ABSTRACT
Osteoarthritis (OA) is a chronic disease affecting the cartilage of over 15% of Canadians. Synovial fluid mesenchymal progenitor cells (sfMPCs) are present in joints and are thought to contribute to healing. OA sfMPCs have a greater proliferative ability but decreased chondrogenic potential. However, little is known about the factors influencing/regulating the differences between normal and OA sfMPCs. Recently, our lab has shown that sfMPC chondrogenic differentiation in vitro is favorably biased toward a similar osmotic environment as they experience in vivo. The current study now examines the expression and functionality of a variety of ion channels in sfMPCs derived from normal individuals and early OA patients. Results indicated that there is differential ion channel regulation at the functional level and expression level in early OA sfMPCs. All ion channels were upregulated in early OA compared to normal sfMPCs with the exception of KCNMA1 at the mRNA level. At the protein level, TRPV4 was over expressed in early OA sfMPCs, while KCNJ12 and KCNMA1 were unchanged between normal and early OA sfMPCs. At the functional level, the inward rectifying potassium channel was under expressed in early OA sfMPCs, however the membrane potential was unchanged between normal and early OA sfMPCs. In the synovial environment itself, a number of differences in ion concentration between normal and early OA synovial fluid were observed. These findings suggest that normal and OA progenitor cells demonstrate functional differences in how they interact with the synovial ion environment.
Keywords: cartilage, ion channel, osteoarthritis, synovial progenitor cells, synovial fluid
Introduction
Osteoarthritis (OA) is a chronic disease characterized by progressive articular cartilage degeneration affecting 1 in 6 Canadians.1 There are currently no disease modifying drugs for the treatment of OA. Diagnosis of OA is typically after a history of joint pain and radiographic changes.2 Although the cartilage has some regenerative capacity, it is very limited and damage cannot be reversed after it has occurred. Treatment of OA is limited to symptom management and lifestyle changes, eventually leading to total joint replacement.2 Early diagnosis of the disease by biomarkers (biochemical, genetic, imaging) is an area that is gaining interest in OA research. Early diagnosis may be able to predict the onset of OA before pain and macroscopic cartilage damage has occurred, allowing for better management of the disease and potentially even delayed progression.3
Normally, the synovium plays a role in maintaining health of articular cartilage through nourishment and lubrication, however, the synovium and synovial fluid also contain resident synovial fluid mesenchymal progenitor cells (sfMPCs) that have the ability to differentiate into bone, fat, and cartilage.4 In OA, our lab and others have shown that sfMPCs have increased proliferative but reduced chondrogenic capacities.5,6 OA is a multifaceted disease and many factors may contribute to the change in phenotype between normal and OA sfMPCs. The synovial environment changes physically, chemically, and physiologically with injury or the onset of disease.7,8 Additionally, inflammation is a core factor in OA and as the disease progresses cytokines, chemokines and other factors that drive the deterioration of the joint are expressed.9,10 The physical environment might also play a role in the regulation of sfMPC phenotype. The synovial fluid osmolality of OA joints is significantly lower (∼280mOsm) compared to healthy joints (∼400mOsm).11 Changes in osmolality have been shown to regulate gene expression of Sox9, a central transcription factor during chondrogenesis.12,13,14 It was recently shown in our lab that sfMPCs undergo efficient chondrogenic differentiation in vitro in a similar osmotic environment as they experienced in vivo.11 In that study, sfMPCs were grown in vitro in hyper- and hypo-osmotic conditions and while changes in chondrogenic gene expression occurred, no cell volume changes were observed. This led us to believe that ion channels may be playing a role in the behavior of sfMPCs.
Ion channels are an essential component of the cell membrane that controls ion movement in and out of the cell and therefore may play a role in the response to osmotic changes associated with OA synovial fluid. These channels have been shown to play an important role in a variety of cell regulating processes.15-16 In specific regards to chondrocytes, potassium channels are involved in mechanotransduction, cell volume regulation, apoptosis and chondrogenesis.17 Ion channels have also been shown to respond to osmotic stress in chondrocytes as well, in particular, TRPV4 has been shown to respond to the early stages of hypo-osmotic stress in chondrocytes.18 and it has also been linked to the expression of Sox9, a key regulator of chondrogenesis.19
The chondrocyte channelome has been a focus of substantial study since chondrocytes have been historically the cell target of choice in OA. Recently the progenitor and stem cell channelome has been gaining interest for the investigation of these cell types as targets in multiple diseases. Human Mesenchymal stem cells (hMSCs) express a wide variety of ion channels that are implicated in different physiological functions. Potassium channels cooperate with calcium and chloride channels in regulating the calcium transient and volume oscillations that accompany the cell cycle.20 Potassium channel types control cell anchorage with stromal matrix and cell migration as well as release of paracrine growth factors.20
Little is known about the changes in ion channels during differentiation from MSCs to mature chondrocytes. A study done in 2005 aimed to functionally characterize ion channels in human Mesenchymal stem cells isolated from bone marrow.21 They found 3 outward currents, a Calcium-activated potassium channel, a transient outward K+ current, and a delayed rectifier K+ current.21 More recently, a review was written comparing calcium channels in mature chondrocytes, differentiating MSCs and undifferentiated MSCs.22 Voltage operated calcium channels (VOCCs) are found in both differentiating MSCs and mature chondrocytes, while the purinergic ligand operated Ca2+ receptor/channels (P2Y) and TRPV4 channels are found in all 3 cell types.22
Although ion channels have been investigated for use as targeting drugs for chronic diseases such as cancer,23,24,25 little is known about their role in MSCs and to our knowledge no studies have looked at the ion channels in sfMPCs. Understanding the changes in channelome between diseased and normal sfMPCs could lead to novel pharmaceutical targets for degenerative joint diseases such as OA. Therefore, this study was undertaken to investigate the expression and functionality of a variety of ion channels in sfMPCs derived from normal individuals and patients with clinically diagnosed early OA.
