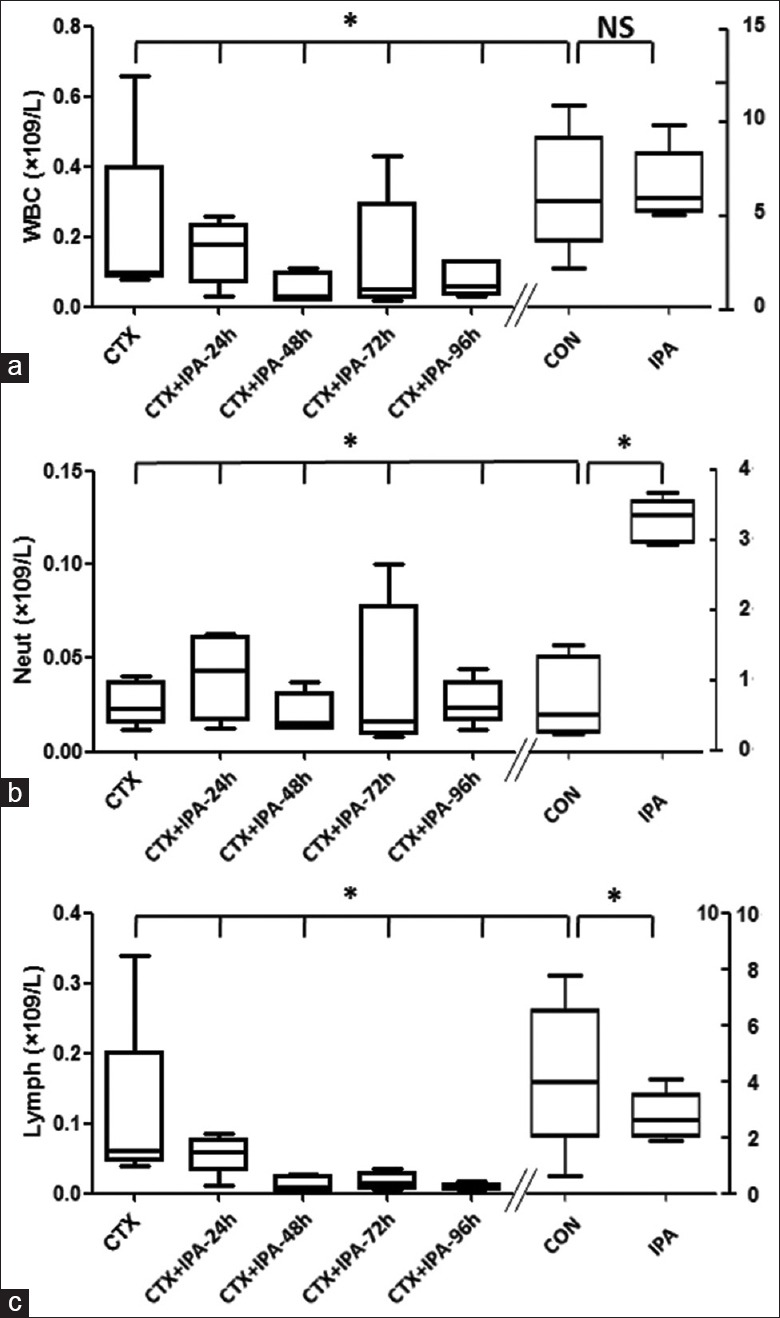
White blood cell, neutrophil, and lymphocytes in the blood of animal models (n = 5 each group). (a) Total white blood cell count. (b) Neutrophil count. (c) Lymphocytes count. Vertical axes: cell number (×109/L). Horizontal axes: Groups *P < 0.05.
Tissue culture and histology
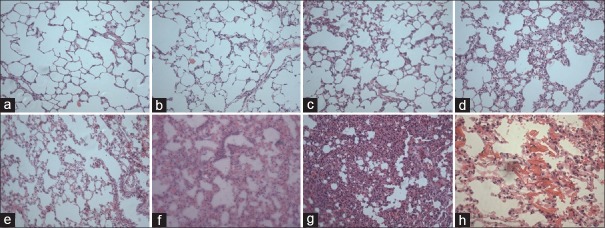
Viability of the conidia was examined by culturing infected lung tissue. Viable A. fumigatus was positively cultured in both IPA and CTX + IPA groups, while it was negative in control and CTX groups (data not shown). Histological examination indicated that lung tissue structure was intact in normal [Figure 2a] and CTX-treated rat lungs [Figure 2b]. In contrast, infiltration of inflammatory cells, blood congestion, and interstitial lung tissue injury was found in the rat lungs of IPA-infected [Figure 2c] or CTX + IPA infection for 24 h [Figure 2d]. At 48 h after CTX + IPA infection, congestion, hemorrhage, and hyphae were seen in the interstitial lung tissue [Figure 2e and 2h]. At 72 h and 96 h after CTX + IPA infection, severe alveolar injury, thickness of interstitial tissue, and alveolar infiltration were further noticed [Figure 2f and 2g, respectively].
