INTRODUCTION
Fluid challenge is a common diagnostic method to help the physician determine fluid responsiveness, which is an important component of fluid management in critically ill patients.[1] Raising legs of a patient induces the transfer of a variable amount of blood (approximately 200–300 ml) contained in the venous reservoir from the limb to central venous compartment. According to Franck-Starling curve, this transient increase of preload might lead to an increase in cardiac output (CO) in future responders resulting from their preload-reserve status. Many clinical studies have validated passive leg raising (PLR), and the advantage of PLR is attractive in Intensive Care Unit (ICU). Recently, PLR has been suggested as a simple and potential method to predict fluid responsiveness, which is similar to an “auto-fluid challenge” without a drop of fluid.[2] However, one study revealed poor application of PLR in the real world.[3] We acknowledged that the lack of education on PLR would result in the current practice. On the other hand, the application of PLR might be not simple in clinical practice, and the holy grail of fluid responsiveness still needs to be discovered.[4] The standard of PLR has not been established, and some questions of PLR merit discussion.
The points of concern for PLR include the following: the indication and contraindication of PLR; the choice of initial position: starting from a semi-recumbent position or supine position for PLR; how to interpret and apply the changes of CO, blood pressure, and central venous pressure (CVP) to identify fluid response during PLR; and how to identify spontaneous variation and sympathetic stimulation during PLR. We insist that these issues are worthy of consideration and clarification. Therefore, we briefly reviewed the valuable literature and provided our viewpoints on these controversies. In addition, corresponding points regarding the use of PLR in clinical practice are summarized in this article.
PASSIVE LEG RAISING INDICATIONS AND CONTRAINDICATIONS
The essential of PLR is to assist the physician to identify fluid responsiveness, which is equal to the fluid challenge. Hypotension is the most common indication to trigger the fluid challenge in ICU. Hence, the theoretic indications of PLR include the following: (1) the presence of unstable hemodynamic status or poor tissue perfusion: hypotension, tachycardia, low CO, oliguria, poor peripheral perfusion (skin mottling, peripheral perfusion index <0.6, capillary refill time >4 s, poor response of transcutaneous partial pressure of oxygen to 1.0 fraction of inspiration O2[FiO2]), high lactate level, and low lactate clearance;[5,6,7] (2) the above clinical problems might benefit from fluid infusion according to the clinical decision, and fluid responsiveness is still undetermined. Some would argue that PLR would be unnecessary if the fluid challenge test is implemented. However, only approximately 50% of fluid challenge results were positive in ICU department. In other words, one-half of fluid challenges should be avoided in critically ill patients.[1] Importantly, PLR could provide valuable information regarding fluid responsiveness without fluid infusion, which would offer great benefits in patients with cardiac dysfunction or lung edema at a high risk of fluid infusion. Moreover, we think that the investigations should focus on the efficacy of PLR for reducing the invalidated fluid challenge in future, which would help promote PLR.
It should be noted that PLR is not suitable for all clinical conditions. First, venous return would decrease in patients with abnormal abdominal pressure or who are wearing elastic compression stockings during PLR, and the ability of PLR for predicting fluid response in these conditions might be invalidated. Second, PLR should be avoided in patients with pain or agitation conditions or patients who recently received extra stimulation for a brief duration of time. The effect of extra stimulation on hemodynamics would also impact the performance of PLR, especially when the stimulation is caused by PLR. Third, head trauma patients might be inappropriate candidates for PLR as this test could increase intracranial pressure.
