Abstract
Background:
The expression of dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) in renal tubular epithelial cells has been thought to be highly correlated with the occurrence of several kidney diseases, but whether it takes place in renal tissues during hemorrhagic shock (HS) is unknown. The present study aimed to investigate this phenomenon and the inhibitory effect of Vitamin C (VitC).
Methods:
A Sprague–Dawley rat HS model was established in vivo in this study. The expression level and location of DC-SIGN were observed in kidneys. Also, the degree of histological damage, the concentrations of tumor necrosis factor-α and interleukin-6 in the renal tissues, and the serum concentration of blood urea nitrogen and creatinine at different times (2–24 h) after HS (six rats in each group), with or without VitC treatment before resuscitation, were evaluated.
Results:
HS induced DC-SIGN expression in rat tubular epithelial cells. The proinflammatory cytokine concentration, histological damage scores, and functional injury of kidneys had increased. All these phenomena induced by HS were relieved when the rats were treated with VitC before resuscitation.
Conclusions:
The results of the present study illustrated that HS could induce tubular epithelial cells expressing DC-SIGN, and the levels of proinflammatory cytokines in the kidney tissues improved correspondingly. The results also indicated that VitC could suppress the DC-SIGN expression in the tubular epithelial cells induced by HS and alleviate the inflammation and functional injury in the kidney.
Keywords: Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Nonintegrin, Hemorrhagic Shock, Renal Injury, Tubular Epithelial Cells, Vitamin C
INTRODUCTION
Bleeding induced by trauma often results in hemorrhagic shock (HS).[1] Major surgery, aorta aneurysm rupture, tumor rupture, or gastrointestinal bleeding can also lead to HS. Previous reports found that approximately one-third of patients with traumatic HS developed acute kidney injury (AKI),[2] which was associated with poorer clinical outcomes including prolonged period of hospitalization and development of chronic kidney disease.[3] It is reported that patients with evidence of AKI within 2 days following trauma had a 19-fold increased risk of developing multiorgan failure and a 6-fold increase in mortality.
The kidney is very sensitive to hypoperfusion. Hypoxia may limit the use of lactate by the kidney for anaerobic metabolism.[4] A report also showed that AKI following HS was associated with the activation of immune system.[5] The phenomenon of epithelial cell transdifferentiation has become the focus of researchers as it is closely related to the pathogenesis of many human diseases such as cardiac fibrosis and tumors. The epithelial cells also could perform epithelial–dendritic cell (DC) transformation by expressing dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN). After DC-SIGN expression, the epithelial cells gain the abilities of secreting inflammatory factors, presenting antigens, activating T-cells, and initiating inflammatory reactions.[6,7] Because a number of studies have demonstrated that DC-SIGN expression in tubular epithelial cells was highly correlated with the outcome of several kidney diseases[8] and DCs participate in the occurrence and development of AKI induced by ischemia,[9,10,11] it was hypothesized that DC-SIGN expression in the tubular epithelial cells could be induced by HS and played a critical role in the development of AKI.
Vitamin C (VitC) could alleviate multiple organ injury and inhibit inflammatory responses under various ischemia/reperfusion conditions such as sepsis, cardiac arrest, and shock.[12] The organ-protective effect of VitC has been related to the attenuation of inflammatory response, neutrophil infiltration, cell apoptosis, and induction of heme oxygenase-1.[13,14] Studies on the relationship between DC-SIGN and VitC are scarce. Our previous study showed that VitC maintained glycogen synthase kinase-3β-S9 activity, attenuated the suppression of E-cadherin caused by hypoxia, and ultimately decreased DC-SIGN expression in rat intestine epithelium cells induced by HS;[15] hence, we hypothesized that VitC could also suppress the DC-SIGN expression in renal tubular epithelial cells.
Using a rat HS model, the present study demonstrated that HS induced DC-SIGN expression in rat renal tubular epithelial cells and VitC suppressed this phenomenon.
METHODS
Animals
Outbreed adult Sprague–Dawley (SD) rats (male, 6–7 weeks old, 250–300 g) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and treated in strict accordance with the guidelines for the care and use of laboratory animals established by the Animal Use and Care Committee of the Shanghai Committee.
