Hyaluronan (sodium hyaluronate, HA) is a very long, anionic linear polymer ubiquitously present in the extracellular matrix (ECM) of mammals, where it forms loose and elastic matrices. Due to its particular rheological properties, HA confers elastoviscosity to all tissues. In addition, HA interacts through hydrogen bonds, van der Waals forces and electrostatic interactions with specific proteins called hyaladerins and with membrane receptors, modulating development, morphogenesis, cell migration, apoptosis, cell survival, and inflammation and tumorigenesis.1
HA is also present in the synovial fluid filling the joints, being continuously secreted by the lining cells of the joint synovial membranes. The synovial fluid exerts a lubricant, protective role against mechanical forces acting on the joint, apparently due to its HA content. In osteoarthritis (OA), a degenerative disease of joints causing strong pain, HA of the synovial fluid is degraded and diluted. Forty years ago, Endre A. Balazs,1 championed the replacement of the altered synovial fluid of the osteoarthritic knee by a high-molecular weight (HMW) HA, named ‘viscosupplementation’, as a treatment for OA pain and pioneered the use, first in racehorses and then in humans, of intra-articular injections of HA to treat arthritic pain. The rationale behind was that in healthy joints, HA acts as an elastoviscous filter, buffering the transmission of mechanical forces to nociceptive endings. In osteoarthritis, the altered rheological properties of low-MW HA weaken this filtering effect, thereby facilitating movement-evoked pain.
Experimental evidence in animals and clinical studies in humans provided support to the tenet that intra-articular injection of HA effectively reduces activity in joint nociceptor nerves and osteoarthritic pain.2,3 However, the molecular mechanisms underlying these effects were not fully clear. It was shown4 that the opening probability of stretch-activated ion channels in oocytes is reduced when they are immersed in HMW HA solutions, suggesting that HA molecules interact with the extracellular mechanosensory apparatus, modulating the opening of stretch-activated channels.
However, the analgesic effects of HA are more marked in inflamed than in intact knee-joints, suggesting that HA may also interact with inflammatory molecules and/or with their molecular targets in nociceptive terminals. TRPV1, a non-selective cationic channel preferentially expressed in primary nociceptive neurons, plays a major role in the detection of noxious stimuli by nociceptive nerve endings and in their sensitization by endogenous inflammatory mediators. Accordingly, TRPV1 has been implicated in pain caused by endogenous chemical agents released during long-term systemic painful arthritis.5 Due to its role as ‘molecular integrator’ of noxious stimuli this channel appears as a promising target for HA analgesic actions; noteworthy, extracellular HA modulates the activity of Ca(v)1.2 channels in hippocampal neurons, thereby contributing to the regulation of use-dependent synaptic plasticity associated to memory.6
In a recent study,7 we demonstrated that HA reduces the response of TRPV1 to heat, H+ and capsaicin both in HEK 293 cells transfected with TRPV1 and in cultured DRG and nodose ganglion sensory neurons. After exposure to HA, TRPV1 channels opened less frequently. Moreover, HA decreased the excitability of peripheral nociceptive neurons and joint sensory fibers, thereby reducing their responsiveness to noxious stimuli. HA modulation of channel function was restricted to TRPV1, whereas the activity of TRPA1 and TRPM8 channels, also associated with sensory transduction of chemical noxious and thermal stimuli, was not affected by HA. Behavioral experiments in mice in which endogenous HA was degraded with hyaluronidase, or in TRPV1 null mice further demonstrated that HA diminished nociceptive responses to heat and capsaicin.
HA inhibitory effects on TRPV1 activity suggest a specific binding with the channel protein, thereby modulating its gating. Single-channel recordings showed that inhibition of TRPV1 by HA is mediated through a stabilization of the channel's closed state. A similar blockade mechanism has been proposed for the inhibitory activity of polyclonal antibodies binding to the pre-pore loop of TRPV1. Our in silico docking analysis showed a high probability for an electrostatic-type interaction between HA and a short positively-charged sequence “H+xRG” located in the external pore domain (S5-P-S6), which we confirmed by electrophysiological recordings of TRPV1 mutant channels (TRPV1 K615A/R617A). A reasonable molecular explanation according to the most recent structural model of TRPV18 is that HA, by immobilizing the outer pore loop, impedes the conformational changes of the channel needed for opening, locking the channel in the closed state.