Methods and materials
Patient criteria
Informed consent to participate was obtained by written agreement. The study protocol was approved by the University of Calgary Research Ethics Board. Normal Group: Inclusion criteria for control cadaveric donations were an age of 30 y or older, no history of arthritis, joint injury or surgery (including visual inspection of the cartilage surfaces during recovery), no prescription anti-inflammatory medications, no co-morbidities (such as diabetes/cancer), and availability within 4 hrs of death. The Southern Alberta Organ and Tissue Donation Program (SAOTDP) screens the medical history of every donor including current medication, eliminating individuals with a previous history of joint disease and other co-morbidities (e.g. cancer, diabetes, inflammatory diseases).
OA group
Early OA was diagnosed based on arthroscopic examination; these patients had an Outerbridge score of under grade 2 (a partial-thickness defect with fissures on the surface that do not reach sub-chondral bone or exceed 1.5 cm in diameter).
Cell derivation
The fresh synovial fluid was plated in untreated culture dishes and after 1–2 hrs at 37°C/5%CO2 culture media was added. Culture media consisted of DMEM (Invitrogen # 11965), 10% FBS, 1% Pen/Strep, 1% Non-essential amino acids (NEAA), 0.2% Beta-mercaptoethanol (BME) (all Invitrogen, Carlsbad, CA). Once cells had adhered to the plastic and reached 30–40% confluence, the media was changed and the cells were allowed to reach 60–70% confluence. At this point the cells were dissociated and resuspended in Dulbecco's PBS (DPBS) at 1 million cells/ml. Progenitors were isolated using magnetic separation. First the total cell population was depleted for cell expressing CD3, CD14, CD16, CD19, CD41a, CD56 and Glycophorin A (All Becton, Dickinson and Company (BD), Franklin Lakes, NJ). The resultant cell population was then purified for cells expressing CD90 (BD). After 7–10 days, fibroblast cell populations were present and isolated as described above.
Flow cytometry
The cells were dissociated and resuspended in 500μl of 90% MeOH and left for 5–10 minutes at room temperature. The cells were then centrifuged, the liquid was removed and 500μl of 0.1% Tween 20 was added to permeabilize the cells for 20 minutes at room temperature. The cells were centrifuged again, the liquid was removed, and 50 μl of Tween buffer and 0.5 μg of antibody was added to each tube and incubated in the dark for 30–45 minutes at room temperature. The cells were then washed 3 times with FACs buffer then resuspended in FACs buffer. The cells were then measured using FACs Caliber. The results were analyzed using FlowJo software.
Fura Red/Fluo-4
Cells were dissociated and resuspended in 10μl of 3μM Fluo-4, 6μl Fura Red solution in PBS and incubated in the dark for 45 minutes at 37°C. Cells were washed with media once, spun down and resuspended in 500 μl of media. Cells were incubated in the dark for 20 minutes at room temperature. Cells were measured on an Attune FACs machine and analyzed using Applied Biosystems Attune software. The ratio of Fluo-4 (530 nm) to Fura Red (610 nm) mean fluorescence intensity was determined for each sample.
Electrophysiology
Single cell electrophysiological measurements were made using the patch-clamp technique in the whole cell configuration, with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Patch pipettes were made from borosilicate capillaries using a Narishige pipette puller (Model PP-10). The resistance of the patch electrodes ranged from 2 to 5 MΩ when filled with a solution containing (in mM) K-Asp 100, KCl 20, CaCl20.85, MgCl21, HEPES 10, EGTA 5, Na-ATP 4 (pH was titrated to 7.2 with KOH 1 N). The control extracellulr solution contained (in mM) NaCl 140, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, Glucose 5.5 (pH was titrated to 7.4 with NaOH 1 N). To enhance transmembrane whole-cell currents carried by potassium (K+) ions (for the characterization of K+ channel subtypes), the control extracellular solution was replaced by a K+-rich solution, in which 65 mM NaCl was replaced by equimolar KCl. This yielded a final K+ ion concentration of 70 mM. A Digidata 1440A acquisition system (Molecular Devices) operating at a sampling rate of 2.0 kHz (with filtering at 1 kHz) was used. All experiments were done at room temperature (21°C). Data analysis was carried using OriginPro software (OriginLab, Northampton, MA). Current records were corrected for capacitive transients in the cell-attached mode using the compensation circuit of the amplifier, before obtaining the whole cell configuration.