Figure 2.
Histology of lung tissue stained with H and E. (a) Control animal. (b) Animals treated with CTX. (c) Animals with IPA. (d) Animals with CTX + IPA for 24 h. (e and h) Animals with CTX + IPA for 48 h. (f) Animals with CTX + IPA for 72 h. (g) Animals with CTX + IPA for 96 h. (h) The mycelium of pulmonary aspergillosis. (a-g: original magnification ×20); (h: original magnification ×40). CTX: Cyclophosphamide; IPA: Invasive pulmonary aspergillosis.
Alteration of CD8+ T-cells and memory CD8+ T-cells
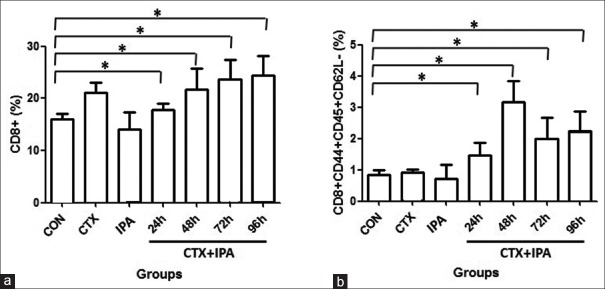
As shown in Figure 3, compared to control (15.95 ± 1.09%) and IPA group (14.07 ± 3.27%), proportion of CD8+ T-cell was significantly increased in the CTX group (21.14 ± 1.91%) and subgroups of CTX + IPA at 24h (17.85 ± 1.16%), 48 h (21.71 ± 4.11%), 72 h (23.64 ± 3.73%), and 96 h (24.41 ± 3.70%) animals (P < 0.05). In addition, CD8+ T-cell was significantly higher in the subgroups of CTX + IPA-72 h and CTX + IPA-96 h than that of CTX + IPA-24 h subgroup (P< 0.05). Similarly, compared to control group (0.85 ± 0.13%) and IPA group (0.70 ± 0.44%), proportion of memory CD8+ T-cells was significantly increased in the subgroups of CTX + IPA-24h (1.45 ± 0.40%), -48 h (3.16 ± 0.69%), -72 h (200 ± 0.66%), and -96 h (2.25 ± 0.61%) animals (P < 0.05).
Figure 3.
Comparison of CD8+ T-cells (a) and memory CD8+ T-cells (b). CD8+ T-cells and memory CD8+ T-cells were sorted by flow cytometry as described in the methods. Vertical axes: percent of CD8+ T-cells (%) or memory CD8+ T-cells (%). Horizontal axes: Groups *P < 0.05.
Alteration of serum mTOR, S6K proteins, eomesodermin, and T-bet proteins
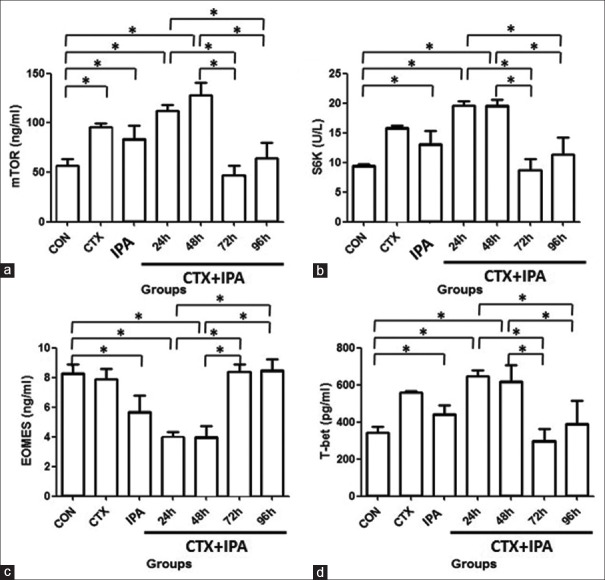
As shown in Figure 4a and 4b, serum level of mTOR and S6K was significantly higher in the groups of CTX-treated (99.13 ± 3.53 ng/ml; 15.73 ± 0.33 U/L) and IPA-infected (83.72 ± 13.44 ng/ml; 13.13 ± 2.18 U/L) rats than that of control (56.53 ± 5.99 ng/ml; 9.5 ± 0.23 U/L) group (P < 0.001). At early stage of CTX + IPA, i.e., 24 h (112.24 ± 5.91 ng/ml; 19.53 ± 0.82 U/L) and 48 h (128.34 ± 12.5 ng/ml; 19.63 ± 0.94 U/L), serum level of mTOR and S6K was higher than that of control (P < 0.001); 72 h (46.68 ± 10.05 ng/ml; 8.71 ± 1.93 U/L) and 96 h (64.65 ± 14.23 ng/ml; 11.32 ± 2.45 U/L) (at later stage of infection), however, serum level of mTOR and S6K was significantly decreased (P < 0.05) and close to the level of control group.
