A 16-year-old girl was administered to our hospital because of primary amenorrhea and solid pelvic mass. She was 158 cm tall and weighed 55 kg. There was no evidence of acanthosis nigricans, acne, hirsutism, goiter, cushingoid features, or Turners stigmata. Examination of secondary sexual characteristics revealed that the breast was small and poorly developed (Tanner's Stage II). Pubic hair was absent while the external genitalia was of female type and had no evidence of clitoromegaly. Ultrasonography of the pelvis showed a solid mass diameter about 9 cm in right adnexal area, a normal uterus with endometrium of 3 mm, and left ovary 1.5 cm × 1.0 cm. Her serum hormonal assay revealed a low estradiol level of 0.155 nmol/L (normal range: 0.200–0.790 nmol/L in follicular phase) in the background of elevated luteinizing hormone level of 25.47 U/L (normal range: 2.12–10.89 U/L in follicular phase), follicle-stimulating hormone level of 51.36 U/L (normal range: 3.85–8.78 U/L in follicular phase), and testosterone level of 3.97 nmol/L (normal range: 0.35–2.60 nmol/L). She was found to have normal progesterone level of 3.77 nmol/L (0.98–4.83 nmol/L in follicular phase) and serum prolactin level of 12.47 ng/ml (3.34–26.72 ng/ml). Serum tumor markers were detected before operation and the results were as following: CA 125 34.20 U/ml, (normal range: 0–35.00 U/ml), CA 199 34.11 U/ml (normal range: 0–39.00 U/ml), alpha-fetoprotein (AFP) >1210.0 ng/ml (normal range: 0–7.0 ng/ml), beta-human chorionic gonadotropin (β-hCG) 12.81 U/L (normal range: 0–2.90 U/L), and lactic dehydrogenase (LDH) 204 U/L (normal range: 109–245 U/L). She did not give any history of abdominal pain, nausea or vomit, no hormonal intake, radiation exposure, chemotherapy, or any central nervous symptoms such as headache or visual disturbances. She has no significant trauma or surgical procedure history. There was no history of childhood tuberculosis or any congenital abnormality or hereditary disease in her family.
At the time of laparoscopy, a solid mass diameter 9 cm in right ovarian area was identified while a small mass about 1 cm in the surface of left ovary was observed. There were no significant abnormalities in uterus and the bilateral fallopian tubes. Salpingo-oophorectomy of the right side was performed and intraoperative frozen section reported a benign tumor. Then, left ovary tumor resection was performed. Postoperative paraffin pathology found a mixed germ cell tumor (GCT) composed of immature teratoma, dysgerminoma [Figure 1], and yolk sac tumor [Figure 2] in right ovary while a mixed tumor composed of GCT and sex cord stromal tumor (mature teratoma and gonadoblastoma) was identified in left ovary. These pathology results were consulted with another famous hospital and got the same diagnosis. The second laparoscopy of staging operation was performed including salpingo-oophorectomy of left side, resection of great omentum, and pelvic and para-aortic lymphadenectomy. The pathologic findings were consistent with the first operation and revealed no metastasis to other area. Immunohistochemical staining results showed inhibin (+), CD99 (focal+), CD117 (focal+), Ki-67 (20%+), PLAP (focal+), OCT-4 (focal+), D2-40 (focal+), GPC3 (−), AFP (−), CD30 (−), and CK (focal+). Until 3 days after second operation, the result of karyotype analysis was out and demonstrated a genotype of pure XY.
Figure 1.