Micro-array analysis
RNA was extracted using Trizol Reagent (Life Technologies, Inc., New York, USA) according to the manufacturer's protocol. Total RNA was purified with RNeasy Plus Micro Kit (Qiagen, Valencia, USA) to remove genomic DNA. The RNA integrity number (RIN) was measured with Agilent RNA 6000 NanoChips on a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA). The quantity was measured with a NanoDrop 1000 (NanoDrop Technologies, Inc., Wilmington, USA). A total of 300 ng of each RNA sample with RIN higher than 9 was labeled with GeneChip Whole Transcript (WT) Sense Target Labeling Assay (Affymetrix, Santa Clara, USA) and hybridized to Affymetrix GeneChip Human Gene 1.0 ST Arrays at 45°C for 16 hours. Arrays were stained and washed on Affymetrix GeneChip Fluidics 450 following the manufacturer's protocol and scanned with an Affymetrix GeneChip Scanner 3000 7G System.
Micro-array data analysis
Array data files were generated with GeneChip® Command Console® Software (AGCC) (Affymetrix, Santa Clara, USA) and statistical significant analysis was carried out on software of GeneSpringTM(Agilent Technologies). The fold change between normal and OA samples was based on the p < 0.05 from a T-test (Asymptotic and Benjamini Hochberg FDR).
Synovial fluid ion analysis
The ASTM D 1976–07 Standard Test Method for Elements in Water by Inductively-Coupled Argon Plasma Atomic Emission Spectroscopy followed. Briefly, synovial fluid and standards were introduced to the ICP system (ThermoFisher iCAP 6400) via a peristaltic pump and nebulizer. The liquid was nebulized into a fine mist in the spray chamber and taken up into the argon plasma, where it was broken into free atoms. As these atoms lose electrons and recombine in the plasma they emit light at characteristic wavelengths for each element. The light emitted was proportional to the concentration of the element and is detected by a charged coupled detector thus allowing the concentration of the elements in each sample to be calculated.
Statistical analysis
Each treatment (cell differentiation) and qPCR was performed in triplicate. Statistical analysis (ANOVA) was performed on qPCR data using GraphPad Prism4 (GraphPad Software) and significance was set at p < 0 .05.
Results
The ion concentration in synovial fluid samples of 2 normal and 2 early OA patients was analyzed using Inductively-Coupled Argon Plasma Atomic Emission Spectroscopy. Ca, Fe, K, Mg, Na, and P were all differentially expressed between the normal and OA samples, with OA samples showing a higher concentration of each ion (Table 1). The normal and OA sfMPCs used in the remainder of the study were isolated from these samples.
Table 1.
Ion concentration in synovial fluid from normal individuals and OA patients.
| Normal | +/− | OA | +/− | |
|---|---|---|---|---|
| Ca (ppm) | 12.747 | 4.849 | 42.086 | 11.243 |
| Fe (ppm) | 0.086 | 0.025 | 0.536 | 0.198 |
| K (ppm) | 36.740 | 20.676 | 118.740 | 83.014 |
| Mg (ppm) | 4.074 | 1.092 | 10.381 | 3.475 |
| Na (ppm) | 714.586 | 67.458 | 1951.686 | 451.700 |
| P (ppm) | 14.577 | 9.349 | 34.628 | 5.600 |
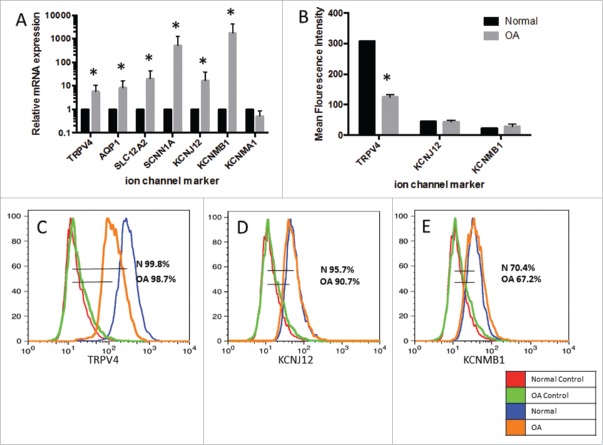
To identify if ion channels were differentially expressed between normal and OA sfMPCs and get a clearer picture of the potential channelome in these cells, microarray analysis was undertaken (Table 2). Thirteen ion channels were differentially expressed between normal and OA sfMPCs (for complete microarray data, see Table S1). Based off of this data and our previous sfMPC osmolality study,11 ion channels might play a role in the change in phenotype and function of osteoarthritic sfMPCs. Therefore, a number of ion channels present that changed in expression between normal and OA sfMPCs were studied in further detail. Transient receptor potential cation channel vanilloid-4 (TRPV4) was chosen because it is a channel known to be sensitive to osmotic changes in chondrocytes. Two potassium channels were studied, an inward rectifier (KCNJ12) and a calcium activated potassium channel (KCNMA1, KCNMB1), which has been shown to increase expression in equine chondrocytes under hypotonic conditions.18 Two channels known for cell volume regulation in chondrocytes were included (AQP1, SLC12A2). Finally, a sodium channel known to be functional in chondrocytes was studied (SCNN1A).26 When quantitative PCR was performed on each of these genes to validate the microarray data, it was found that each ion channel mRNA was upregulated in OA sfMPCs compared to normal sfMPCs with the exception of KCNMA1, which had no change (Fig. 1A).
