Abstract
Seven new prenylated indole alkaloids, Taichunamides A-G (1–7), were isolated from the fungus Aspergillus taichungensis (IBT 19404). Taichunamides A (1) and B (2) contained an azetidine and 4-pyridone units, respectively, and are likely biosynthesized from Notoamide S (22) via (+)-6-epi-Stephacidin A (13). Taichunamides C (3) and D (4) contain an endoperoxide and methylsulfonyl units, respectively. This fungus produced indole alkaloids containing an anti-bicyclo[2.2.2]diazaoctane core, whereas A. protuberus and A. amoenus produced congeners with a syn-bicyclo[2.2.2]diazaoctane core. The plausible biosynthetic pathways of these cores in these three species, which likely arise via an intramolecular hetero Diels-Alder reaction from 22, is discussed.
Keywords: alkaloids, biosynthesis, azetidine, stereochemical diversity, structure elucidation
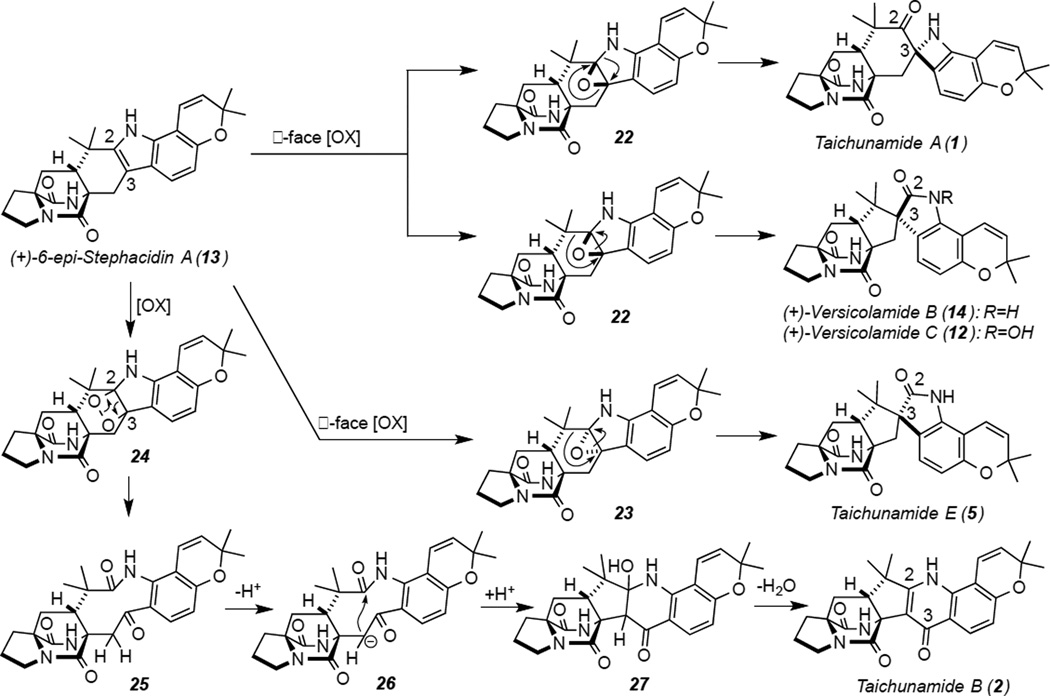
We have been studying the structures, synthesis and biosynthesis of the Notoamides and Stephacidins in the fungi of the genus Aspergillus and found that the marine-derived A. protuberus (MF297-2) produced the (+)-enantiomer of Stephacidin A (16) and the (−)-enantiomer of Notoamide B (15) as major metabolites (Scheme 1).[1] Curiously, A. amoenus (NRRL 35600) was found to produce their respective enantiomers[2] (Scheme 1). We have recently documented that both fungi produced the same enantiomer of (+)-Versicolamide B (14) as a minor metabolite (Scheme 1). The putative biosynthetic precursor to (+)-Versicolamide B (14), 6-epi-Stephacidin A (13), was isolated from A. amoenus as an enantiomeric mixture enriched with the (−)-isomer.[3] These stereochemical observations suggested that this fungus produced both enantiomers of 6-epi-Stephacidin A (13) and contain a highly enantio-discriminating oxidase, which converts only (+)-6-epi-Stephacidin A (13) into (+)-Versicolamide B (14), but not for (−)-13. The characteristic and unique bicyclo[2.2.2]diazaoctane core common to this family of prenylated indole alkaloids are most likely biosynthesized via an intramolecular hetero Diels-Alder (IMDA) reaction. The metabolite profiles of these fungi currently indicates that the biosynthesis of these stereochemically distinct metabolites plausibly proceeds via the pathways a (main) and b (minor) in A. protuberus and c (main) and b/d (minor) in A. amoenus. The recent isolation of a plausible precursor, Notoamide S (22), for these metabolites from A. amoenus provided strong support for this proposal.