Abstract
Background:
Acute lung injury (ALI) is a common complication of sepsis that is associated with high mortality. Intracellular Ca2+ overload plays an important role in the pathophysiology of sepsis-induced ALI, and cyclic adenosine diphosphate ribose (cADPR) is an important regulator of intracellular Ca2+ mobilization. The cluster of differentiation 38 (CD38)/cADPR pathway has been found to play roles in multiple inflammatory processes but its role in sepsis-induced ALI is still unknown. This study aimed to investigate whether the CD38/cADPR signaling pathway is activated in sepsis-induced ALI and whether blocking cADPR-mediated calcium overload attenuates ALI.
Methods:
Septic rat models were established by cecal ligation and puncture (CLP). Rats were divided into the sham group, the CLP group, and the CLP+ 8-bromo-cyclic adenosine diphosphate ribose (8-Br-cADPR) group. Nicotinamide adenine dinucleotide (NAD+), cADPR, CD38, and intracellular Ca2+ levels in the lung tissues were measured at 6, 12, 24, and 48 h after CLP surgery. Lung histologic injury, tumor necrosis factor (TNF)-α, malondialdehyde (MDA) levels, and superoxide dismutase (SOD) activities were measured.
Results:
NAD+, cADPR, CD38, and intracellular Ca2+ levels in the lungs of septic rats increased significantly at 24 h after CLP surgery. Treatment with 8-Br-cADPR, a specific inhibitor of cADPR, significantly reduced intracellular Ca2+ levels (P = 0.007), attenuated lung histological injury (P = 0.023), reduced TNF-α and MDA levels (P < 0.001 and P = 0.002, respectively) and recovered SOD activity (P = 0.031) in the lungs of septic rats.
Conclusions:
The CD38/cADPR pathway is activated in the lungs of septic rats, and blocking cADPR-mediated calcium overload with 8-Br-cADPR protects against sepsis-induced ALI.
Keywords: Acute Lung Injury, Calcium Overload, Cyclic Adenosine Diphosphate Ribose, Sepsis
INTRODUCTION
Acute lung injury (ALI) is among the major causes of mortality in septic patients.[1] Intracellular Ca2+ overload is an important mechanism for sepsis-induced ALI, which causes proinflammatory cytokine release,[2] mitochondrial dysfunction,[3] oxidative stress,[4] and vascular barrier impairment.[5] The cluster of differentiation 38 (CD38)/cyclic adenosine diphosphate ribose (cADPR) pathway is an important regulator of intracellular Ca2+ mobilization.[6] CD38 is the major adenosine diphosphate (ADP)-ribosyl cyclase in mammals and uses nicotinamide adenine dinucleotide (NAD+) to produce ADPR and a small portion of cADPR.[7] cADPR then regulates Ca2+ mobilization by activating calcium-induced calcium release[6] or promotes Ca2+ entry via the transient receptor potential cation channel M2.[8]
The CD38/cADPR pathway has been found to play roles in various physiological and pathological processes including inflammation, cell proliferation, neuronal differentiation, cardiogenesis, Ca2+ sparks in cardiomyocytes, insulin release, and diabetes.[6] Recently, more attention was paid to the important roles of this pathway in multiple inflammatory processes. The CD38/cADPR pathway was found to be involved in the adhesion and chemotaxis of immune cells during inflammation.[9,10] Proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and interferon-γ could enhance CD38 expression and ADP-ribosyl cyclase activity in human airway smooth muscle cells.[11,12] CD38 expression was significantly upregulated in IL-1β- or human immunodeficiency virus-1-activated human astrocytes, and CD38 knockdown could reduce proinflammatory cytokine production.[13] Blocking the CD38/cADPR pathway with 8-bromo-cyclic adenosine diphosphate ribose (8-Br-cADPR), which is a specific inhibitor of cADPR, could reduce intracellular Ca2+, [10,14,15] reactive oxygen species (ROS),[16,17] proinflammatory cytokines,[14,15] and attenuate tissue injury such as neuroinflammation,[18] cardiac ischemia/reperfusion injury[19] and airway hyper-responsiveness.[11,20] However, the role of the CD38/cADPR pathway in sepsis-induced ALI is still unknown. In this study, we looked into the role of this pathway in sepsis-induced ALI and evaluated the effect of blocking cADPR-mediated calcium overload on lung injury.