Table 2.
Ion channel expression data from microarray data collected from normal and OA sfMPCs.
| Gene Symbol | Name | Fold-Difference |
|---|---|---|
| SLC40A1 | Solute Carrier Family 40 (Iron-Regulated Transporter), member 2 | 26.281 |
| KCND2 | potassium voltage-gated channel, Shal-related subfamily, member 2 | 9.370 |
| SLC7A11 | Solute Carrier Family 7 (anionic amino acid transporter light chain, xc-system), member 11 | 7.868 |
| KCNJ6 | potassium inwardly-rectifying channel, subfamily J, member 6 | 6.870 |
| CLIC2 | chloride intracellular challel 2 | 5.994 |
| SLC2A12 | Solute Carrier Family 2 (facilitated glucose transporter), member 12 | 4.853 |
| CLCA2 | chloride channel accessory 2 | 4.675 |
| SLC1A3 | Solute Carrier Family 1 (glial high affinity glutamate transporter), member 3 | 4.327 |
| SLC19A3 | Solute Carrier Family 19 (thiamine transporter), member 3 | 4.169 |
| SLC7A5 | Solute Carrier Family 7 (amino acid transporter light chain, L system), member 5 | 4.050 |
| SLC26A4 | Solute Carrier Family 26 (anion exchanger), member 4 | 3.110 |
| NALCN | sodium leak channel, non-selective | 3.123 |
| SLC14A1 | Solute Carrier Family 14 (urea transporter), member 1 (Kidd blood group) | 5.904 |
Figure 1.
Characterization of ion channel expression. mRNA expression of ion channel markers in OA sfMPC lines (n=2) normalized to the average mRNA expression of each ion channel marker in sfMPCs from normal patients (n=2) (A). Flow cytometry data for OA compared to normal patient. Mean fluorescence intensity of each sample (B). Histograms and percent expression of TRPV4 (C), KCNJ12 (D), and KCNMB1 (E).
Flow cytometry was performed on TRPV4, KCNJ12, and KCNMB1 to determine if the protein product of the genes analyzed was expressed on the sfMPCs and if so, would it correlate with the gene expression results. sfMPCs derived from the OA patients showed a decrease in TRPV4 compared to the normal sfMPCs (Fig. 1B–C), while there was a no change in KCNJ12, and KCNMB1 in OA lines compared to the normal control (Fig. 1B–E).
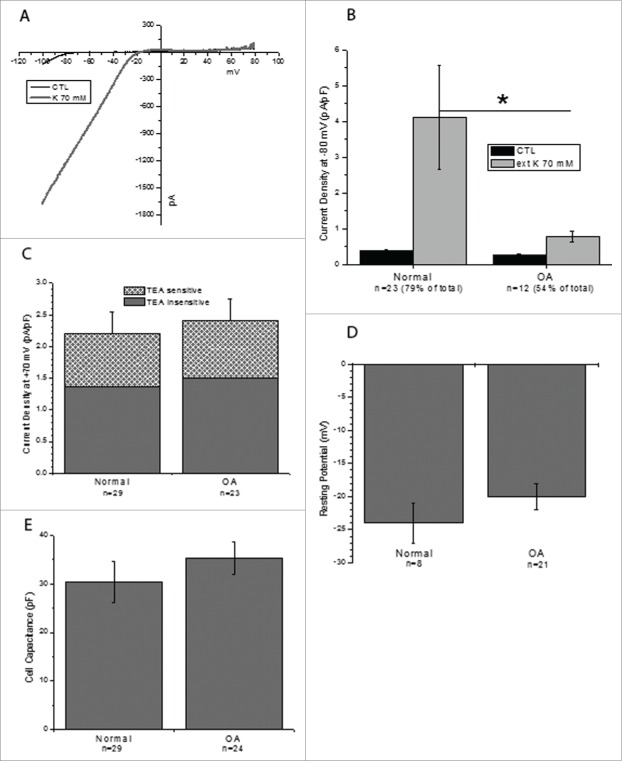
The functionality of the potassium ion channels was then assayed using electrophysiology. Cells from the same 4 cell lines used for the previous experiments were studied. Interestingly, the potassium inward rectifier channel was activated in 79% of normal cells (n = 23) and 54% of OA cells (n=12) when treated with 70 mM potassium extracellular fluid (Fig. 2A–B). The density of the inward current (measured at −80 mV in the cells expressing the inward rectifier) was also lower in OA cells as compared to the normal ones: 0.37±0.05 pA/pF (normal) vs. 0.26±0.04 pA/pF (OA) in control conditions and 4.11±1.46 pA/pF (normal) vs. 0.77±0.15 pA/pF (OA) in the presence of 70 mM external K+. The potassium channel blocker TEA was used to assess the contribution of the large conductance calcium activated potassium channel (KCNMA1/KCNMB1) to the outward current (Fig. 2C). TEA reduced outward currents (measured at +70 mV) by 38% in both cell types. A residual outward current remained at +70 mV during TEA blocking, possibly indicating functional expression of chloride channels (Fig. 2C). Although there was a functional difference between the 2 lines, the resting potential (−20 to –25mV) and cell capacitance (30–35 pF) of the cells did not change significantly between the normal and OA cells (Fig. 2D–E). The resting potential observed is higher than reported literature in chondrocytes (−10 mV).27
Figure 2.