[3] Most of the alkaloids thus far identified containing the bicyclo[2.2.2]diazaoctane ring system, possess a syn-relative configuration at C6 (see Stephacidin numbering). Distinct, opposite enantiomers of Notoamide B (15) and Stephacidin A (16) are among the main metabolites from A. protuberus and A. amoenus, and Versicolamide B (14) was the first alkaloid within this family to be identified containing the corresponding anti-relative configuration at C6. Cai et al. recently isolated natural alkaloids with the anti-relative configuration, namely, (+)-Versicolamide B (14) and (+)-6-epi-Stephacidin A (13), from A. taichungensis ZHN-7-07[4] (syn- and anti-relationship is based on the H-21 and bridging amide C-18/N-19). With these stereochemical differences as a perspective, we have been interested in elucidating the biosynthetic machinery of the producing organisms that gives rise to the specific relative and absolute configurations observed. As part of that effort, we have carried out the isolation, structural elucidation and stereochemical assignments of structurally unprecedented alkaloids produced by A. taichungensis (IBT 19404) and herein suggest a possible biosynthetic pathway of seven new indole alkaloids that we have named the Taichunamides A-G (1–7).
Scheme 1.
Proposed facial specificities of IMDA reactions for metabolites in A. protuberus (circles), A. amoenus (triangles), and A. taichungensis (squares). Major and minor metabolites in each fungus are represented with large and small symbols, respectively.
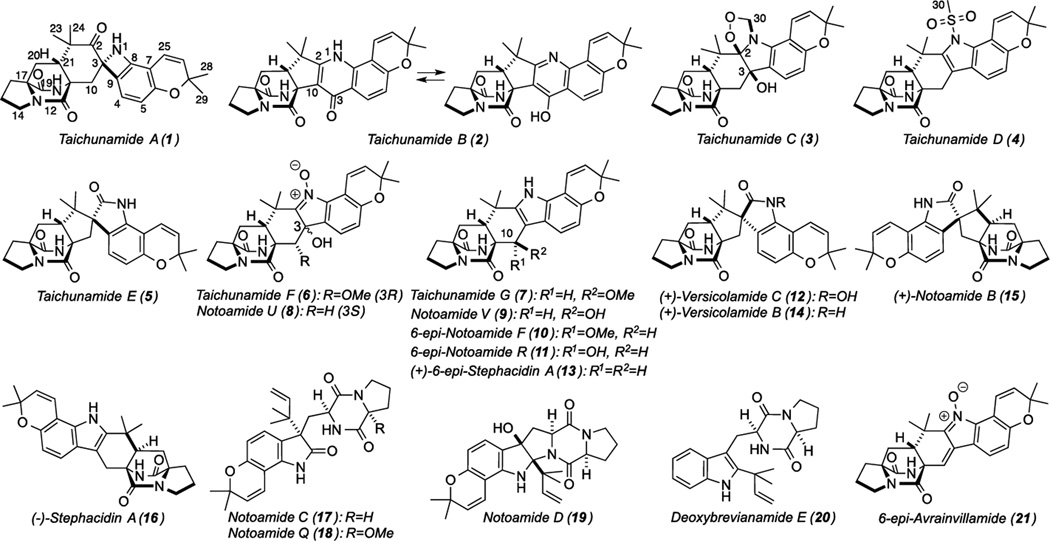
The fungus was cultured on a rice medium and extracted with n-BuOH. The extract was purified to afford 1–7 and fourteen known derivatives (8–21).[5] Of these, compounds 8–12 were previously obtained by the photo-conversion from (+)-6-epi-Stephacidin A (13)[4] and herein, we have isolated these compounds directly from the fungus for the first time.