METHODS
Materials and reagents
Alcohol dehydrogenase, NAD, ADP-ribosyl cyclase, nicotinamide, resazurin, diaphorase, bovine serum albumin (BSA), 3-4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), bicine, riboflavin 5’-mono-phosphate (FMN), phenazine ethosulfate (PES), ethylenediaminetetraacetic acid (EDTA)-Na, alcohol dehydrogenase (ADH), and 8-Br-cADPR were purchased from Sigma-Aldrich (St. Louis, MO, USA). cADPR and CD38 antibody were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Phosphodiesterase I was obtained from Worthington Biochemicals (Lakewood, CA, USA). Fluo-3/acetoxymethyl ester (Fluo-3/AM) was purchased from Solarbio (Beijing, China), and the other reagents were purchased from North Bell (Changsha, China).
Rat model of sepsis
This study was approved by the Ethical Committee of the Laboratory Animal Research Center of Central South University and complied with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Humans for studies involving experimental animals. Male Sprague-Dawley rats (SCXK [Xiang] 2011-0002) with a mean weight of 200 g were used. Rat models of sepsis were established by cecal ligation and puncture (CLP) as previously described.[21] Briefly, rats were fasted overnight before the day of surgery and anesthetized by intraperitoneally injecting 0.3 ml/100 g of 10% chloral hydrate. The lower abdomen was incised and the cecum was ligated with 2-0 surgical silk, pierced with an 18-gauge needle, gently compressed until fecal matter was extruded, and returned to the abdominal cavity. The abdomen was then completely closed with 2-0 surgical silk. About 10 ml of normal saline solution were administered subcutaneously immediately after surgery for volume resuscitation. Rats were randomly divided into the sham group, the CLP group (including four different time points groups: 6, 12, 24, and 48 h), and the CLP+ 8-Br-cADPR group (n = 12). The CLP+ 8-Br-cADPR group was injected with 1 µmol/kg 8-Br-cADPR dissolved in normal saline solution through the tail vein immediately after CLP surgery.[22] Rats were killed after anesthesia, and the lungs were collected and frozen in liquid nitrogen or were perfused and fixed for histology studies.
Nicotinamide adenine dinucleotide assay
Intracellular NAD+ levels were measured by an enzyme cycling method as described previously.[22,23] Briefly, lung tissues were pulverized into a powder in liquid nitrogen, resuspended in 100 mmol/L HCl, put on ice for 10 min, and then centrifuged at 12,000 ×g. The acid-soluble fractions were neutralized with 100 mmol/L KOH. The samples were mixed with 0.83 mg/ml BSA, 100 mmol/L bicine, 4.17 mmol/L EDTA-Na, 0.50 mol/L ethanol, 0.42 mmol/L MTT, 1.66 mmol/L PES, and 2 U ADH in 96 well plates. The 96-well plates were incubated at 30°C for 30 min in the dark. Absorbances were measured at 550 nm, and NAD+ concentrations were calculated according to the standard curves of purified NAD.
Cyclic adenosine diphosphate ribose assay
cADPR levels were measured by an enzyme cycling method as described previously.[22,24] Briefly, lung tissues were pulverized into a powder in liquid nitrogen, extracted with 0.6 mol/L perchloric acid at 4°C, mixed with 1:4 volume of an organic solution of 1,1,2-trichlorotrifluoroethane and tri-n-octylamine (3:1), vortexed for 1 min, and put on ice until the aqueous phase separated from the organic phase. The aqueous layer was collected and adjusted to a pH of 8 with 20 mmol/L sodium phosphate. The samples were then mixed with 0.0625 U/ml NADase in 2.5 mmol/L MgCl2, 12.5 U/ml alkaline phosphatase, 0.44 U/ml nucleotide pyrophosphatase, and 20 mmol/L sodium phosphate, pH 8.0, and incubated overnight at 37°C. After filtering with Multiscreen Assay System filtration plates (Millipore, Billerica, MA, USA), 0.1 ml cADPR or the samples and 50 µl of a reagent containing 30 mmol/L nicotinamide, 0.3 µg/ml ADP-ribosyl cyclase, and 100 mmol/L sodium phosphate, pH 8.0, were mixed and incubated for 15 min at room temperature. The samples were then mixed with 100 µg/ml ADH, 10 µmol/L FMN, 20 µmol/L resazurin, 10 mmol/L nicotinamide, 10 µg/ml diaphorase, 2% ethanol, 0.1 mg/ml BSA, and 100 mmol/L sodium phosphate, pH 8.0, and incubated in the dark for 4 h at room temperature. Fluorescence was measured with a fluorescence plate reader at an excitation of 544 nm and an emission of 590 nm. cADPR concentrations were calculated according to the standard curves of purified cADPR.
