A key property of many synapses in the nervous system is their ability to trigger fast fusion of transmitter-containing vesicles at specialized release sites (active zones; AZs). This raises the question of how such remarkable temporal fidelity and speed can be achieved at the level of individual AZs. Recent experimental and modeling studies have shown that this temporal tuning might, in significant part, be due to the structural arrangement of the vesicle release machinery at single vesicle release sites which includes voltage-gated calcium channels (VGCCs) and synaptic vesicle associated proteins. The single-vesicle release sites themselves might function in an unreliable manner, i.e. release transmitter with low probability.1-3 Here, the number and coupling of VGCCs to synaptic vesicles and the spatio-temporal dynamics of the cytoplasmic cloud of calcium ions following stochastic channel opening has emerged as a key property shaping the presynaptic response after stimulation. In fact, recent evidence has shown that at many fast transmitting presynaptic terminals, only a small number of VGCCs are closely associated with synaptic vesicles via interactions with AZ proteins.2,4,6 At many of these synapses, vesicle release appears to be triggered by the spatially localized and largely non-overlapping single channel-derived cloud of calcium ions (nanodomain) after channel opening.1,3,7 In addition, since VGCC open probability during a brief action potential (p0) is often low, vesicle release is unreliable. For example, at the frog NMJ, VGCCs open following action potential stimulation with p0 = 0.2, and individual vesicles have a fusion probability of around 0.06.3 Such nanodomain coupling and unreliable release has been found at a variety of other synapses.1, 7
Thus, the question arises if nanodomain coupling (Fig. 1) is a general mechanism for presynaptic vesicle release, or rather a feature of a number of specialized synapses only. This is where the current study by Stanley8 provides important new insight using an innovative approach called graphical modeling. Stanley's approach provides a simple method to estimate synaptic vesicle release probability based on basic geometrical constraints. In particular, it allows one to estimate the contribution of release triggered by calcium ions from only a single VGCC. Applying their graphical modeling approach to model release sites with increasing numbers of VGCCs, Stanley shows that as long as the channel open probability is low, nanodomain-triggered synaptic vesicle fusion predominates even if large clusters of VGCCs are present. In particular, Stanley finds that in the case of VGCC clustering, individual remote channels can contribute substantially to vesicle release. Since VGCCs at most mature (adult) fast-transmitting synapses studied to date were found to have a low open probability during an action potential, Stanley's findings provide new evidence that nanodomain-gated synaptic vesicle fusion may be a general mechanism both in the CNS and the peripheral nervous system. Notable exceptions to this rule may be developing synapses, as well as some specialized systems such as the hippocampal mossy fiber synapse.
Figure 1.
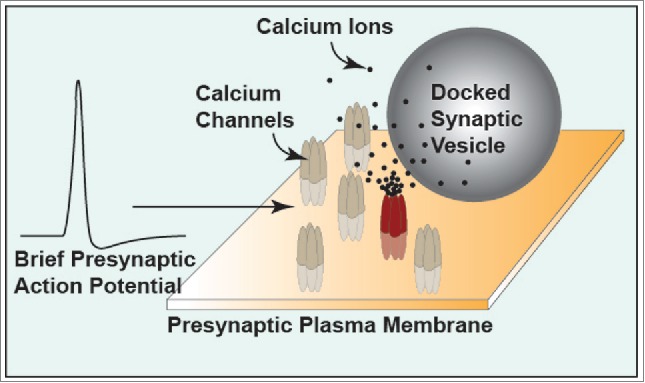
Diagram illustrating non-overlapping nanodomain calcium gating of synaptic vesicle fusion driven by brief action potential depolarizations that open a small fraction of available calcium channels in the active zone. In this diagram, the action potential has only opened one channel (red) which provides a local nanodomain of calcium ions (black dots).
What might be the advantages of building fast synapses with a collection of nanodomain-coupled low probability release sites? As Stanley points out, such an arrangement might minimize vesicle depletion, conserving resources. It might also provide the required temporal fidelity and reduce stochastic jitter. In fact, a recent study has shown that removing RIM binding protein in cultured hippocampal neurons or the calyx of Held, a key component for tethering VGCCs close to synaptic vesicles, leads to a severe loss of these synapses' temporal fidelity.2 Reliance on nanodomain coupling may also be energetically more efficient since it minimizes the resources required to maintain calcium homeostasis. It is also interesting to consider the role of nanodomain coupling in short-term synaptic plasticity. For example, a recent study in the frog NMJ3 has shown that while synaptic vesicles are predominately triggered to fuse by calcium ions emanating from the single closest calcium channel, a small fraction of vesicle-bound calcium ions originate from other VGCCs nearby. This sub-threshold level of calcium binding to vesicles might play an important role in vesicle priming and plasticity. In summary, the paper by Stanley8 provides the latest evidence pointing to nanodomain coupling as a fundamental mode of operation in many, if not most, fast transmitting synapses.
Funding
Funding was provided by the National Institutes of Health (grant id NS090644).
References
- [1].Tarr T, et al.. TINS 2013; 36:14; PMID:23102681; http://dx.doi.org/ 10.1016/j.tins.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Acuna C, et al.. Neuron 2015; 87:1234; PMID:26402606; http://dx.doi.org/ 10.1016/j.neuron.2015.08.027 [DOI] [PubMed] [Google Scholar]
- [3].Luo F, et al.. J. Neurophys 2015; 113:2480; PMID:25652927; http://dx.doi.org/ 10.1152/jn.00879.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoppa M, et al.. Nature 2012; 486:122; PMID:22678293; http://dx.doi.org/ 10.1038/nature11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yunyun H, et al.. J. Neurophys. 2015; 113:255; PMID:25343783; http://dx.doi.org/ 10.1152/jn.00488.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calloway N, et al.. eLife 2015; PMID:26196145; http://dx.doi.org/ 10.7554/eLife.07728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eggermann E, et al.. Nat Rev Neurosci 2011; 13:7–21; PMID:22183436; http://dx.doi.org/ 10.1038/nrn3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanley EF. Single calcium channel domain gating of synaptic vesicle fusion at fast synapses; analysis by graphic modeling. Channels 2015; 9(5):324-33; PMID:26457441; http://dx.doi.org/ 10.1080/19336950.2015.1098793. [DOI] [PMC free article] [PubMed] [Google Scholar]