CHOICE OF INITIAL POSITION: SEMI-RECUMBENT OR SUPINE POSITION
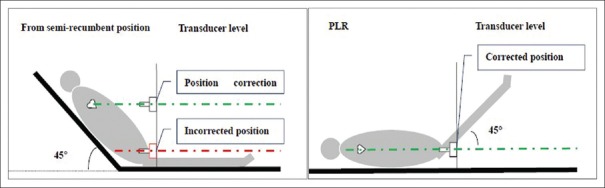
When PLR is initiated from a semi-recumbent position, more venous return and reduced stimulation of the hip joint is noted. Therefore, starting PLR from a semi-recumbent position would better predict fluid response compared with starting PLR from a supine position.[8] However, some points should be kept in mind when starting from a semi-recumbent position. First, this maneuver would lead to a variation of the blood pressure transducer position, which is referred to as the heart position (this relationship between heart position and change in pressure transducer position when PLR is initiated from the semi-recumbent position is presented in Figure 1) during PLR, which is easily ignored.[9] The variation of arterial transducer position can result in incorrect measurements of pulse contour waveform-derived parameters. Our study found when the transducer's vertical distance was >10 cm from the phlebostatic axis, the changes in continuous cardiac index reached statistical significance, and the change in the continuous cardiac index was >5% at the 15 cm variation of transducer and approximately 10% at 20 cm.[10,11] Some might argue that we could attach the transducer to a fixed level associated with the heart, but this practice might be difficult for femoral blood pressure monitoring and might require some special attached device. Second, the safety of tracheal tube or venous or arterial lines should be monitored when PLR is initiated from a semi-recumbent position. Third, the patient is required to maintain a constant angle (135°) between trunk and leg to avoid stimulation of the hip joint when starting from a semi-recumbent position and a suitable bed might be necessary to complete this maneuver. From a practical application aspect, the above limitations would make PLR difficult.
Figure 1.
The relationship between heart position and change in pressure transducer level when PLR is initiated from the semi-recumbent position. PLR: Passive leg raising.
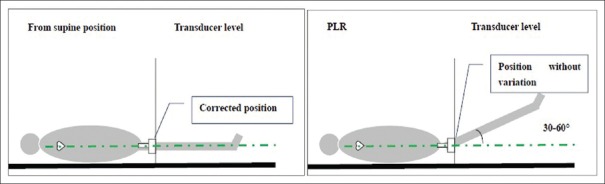
However, PLR might be easier when starting from a supine position, which is without the variation of pressure transducer position (this relationship between heart position and change in pressure transducer position when PLR is initiated from the supine position is shown in Figure 2). Some studies also supported the validation of PLR from the supine position.[12,13,14,15] In addition, a meta-analysis revealed no significant differences in the predictive performance between PLR starting from a supine versus a semi-recumbent position.[16] Thus, when unable to initiate PLR from a semi-recumbent position, a supine position still is worthy of consideration.
Figure 2.
The relationship between heart position and pressure transducer level when PLR is performed from the supine position. PLR: Passive leg raising.
INTERPRETATION OF THE CHANGES OF CARDIAC OUTPUT, ARTERIAL PULSE PRESSURE, AND CENTRAL VENOUS PRESSURE DURING PASSIVE LEG RAISING
Because the effect of PLR on CO is short and transient, a real-time evaluation of CO would be important to identify rapid variations of CO during PLR. Pulse contour analysis, esophageal Doppler, transthoracic echocardiography, and bioreactance are the most frequent used measurement techniques for flow parameters. PLR-induced change in flow parameters always involves CO, cardiac index, stroke volume index, or aortic blood flow. The meta-analysis revealed a reliable performance of PLR-induced change in flow parameters to predict fluid responsiveness. The cutoff value of PLR-induced change in flow parameters to discriminate fluid responders from nonresponders was generally between 8% and 15%.[17,18]
Given the limited measurement techniques for flow parameters, the change of pulse pressure (PP) is also attracting attention during PLR in clinical practice. The ability of CO translated into blood pressure is poor in patients with low vascular tone/systemic vascular resistance and even in the fluid responder [Figure 3]. Studies have demonstrated that the reliability of blood pressure as a surrogate of stroke volume was poor,[19] but blood pressure remains the most widely used parameter to assess the response of fluid challenge.[3] Lakhal et al.[20] demonstrated that a substantial increase in invasive PP (23%) or noninvasive PP (35%) reliably detected a fluid response, and a nonmarked increase in PP (4–5%) indicated a lack of a fluid response. A recent meta-analysis demonstrated that the predictive value of a change in PP on PLR was inferior to a PLR-induced change in a flow variable (sensitivity of 58% [95% confidence interval (CI), 44–70] and specificity of 83% [95% CI, 68–92] vs. sensitivity of 85% [95% CI, 78–90] and specificity of 92% [95% CI, 87–94], P < 0.001).[18] When PLR effects are assessed by changes in PP, the specificity of PLR test remains acceptable but its sensitivity would be poor. In other words, PLR-induced changes in PP remain an alternative with lower predictive ability. The threshold of PLR-induced change in PP% for predicting fluid response ranges from 9% to 12%.[17,18]
Figure 3.