The rats were housed in standard cages with 12:12-h light/dark cycle and controlled temperature (25.0 ± 0.5°C). They were maintained on a standard diet and freely available water. They were anesthetized with sodium pentobarbital (Merck KGaA, Darmstadt, Germany) before surgery and sacrifice. The animal surgical procedures were approved by the Institutional Animal Care and Use Committee at the Shanghai Jiao Tong University, Shanghai, China (Permit Number: SCXK [Shanghai] 2008-0016).
Hemorrhagic shock model
A pressure-controlled HS model was established according to the method described by Umeda et al.[16] with modifications. The left and right femoral arteries were dissected after the rats were anesthetized with sodium pentobarbital (50 mg/kg of body weight). A heparinized polyethylene tube was inserted into the left femoral artery of the rat to monitor the blood pressure using PowerLab 15T (ADInstruments, Sydney, Australia), and another tube was inserted into the right femoral artery to withdraw the blood. The body temperature of each rat was maintained at 37°C. The blood pressure and heart rate were monitored constantly. By withdrawing blood using a heparinized syringe, HS was initiated when the mean arterial pressure (MAP) of 35 mmHg was achieved in about 15 min. Then, this MAP was maintained for 1 h by further blood withdrawal or reinfusing the withdrawn blood (average bleeding volume = 6.5 ml). The rats were resuscitated by reinfusing the blood, and infusing Ringer's lactate (average volume = 7 ml) until the MAP reached the baseline level in 15 min. Sham surgery consisted of the same procedure except blood collection and resuscitation.
Experimental design
Forty-two rats were randomly divided into seven groups (6 rats/group): sham, HS groups (HS 2 h, HS 6 h, and HS 24 h), and HS + VitC groups (HS + VitC 2 h, HS + VitC 6 h, and HS + VitC 24 h). The specific protocol of the study is shown in Figure 1. The rats in HS and HS + VitC groups took HS operation; rats in sham group took sham surgery. VitC (100 mg/kg) was administered to the rats in HS + VitC groups through intraperitoneal injections before resuscitation, normal saline were used in sham and HS groups. The rats were sacrificed 2 (HS 2 h, HS + VitC 2 h groups), 6 (HS 6 h, HS + VitC 6 h groups), and 24 (HS 24 h, HS + VitC 24 h groups) h after HS to obtain blood samples and kidneys, respectively. The DC-SIGN protein levels in the kidney were observed. The histological changes, levels of inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in the kidneys, the blood urea nitrogen (BUN), and creatinine (Cre) were assessed.
Figure 1.
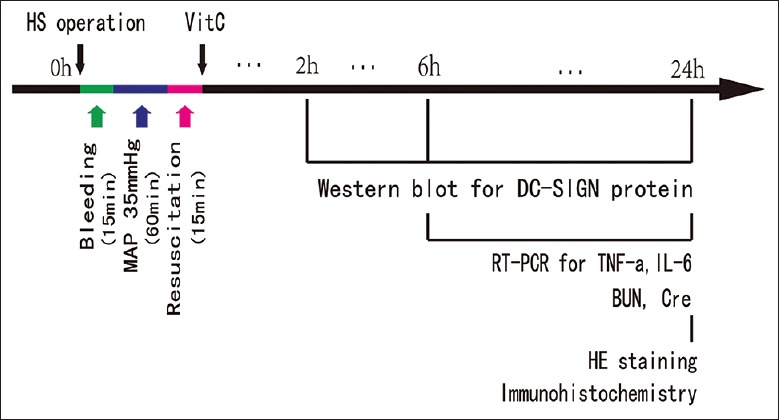
The schematic diagram of the main protocol. HS: Hemorrhagic shock; VitC: Vitamin C; HE: Hematoxylin and eosin; MAP: Mean arterial pressure; BUN: Blood urea nitrogen.
Western blotting
The protein samples were loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel for electrophoresis. Then, they were transferred to Hybond-P polyvinylidene membranes (Millipore, CA, USA). After blocking the nonspecific binding site, the membranes were incubated with primary antibodies of DC-SIGN (1:200, Santa Cruz Biotechnology, CA, USA) and glyceraldehyde-3-phosphate dehydrogenase (GADPH) (1:10,000, BioVision, NJ, USA) overnight at 4°C. The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The signals were detected using the enhanced chemiluminescence method, and the results were visualized using an ImageQuant LAS400 digital imaging system (GE Co., USA).