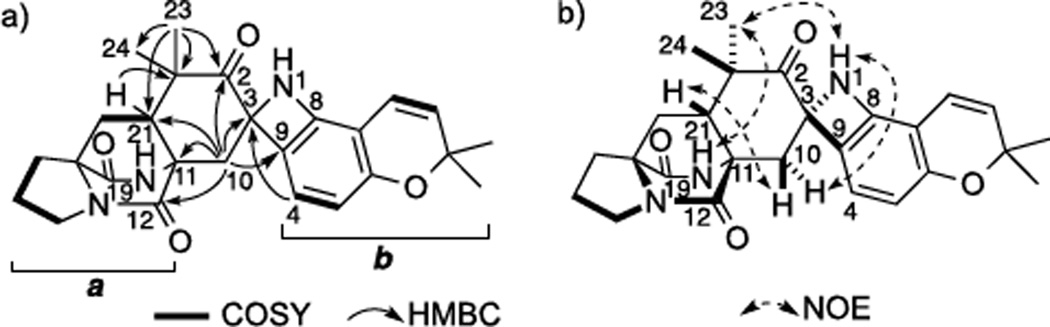
Taichunamide A (1) has the molecular formula C26H29N3O4, which was established by HRESIMS. The 1H and 13C NMR spectra in DMSO-d6 (Table S1) were similar to those of Versicolamide B (14) and indicated the presence of a bicyclo[2.2.2]diazaoctane core comprising proline (a unit in Figure 2a) and a 5,6-disubstituted 2,2-dimethyl-2H-chromene (b unit) also present in 14. The presence of a 2,2-dimethylcyclohexanone ring fused with an a unit was indicated by the presence of a ketone carbon (δC 190.7 (C-2)) and two singlet methyl groups (δH 1.35, δC 19.7 (C-23); δH 1.21, δC 27.2 (C-24)) along with HMBC correlations, H3-23/C-2, C-21, C-22, and C-24, H2-10/C-2, C-3, C-11, C-12, and C-21, and H-21/C-22. The direct connection between C-9 in the b unit and the quaternary carbon C-3 was shown by HMBC correlations, H-4/C-3 and H-10/C-9. The chemical shifts of C-3 (δC 81.3) and C-8 (δC 147.9) revealed that the two carbons were linked through the remaining portion NH, which resulted in the formation of an azetidine ring. Two exchangeable hydrogen signals were observed at δ 6.29 (s) and 7.54 (s). The latter signal showed HMBC correlations with C-11, C-12, and C-17, which indicated that the signal was H-19 and the former signal was H-1. The relative configuration of 1 was established by NOE correlations, H-1/H-10 (δ 2.63), H-1/H3-23 (δ 1.35), H3-23/H-19, and H-21/H-10 (δ 1.71), which showed that H-1, H-10 (δ 2.63), H-19, and H3-23 (δ 1.35) were on the same side and H-21 and H-10 (δ 1.71) were located on the opposite side (Figure 2b, see Figure S1). The Cotton effect at 225–250 nm arises from an n-π* transition of the dioxopiperazine moiety, which is diagnostic of the bicyclo[2.2.2]diazaoctane dioxopiperazine core.[6] The ECD spectra of 1 showed a positive Cotton effect around 225 nm, and thus the absolute configuration of 1 was assigned as 3S,11S,17S,21R.
Figure 2.
COSY and key HMBC correlations (a) and key NOE correlations (b) for 1.