Electrophysiology of normal and OA sfMPCs. Voltage clamp data from normal (n = 29 from 2 patients) and OA (n = 22 from 2 patients). The inward rectifying current was active in 79% of normal cells and 54% of OA cells. (A) Representative example of the current ramp seen in cells expressing functional inward rectifier channels before and after 70 mM potassium solution was added. (B) Inward current density was lower in OA cells than in normal cells (*p < 0 .05) (C) Outward current density decreased 38%with the addition of TEA in both cell lines lines. (D) The resting potential and cell capacitance (E) of each line was not significantly changed.
The relationship between TRPV4 and osmolality of the growth media was then studied. mRNA expression of TRPV4 was measured in OA and normal sfMPCs and normalized to the lowest osmolality (264 mOsm) in the respective cell line. The OA cell lines show a parabolic response to the different osmolality treatments, with the mid range osmolalities showing the highest expression of TRPV4 (Fig. 3A). The normal cell lines did not show any response to the change in osmolality of the growth media (Fig. 3B). The Fluo-4/Fura Red ratio was then used to determine the intracellular Ca2+ level of the normal and OA cells. Once again, the parabolic response to osmolality was found in the OA cell lines (Fig. 3C, E) and not in the normal cell lines (Fig. 3D, F).
Figure 3.
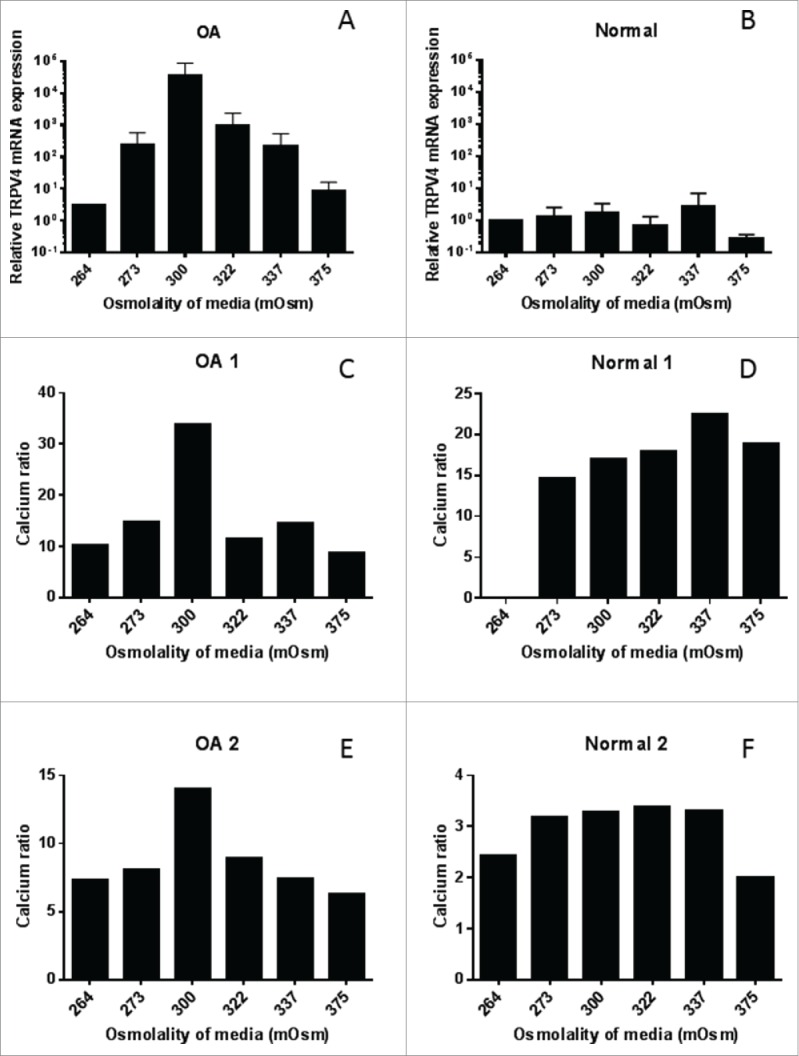
TRPV4 expression and activity in sfMPCs. TRPV4 mRNA expression corresponds to media osmolality conditions 264–375 mOsm in OA (A) but not in normal cells lines (B). Flow cytometry intracellular calcium ratio (Fluo-4/Fura red) corresponds to media osmolality conditions in OA (C,E) but not in normal (D,F) cell lines.