Western blotting
Lung tissues were lysed with RIPA buffer, sonicated on ice, and centrifuged at 200 ×g for 20 min at 4°C. The samples were then boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer for 5 min, electrophoresed on 10% SDS-PAGE, and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Membranes were probed with primary antibodies overnight at 4°C and then with HRP-conjugated secondary antibodies. Bands were visualized with the enhanced chemiluminescence prime (GE Healthcare, Marlborough, MA, USA).
Calcium assay
Intracellular Ca2+ levels were measured with the Ca2+ indicator Fluo-3/AM as described previously.[25] Lungs were chopped into small pieces in cold D-Hanks solution and digested with 0.25% trypsin-EDTA at 37°C for 20 min with shaking. The undigested tissue was removed through a 200 µm mesh nylon. Isolated cells were incubated with 5 µmol/L Fluo-3/AM for 30 min at 37°C in the dark and then washed twice with cold D-Hanks solution. Green fluorescence at 526 nm was measured by flow cytometry (Beckman Coulter, Brea, CA, USA).
Tissue fixation and sectioning for hematoxylin-eosin staining
Rats were killed after anesthesia and perfused through the left ventricles with 200 ml normal saline followed by 100 ml of 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS). Lungs were removed, fixed in 4% paraformaldehyde, 0.1% diethylprocarbonate, and 0.1 mol/L PBS pH 7.0–7.6 for 30 min, dehydrated with increasing concentrations of ethanol for 30 min, cleared in xylene twice for 30 min, embedded in paraffin wax at 55°C with a copper mold, sectioned at a thickness of 5 µm, and mounted on slides. Slides were incubated at 60°C overnight, dewaxed in xylene twice for 2 min, serially rehydrated in 100%, 95%, 90%, 85%, and 75% ethanol twice for 5 min, washed with PBS, and stained with hematoxylin-eosin.[22,26]
Histological injury scores
Lung injury scores were determined according to previously established criteria[27] with slight modifications. The degree of edema, alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, atelectasis, necrosis, and hyaline membrane formation was scored as follows: 0 = none; 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; and 4 = 76–100%.
Tumor necrosis factor-α, malondialdehyde, and superoxide dismutase activity assays
Malondialdehyde (MDA), superoxide dismutase (SOD) activity, and TNF-α levels were measured with a thiobarbituric acid assay kit (Solarbio, Beijing, China), a xanthine and xanthine oxidase reaction system, and an enzyme-linked immunosorbent assay kit (Neobioscience, Shenzhen, China), respectively, following manufacturer's instructions. Absorbances at 532 nm (MDA) and 450 nm (SOD and TNF-α) were measured with a microplate reader (Bio-Tek, Shanghai, China). The results were calculated according to the manufacturer's instructions and normalized to total protein concentration.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Differences between groups were analyzed by one-way analysis of variance (ANOVA) and post hoc with SPSS 19.0 (International Business Machines Corp., Armonk, NY, USA). A value of P < 0.05 was considered significant.