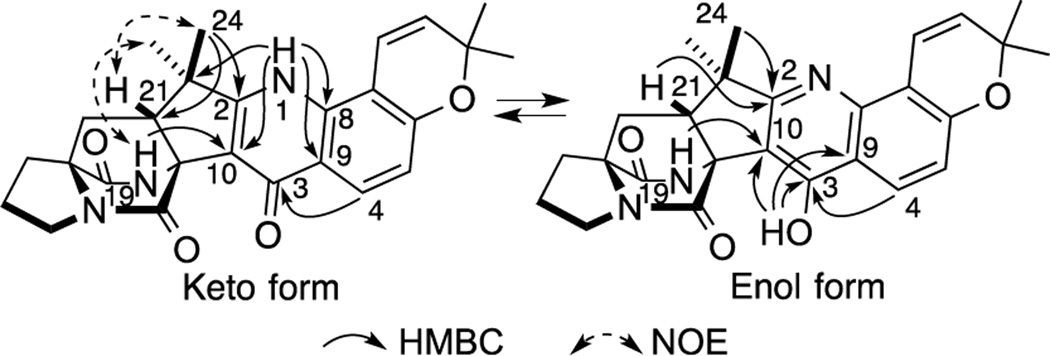
Taichunamide B (2) showed a protonated molecular ion at m/z 446.2064, which indicated a molecular formula C26H27N3O4. The 1H NMR spectrum in DMSO-d6 (Table S2) indicated that 2 was an equilibrium mixture of two entities in the ratio of 3:1. An analysis of 2D NMR spectra revealed that 2 contained a 5,6-disubstituted 2,2-dimethyl-2H-chromene and bicyclo[2.2.2]diazaoctane moieties as observed in 1. HMBC correlations, H-4 (δH 7.92)/C-3 (δC 172.8), H-1 (δH 10.42)/C-8 (δC 135.7), C-9 (δC 121.1), C-10 (δC 119.9), and C-22 (δC 43.7), H3-24 (δH 1.41)/C-2 (δC 164.1) and C-21 (δC 53.5), and H-19 (δH 8.47)/C-10, revealed that the major tautomer of 2 comprised a 4-pyridone ring (Figure 3a). On the other hand, HMBC correlations, 3-OH (δH 11.27)/C-3 (δC 157.3), C-9 (δC 114.4), and C-10 (δC 107.4), H-4 (δH 7.96)/C-3, and H-19 (δH 8.93)/C-10, H3-24 (δH 1.30) and H-21 (δH 2.15)/C-2 (δC 174.2), secured the presence of a 4-pyridol ring as the minor tautomer (Figure 3). Thus, 2 exists as an equilibrium mixture of keto-enol tautomers in DMSO-d6. Curiously, the ratio of the keto and enol forms is highly solvent-dependent. A single keto form is evident in CD3OD and a single enol form is apparent in acetone-d6 (Table S3). NOE correlations, H-21/H3-24 and H-19/H3-23, in the keto form (Figure 3, see Figure S1) and ECD spectrum established the 11R,17S,21R configuration.
Figure 3.
Key HMBC and NOE correlations for 2.
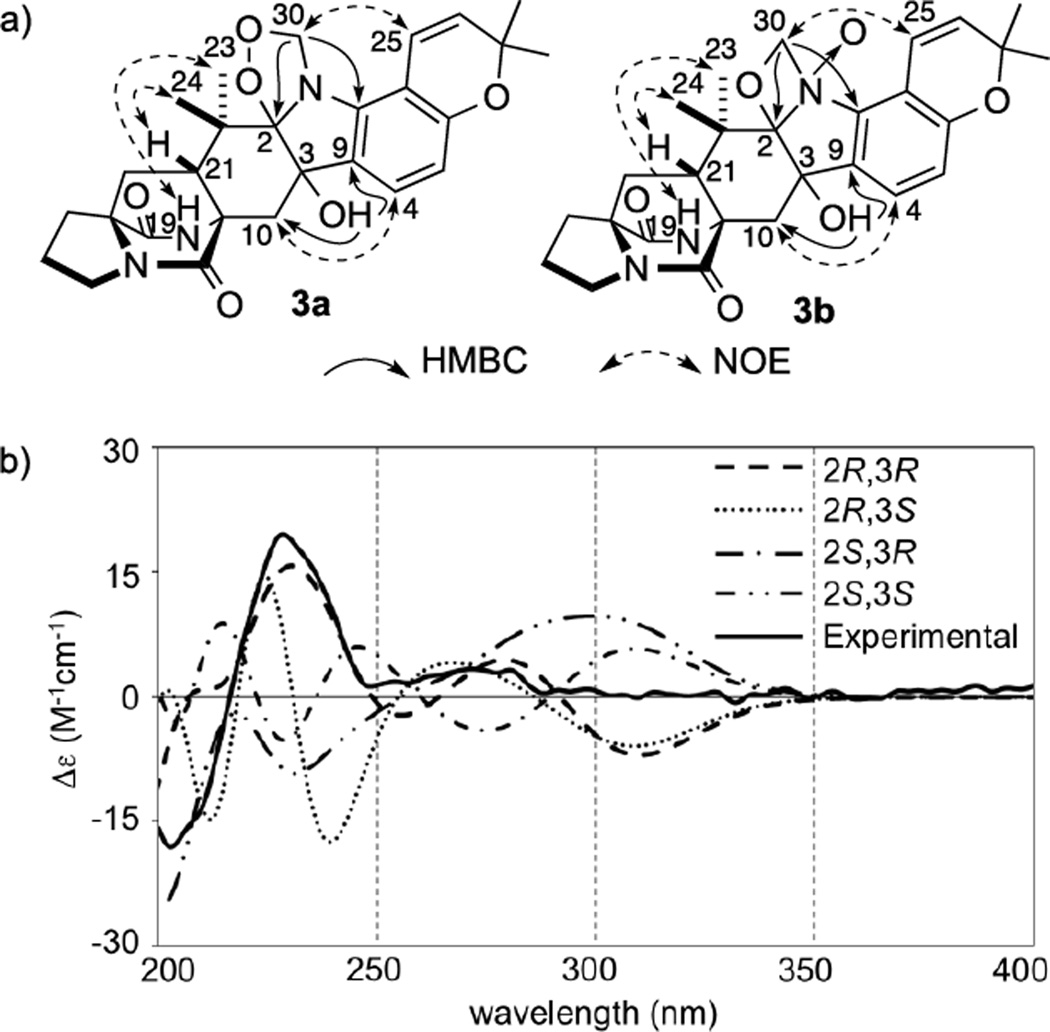
The molecular formula of Taichunamide C (3) was established by HRESIMS to be C27H31N3O6, indicating a CH3O3 unit more than that of 6-epi-Stephacidin A (13). Analysis of 2D NMR spectra (Table S4) indicated that the structure of 3 was similar to 13. Carbon chemical shifts of C2 (δ 107.2) and C3 (δ 73.2) suggested that the olefinic carbons, C2 and C3 in 13 were replaced with oxygen-bearing carbon atoms in 3. The presence of a hydroxyl group at C-3 was determined by HMBC correlations from 3-OH (δH 5.75) to C-9 (δC 130.1) and C-10 (δC 39.1) (Figure 4a). Geminal hydrogens (δH 4.65 and 4.57, H2-30) of the isolated methylene (δC 88.4, C-30) showed HMBC correlations with C-2 (δC 107.2) and C-8 (δC 139.8), which clearly indicated that the