Figure 4.
Serum levels of mTOR (a), S6K (b), EOMES (c), and T-bet (d). Serum was harvested, and protein levels of mTOR, S6K, EOMES, and T-bet were quantified by enzyme-linked immunosorbent assay as described in the methods. Vertical axes: Amount of mTOR (ng/ml) or S6K (U/L) or EOMES (ng/ml) or T-bet (pg/ml). Horizontal axes: Groups *P < 0.05. EOMES: Eomesodermin.
As shown in Figure 4c and 4d, compared to control group (8.38 ± 0.51 ng/ml; 340.03 ± 26.32 pg/ml), serum level of EOMES (P < 0.001) and T-bet (P < 0.05) was significantly higher in the groups of IPA (5.69 ± 1.07 ng/ml; 443.03 ± 48.1 pg/ml) and CTX + IPA at 24 h (3.97 ± 0.35 ng/ml; 647 ± 33.03 pg/ml) and 48 h (4.00 ± 0.74 ng/ml; 619.23 ± 87.44 pg/ml). Serum level of EOMES and T-bet in the groups of CTX + IPA-72 h (8.38 ± 0.54 ng/ml; 299.64 ± 63.07 pg/ml) and -96 h (8.4 ± 0.7 ng/ml; 398.02 ± 109.22 pg/ml), however, was significantly lower than that in the groups of CTX + IPA-24 h or -48 h (P < 0.001).
Correlation between mTOR or S6K and eomesodermin or T-bet
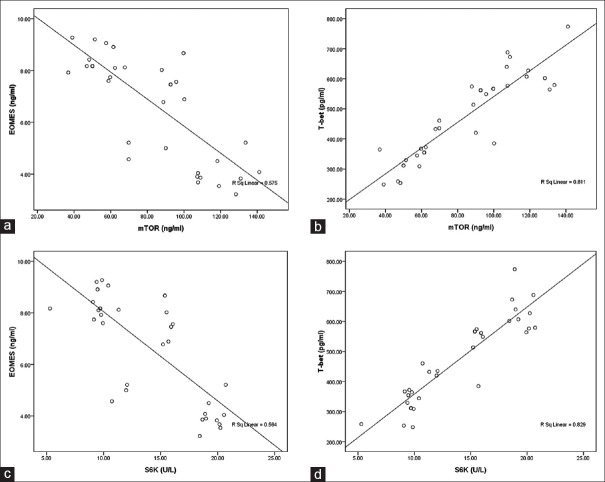
Pearson's correlation analysis indicated serum mTOR was significantly correlated with T-bet (r = 0.901, P < 0.001), but negatively and significantly correlated with EOMES [r = −0.758, P < 0.001, Figure 5a and 5b]. Similarly, serum level of S6K was significantly correlated with T-bet (r = 0.91, P < 0.001), but negatively and significantly correlated with EOMES [r = −0.751, P < 0.001, Figure 5c and 5d].
Figure 5.
Correlation between mTOR, S6K, EOMES, and T-bet. (a) mTOR and EOMES. (b) mTOR and T-bet. (c) S6K and EOMES. (d) S6K and T-bet. EOMES: Eomesodermin.
DISCUSSION
In the current study, we demonstrated that CTX-induced immune suppression resulted in significant decrease of lymphocytes. Ratio of CD8+ T-cells memory CD8+ T-cells was significantly increased in early stage, but decreased in the late stage of A. fumigatus-infection in the animals of CTX-induced immune suppression (CTX + IPA). Furthermore, serum level of mTOR and S6K was also altered in consistence with the alteration of CD8+ T-cell number and was significantly correlated with the level of T-bet or negatively but significantly correlated with EOMES.