Discussion
Synovial fluid has long been analyzed for properties such as protein concentration, pH, viscosity, and color, which can indicate signs of disease to varying degrees. Ion concentrations have not been extensively studied. One study by Yielding et al in 1954 compared the concentrations of potassium and sodium in normal and diseased synovial fluid using a Beckman DU flame photometer.28 This study did not find a significant difference in normal (Na 3130.3 ppm 37.49, K 156 pm 9.75) and OA (Na 3148.7 ppm 44.85, K 159.9 ppm 3.9) synovial fluid ion concentrations. Both the sodium and potassium results from the Yielding study are much higher than the results obtained from our study (Na: normal 714.59 ppm 67.46, OA 1951.69 ppm 451.70, and K: normal 36.74 ppm 20.68, OA 118.74 ppm 83.01), however, they are on the same magnitude. Another study published by Hoshika et al in 1992 also analyzed the ion concentrations of normal and OA synovial fluid samples.29 This study used Particle-Induced X-Ray Emission (PIXE) in order to quantify the ion concentration present. This study looked at Cl, K, Ca, Mn, Fe, Cu, Zn, and Br concentrations in 3 OA samples and 1 normal sample. They found that OA samples had lower concentrations of all ions except for Cu and Zn. The differences observed between our study and the previous studies could be explained by sample handling and/or technique used to quantify the ions, however, it is equally possible that the same sample sizes in the studies could be an issue.
Differences in ions present in the synovial fluid could play a role in the ion channel activity of sfMPCs. It is difficult to distinguish whether the changes in ion concentration of the synovial fluid are the cause or result of the onset of OA. It is important to note that, while the cells used for this study are from the same patients as the synovial fluid analyzed, cells were cultured over multiple passages in the same conditions for normal and OA cells. Overall, the results of this paper suggest that there is differential ion channel regulation at the functional level, the protein level, and at the genetic level in OA compared to normal sfMPCs that is maintained after in vitro culturing. At the functional level, the potassium inward rectifying channel is clearly under-expressed in the OA cells compared to the normal cells. This is demonstrated by fewer OA cells expressing the channel compared to normal cells and by a lower current density in the OA cells that do express the channel. Based on these results it was interesting that there was no difference in the expression of KCNJ12 between the OA and normal cells when analyzed by flow cytometry. KCNJ12 (Kir2.2) is one of 15 different potassium inward rectifier subunits belonging to 7 subfamilies.30 We chose to look at the Kir 2.2 protein and gene because it is in the subfamily of inward rectifiers that are constitutively active and exhibit a steep inward rectification, as we have seen in the voltage clamp experiments.31 Another explanation for the unchanged Kir2.2 protein may be that there is a problem with embedding the protein into the membrane of the diseased cells, therefore decreasing the functionality of the channel while maintaining a similar amount of expression compared to normal cells.
Inward rectifier potassium channels are known to be involved in the regulation of cell membrane potential in articular chondrocytes.17 Although there was a functional difference between the potassium inward rectifying channel in OA and normal cells, it is interesting that the membrane potential was not significantly different between the 2 cell lines. This indicates that there is another type of regulation occurring in the OA cells to compensate for the difference of inward rectifying potassium channels that we see functionally. It is unclear if this other regulation is the cause or effect of the under-expression of the potassium inward rectifying channel. To our knowledge, there is no published data on the presence or function of the potassium inward rectifying channel in arthritic chondrocyte progenitor cells. However, these channels are known to be involved in the regulation of resting membrane potential, control of excitability in excitable cell types, and the generation of prolonged action potentials in cardiomyocytes.31
At the protein level, TRPV4 seems to be under expressed in OA cells compared to normal cells, while both potassium channel proteins remain unchanged. TRPV4 has been shown to be involved in the regulation of the chondrogenic transcription factor SOX9.32 SOX9 has been shown to activate the production of collagen II in mesenchymal stem cells during chondrogenesis.33 The down regulation of TRPV4 in OA cells may be an indication that these cells do not produce as much SOX9 as normal cells, inhibiting differentiation into chondrocytes and further progressing the disease state. Because TRPV4 is a calcium ion channel that is known to be sensitive to osmotic changes in the joint,34 we looked at the effect of changes of culture medium osmolality. Intracellular calcium levels appeared to change with the osmolality of the culture medium in OA cells concurrently with TRPV4 levels. There was very little change in the calcium levels of the normal patient when culture medium was changed; again, this result corresponded to the TRPV4 levels.
At the mRNA level, all of the channels studied were over-expressed in OA cells compared to normal cells with the exception of KCNMA1. This overexpression may be occurring because the channels are not being transcribed properly, revealing a transcriptional channelopathy in the arthritic cell line.35 Another possible explanation for this change is the difference in osmolality of the culture media and the in vivo environment of the OA cells. Our lab has shown that OA and normal sfMPCs proliferate and differentiate best in osmolality media most similar to their in vivo environment.11 For OA cells, this is a lower osmolality (∼280 mOsm) than the typical MSC culture media (∼300 mOsm) used for these experiments.