Real-time polymerase chain reaction
Total RNA was isolated from the frozen kidneys; 2 mg of RNA was used for reverse transcription (RT). The RT reaction was conducted using reverse transcription kits (Takara Bio Inc., Shiga, Japan). The RT-polymerase chain reaction (PCR) was performed using SYBR Premix Ex Taq (Takara Bio Inc.). The primers used were as follows: TNF-α, forward primer5’ CCCAATCTGTGTCCTTCTAACT 3’, backward primer 5’ CACTACTTCAGCGTCTCGTGT 3’; IL-6, forward primer 5’ CAAAGCCAGAGTCATTCAAGC 3’, backward primer 5’ GGTCCTTAGCCACTCCTTCTGT 3’; and GAPDH, forward primer 5’ TTCATTGACCTCAACTACAT 3’, backward primer5’ GAGGGGCCATCCACAGTCTT 3’. The initial denaturation step of PCR was 10 s at 95°C, then the thermal cycling conditions were 40 cycles with 5 s at 95°C for annealing and 20 s at 60°C for elongation on a real-time PCR system (7500, Applied Biosystems, CA, USA). The relative mRNA expression levels were normalized to GADPH and calculated using the ΔΔCt method, and the mRNA levels of TNF-α and IL-6 in renal tissues were expressed relative to the sham group.
Immunohistochemical staining
Renal tissue samples were fixed in 10% phosphate-buffered saline (PBS)-buffered formaldehyde solution at room temperature for 1 week, then were dehydrated using graded ethanol and embedded in paraffin wax (Sherwood Medical, Mahwah, USA). For immunohistochemistry, the renal tissue slices were pretreated for 30 min with 1% H2O2/PBS followed by 1 h with 1% bovine serum albumin, 5% fetal bovine serum, and 0.2% Triton X-100, and then incubated overnight at 4°C with DC-SIGN primary antibodies (1:200; Santa Cruz Biotechnology). Normal mouse serum was used for nonspecific control staining. Then, the slices were further incubated with biotinylated secondary antibody for 1 h. The images were collected using an AxioVision microscope (Carl Zeiss, Milan, Italy), and all analyses were performed blindly.
Histological studies
After deparaffinization and dehydration, the sections were stained with hematoxylin and eosin and studied using an AxioVision microscope. The histological changes were scored in a blinded manner by two independent pathologists. All of the evaluations were performed on six fields per se ction under 100 × magnification. The severity of renal tubular injury was scored by estimating the percentage of tubules in the cortex or the outer medulla that showed epithelial necrosis or had luminal necrotic debris, tubular dilation, and hemorrhage: 0 - none; 1 - <5%; 2 - 5–25%; 3 - 25–75%; and 4 - >75%.[13]
Analysis of serum biochemical indicators
Immediately after collection, the arterial blood samples were centrifuged at 3000 ×g for 15 min to obtain the serum. The serum levels of BUN and Cre were measured using an automatic biochemical analyzer (UniCel DxC 800, Beckman Coulter, CA, USA).
Statistical analysis
All values were expressed as the mean ± standard error (SE) of the mean. The unpaired Student's t-test and one-way analysis of variance (ANOVA) followed by the Tukey's test were used for statistical comparisons. All the statistical calculations were performed using SPSS, version 19.0 (IBM, IL, USA). The differences with P < 0.05 were considered statistically significant.
RESULTS
Hemorrhagic shock-induced dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin expression in rat renal tubular epithelial cells, and Vitamin C inhibited this phenomenon
The effects of VitC on DC-SIGN protein levels in kidneys of SD rat of HS model were investigated using Western blot analysis [Figure 2]. The rats underwent HS operation (HS 2 h, HS 6 h, and HS 24 h) displayed higher DC-SIGN protein levels in kidneys compared with Sham rats (P < 0.05). The rats underwent both HS operation and VitC treatment (HS + VitC 2 h, HS + VitC 6 h, and HS + VitC 24 h) displayed obviously lower DC-SIGN levels than rats only underwent HS operation, but they were still higher than that of Sham rats (P < 0.05).