RESULTS
Nicotinamide adenine dinucleotide, cyclic adenosine diphosphate ribose, cluster of differentiation 38, and intracellular calcium levels in the lungs of septic rats increased significantly at 24 h after cecal ligation and puncture surgery
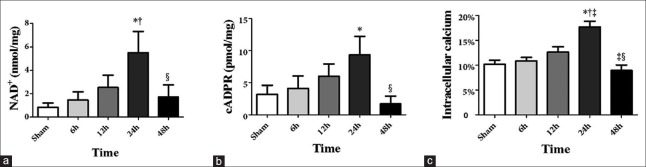
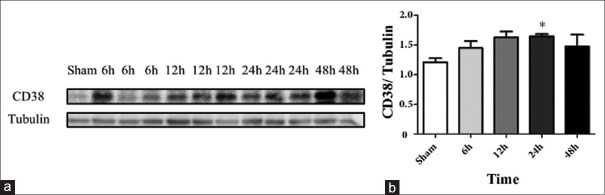
NAD+, cADPR, and intracellular Ca2+ levels in the lungs of septic rats increased slightly as early as 6 h after CLP surgery, peaked at 24 h, and returned to baseline levels by 48 h [Figure 1]. CD38 expression was also significantly upregulated at 24 h after CLP surgery (P = 0.046) [Figure 2].
Figure 1.
NAD+, cADPR, and Ca2+ levels in the lungs of septic rats (a-c). *P < 0.05 compared to the sham group. †P < 0.05 compared to 6 h. ‡P < 0.05 compared to 12 h. §P < 0.05 compared to 24 h. NAD: Nicotinamide adenine dinucleotide; cADPR: Cyclic adenosine diphosphate ribose.
Figure 2.
CD38 expression in the lungs of septic rats (a-b). (a) Western blotting of CD38 and tubulin in the lungs of septic rats. (b) Densitometry of Western blotting of CD38 and tubulin (n = 3). *P < 0.05 compared to the sham group. CD38: Cluster of differentiation 38.
Intracellular calcium overload was inhibited by 8-bromo-cyclic adenosine diphosphate ribose in the lungs of septic rats
Rats were divided into the sham group, the CLP group, and the CLP+ 8-Br-cADPR group. The intracellular Ca2+ level in the lungs of the CLP group was significantly higher than that in the sham group (P = 0.006). 8-Br-cADPR treatment significantly reduced intracellular Ca2+ levels in the lungs of septic rats (P = 0.007) [Figure 3].
Figure 3.
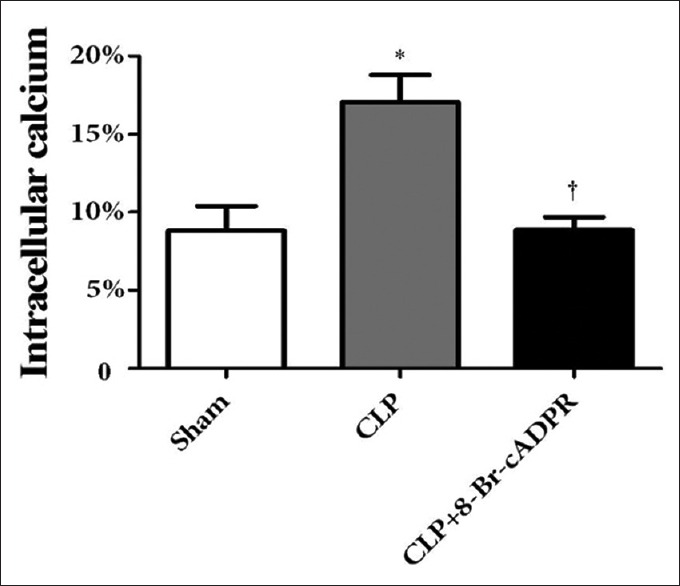
Effect of 8-Br-cADPR on intracellular Ca2+ levels (n = 3). *P < 0.05 compared to the sham group. †P < 0.05 compared to the CLP group. 8-Br-cADPR: 8-bromo-cyclic adenosine diphosphate ribose; CLP: Cecal ligation and puncture.