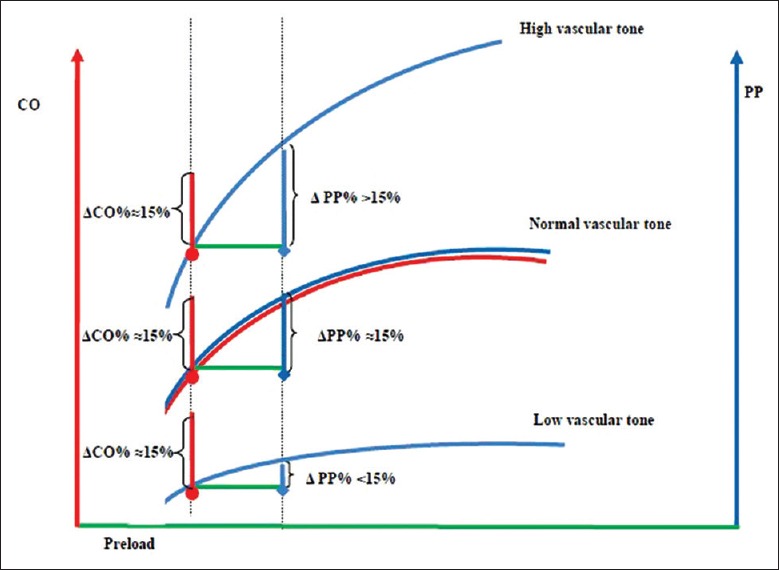
Schematic representation of the cardiac output/arterial pulse pressure relationship of a high, normal, and low vascular tone in a fluid responsive condition (fluid responsiveness is defined as an increase of 15% in cardiac output by a preload stimulation according to Franck-Starling curve). CO: Cardiac output; PP: Arterial pulse pressure; ΔCO: Change of cardiac output in response to preload stimulation; ΔPP: Change of arterial pulse pressure in response to preload stimulation.
In addition, the change of CVP also should be emphasized during PLR. The study showed that CVP measurements could improve the accuracy of leg raising-induced change in PP to predict fluid responsiveness.[14] The area under the receiver-operating characteristic curve was 0.91 for PLR-induced change in PP% to predict fluid response in case of PLR-induced change in CVP >2 mmHg (1mmHg=0.133kPa), and the cutoff of PP% was 8%.[14] For the noninvasive blood pressure, the threshold was 9% to determine fluid responders when PLR-induced change in CVP >2 mmHg.[21]
Moreover, CVP is always used as the safe limitation in volume challenge. Therefore, the assessment of PLR-induced change in CVP could provide more information for fluid management rather than fluid responsiveness. For example, if PLR causes a high increase of CVP (>5 cmH2O [1 cmH2O = 0.098 kPa] according to the “2–5” rules for CVP), further infusion of fluid might be unsafe. In other words, a PLR-induced change in CVP >5 cmH2O suggests the cardiac dysfunction and volume intolerance. Furthermore, CVP monitoring could provide information regarding volume tolerance and cardiac function during PLR.