Figure 2.
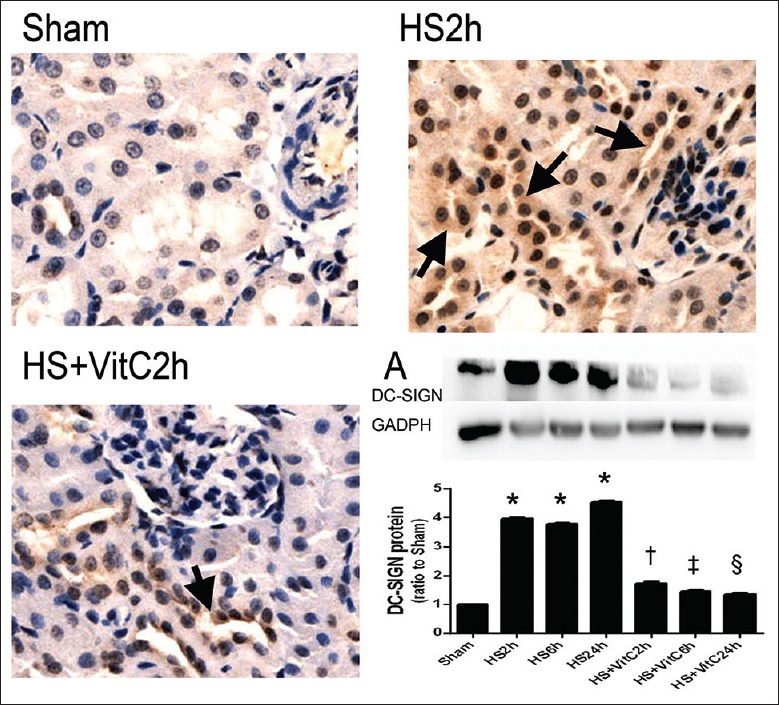
HS induced DC-SIGN expression in renal tubular epithelial cell and VitC suppressed this induction. Immunohistochemistry staining was used to detect DC-SIGN protein expression in the kidney samples. Original magnification: ×200. The arrows indicate the DC-SIGN positive cells. A: The western blot analysis for DC-SIGN protein levels in rat kidneys. Data are mean ± SEM, n = 6/group. *P < 0.05 compared to Sham, †P < 0.05 compared to HS2h and Sham, ‡P < 0.05 compared to HS6h and Sham, §P < 0.05 compared to HS24h and Sham. HS: Hemorrhagic shock; VitC: Vitamin C; DC-SIGN: Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin.
The immunohistochemical analysis showed little DC-SIGN protein in sham rat kidneys. The DC-SIGN expression level in renal tubular epithelial cells increased markedly in rats underwent HS operation. This increasing trend of DC-SIGN expression was suppressed in the HS + VitC groups.
Vitamin C relieved the hemorrhagic shock-related histological injury in rat kidneys
Obvious pathological damages, including renal tubular epithelial cell edema, necrosis, renal tubular dilation, and hemorrhage, were observed in the HS groups on investigating the histological injury of the kidneys. Compared to the HS groups, pathological damages were suppressed in the HS + VitC groups.
The histological changes were demonstrated and compared by the injury scores [Figure 3]. The rats in HS groups (HS 6 h and HS 24 h) displayed higher injury scores in kidneys compared with Sham rats (P < 0.05). The rats in HS + VitC groups (HS + VitC 6 h and HS + VitC 24 h) displayed lower injury scores than rats in HS groups, but they were still higher than that of Sham rats (P < 0.05).
Figure 3.