Lung injury in septic rats was attenuated with 8-bromo-cyclic adenosine diphosphate ribose
In the sham group, there was slight interstitial edema and inflammatory cell infiltration, but the structural of the alveolus was preserved [Figure 4a]. In contrast, in the CLP group, there was obvious alveolar/interstitial edema and inflammatory cell infiltration [Figure 4b]. In the CLP+ 8-Br-cADPR group, alveolar/interstitial edema was attenuated, and inflammatory cell infiltration was reduced compared with the CLP group [Figure 4c]. Histological injury scores revealed significant differences between the CLP and sham groups (P = 0.002), and significant differences between the CLP and CLP+ 8-Br-cADPR groups (P = 0.023), which indicated that treatment with 8-Br-cADPR attenuated sepsis-induced ALI [Figure 4d].
Figure 4.
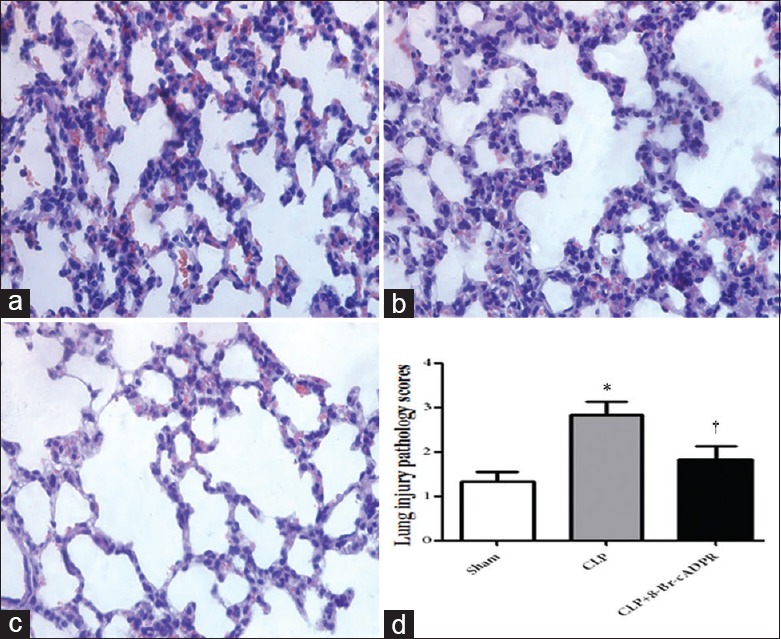
Representative Hematoxylin and Eosin (HE) staining images for the sham group (a), CLP group (b), and CLP+ 8-Br-cADPR group (c). (d) Histologic lung injury scores (n = 6). *P < 0.05 compared to the sham group. †P < 0.05 compared to the CLP group (Original magnification ×400). 8-Br-cADPR: 8-bromo-cyclic adenosine diphosphate ribose; CLP: Cecal ligation and puncture.
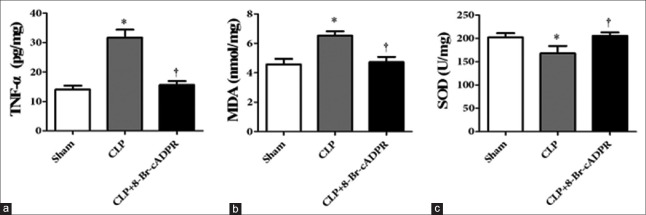
Tumor necrosis factor-α and malondialdehyde levels were reduced, and superoxide dismutase activity was recovered by 8-bromo-cyclic adenosine diphosphate ribose in the lungs of septic rats
TNF-α and MDA levels were significantly increased (P < 0.001 and P = 0.001, respectively), and SOD activity was significantly decreased (P = 0.048) in the lungs of the CLP group compared with the sham group. Treatment with 8-Br-cADPR reduced TNF-α and MDA levels (P < 0.001 and P = 0.002, respectively) and recovered SOD activity (P = 0.031) in the lungs of septic rats [Figure 5].
Figure 5.
TNF-α, MDA, and SOD activities in the lungs of septic rats (n = 6) (a-c). *P < 0.05 compared to the sham group. †P < 0.05 compared to the CLP group. TNF: Tumor necrosis factor; MDA: Malondialdehyde; SOD: Superoxide dismutase; CLP: Cecal ligation and puncture; 8-Br-cADPR: 8-bromo-cyclic adenosine diphosphate ribose.