Ion channels are being researched as drug targets for many diseases, including potassium and calcium channels.24,36-38 Specifically, TRP channels are being studied for treatment of musculoskeletal diseases.39 Understanding the cellular response to changes in external conditions associated with disease may lead to novel pharmaceutical treatments or early diagnosis biomarkers for osteoarthritis in the future.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the Southern Alberta Organ and Tissue Donation Program and Dr. Cyril Frank for acquiring the tissues used to derive the cells lines described in this study.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC grant # RGPIN-2014–04586) and the Canada Research Chairs Program.
References
- [1].The Arthritis Society Arthritis Facts and Figures. 2015. [Online]. Available: http://www.arthritis.ca/facts [Google Scholar]
- [2].Sinusas K. Osteoarthritis: Diagnosis and Treatment. Am Fam Physician 2012; 85(1):49-56; PMID:22230308 [PubMed] [Google Scholar]
- [3].Kandahari AM, Yang X, Dighe AS, Pan D, Cui Q. Recognition of Immune Response for the Early Diagnosis and Treatment of Osteoarthritis. 2015; 2015:192415; PMID:26064995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP.. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 2001; 44(8):1928-42; PMID:11508446; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [5].Krawetz RJ, Wu YE, Martin L, Rattner JB, Matyas JR, Hart DA. Synovial fluid progenitors expressing CD90+ from normal but not osteoarthritic joints undergo chondrogenic differentiation without micro-mass culture. PLoS One 2012; 7(8):e43616; PMID:22952721; http://dx.doi.org/ 10.1371/journal.pone.0043616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, et al.. Human Mesenchymal Stem Cells in Synovial Fluid Increase in the Knee with Degenerated Cartilage and Osteoarthritis 2012; 30(6):943-9 [DOI] [PubMed] [Google Scholar]
- [7].Shanreld S, Campbell P, Baumgarten M, Bloebaum R, Sarmiento A Synovial fluid osmolality in osteoarthritis and rheumatoid arthritis. Clin Orthop Relat R. 1988; 235:289–295. [PubMed] [Google Scholar]
- [8].Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 2001; 3:107-13; PMID:11178118; http://dx.doi.org/ 10.1186/ar148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Adams SB, Setton LA, Kensicki E, Bolognesi MP, Toth AP, Nettles DL. Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis Cartilage 2012; 20:64-7; PMID:22063369; http://dx.doi.org/ 10.1016/j.joca.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vangsness CT, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis–a pilot study. Bull NYU Hosp Jt Dis 2011; 69:122-7; PMID:22035391 [PubMed] [Google Scholar]
- [11].Bertram KL, Krawetz RJ. Osmolarity regulates chondrogenic differentiation potential of synovial fluid derived mesenchymal progenitor cells. Biochem Biophys Res Commun 2012; 422:455-61; PMID:22579684; http://dx.doi.org/ 10.1016/j.bbrc.2012.05.015 [DOI] [PubMed] [Google Scholar]
- [12].Tew SR, Peffers MJ, McKay TR, Lowe ET, Khan WS Hardingham TE, Clegg PD. Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am J Physiol Cell Physiol 2009; 297:C898-906; PMID:19657054; http://dx.doi.org/ 10.1152/ajpcell.00571.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peffers MJ, Milner PI, Tew SR, Clegg PD. Regulation of SOX9 in normal and osteoarthritic equine articular chondrocytes by hyperosmotic loading. Osteoarthritis Cartilage 2010; 18:1502-8; PMID:20800688; http://dx.doi.org/ 10.1016/j.joca.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tew SR, Vasieva O, Peffers MJ, Clegg PD. Post-transcriptional gene regulation following exposure of osteoarthritic human articular chondrocytes to hyperosmotic conditions. Osteoarthritis Cartilage 2011; 19:1036-46; PMID:21640843; http://dx.doi.org/ 10.1016/j.joca.2011.04.015 [DOI] [PubMed] [Google Scholar]
- [15].Hebert SC. General Principles of the Structure of Ion Channels. Am J Med 1998; 104:87-98; PMID:9528724; http://dx.doi.org/ 10.1016/S0002-9343(97)00358-6 [DOI] [PubMed] [Google Scholar]
- [16].Endo M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J Pharmacol Sci 2006; 100:519-24; PMID:16702757; http://dx.doi.org/ 10.1254/jphs.CPJ06004X [DOI] [PubMed] [Google Scholar]
- [17].Mobasheri A, Lewis R, Ferreira-Mendes A, Rufino A, Dart C, Barrett-Jolley R. Potassium channels in articular chondrocytes. Channels (Austin) 2012; 6:416-25; PMID:23064164; http://dx.doi.org/ 10.4161/chan.22340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hdud IM, Mobasheri A, Loughna PT. Effect of osmotic stress on the expression of TRPV4 and BKCa channels and possible interaction with ERK1/2 and p38 in cultured equine chondrocytes. Am J Physiol Cell Physiol. 2014; 306:C1050-7; PMID:24671100; http://dx.doi.org/ 10.1152/ajpcell.00287.2013 [DOI] [PubMed] [Google Scholar]