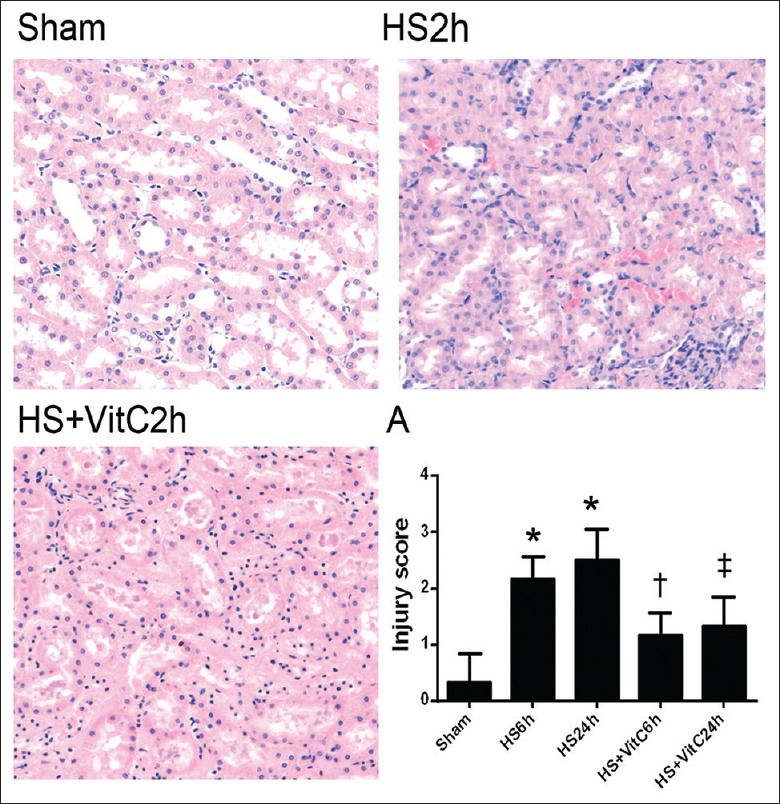
VitC relieved HS-related histological injury in the kidneys. The kidney samples were stained with H and E, ×100. A: The histological injury scores of rat kidneys. Data are mean ± SEM, n = 6/group, *P < 0.05 compared to Sham, †P < 0.05 compared to HS 6 h and Sham, ‡P < 0.05 compared to HS 24 h and Sham. HS: Hemorrhagic shock; VitC: Vitamin C.
Vitamin C relieved the hemorrhagic shock-related inflammatory response and functional injury in kidneys
The effects of HS and VitC on the inflammatory response were investigated in the rat kidneys by testing the concentration of proinflammatory cytokines in the renal tissues. The concentrations of TNF-α [Figure 4a] and IL-6 [Figure 4b] were significantly elevated 6 and 24 h after HS (HS 6 h, and HS 24 h) (P < 0.05). However, when the rats underwent HS operation were treated with VitC before resuscitation (HS + VitC 6 h, and HS + VitC 24 h), the increasing trend of TNF-α and IL-6 concentrations in rat kidneys induced by HS was suppressed (P < 0.05).
Figure 4.
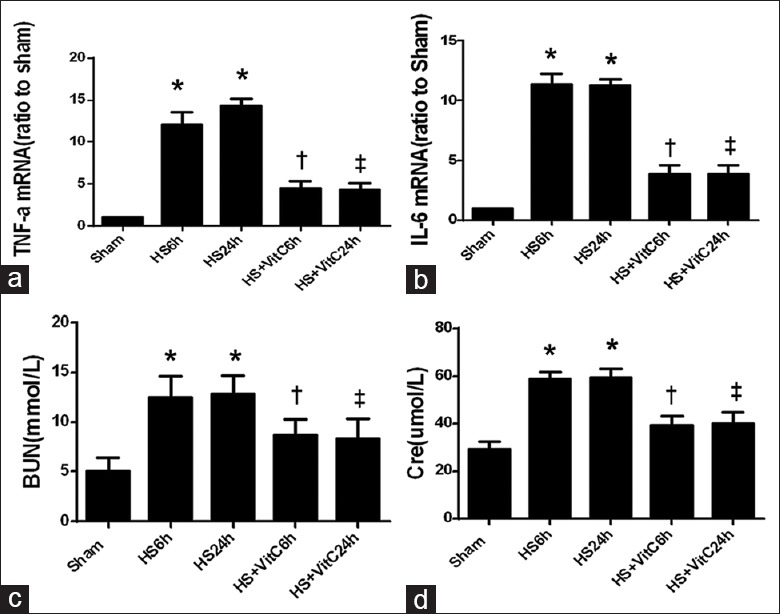
VitC relieved HS-related inflammatory responses and function injury in the kidneys. (a) The TNF-α mRNA levels in rat kidneys. (b) The IL-6 mRNA levels. (c) The plasma concentration of BUN. (d) The plasma concentration of Cre. Data are mean ± SEM, n = 6/group, *P < 0.05 compared to Sham, †P < 0.05 compared to HS 6 h and Sham, ‡P < 0.05 compared to HS 24 h and Sham. HS: Hemorrhagic shock; VitC: Vitamin C; SEM: Standard error of mean; TNF-α: Tumor necrosis factor-α; IL: Interleukin; BUN: Blood urea nitrogen; Cre: Creatinine.