methylene was directly attached to N-1. Since C-30 was observed in low field, it may be attached to an oxygen atom. Considering the remaining two oxygen atoms in the molecular formula, there were two plausible possibilities for the structure of 3, namely peroxide 3a or N-oxide 3b (Figure 3a). NOE correlations, H-19/H3-23 (δ 1.35) and H-21/H3-24 (δ 0.87) (Figure 4a, see Figure S1), and the positive Cotton effect at 225 nm permits the stereochemical assignment as 11S,17S,21R. In order to establish the stereochemistry of C-2 and C-3, a low energy conformational search was quantum-mechanically conducted at the DFT level in Spartan’14 using four possible configurations, 2R,3R, 2R,3S, 2S,3R, and 2S,3S, for 3a and 3b (see Table S9). Although NOE correlation was observed for H-25/H-30 (δ 4.57), its calculated distance in 3b was over 3 Å in every configuration, and therefore 3b was excluded. Since the calculated distances are all sufficiently proximal in every configuration of 3a, computer simulation of ECD spectra were performed. As shown in Figure 4b, the calculated spectrum of 2R,3R-3a matched the experimental spectrum, and consequently the structure of 3 was determined to be 2R,3R,11S,17S,21R-3a.
Figure 4.
a) Key HMBC and NOE correlations for two possible structures of 3 and b) experimental ECD spectrum of 3 along with calculated ECD spectra of 2R,3R-, 2R,3S-, 2S,3R-, and 2S,3S-3a after optimization at B3LYP/6-31G* level.
Taichunamide D (4) has a molecular formula C27H31N3O5S, which was established by HRESIMS. Although 1H and 13C NMR spectra of 4 (Table S5) were almost superimposable with those of 6-epi-Stephacidin A (13), the presence of a singlet methyl group (δH 2.55 and δC 33.8, C-30), which showed no HMBC correlation, and the absence of an exchangeable hydrogen (δH 10.47, br s, NH) were evident. HRESIMS suggested the presence of an additional SO2 in 4 and 1H and 13C chemical shifts of the methyl group indicated that 4 was the corresponding N-methylsulfonyl derivative of (+)-6-epi-Stephacidin A (13), which was supported by the absorption bands at 1361 and 1179 cm−1 arising from asymmetric and symmetric SO2 stretching, respectively, in the IR spectrum along with the ECD and NOE spectra (Figure S1).