CD8+ T-cells play an important role in adaptive immunity. Following infection, CD8+ T-cells undergo proliferation and differentiation under stimulation of antigens, co-stimulators and cytokines such as interferon-γ.[13] Differentiation of CD8+ T-cell is regulated by several transcription factors and signal transduction pathways. Ratio and number of different phenotypes of CD8+ T-cells determine the function of CD8+ T-cells.[14] It has been reported that different pathogens may induce CD8+ T-cells into different phenotypes, which closely related with host defense mechanism in the secondary infection.[15,16] Immunocompromised patient is highly in risk to severe infection and secondary infection. It is crucial for these patients to have an immune response in the early stage of infection in order to develop adaptive immunity in the late phase of infection. Memory CD8+ T-cells are believed to play an important role in the development of adaptive immunity. Consistent with this concept, the current study demonstrated that CD8+ T-cells were significantly increased following the immune suppression by CTX and A. fumigatus -infection, especially memory CD8+ T-cells were significantly increased 48 h after fungal infection in the immunocompromised animals, suggesting resident CD8+ T-cells play an important role in forming adaptive immunity in response to a fungal infection. At 72 h and 96 h after fungal infection, however, ratio and number of CD8+ T-cells were gradually decreased, indicating re-population of effector cells from memory CD8+ T may require longer time after the contraction of early stage effector cells.
mTOR plays an important role in regulating T-cell immune function. Studies indicated that mTOR contribute to proliferation and differentiation of T-cells in response to antigen stimulation[17,18] and that mTOR modulates expression of TCR, CD28, and programmed death 1, by which mechanism, mTOR regulates antigen recognition and adaptive immune reaction of T-cells.[19,20] In the current study, the serum level of mTOR and S6K was significantly increased in the rats treated with CTX with or without fungal infection (CTX + IPA-24 h and -48 h), suggesting mTOR signal transduction pathway was activated in the animals with immune suppression and fungal infection.
Studies have demonstrated that several intracellular signal transduction pathways and transcription factors including pathways of tyrosine kinase, anti-inflammatory signal, and mitochondrial apoptosis involve in regulating T-cell differentiation.[19,20,21,22,23,24] In addition to these signal transduction pathways, it is also likely that mTOR involve in immune activation and regulation following infection. In this content, mTOR signaling pathway also acts as an important integrator of nutrient-sensing pathways, which in turn control and coordinate the metabolism of the cells according to this need to proliferate or functionally differentiate. Interestingly, the current study found that serum level of mTOR and S6K was increased in early stage of fungal infection (24 h and 48 h), but significantly decreased at late stage (72 h and 96 h), suggesting mTOR and S6K may be involved in re-populating effector T-cells from memory CD8+ T-cells after the contraction of early stage effector cells. Mechanism of this retraction of mTOR and S6K level, however, remains to be further defined.
T-bet and EOMES are a family of T-box and regulate effector T-cell differentiation. T-bet is known to regulate Th1 cell differentiation and inflammatory cytokine production, while EOMES involves in modulating CD8+ T differentiation in the late phase of infection.[14] Similar to mTOR, these two transcription factors may also be involved in regulating immune response following Aspergillus infection. There had also been identified that mTOR as the central regulator of transcriptional programs that determine effector and/or memory cell fates in CD8+ T-cells.[12] The findings of the current study demonstrated that T-bet was significantly increased in the immunocompromised animals with a fungal infection, especially at 48 h after infection. In contrast, EOMES was significantly decreased after 24 h and 48 h of infection. Furthermore, we found that mTOR and S6K were significantly and negatively correlated with EOMES, while they were significantly correlated with T-bet. These findings suggested that mTOR might modulate immune cell differentiation through the transcription factors T-bet and EOMES.
There are some limitations of our study. First, although we found that mTOR/S6K levels positively correlated with T-bet and negative correlated with the EOMES, the mechanism of this phenomenon remains unclear. It is still unknown whether we can regulate the activity of molecular signaling pathways to establish the immunity adaption. It is necessary to reveal that the effect of inhibiting the corresponding mTOR/S6K pathway on CD8 memory cells. Second, the detected CD8 memory T-cells are smaller than we originally expected, which may be due to the immunosuppressive animal model or other specific treatment for this study. However, the clear mechanism needs further be studied.