- [19].Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum 2009; 60:3028-37; PMID:19790068; http://dx.doi.org/ 10.1002/art.24799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pillozzi S, Becchetti A. Ion channels in hematopoietic and mesenchymal stem cells. Stem Cells Int 2012; 2012:217910; PMID:22919401; http://dx.doi.org/ 10.1155/2012/217910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li GR, Sun H, Deng X, Lau CP. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells 2005; 23:371-82 [DOI] [PubMed] [Google Scholar]
- [22].Matta C, Zakany R. Calcium signalling in chondrogenesis: implications for cartilage repair. Front. Biosci. (Schol. Ed) 2013; 5:305-24; PMID:23277053 [DOI] [PubMed] [Google Scholar]
- [23].Kaczorowski GJ, McManus OB, Priest BT, Garcia ML. Ion channels as drug targets: the next GPCRs. J Gen Physiol 2008; 131:399-405; PMID:18411331; http://dx.doi.org/ 10.1085/jgp.200709946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verkman AS, Galietta LJV. Chloride channels as drug targets. Nat Rev Drug Discov 2009; 8:153-71; PMID:19153558; http://dx.doi.org/ 10.1038/nrd2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li M, Xiong ZG. Ion channels as targets for cancer therapy. Int J Physiol Pathophysiol Pharmacol 2011; 3:156-66; PMID:21760973 [PMC free article] [PubMed] [Google Scholar]
- [26].Lewis R, Feetham CH, Barrett-jolley R. Cell Volume Regulation in Chondrocytes. Cell Physiol Biochem 2011; 28(6):1111-22 [DOI] [PubMed] [Google Scholar]
- [27].Lewis R, Asplin KE, Bruce G, Dart C, Mobasheri A, Barrett-Jolley R. The role of the membrane potential in chondrocyte volume regulation. J Cell Physiol 2011; 226:2979-86; PMID:21328349; http://dx.doi.org/ 10.1002/jcp.22646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yielding KL, Platt D, Holley HL. Synovial Fluid. I. Comparison of Sodium and Potassium Concentrations in Normal and Diseased Joint Fluid. Exp Biol Med 1954; 85:665-7; http://dx.doi.org/ 10.3181/00379727-85-20986 [DOI] [PubMed] [Google Scholar]
- [29].Hoshika Y, Yukawa M. Determination of elemental compositions in synovial fluids from arthritis patients by PIXE. Int J PIXE 1992; 2:511-9; http://dx.doi.org/ 10.1142/S0129083592000555 [DOI] [Google Scholar]
- [30].Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 2010; 90:291-366; PMID:20086079; http://dx.doi.org/ 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- [31].Hugnot JP, Pedeutour F, Le Calvez C, Grosgeorge J, Passage E, Fontes M, Lazdunski M. The Human Inward Rectifying K / Channel Kir 2. Two (KCNJ12) Gene: Gene Structure, Assignment to Chromosome 17p11. One, and Identification of a Simple Tandem Repeat Polymorphism Genomics 1997; 2:113-6 [DOI] [PubMed] [Google Scholar]
- [32].Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, Shiojiri S, Tashiro K, Suzuki Y, Nishimura R, et al.. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem 2007; 282:32158-67; PMID:17804410; http://dx.doi.org/ 10.1074/jbc.M706158200 [DOI] [PubMed] [Google Scholar]
- [33].Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS, “SOX9 directly regulates the type-II collagen gene.,” Nat Genet 1997; 16:174-8; PMID:9171829; http://dx.doi.org/ 10.1038/ng0697-174 [DOI] [PubMed] [Google Scholar]
- [34].Itoh Y, Hatano N, Hayashi H, Onozaki K, Miyazawa K, Muraki K. An environmental sensor, TRPV4 is a novel regulator of intracellular Ca2+ in human synoviocytes. Am J Physiol Cell Physiol 2009; 297:C1082-90; PMID:19759329; http://dx.doi.org/ 10.1152/ajpcell.00204.2009 [DOI] [PubMed] [Google Scholar]
- [35].Waxman SG. Transcriptional channelopathies: an emerging class of disorders 2001; 2:652-9; PMID:11533733 [DOI] [PubMed] [Google Scholar]
- [36].Kaczorowski GJ, McManus OB, Priest BT, Garcia ML. Ion channels as drug targets: the next GPCRs. J Gen Physiol 2008; 131:399-405; http://dx.doi.org/ 10.1085/jgp.200709946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gottlieb P, Suchyna T, Ostrow L, Sachs F. Mechanosensitive Ion Channels as Drug Targets. Curr Drug Target -CNS Neurol Disord 2004; 3:287-95; http://dx.doi.org/ 10.2174/1568007043337283 [DOI] [PubMed] [Google Scholar]
- [38].Curran ME. Potassium ion channels and human disease: phenotypes to drug targets?. Curr Opin Biotechnol 1998;9:565-72; PMID:9889143; http://dx.doi.org/ 10.1016/S0958-1669(98)80133-X [DOI] [PubMed] [Google Scholar]
- [39].Nilius B, Szallasi A, Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 2014; 66:676-814; PMID:24951385; http://dx.doi.org/ 10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.