DISCUSSION
Recent studies have reported that the CD38/cADPR signaling pathway is involved in airway inflammation and hyper-responsiveness.[11,12,20,28,29] When stimulated by cytokines such as TNF-α or IL-13, CD38 expression, and ADP-ribosyl cyclase activity are significantly enhanced in human airway smooth muscle cells.[11,12,20,28] CD38 knockdown or cADPR inhibition with 8-Br-cADPR can attenuate airway hyper-responsiveness.[11,28] Possible mechanisms by which the CD38/cADPR pathway is involved in inflammation include its regulatory effect on calcium homeostasis,[6,8,29] the adhesion and chemotaxis of immune cells,[9,30] the release of proinflammatory cytokines and chemokines,[12,18] and the production of ROS.[16,17] However, little is known about its function in sepsis-induced ALI. In our previous study, the NAD+/CD38/cADPR/Ca2+ signaling pathway was found to be activated in the heart, liver, and kidneys of septic rats, and blocking this pathway with 8-Br-cADPR protected these organs against sepsis-induced injury.[22] Thus, we hypothesized that this pathway also is activated and plays important roles in sepsis-induced ALI. In this study, we found that NAD+, CD38, cADPR, and intracellular Ca2+ levels were all significantly increased in the lungs of septic rats at 24 h after CLP surgery.
We then administered 8-Br-cADPR by intravenous injection to septic rats immediately after CLP surgery. The dose was chosen according to our previous study.[22] We found that intracellular Ca2+ overload was significant in the lungs of septic rats, and treatment with a specific inhibitor of cADPR, 8-Br-cADPR, effectively inhibited Ca2+ overload, which indicated that cADPR plays a major role in sepsis-induced calcium overload. Treatment with 8-Br-cADPR significantly attenuated lung injury, reduced TNF-α and MDA levels, and recovered SOD activity in the lungs of septic rats, which suggest that blocking cADPR-mediated calcium overload protected septic rats from sepsis-induced ALI.
Recent studies have reported many new potential therapies for sepsis. For example, Toner et al.[31] reported that aspirin could attenuate sepsis and acute respiratory distress syndrome by inhibiting cyclooxygenase, nuclear factor kappa B, nitric oxide, and lipoxin production. Low-dose exogenous carbon monoxide exposure protected against sepsis in animal models by inhibiting inflammatory responses and promoting host defense mechanism.[32]β-blockers could help maintain a favorable hemodynamic profile, reduce proinflammatory cytokines, and improve survival in clinical and animal studies.[33,34] Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, could attenuate cytokine levels and improve survival in CLP mice models.[35] Our study found that 8-Br-cADPR is also a new potential antiseptic agent that reduces intracellular calcium overload, TNF-α production, and oxidative stress.
Our study had some limitations. First, antibiotics were not administered as they would have been in the current clinical setting. Second, we did not examine the effects of 8-Br-cADPR alone, and we detected only limited effects of 8-Br-cADPR in septic rats, for example, we only measured TNF-α as a representative proinflammatory cytokine, which has been reported to have the greatest effect on the CD38/cADPR pathway in airway hyper-responsiveness.[11,28] The other functions of 8-Br-cADPR, its roles in other organs, its safety and toxicology require further study. Third, as the samples used for the test were collected from rat lung tissues, the data presented relatively larger variance compared to cell samples.
In conclusion, we found that the CD38/cADPR pathway was activated in the lungs of septic rats, and blocking cADPR-mediated calcium overload with 8-Br-cADPR protected against sepsis-induced ALI.