Taken together, serum levels of mTOR, S6K, T-bet, and EOMES were significantly altered in the immunocompromised rats with IPA. Consistent with the alteration of these molecules, number and proportion of memory CD8+ T-cells were also significantly altered in a time-dependent manner. These findings indicated that mTOR modulate immune cell proliferation and differentiation through EOMES and T-bet in the immunocompromised animals with IPA. Whether the level of mTOR and its downstream components is altered in CD8+ T-cells in the animal model of the current study, however, remains to be further investigated.
Financial support and sponsorship
The work was supported by the Beijing Municipal Natural Science Foundation (No. 7152119) and Special Project Funds for Clinical Research of Chinese Medical Association (No. 14030250562).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–65. doi: 10.1128/CMR.00055-08. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nucci M, Marr KA. Emerging fungal diseases. Clin Infect Dis. 2005;41:521–6. doi: 10.1086/432060. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 3.Alexander-Miller MA. High-avidity CD8+T cells: Optimal soldiers in the war against viruses and tumors. Immunol Res. 2005;31:13–24. doi: 10.1385/IR:31:1:13. doi: 10.1385/IR:31:1:13. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N, Bevan MJ. CD8(+) T cells: Foot soldiers of the immune system. Immunity. 2011;35:161–8. doi: 10.1016/j.immuni.2011.07.010. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefrançois L, Obar JJ. Once a killer, always a killer: From cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–18. doi: 10.1111/j.0105-2896.2010.00895.x. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui N, Wang H, Long Y, Liu D. CD8(+) T-cell counts: An early predictor of risk and mortality in critically ill immunocompromised patients with invasive pulmonary aspergillosis. Crit Care. 2013;17:R157. doi: 10.1186/cc12836. doi: 10.1186/cc12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui N, Wang H, Long Y, Liu D. Altered CD8(+) T-cell counts as an early predictor of prognosis in critically ill immunocompromised patients with invasive pulmonary aspergillosis. Chin Med J (Engl) 2014;127:36–42. doi: 10.3760/cma.j.issn.0366-6999.20131095. [PubMed] [Google Scholar]
- 8.Cobbold SP. The mTOR pathway and integrating immune regulation. Immunology. 2013;140:391–8. doi: 10.1111/imm.12162. doi: 10.1111/imm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 10.McLane LM, Banerjee PP, Cosma GL, Makedonas G, Wherry EJ, Orange JS, et al. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J Immunol. 2013;190:3207–15. doi: 10.4049/jimmunol.1201556. doi: 10.4049/jimmunol.1201556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaëlsson J, et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+T cells in HIV infection. PLoS Pathog. 2014;10:e1004251. doi: 10.1371/journal.ppat.1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoycheva D, Deiser K, Stärck L, Nishanth G, Schlüter D, Uckert W, et al. IFN-g regulates CD8+memory T cell differentiation and survival in response to weak, but not strong, TCR signals. J Immunol. 2015;194:553–9. doi: 10.4049/jimmunol.1402058. doi: 10.4049/jimmunol.1402058. [DOI] [PubMed] [Google Scholar]
- 14.Lefrançois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–23. doi: 10.1038/nri1866. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 15.Plumlee CR, Sheridan BS, Cicek BB, Lefrançois L. Environmental cues dictate the fate of individual CD8+T cells responding to infection. Immunity. 2013;39:347–56. doi: 10.1016/j.immuni.2013.07.014. doi: 10.1016/j.immuni.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–105. doi: 10.4049/jimmunol.166.2.1097. doi: 10.4049/?jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–77. doi: 10.1038/ni.1743. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+regulatory T cells. Nat Immunol. 2010;11:618–27. doi: 10.1038/ni.1884. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 19.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–72. doi: 10.1038/nm.3105. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. doi: 10.1016/j.cell.2013.05.016. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Gräf P, et al. Disparate individual fates compose robust CD8+T cell immunity. Science. 2013;340:630–5. doi: 10.1126/science.1235454. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach C, Rohr JC, Perié L, van Rooij N, van Heijst JW, Velds A, et al. Heterogeneous differentiation patterns of individual CD8+T cells. Science. 2013;340:635–9. doi: 10.1126/science.1235487. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 23.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winstead CJ, Weaver CT. Dwelling on T cell fate decisions. Cell. 2013;153:739–41. doi: 10.1016/j.cell.2013.04.026. doi: 10.1016/j.cell.2013.04.026. [DOI] [PubMed] [Google Scholar]