IDENTIFY THE SPONTANEOUS VARIATION AND SYMPATHETIC STIMULATION DURING PASSIVE LEG RAISING
It is important to identify the spontaneous variation and sympathetic stimulation during PLR, which is the basis for the correct judgment of PLR. Spontaneous variation and sympathetic stimulation are involved in the rules of PLR, but it is unclear how we can determine these factors.[2] Given that spontaneous variation also could affect the change in posture, the reassessment of CO could not determine the preload effect or spontaneous variation when the patients return to the baseline position. We suggested that the baseline spontaneous variation of hemodynamic data should be evaluated before PLR. In addition, the change of heart rate (HR) has been used to reflect sympathetic tone. The 5% variation of hemodynamic data might be accepted as a physiologic variation in clinical practice. Thus, we suggested a large variation (more than ± 5%) of HR evoked by the PLR maneuver might indicate the invalidation of PLR, which might suggest the presence of sympathetic stimulation. Pain stimulation merits attention especially in patients subject to hip or extensive lower leg surgery and various gynecologic and urologic operations during PLR. Recently, Klijn et al.[22] found that sublingual functional capillary density was the best parameter to use when evaluating fluid responsiveness independent of changes in sympathetic tones, and this finding required further study.
In summary, we proposed some important points regarding the use of PLR in clinical practice as follows: (1) PLR should be performed in relatively peaceful conditions without external stimulation during a short period. This procedure should be avoided in patients with abnormal abdominal pressure or brain trauma or those wearing elastic compression stockings; (2) When PLR initiated from a semi-recumbent position, care should be taken to maintain the arterial pressure transducer at heart level and assess the safety of tubes; (3) When starting from a semi-recumbent position, the patient should maintain a constant angle (135°) between the trunk and leg to avoid the stimulation of hip joint during PLR; (4) PLR performance starting from a semi-recumbent position is superior to starting from a supine position, but the latter appears to be easier; (5) If it is not possible to start from a semi-recumbent position, PLR initiation from a supine position merits consideration; (6) The PLR-induced change in flow variables is superior to the PLR-induced change in PP for predicting fluid responsiveness; (7) For the PLR-induced change in PP% to predict fluid responsiveness, the specificity of the PLR test remains acceptable, but its sensitivity might be poor; (8) In case of PLR-induced change in CVP >2 mmHg, the PLR-induced change in PP% would be reliable to identify the fluid responder; (9) The PLR-induced change of CVP is helpful in determining tolerance of volume and cardiac function; (10) A PLR-induced change in HR of >5% indicates the presence of sympathetic stimulation, and the PLR information for predicting fluid response would be invalidated.
In conclusion, PLR is a potential and attractive method for assessing fluid status in critically ill patients. Before the era of “PLR for fluid responsiveness,” more attention should be paid and more education should be provided regarding the details of PLR in clinical practice.
Financial support and sponsorship
This work was supported by the grant from Beijing Excellent TalentsTrain Project (No. 2015000020124G072).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.He H, Liu D. Fluid bolus therapy is a medical therapy or a diagnostic method? Crit Care. 2015;19:360. doi: 10.1186/s13054-015-1078-3. doi: 10.1186/s13054-015-1078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monnet X, Teboul JL. Passive leg raising: Five rules, not a drop of fluid! Crit Care. 2015;19:18. doi: 10.1186/s13054-014-0708-5. doi: 10.1186/s13054-014-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: The FENICE study:A global inception cohort study. Intensive Care Med. 2015;41:1529–37. doi: 10.1007/s00134-015-3850-x. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichet J, Cariou A. Passive leg raising: Good for everyone? Crit Care Med. 2010;38:989–90. doi: 10.1097/CCM.0b013e3181cc159b. doi: 10.1097/CCM.0b013e3181cc159b. [DOI] [PubMed] [Google Scholar]