Also, the changes in serum concentrations of BUN and Cre were observed as they represented the renal function. The plasma concentration of BUN [Figure 4c] and Cre [Figure 4d] significantly increased 6 and 24 h after HS (P < 0.05). VitC alleviated the increasing trend of BUN and Cre induced by HS (P < 0.05).
DISCUSSION
The present study showed that HS upregulated DC-SIGN expression in renal tubular epithelial cells, increased proinflammatory cytokine concentrations in kidneys, and renal functional injury. Moreover, VitC significantly inhibited HS-induced DC-SIGN expression in renal tubular epithelial cells, alleviated the inflammatory response in kidneys and renal functional injury.
The primary function of epithelial cells is to protect the underlying tissue from the external environment. However, they also regulate the inflammatory response and immunity. Under critical conditions, they can express immune molecules such as Toll-like receptors, complement receptors, and costimulatory molecules to regulate T lymphocyte activity. Moreover, recent studies have shown that the renal tubular epithelial cells could participate in aggravating inflammation by expressing DC-SIGN and gaining functions of DCs.[6,7,8]
DCs are the most important antigen-presenting cells. They can influence the systemic inflammatory response by secreting massive inflammatory factors, regulating cell apoptosis, and activating T-cells.[17,18] DC-SIGN is a pattern recognition receptor and adhesion receptor of DCs. It is important for the maturation and migration of DCs and mediates the antigen internalization and T-cell-activating functions of DCs.
It has been reported that DC-SIGN expression in epithelial cells plays an important role in the occurrence and development of many chronic inflammatory diseases, such as Crohn's disease, ulcerative colitis, and Helicobacter pylori-induced gastritis.[6,7] Our previous study showed that anoxic culture could induce intestinal epithelial cell-6 (IEC-6) expressing DC-SIGN, and the inflammatory factors secreted by IEC-6 obviously increased.[15] Zhou reported that the injured human renal tubular epithelial cells could express DC-SIGN and gain the functions of DCs to participate in the initiation of renal tubular–interstitial inflammation and renal fibrosis. When DC-SIGN expression in tubular epithelial cells was inhibited using anti-P-selectin lectin-epidermal growth factor domain monoclonal antibody, the inflammation and injury in renal tissues were alleviated.[19]
The results of this study illustrated that HS could also induce tubular epithelial cells expressing DC-SIGN, the levels of proinflammatory cytokines in the kidney tissues, and renal functional injury improved correspondingly. This study reported a novel finding about DC-SIGN expression in the renal tubular epithelial cells induced by HS.
The tubular epithelial cells are the most vulnerable cells in the kidney during HS.[20] The present results showed that DC-SIGN was expressed markedly in tubular epithelial cells, coinciding with the site with the most serious damage during ischemia-reperfusion injury (IRI) and the location where DC-SIGN was expressed during the initiation of renal tubular–interstitial inflammation and renal fibrosis as reported by Zhou et al.[8]
In the mouse multiple organ dysfunction syndrome (MODS) models set up by an intraperitoneal injection of yeast polysaccharide, Lu et al. observed that the increase in the quantity and activity of DCs played an important role in the development of systemic inflammatory response syndrome (SIRS) and MODS.[21,22] Reports have shown that after IRI during kidney transplantation, DCs in the kidney increased obviously despite no sign of acute rejection.[9,10,11,23] Some studies showed that after activation by danger-associated molecular patterns or pathogen-associated molecular patterns, DCs became key initiators of the innate immune system in kidney IRI to induce kidney injury as they could activate neutrophils, monocytes/macrophages, natural killer cells, and natural killer T-cells.[24,25,26] Otherwise, DCs were the predominant TNF-secreting cells in early IRI-induced AKI.[27] This study found that epithelial cells could express DC-SIGN after HS, consistent with the phenomenon that DCs increased in the early period of SIRS and MODS.