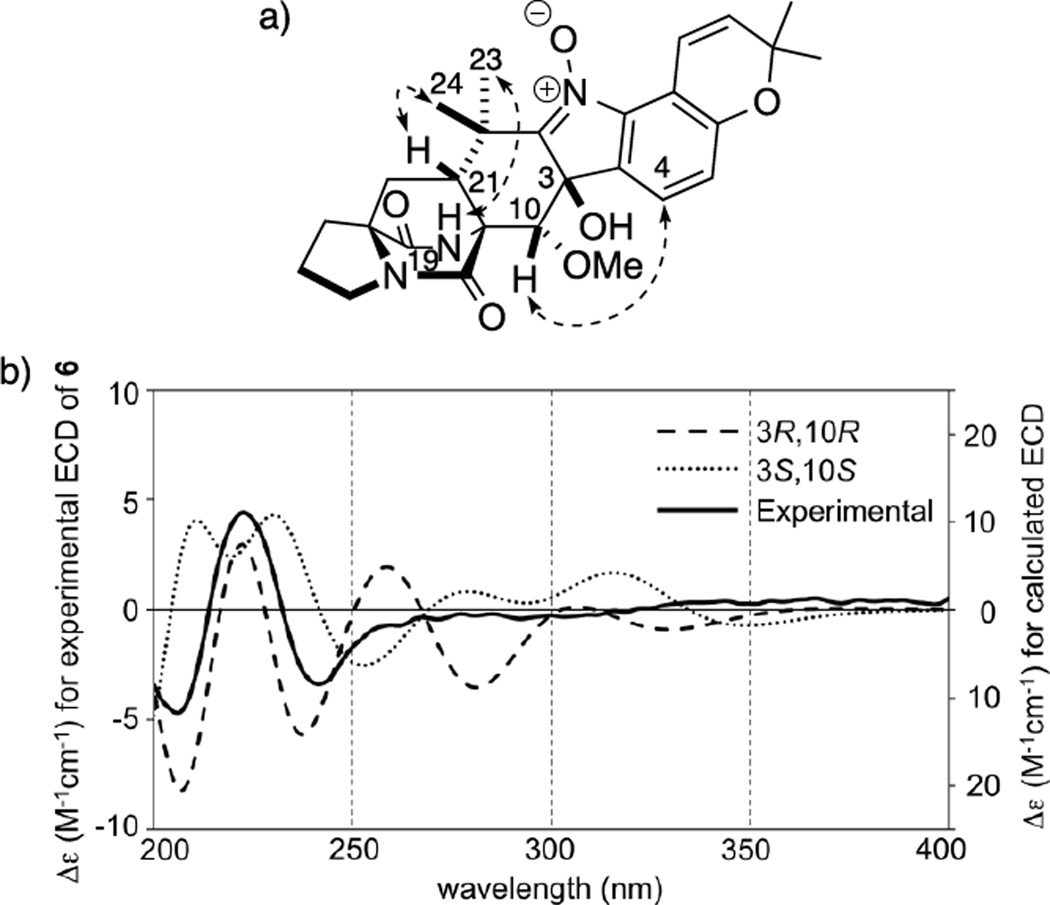
1H and 13C NMR spectra of Taichunamide F (6) were similar to those of Notoamide U[4] (8) and showed the presence of a methoxyl residue (δH 3.02, δC 61.6) (Table S7). HMBC correlations from the hydrogen to C-10 (δC 76.4) indicated that the methoxy group was attached to C-10. Although NOE correlations, H-19/H3-23 (δ 1.29) and H-21/H3-24 (δ 1.30) (Figure 5a, see Figure S1), and the positive Cotton effect at 225 nm showed 11S,17S,21R-configuration, the configurations of C-3 and C-10 could not be determined by spectroscopic data. A low energy conformational search was then conducted at DFT level in Spartan’14 using the four possible isomers, 3R,10R-, 3R,10S-, 3S,10R-, and 3S,10S-6. Although a NOE cross peak was observed for H-4/H-10, its calculated distances in 3R,10S- and 3S,10R-6 were beyond 3 Å (see Table S10), which indicated that these configurations were excluded. Computer simulation of the ECD spectra for 3R,10R- and 3S,10S-6 were performed and the spectra for 3R,10R-6 matched well with the experimental spectrum (Figure 5b). Therefore, the structure of 6 was assigned to be 3-epi-10R-methoxy derivative of 8.
Figure 5.
a) Key NOE correlations of 3R,10R-6, and b) experimental ECD spectrum of 6 along with calculated ECD spectra of 3R,10R- and 3S,10S-6 after optimization at B3LYP/6-31G* level.