Financial support and sponsorship
This study was funded by the National Natural Science Foundation of China (No. 81501649, No. 81401099), and the Science and Technology Project of Hunan Province (No. 14JJ7010).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Gill SE, Rohan M, Mehta S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo. Respir Res. 2015;16:109. doi: 10.1186/s12931-015-0266-7. doi: 10.1186/s12931-015-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Németh ZH, Haskó G, Szabó C, Salzman AL, Vizi ES. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull. 1998;46:257–61. doi: 10.1016/s0361-9230(98)00005-7. doi: 10.1016/S0361-9230(98)00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun SM, Marechal X, Montaigne D, Bouazza Y, Decoster B, Lancel S, et al. Prevention of endotoxin-induced sarcoplasmic reticulum calcium leak improves mitochondrial and myocardial dysfunction. Crit Care Med. 2008;36:2590–6. doi: 10.1097/CCM.0b013e3181844276. doi: 10.1097/CCM.0b013e3181844276. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Bowling WM, Karl IE, Osborne DF, Flye MW. Calcium antagonists inhibit oxidative burst and nitrite formation in lipopolysaccharide stimulated rat peritoneal macrophages. Shock. 1997;8:170–8. doi: 10.1097/00024382-199709000-00004. doi: 10.1097/00024382-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123:887–902. doi: 10.1172/JCI65647. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei W, Graeff R, Yue J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J Biol Chem. 2014;5:58–67. doi: 10.4331/wjbc.v5.i1.58. doi: 10.4331/wjbc.v5.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graeff R, Liu Q, Kriksunov IA, Hao Q, Lee HC. Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J Biol Chem. 2006;281:28951–7. doi: 10.1074/jbc.M604370200. doi: 10.1074/jbc.M604370200. [DOI] [PubMed] [Google Scholar]
- 8.Yu PL, Zhang ZH, Hao BX, Zhao YJ, Zhang LH, Lee HC, et al. A novel fluorescent cell membrane-permeable caged cyclic ADP-ribose analogue. J Biol Chem. 2012;287:24774–83. doi: 10.1074/jbc.M111.329854. doi: 10.1074/jbc.M111.329854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–32. doi: 10.1016/B978-0-12-385526-8.00010-2. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partida-Sánchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–16. doi: 10.1038/nm1101-1209. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J. 2003;17:452–4. doi: 10.1096/fj.02-0450fje. doi: 10.1096/fj.02-0450fje. [DOI] [PubMed] [Google Scholar]
- 12.Sathish V, Thompson MA, Sinha S, Sieck GC, Prakash YS, Pabelick CM. Inflammation, caveolae and CD38-mediated calcium regulation in human airway smooth muscle. Biochim Biophys Acta. 2014;1843:346–51. doi: 10.1016/j.bbamcr.2013.11.011. doi: 10.1016/j.bbamcr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kou W, Banerjee S, Eudy J, Smith LM, Persidsky R, Borgmann K, et al. CD38 regulation in activated astrocytes: Implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res. 2009;87:2326–39. doi: 10.1002/jnr.22060. doi: 10.1002/jnr.22060. [DOI] [PubMed] [Google Scholar]
- 14.Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, et al. Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol. 2007;179:7827–39. doi: 10.4049/jimmunol.179.11.7827. doi: 10.4049/jimmunol.179.11.7827. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S, Walseth TF, Borgmann K, Wu L, Bidasee KR, Kannan MS, et al. CD38/cyclic ADP-ribose regulates astrocyte calcium signaling: Implications for neuroinflammation and HIV-1-associated dementia. J Neuroimmune Pharmacol. 2008;3:154–64. doi: 10.1007/s11481-008-9105-7. doi: 10.1007/s11481-008-9105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D, Elner SG, Chen X, Field MG, Petty HR, Elner VM. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:6026–34. doi: 10.1167/iovs.10-7023. doi: 10.1167/iovs.10-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Jin S, Yi F, Xia M, Dewey WL, Li PL. Local production of O2- by NAD(P)H oxidase in the sarcoplasmic reticulum of coronary arterial myocytes: CADPR-mediated Ca2+regulation. Cell Signal. 2008;20:637–44. doi: 10.1016/j.cellsig.2007.11.013. doi: 10.1016/j.cellsig.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe CU, Lardong K, Gelderblom M, Ludewig P, Leypoldt F, Koch-Nolte F, et al. CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia. PLoS One. 2011;6:e19046. doi: 10.1371/journal.pone.0019046. doi: 10.1371/journal.pone.0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie GH, Rah SY, Yi KS, Han MK, Chae SW, Im MJ, et al. Increase of intracellular Ca(2+) during ischemia/reperfusion injury of heart is mediated by cyclic ADP-ribose. Biochem Biophys Res Commun. 2003;307:713–8. doi: 10.1016/s0006-291x(03)01240-3. doi: 10.1016/S0006-291X(03)01240-3. [DOI] [PubMed] [Google Scholar]