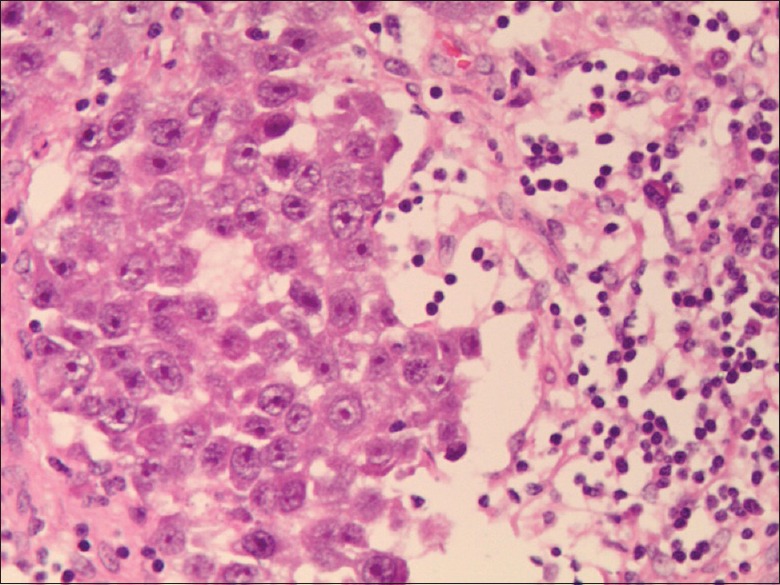
Dysgerminoma. H and E staining, original magnification ×20.
Figure 2.
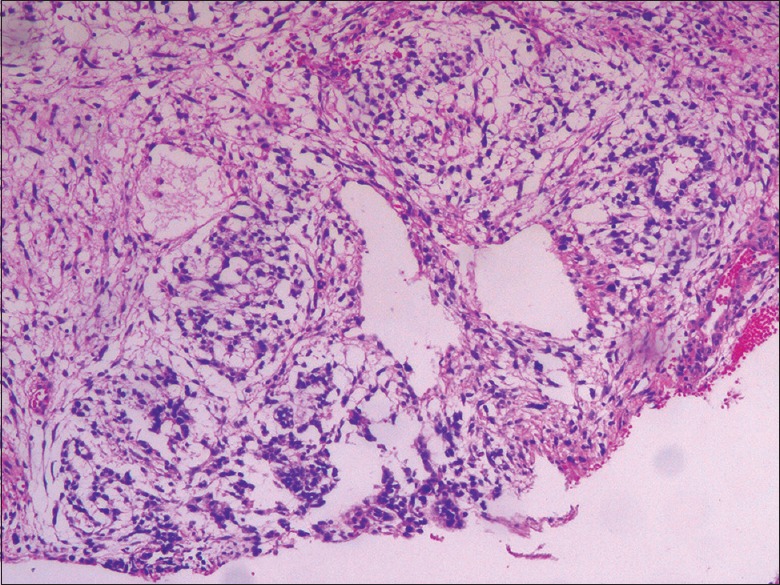
Yolk sac tumor. H and E staining, original magnification ×20.
Adjuvant chemotherapy was performed with 6 cycles of cisplatin/etoposide/bleomycin from April 2015 to September 2015. The patient's serum AFP decreased to 24.83 ng/ml 3 days after second operation and decreased to normal after first chemotherapy and maintained normal during 6 cycles of chemotherapy. Her serum β-hCG decreased to normal after second operation and maintained normal during the whole chemotherapy. The patient is back to school now and followed up every 3 months without abnormal findings. If there is no relapse 6 months after the operation, hormone replacement therapy (HRT) will be suggested.
Swyer's syndrome was first described by Gim Swyer in 1955. Affected individuals have an XY karyotype but the external and internal genitalia is of the female type.[1] It is estimated that 15% of complete sporadic 46, XY sex reversal cases (pure gonadal dysgenesis) are due to Sex-detemining Region Y (SRY) point mutations, another 15% are due to SRY deletions from aberrant X/Y recombinations, but almost 70% of all cases have unknown origin.[2] It is an extremely rare condition.
We made a diagnosis of Swyer's syndrome for this patient because she was a normal statured girl with primary amenorrhea, with clinical features of sexual infantilism, whose genotype was pure XY. Since the genotype result was reported later than the operation time, we only did salpingo-oophorectomy of the right side at first laparoscopy. In fact, it is reasonable to perform the operation after the genotype result reported, if that was the case, bilateral salpingo-oophorectomy should be performed at the same operation and the second operation could be saved.