VitC has been shown to act as a prooxidant that generates reactive oxygen species at high concentrations but in the study of Zhao et al.,[14] the results from pathological evaluation showed that a dose of 100 mg/kg VitC of body weight in vivo was nontoxic to rats. Studies on the relationship between DC-SIGN expression and VitC are limited. Our previous study showed that VitC could suppress the DC-SIGN expression in rat intestine epithelial cells induced by HS.[15] The results of this study indicated that VitC could also suppress the DC-SIGN expression in the tubular epithelial cells induced by HS.
There is a limitation in our study. We did not inhibit or upregulate DS-SIGN expression in advance to confirm that VitC protected rat kidney against HS injury by inhibiting DS-SIGN expression. Further studies are needed to validate our results.
In conclusion, our results showed that HS induced DC-SIGN expression in the tubular epithelial cells, increased the proinflammatory factor secretion, aggravated kidney histological damage, and functional injury. VitC attenuated these phenomena induced by HS.
These findings provide new ways of studying the mechanism through which HS induces AKI, and how VitC exerts organ protective effects. These findings provide evidence that VitC might be applicable in critical situations.
Financial support and sponsorship
This study was financially supported by the National Natural Science Fundation of China (No. 81501643) and the research foundation of Shanghai Municipal Health Bureau (No. 20124438).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Prof. Jian-Qing Ding for providing the expertise and excellent training with regard to experimental techniques, the staffs of Shanghai Institute of Traumatology and Orthopaedics for their technical support.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: Latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes E, Antunes R, Dias C, Araújo R, Costa-Pereira A. Acute kidney injury in severe trauma assessed by RIFLE criteria: A common feature without implications on mortality? Scand J Trauma Resusc Emerg Med. 2010;18:1. doi: 10.1186/1757-7241-18-1. doi: 10.1186/1757-7241-18-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bihorac A, Delano MJ, Schold JD, Lopez MC, Nathens AB, Maier RV, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg. 2010;252:158–65. doi: 10.1097/SLA.0b013e3181deb6bc. doi: 10.1097/SLA.0b013e3181deb6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelimarkka O. Renal oxygen and lactate metabolism in hemorrhagic shock. An experimental study. Acta Chir Scand Suppl. 1984;518:1–44. [PubMed] [Google Scholar]
- 5.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. doi: 10.1111/j1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 6.Zeng JQ, Xu CD, Zhou T, Wu J, Lin K, Liu W, et al. Enterocyte dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin expression in inflammatory bowel disease. World J Gastroenterol. 2015;21:187–95. doi: 10.3748/wjg.v21.i1.187. doi: 10.3748/wjg.v21.i1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Lin K, Zeng J, Liu W, Yang F, Wang X, et al. Role of DC-SIGN in Helicobacter pylori infection of gastrointestinal cells. Front Biosci (Landmark Ed) 2014;19:825–34. doi: 10.2741/4250. doi: 10.2741/4250. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T, Li X, Zou J, Cai M, Sun G, Zhang Y, et al. Effects of DC-SIGN expression on renal tubulointerstitial fibrosis in nephritis. Front Biosci (Landmark Ed) 2009;14:3814–24. doi: 10.2741/3490. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, et al. Dendritic cells tolerized with adenosine A2AR agonist attenuate acute kidney injury. J Clin Invest. 2012;122:3931–42. doi: 10.1172/JCI63170. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penfield JG, Wang Y, Li S, Kielar MA, Sicher SC, Jeyarajah DR, et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 1999;56:1759–69. doi: 10.1046/j.1523-1755.1999.00741.x. doi: 10.1046/j.1523-1755.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, et al. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–7. