Introduction
Investigations into reversible cysteine acylation (S-palmitoylation) have enjoyed a renaissance recently, thanks to numerous techniques that have improved the sensitivity of detection above the traditional use of 3H-palmitate. Often considered a largely static modification necessary only for traffic of membrane proteins through the secretory pathway, an appreciation is now growing that S-palmitoylation dynamically regulates the landscape of the cell surface in unexpected ways, including the formation and endocytosis of protein/lipid domains, as well as directly modulating ion channel and transporter function.
S-palmitoylation and NCX1 Inactivation
The electrogenic Na/Ca exchanger, NCX, mediates bidirectional Ca movements that are highly sensitive to changes in Na gradients in many cells. In cardiac muscle NCX1 is responsible for Ca removal during diastole, but currents generated by NCX1 in the heart can cause arrhythmogenic delayed afterdepolarizations, and activation of reverse mode NCX1 following reoxygenation of ischemic tissue causes Ca overload and myocyte necrosis.1
In our recent paper2 we show that NCX1 is substantially S-palmitoylated in all tissues investigated. Palmitoylation occurs in the Golgi at a single cysteine (C739) in the NCX1 regulatory intracellular loop, such that ∼60% of the NCX1 in ventricular muscle is palmitoylated. In HEK cells the delivery of unpalmitoylatable NCX1 to the cell surface is indistinguishable from wild type. The normal forward and reverse transport modes of NCX1 are unaffected by palmitoylation, but NCX1 inactivation is profoundly influenced by palmitoylation.
NCX1 inactivation decreases myocyte Na load during metabolic stress as part of a shift of Ca handling to internal recycling from turnover across the sarcolemma. Inactivation is promoted by a variety of cellular conditions, including Na overload, Ca depletion and PIP2 depletion. We measured NCX1 activity following chelation of intracellular Ca and after depleting or chelating PIP2, and found substantial NCX1 activity in cells expressing unpalmitoylatable NCX1 when wild type NCX1 inactivated. Hence palmitoylation is required for efficient NCX1 inactivation.
NCX1 inactivation involves an interaction of the exchanger inhibitory peptide (XIP, residues 219–238 at the N-terminus of the NCX1 regulatory intracellular loop) with a distal region of this loop. Anionic phospholipids such as PIP2 oppose NCX1 inactivation by binding to XIP.3 Our data therefore suggest that the functional effect of XIP on NCX1 is enhanced by NCX1 palmitoylation at C739: perhaps by a direct influence on the tertiary structure of the loop to facilitate XIP binding. Historically NCX inactivation is not observed in all experimental models, and our data suggests an explanation for this controversy: it may reflect differences in NCX palmitoylation in these models. Indeed, given that ∼60% of NCX1 is palmitoylated in ventricular muscle, the remaining 40% must be rather insensitive to inactivation. The functional implications for this remain to be established: clearly, it will be important to determine the mechanism(s) behind NCX1 depalmitoylation, which presumably occurs after its delivery to the cell surface.
S-palmitoylation and the Regulation of Cardiac Na Transporters
Massive endocytosis (MEND) is a recently described, adapter-independent form of endocytosis in which up to 70% of the cell surface membrane can be rapidly internalised following cell stressors such as Ca overload.4 Dynamic palmitoylation at the cell surface by the acyl transferase DHHC5 promotes MEND following mitochondrial stress, probably as a result of the clustering of palmitoylated proteins in lipid-ordered domains.5 In our recent study2 the presence of an abundant palmitoylated protein at the cell surface (wild type but not unpalmitoylatable NCX1) was sufficient to accelerate MEND when adapter-independent endocytosis was initiated by G protein activation (Fig. 1). This mirrors the influence of the palmitoylated Na pump accessory protein phospholemman on MEND,4,5 suggesting MEND is a ‘cargo-dependent’ phenomenon in which palmitoylated proteins promote the formation of lipid-protein domains at the cell surface to trigger endocytosis.
Figure 1.