Compared with ovarian epithelial tumors, ovarian germ cell tumors are rare but occur commonly in a younger population. GCT account for over 60% of the ovarian neoplasms in children and adolescents, one-third of these tumors are malignant. The main serum markers of GCT are AFP, the beta subunit of β-hCG, and LDH. It is suggested to measure these three serum tumor markers preoperatively in any woman <40 years with suspected ovarian cancer.[3]
Considering the highly malignance of yolk sac tumor and mixed histology of the right ovary, as well as the patient's positive tumor markers before first surgery, pelvic and para-aortic lymphadenectomy were performed at second operation and chemotherapy consisting of cisplatin/etoposide/bleomycin (BEP) was administered after surgery. According to 2015 edition of NCCN guidelines for malignant germ cell tumors (MGCTs), when the prior surgery was incompletely surgically staged, the pathology of the tumor should be considered for decision of the second operation. If the tumor is embryonal, or endodermal sinus tumor (yolk sac tumor), or Grade 2–3 immature teratoma, or mixed histological tumor, the results of imaging and tumor makers are deciding factors. When the patient has positive imaging and positive tumor markers, if she is fertility desired, fertility-sparing surgery and comprehensive staging are suggested. While if she has no fertility desire, completion staging surgery with possible tumor reductive surgery is suggested. When the patient has negative imaging, the second surgery could be saved regardless of the tumor markers’ results. After operation, chemotherapy is suggested for all MGCTs excluding Stage I dysgerminoma or Stage I Grade 1 immature teratoma. Up to date, the primary chemotherapy for MGCTs is BEP regimen. Etoposide/carboplatin is alternative for selected patients with Stage IB–III dysgerminoma.[4]
Patients with Sywer's syndrome usually present in adolescence with primary amenorrhea and lack of secondary sexual characteristics. They show a high predisposition to ovarian cancer with the most frequent observed histotypes are gonadoblastomas and dysgerminomas, followed by Brenner tumors, malignant teratomas, and mixed endodermal sinus tumors.[2,5] The lifetime risk of gonadal tumors is in range of 15–35%.
Given the high oncogenic potential of the disease, bilateral oophorectomy should be seriously taken into account in this condition as a prophylactic and therapeutic treatment.[1,2,3] These patients could have a normal life expectancy, provided that they have undergone bilateral gonadectomy without malignant change. They need to be on lifelong HRT and could have normal sexual relations and theoretically they can conceive using donor oocytes and artificial reproductive techniques.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Culha C, Ozkaya M, Serter R, Sahin I, Aydin B, Aral Y. Swyer's Syndrome: In a fifty-year-old female. J Obstet Gynaecol India. 2012;62:571–4. doi: 10.1007/s13224-011-0100-1. doi: 10.1007/s13224-011-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchina E, Gambera A, Spinelli E, Clerici P, Scagliola P, Sartori E, et al. Identification of a new mutation in the SRY gene in a 46,XY woman with Swyer syndrome. Fertil Steril. 2009;91:932, e7–11. doi: 10.1016/j.fertnstert.2008.07.1722. doi: 10.1016/j.fertnstert. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal P, Kehoe S. Serum tumour markers in gynaecological cancers. Maturitas. 2010;67:46–53. doi: 10.1016/j.maturitas.2010.04.017. doi: 10.1016/j.maturitas.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Ovarian Cancer. [Last accessed on 2016 Mar 21]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#ovarian .
- 5.Morsy AH, al-Fadly A, Mokhtar S, el-Aasar EM, Farag TI. Swyer syndrome: An unusual presentation. Int J Gynaecol Obstet. 1995;49:185–6. doi: 10.1016/0020-7292(94)02334-u. doi: 10.1016/0020-7292(94)02334-U. [DOI] [PubMed] [Google Scholar]