The structure determination of other new compounds, Taichunamides E (5) and G (7), is described in the Supporting Information. Regarding preliminary biological activity, Taichunamide F (6) and 6-epi-Avrainvillamide (21) were found to inhibit the chymotrypsin-like activity of the proteasome by 81 and 95%, respectively, at the concentration of 10 µM. However, other compounds were inactive at this concentration.
The new alkaloids described herein, include unprecedented structures, in particular, Taichunamides A, B, and C (1–3) all constitute hitherto unknown systems derived from tailoring of the tryptophan moiety. Thus, the biosynthesis of these novel compounds constitutes a series of fascinating bond constructions. We suggest in Scheme 2, plausible biosynthetic pathways for the construction of these natural compounds. The most plausible biosynthetic precursor is (+)-6-epi-Stephacidin A (13), a main metabolite in this fungus, which would afford 1 and (+)-Versicolamide B (14)/(+)-Versicolamide C (12) as minor and major metabolites, respectively, through β-face oxidation followed by distinct pinacol rearrangements. This hypothesis is consistent with the stereochemical configuration at C-3 of 1, which was determined by NOE correlations and ECD spectra. On the other hand, Taichunamide E (5) palusibly arises via α-face oxidation followed by a pinacol rearrangement. Compared with β-face oxidation, α-face oxidation is more sterically demanding with N-19, reflected by the metabolite ratios of (+)-14 (15.9 mg)/(+)-12 (240 mg) versus 5 (0.43 mg). Although 5 was identified as a minor product along with 12,[7] this is the first report of its isolation from the fungal culture. The 4-pyridone unit in 2 would reasonably arise by singlet oxygen reaction at the indole 2,3-position of (+)-13, followed by cyclization (Scheme 2). A wide structural array of prenylated indole alkaloids have been isolated from the genera of Aspergillus and Penicillium to date,[1,2,8,9b] yet their respective carbon frameworks are very unique. Relatively few natural products containing an azetidine ring are known,[9] and to our knowledge, Taichunamide A (1) is the first naturally occurring spiro-indole-derived metabolite bearing an azetidine moiety. Taichunamide D (4) was found to contain a 1-methylsulfonyl group. To the best of our knowledge, this is the first report of the isolation of 1-methylsulfonylindole alkaloid from natural sources. In this study, we isolated (+)-Versicolamide C (12, 240 mg) and (+)-6-epi-Stephacidin A (13, 160 mg) as major metabolites and (+)-Notoamide B (15, 0.77 mg) and (−)-Stephacidin A (16, 0.82 mg) as minor metabolites from A. taichungensis (IBT 19404), which are most plausibly biosynthesized via pathways b and c (Scheme 1). Previously, we reported that A. protuberus (MF297-2) produced (+)-Stephacidin A (16) and (−)-Notoamide B (15) as major metabolites[1] and A. amoenus (NRRL 35600) produced their respective enantiomers.[2] Comparing the metabolite profiles of these three ancestrally-related (orthologous) species clearly reveals the distinct yet subtle stereochemical diversity interrogations being conducted within this genus that involves both enantiodivergent and diastereodivergent biosynthetic constructions. We have previously reported the high sequence homology between the genes encoding the biosynthetic enzymes in this genus. However, the underlying biochemical bases for the stereochemical divergencies observed remains to be elucidated at high resolution.[10] The structurally new and unique metabolites recorded here, are likely just a tantalizing microcosm of secondary metabolite tailoring leading to unprecedented structural diversity from a relatively small pool of primary metabolite building blocks. Future efforts of our laboratories are directed at further elucidating the breadth of structural diversity extant within the Aspergillus genus, and that of closely related marine and terrestrial fungi.
Scheme 2.
Possible biogenetic pathways of 1, 2, 5, and 12/14 from 13.
Supplementary Material
Figure 1.
Structures of prenylated indole alkaloids from Aspergillustaichungensis (IBT 19404).
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (Nos. 23108518 and 25108719 to S.T. and 24710252 to H.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Financial support from the National Institutes of Health (R01 CA070375 to R.M.W. and D.H.S.) is gratefully acknowledged.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.2015xxxxx.
Contributor Information
Ippei Kagiyama, Graduated School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Kumamoto 862-0973 (Japan).