Our work unveiled a hitherto ignored mechanism of control of TRPV1 open probability through the interaction with HA, an important EM component, and provides a novel explanation for the analgesic effects of intra-articularly injected HA. TRPV1 is the canonical polymodal nociceptive neuron channel, but is also expressed by a variety of other types of neural and non-neural cells. The possibility that some functional aspects of these cells are modulated by HA, ubiquitously present in their extracellular matrix, is worth to be considered (Fig. 1).
Figure 1.
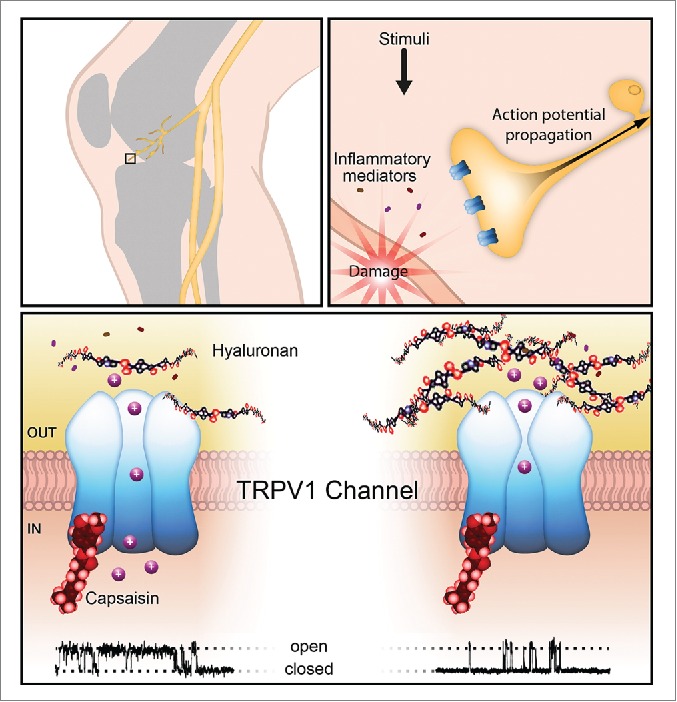
Joints are innervated by nerve endings expressing TRPV1 channels (upper panels). As shown in the lower panel, increase in the density of HA molecules interacting with the TRPV1 channel reduces its opening probability, as shown in the single-channel activity recordings, thereby decreasing nerve impulse activity in nociceptor endings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Balazs E.A, et al.. Hyaluronan: Structure, Metabolism, Biological Activities, Therapeutic Applications, Matrix Biology Institute, Edgewater, NJ, USA: 2005; ISBN 0-9771359-0-X [Google Scholar]
- [2].Pozo MA, et al.. Exp Brain Res 1997; 116:3-9; PMID:9305809; http://dx.doi.org/ 10.1007/PL00005742 [DOI] [PubMed] [Google Scholar]
- [3].Gomis A, et al.. Arthritis Rheum 2004; 50:314-26; PMID:14730630; http://dx.doi.org/ 10.1002/art.11421 [DOI] [PubMed] [Google Scholar]
- [4].De la Peña E, et al.. Pain 2002; 99:501-8; PMID:12406526 [DOI] [PubMed] [Google Scholar]
- [5].Kelly S. et al.. Ann Rheum Dis 2015; 2074:252-9; http://dx.doi.org/ 10.1136/annrheumdis-2013-203413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kochlamazashvili G, et al.. Neuron 2010; 67:116-28; PMID:20624596; http://dx.doi.org/ 10.1016/j.neuron.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caires R. et al.. Nat Commun 2015; 6:8095; PMID:26311398; http://dx.doi.org/ 10.1038/ncomms9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cao E. et al.. Nature 2013; 504:113-8; PMID:24305161; http://dx.doi.org/ 10.1038/nature12823 [DOI] [PMC free article] [PubMed] [Google Scholar]