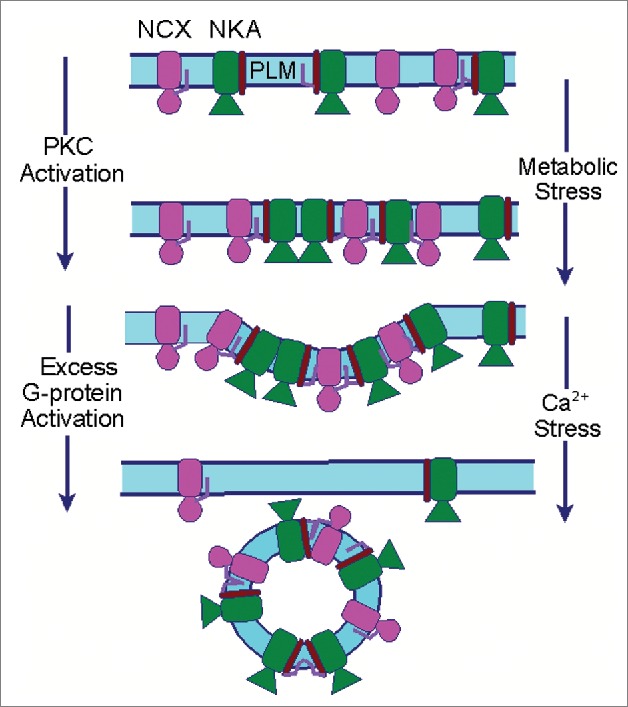
Palmitoylation of cardiac Na transporters can modify their function, physical interactions, and removal from the sarcolemma by domain-dependent endocytosis.
Many mechanistic questions about this form of endocytosis, including the actions of G-proteins and Ca, remain to be resolved. It is likely relevant that attachment of palmitate increases integral membrane protein association with lipid-ordered domains (probably because the saturated palmitoyl chain has a high affinity for the ordered lipid environment).6 Intriguingly, the acyl group of a palmitoylated protein prefers to insert into curved rather than planar membranes, because it is easier for the acyl chain to penetrate between phospholipid head groups.7 This implies that palmitoylated proteins will naturally cluster in invaginated (curved) lipid-ordered domains (such as those that form during endocytosis). The ordering of membrane proteins with bulky cytoplasmic domains, such as the Na pump and NCX, would further promote the development of curvature.
The cardiac myocyte surface membrane is notably far from planar, with abundant caveolae (in which palmitoylated proteins are enriched8), and curved junctions between the t-tubular and sarcolemmal compartments. Even in the absence of MEND-inducing stressors palmitoylation may therefore influence surface membrane microdomain localization as well as inactivation of a population of cardiac NCX1, with the potential to generate regional differences in NCX1 regulation within a myocyte. This alone has significant implications for regulation of local and global myocyte Ca handling. Since ion transporter palmitoylation also appears to influence the formation of surface membrane microdomains and their internalization via the MEND pathway, membrane protein palmitoylation clearly plays a major role in the regulation of cardiac function in both health and disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Venetucci LA, et al.. Na/Ca exchange: regulator of intracellular calcium and source of arrhythmias in the heart. Ann N Y Acad Sci 2007; 1099:315-25; PMID:17446473; http://dx.doi.org/ 10.1196/annals.1387.033 [DOI] [PubMed] [Google Scholar]
- [2].Reilly L, et al.. Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling. FASEB J 2015; pii: fj.15–276493; PMID:26174834; http://dx.doi.org/ 10.1096/fj.15-276493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He Z, et al.. Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 2000; 278:C661-6; PMID:10751315 [DOI] [PubMed] [Google Scholar]
- [4].Hilgemann DW, et al.. Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts. eLife 2013; 2:e01293; http://dx.doi.org/ 10.7554/eLife.01293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lin MJ, et al.. Massive palmitoylation-dependent endocytosis during reoxygenation of anoxic cardiac muscle. eLife 2013; 2:e01295; http://dx.doi.org/ 10.7554/eLife.01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levental I, et al.. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A 2010; 107:22050-4; http://dx.doi.org/ 10.1073/pnas.1016184107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Larsen JB, et al.. Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases. Nat Chem Biol 2015; 11:192-4; http://dx.doi.org/ 10.1038/nchembio.1733 [DOI] [PubMed] [Google Scholar]
- [8].Wypijewski KJ, et al.. Identification of caveolar resident proteins in ventricular myocytes using a quantitative proteomic approach: dynamic changes in caveolar composition following adrenoceptor activation. Mol Cell Proteomics 2015; 14:596-608; http://dx.doi.org/ 10.1074/mcp.M114.038570 [DOI] [PMC free article] [PubMed] [Google Scholar]