Dr. Hikaru Kato, Graduated School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Kumamoto 862-0973 (Japan)
Dr. Tatsuo Nehira, Graduated School of Integrated Arts and Sciences, Hiroshima University, 1-7-1 Kagamiyama, Higashi-hiroshima 739-8521 (Japan)
Prof. Jens C. Frisvad, Section for Eukaryotic Biotechnology, Departments of System Biology, Building 221, Technical University of Denmark, 2800 Kongens Lyngby (Denmark)
Prof. David H. Sherman, Life Sciences Institute and Departments of Medicinal Chemistry, Chemistry, Microbiology & Immunology, The University of Michigan, 210 Washtenaw Avenue, Ann Arbor, MI 48109-2216 (USA)
Prof. Robert M. Williams, Department of Chemistry, Colorado State University, 1301 Center Avenue, Fort Collins, CO 80523 (USA) and Department of Chemistry, Colorado State University, 1301 Center Avenue, Fort Collins, CO 80523 (USA)
Prof. Sachiko Tsukamoto, Graduated School of Pharmaceutical Sciences, Kumamoto University, 5-1 Oe-honmachi, Kumamoto 862-0973 (Japan)
References
- 1.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew. Chem. Int. Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. Angew. Chem.2007, 119, 2304–2306. [DOI] [PubMed] [Google Scholar]
- 2.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew. Chem. Int. Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. Angew. Chem. 2008, 120, 3629–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato H, Nakahara T, Sugimoto K, Matsuo K, Kagiyama I, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S. Org. Lett. 2015;17:700–703. doi: 10.1021/ol5037198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai S, Luan Y, Kong X, Zhu T, Gu Q, Li D. Org. Lett. 2013;15:2168–2171. doi: 10.1021/ol400694h. [DOI] [PubMed] [Google Scholar]
- 5.a) 8–12 and 21: reference 4; b) 17 and 19: reference 1; c) 18: Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. J. Nat. Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. d) 20: Steyn PS. Tetrahedron Lett. 1971;12:3331–3334.
- 6.Williams RM, Kwast E, Coffman H, Glinka T. J. Am. Chem. Soc. 1989;111:3064–3065. [Google Scholar]
- 7.Miller KA, Tsukamoto S, Williams RM. Nat. Chem. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Brevianamides: Steyn PS. Tetrahedron. 1973;29:107–120. b) Marcfortines: Prange T, Billion M-A, Vuilhorgne M, Pascard C, Polonsky J. Tetrahedron Lett. 1981;22:1977–1980. c) Paraherquamides: Blanchflower SE, Banks RM, Everet JR, Reading C. J. Antibiot. 1993;46:1355–1363. doi: 10.7164/antibiotics.46.1355. d) Okaramines: Murao S, Hayashi H, Takiuchi K, Arai M. Agric. Biol. Chem. 1989;53:461–469. e) Sclerotiamide: Authrine C, Gloer JB. J. Nat. Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. f) Notoamides: sukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J. Nat. Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. g) Apsperparalines: Hayashi H, Nishimoto Y, Nozaki H. Tetrahedron Lett. 1997;38:5655–5658. h) Avrainvillamide (CJ-17,665): Sugie Y, Hirai H, Inagaki T, Ishiguro M, Kim Y-J, Kojima Y, Sakakibara T, Sakemi S, Sugiura A, Suzuki Y, Brennan L, Duignan J, Huang LH, Sutcliffe J, Kojima N. J. Antibiot. 2001;54:911–916. doi: 10.7164/antibiotics.54.911. i) Stephacidins: Qian-Cutrone J, Haung S, Shu Y-Z, Vyas D, Fairchild C, Menendez Z, Krampitz K, Dalterio R, Klohr SE, Gao Q. J. Am. Chem. Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. j) Malbrancheamides: Martinez-Luis S, Rodriguez R, Acevedo L, Gonzalez MC, LiraRocha A, Mata R. Tetrahedron. 2006;62:1817–1822.
- 9.a) Kobayashi J, Cheng J-F, Ishibashi M, Wälchli MR, Yamamura S, Ohizumi Y. J. Chem. Soc. Perkin Trans. 1. 1991:1135–1137. [Google Scholar]; b) Hayashi H, Takiuchi K, Murao S, Arai M. Agric. Biol. Chem. 1988;52:2131–2133. [Google Scholar]; c) Kitajima M, Kogure N, Yamaguchi K, Takayama H, Aimi N. Org. Lett. 2003;5:2075–2078. doi: 10.1021/ol0344725. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Srinivasan K, Tran H, Yu F, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. Med Chem Comm. 2012;3:987–996. doi: 10.1039/C2MD20029E. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.