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton GJ, Van PY, Differding JA, Kremenevskiy IV, Spoerke NJ, Sambasivan C, et al. Lyophilized plasma with ascorbic acid decreases inflammation in hemorrhagic shock. J Trauma. 2011;71:292–7. doi: 10.1097/TA.0b013e31821f4234. doi: 10.1097/TA.0b013e31821f4234. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Fei J, Chen Y, Ying YL, Ma L, Song XQ, et al. Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complement Altern Med. 2014;14:442. doi: 10.1186/1472-6882-14-442. doi: 10.1186/1472-6882-14-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, Fei J, Chen Y, Ying YL, Ma L, Song XQ, et al. Pharmacological preconditioning with Vitamin C attenuates intestinal injury via the induction of heme oxygenase-1 after hemorrhagic shock in rats. PLoS One. 2014;9:e99134. doi: 10.1371/journal.pone.0099134. doi: 10.1371/journal.pone.0099134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Chen Y, Song X, Wang L, Zhao B, Yang Z, et al. Vitamin C attenuates hemorrhagic hypotension induced epithelial-dendritic cell transformation in rat intestines by maintaining GSK-3ßactivity and E-cadherin expression. Shock. 2016;45:55–64. doi: 10.1097/SHK.0000000000000486. doi: 10.1097/SHK.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 16.Umeda K, Takahashi T, Inoue K, Shimizu H, Maeda S, Morimatsu H, et al. Prevention of hemorrhagic shock-induced intestinal tissue injury by glutamine via heme oxygenase-1 induction. Shock. 2009;31:40–9. doi: 10.1097/SHK.0b013e318177823a. doi: 10.1097/SHK.0b013e318177823a. [DOI] [PubMed] [Google Scholar]
- 17.Chen ML, Tsai TC, Wang LK, Lin YY, Tsai YM, Lee MC, et al. Risperidone modulates the cytokine and chemokine release of dendritic cells and induces TNF-a-directed cell apoptosis in neutrophils. Int Immunopharmacol. 2012;12:197–204. doi: 10.1016/j.intimp.2011.11.011. doi: 10.1016/j.intimp.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 18.García-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: The true regulators of immune homeostasis. Immunol Rev. 2009;230:22–37. doi: 10.1111/j.1600-065X.2009.00786.x. doi: 10.1111/j.1600-065X.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- 19.Cai M, Wu J, Mao C, Ren J, Li P, Li X, et al. A Lectin-EGF antibody promotes regulatory T cells and attenuates nephrotoxic nephritis via DC-SIGN on dendritic cells. J Transl Med. 2013;11:103. doi: 10.1186/1479-5876-11-103. doi: 10.1186/1479-5876-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toda N, Takahashi T, Mizobuchi S, Fujii H, Nakahira K, Takahashi S, et al. Tin chloride pretreatment prevents renal injury in rats with ischemic acute renal failure. Crit Care Med. 2002;30:1512–22. doi: 10.1097/00003246-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Lu JY, Wang XH, Sun Y. Study on pathological changes in thymus dendritic cells and their role in multiple organ dysfunction syndrome rat and their effect on pathogenesis (in Chinese) Chin Crit Care Med. 2001;13:675–8. doi: 10.3760/j.issn1003-0603.2001.11.011. [Google Scholar]
- 22.Lu JY, Li ZH, Wang XH, Yang Y, Li L. Changes in spleen dendritic cells in multiple organ dysfunction syndrome in mice (in Chinese) Chin Crit Care Med. 2006;18:24–7. [PubMed] [Google Scholar]
- 23.Loverre A, Capobianco C, Stallone G, Infante B, Schena A, Ditonno P, et al. Ischemia-reperfusion injury-induced abnormal dendritic cell traffic in the transplanted kidney with delayed graft function. Kidney Int. 2007;72:994–1003. doi: 10.1038/sj.ki.5002468. doi: 10.1038/sj.ki.5002468. [DOI] [PubMed] [Google Scholar]
- 24.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–30. doi: 10.1016/j.imlet.2008.11.017. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Rosin DL, Okusa MD. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22:416–25. doi: 10.1681/ASN.2010040430. doi: 10.1681/ASN.2010040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlichting CL, Schareck WD, Weis M. Renal ischemia-reperfusion injury: New implications of dendritic cell-endothelial cell interactions. Transplant Proc. 2006;38:670–3. doi: 10.1016/j.transproceed.2006.01.059. doi: 10.1016/j.transproceed2006.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–28. doi: 10.1038/sj.ki.5002132. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]