- 20.Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+entry by CD38 in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L378–85. doi: 10.1152/ajplung.00394.2007. doi: 10.1152/ajplung.00394.2007. [DOI] [PubMed] [Google Scholar]
- 21.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng QY, Ai ML, Zhang LN, Zou Y, Ma XH, Ai YH. Blocking NAD+/CD38/cADPR/Ca2+pathway in sepsis prevents organ damage. J Surg Res. 2016;201:480–9. doi: 10.1016/j.jss.2015.11.029. doi: 10.1016/j.jss.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Shah GM, Poirier D, Duchaine C, Brochu G, Desnoyers S, Lagueux J, et al. Methods for biochemical study of poly(ADP-ribose) metabolism in vitro and in vivo. Anal Biochem. 1995;227:1–13. doi: 10.1006/abio.1995.1245. doi: 10.1006/abio.1995.1245. [DOI] [PubMed] [Google Scholar]
- 24.Graeff R, Lee HC. A novel cycling assay for cellular cADP ribose with nanomolar sensitivity. Biochem J. 2002;361(Pt 2):379–84. doi: 10.1042/bj3610379. doi: 10.1042/bj3610379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aton E, Renault T, Gagnaire B, Thomas-Guyon H, Cognard C, Imbert N. A flow cytometric approach to study intracellular-free Ca2+in Crassostrea gigas haemocytes. Fish Shellfish Immunol. 2006;20:493–502. doi: 10.1016/j.fsi.2005.06.008. doi: 10.1016/j.fsi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhang LN, Ai YH, Gong H, Guo QL, Huang L, Liu ZY, et al. Expression and role of neuroglobin in rats with sepsis-associated encephalopathy. Crit Care Med. 2014;42:e12–21. doi: 10.1097/CCM.0b013e3182a63b1a. doi: 10.1097/CCM.0b013e3182a63b1a. [DOI] [PubMed] [Google Scholar]
- 27.Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, et al. Prolonged partial liquid ventilation using conventional and high frequency ventilatory techniques: Gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med. 1997;25:1888–97. doi: 10.1097/00003246-199711000-00030. doi: 10.1097/00003246-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 28.Guedes AG, Deshpande DA, Dileepan M, Walseth TF, Panettieri RA, Jr, Subramanian S, et al. CD38 and airway hyper-responsiveness: Studies on human airway smooth muscle cells and mouse models. Can J Physiol Pharmacol. 2015;93:145–53. doi: 10.1139/cjpp-2014-0410. doi: 10.1139/cjpp-2014-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: Role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L773–88. doi: 10.1152/ajplung.00217.2004. doi: 10.1152/ajplung.00217.2004. [DOI] [PubMed] [Google Scholar]
- 30.Lischke T, Heesch K, Schumacher V, Schneider M, Haag F, Koch-Nolte F, et al. CD38 controls the innate immune response against Listeria monocytogenes. Infect Immun. 2013;81:4091–9. doi: 10.1128/IAI.00340-13. doi: 10.1128/IAI.00340-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care. 2015;19:374. doi: 10.1186/s13054-015-1091-6. doi: 10.1186/s13054-015-1091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahira K, Choi AM. Carbon monoxide in the treatment of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1387–93. doi: 10.1152/ajplung.00311.2015. doi: 10.1152/ajplung.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA. 2013;310:1683–91. doi: 10.1001/jama.2013.278477. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 34.Ackland GL, Yao ST, Rudiger A, Dyson A, Stidwill R, Poputnikov D, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med. 2010;38:388–94. doi: 10.1097/CCM.0b013e3181c03dfa. doi: 10.1097/CCM.0b013e3181c03dfa. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Li Y, Liu B, Liu Z, Chong W, Duan X, et al. Novel pharmacologic treatment attenuates septic shock and improves long-term survival. Surgery. 2013;154:206–13. doi: 10.1016/j.surg.2013.04.003. doi: 10.1016/j.surg.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]