- 5.He H, Long Y, Liu D, Wang X, Zhou X. Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care. 2015;19:330. doi: 10.1186/s13054-015-1057-8. doi: 10.1186/s13054-015-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He HW, Liu DW, Long Y, Wang XT. High central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care. 2016;31:76–81. doi: 10.1016/j.jcrc.2015.10.017. doi: 10.1016/j.jcrc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.He HW, Liu DW, Long Y, Wang XT. The peripheral perfusion index and transcutaneous oxygen challenge test are predictive of mortality in septic patients after resuscitation. Crit Care. 2013;17:R116. doi: 10.1186/cc12788. doi: 10.1186/cc12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: Importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 9.He HW, Liu DW. Passive leg raising: Influence of blood pressure transducer site. Intensive Care Med. 2013;39:1668. doi: 10.1007/s00134-013-2994-9. doi: 10.1007/s00134-013-2994-9. [DOI] [PubMed] [Google Scholar]
- 10.He H, Liu D, Long Y, Wang X, Yu Y, Li X, et al. The relationship between arterial transducer level and pulse contour waveform-derived measurements. Crit Care. 2015;19:31. doi: 10.1186/s13054-015-0745-8. doi: 10.1186/s13054-015-0745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He HW, Liu DW, Long Y, Wang XT, Zhao ML, Lai XL. The effect of variable arterial transducer level on the accuracy of pulse contour waveform-derived measurements in critically ill patients. J Clin Monit Comput. 2015 doi: 10.1007/s10877-015-9756-x. [Epub ahead of print] doi: 10.1007/s10877-015-9756-x. [DOI] [PubMed] [Google Scholar]
- 12.Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002;121:1245–52. doi: 10.1378/chest.121.4.1245. doi: 10.1378/chest.121.4.1245. [DOI] [PubMed] [Google Scholar]
- 13.Lafanechère A, Pène F, Goulenok C, Delahaye A, Mallet V, Choukroun G, et al. Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care. 2006;10:R132. doi: 10.1186/cc5044. doi: 10.1186/cc5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakhal K, Ehrmann S, Runge I, Benzekri-Lefèvre D, Legras A, Dequin PF, et al. Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med. 2010;36:940–8. doi: 10.1007/s00134-010-1755-2. doi: 10.1007/s00134-010-1755-2. [DOI] [PubMed] [Google Scholar]
- 15.Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–8. doi: 10.1007/s00134-007-0642-y. doi: 10.1007/s00134-007-0642-y. [DOI] [PubMed] [Google Scholar]
- 16.Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: Systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–83. doi: 10.1007/s00134-010-1929-y. doi: 10.1007/s00134-010-1929-y. [DOI] [PubMed] [Google Scholar]
- 17.Monnet X, Marik P, Teboul JL. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016 doi: 10.1007/s00134-015-4134-1. [Epub ahead of print] doi: 10.1007/s00134-015-4134-1. [DOI] [PubMed] [Google Scholar]
- 18.Cherpanath TG, Hirsch A, Geerts BF, Lagrand WK, Leeflang MM, Schultz MJ, et al. Predicting fluid responsiveness by passive leg raising: A systematic review and meta-analysis of 23 clinical trials. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001556. [Epub ahead of print] doi: 10.1097/CCM.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 19.Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–8. doi: 10.1007/s00134-011-2457-0. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- 20.Lakhal K, Ehrmann S, Perrotin D, Wolff M, Boulain T. Fluid challenge: Tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive) Intensive Care Med. 2013;39:1953–62. doi: 10.1007/s00134-013-3086-6. doi: 10.1007/s00134-013-3086-6. [DOI] [PubMed] [Google Scholar]
- 21.Lakhal K, Ehrmann S, Benzekri-Lefèvre D, Runge I, Legras A, Dequin PF, et al. Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim. 2012;31:e67–72. doi: 10.1016/j.annfar.2012.01.032. doi: 10.1016/j.annfar.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Klijn E, Niehof S, Groeneveld AB, Lima AP, Bakker J, van Bommel J. Postural change in volunteers: Sympathetic tone determines microvascular response to cardiac preload and output increases. Clin Auton Res. 2015;25:347–54. doi: 10.1007/s10286-015-0286-x. doi: 10.1007/s10286-015-0286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]