Abstract
Purpose
We recently reported that isolated duct segments from rabbit lacrimal gland (LG) were able to secrete fluid in response to secretagogues, which were blocked completely by bumetanide. This suggests the functional involvement of Na+-K+-2Cl− cotransporter (NKCC1) in ductal fluid secretion. Therefore, the aim of this study was to investigate the activity profile of NKCC1 in isolated rabbit LG duct segments.
Methods
Interlobular ducts were isolated from fresh rabbit LG tissue. Microfluorometry with the ammonium (NH4+)–pulse technique was used to elicit pH changes in duct cells, and the rate of bumetanide-sensitive cytosolic acidification after addition of NH4+ was used to quantify the activity of NKCC1.
Results
While basal activity of NKCC1 was undetectable, low cytosolic chloride (Cl−) level and hyperosmotic challenge (390 mOsm) were able to increase the activity of NKCC1. Carbachol (100 μM) had no significant effect on NKCC1 activity. Elevation of cytosolic calcium (Ca2+) level with Ca2+-ionophore (A 23187, 1 μM) did not cause any alteration in the activity of the cotransporter while direct activation of protein kinase C (phorbol myristate acetate, 100 nM) increased its activity slightly but in a significant manner. Addition of either forskolin (10 μM), cell-permeable cAMP analogue (8-bromo cAMP, 100 μM) or vasoactive intestinal peptide (200 nM) resulted in a significant increase in the activity of NKCC1.
Conclusions
These results highlight the functional involvement of NKCC1 in LG duct secretion. These findings may facilitate our understanding of LG function and may contribute to the development of targeted pharmacologic interventions in case of dry eye disease.
Keywords: lacrimal gland, lacrimal gland, duct epithelium, duct epithelium, NKCC1, NKCC1
Preocular tear film is an essential protector of the ocular surface. The bulk of the aqueous component of the tear fluid is produced by the lacrimal gland (LG) which is composed of acinar, ductal, and myoepithelial cells.1 Most earlier research has focused on acinar cells; therefore, our information about the role of the duct system in lacrimal secretion is far from complete.2,3 Earlier and recent reports, however, indicate that duct cells modify potassium (K+) and chloride (Cl−) contents of the primary acinar fluid suggesting the active role of these cells in LG secretion.4–7 Gene expression analysis of LG duct cells has revealed the increased expression of basolateral-to-apical K+ secretion–related transport proteins.8 Cystic fibrosis transmembrane conductance regulator (CFTR), another transporter that is responsible for Cl− transport, was reported in acinar and duct cells, with strong predominance in the ducts.9,10 Recently, experimental evidence of lacrimal duct fluid secretion was provided by our laboratory using videomicroscopic analysis of isolated LG duct segments, providing further support that these duct cells are functionally involved in LG secretion.11 Determining the role of Na+-K+-2Cl− cotransporter (NKCC1) in ductal fluid secretion was suggested by these experiments as forskolin-stimulated fluid secretion was completely blocked by bumetanide, a potent inhibitor of the cotransporter in bicarbonate-free HEPES and in bicarbonate-buffered solutions.
Lacrimal gland fluid production is a Cl− driven secretion mediated by a variety of ion channels and transporters. NKCC1 is an important Cl− accumulating transporter in the basolateral membranes of many mammalian tissues, including LG, salivary glands, tracheal epithelial cells, pancreatic duct cells, and colonic epithelium.12–16 NKCC1 is a furosemide and bumetanide sensitive transporter from the family of cation-Cl cotransporters that mediates concurrent uptake of sodium (Na+), K+, and Cl− in a ratio of 1:1:2; therefore, its action is electroneutral.17,18
There are only very limited studies on the role of NKCC1 in LG secretion. Existence of a furosemide sensitive secretory mechanism in rabbit LG was reported by Dartt et al.,7 providing indirect evidence that coupled transport of Na+ and Cl− has an important role in LG fluid secretion. Recently, NKCC1 expression was reported on the basolateral membranes of LG acinar and duct cells of rat, mice, and rabbit.8,9,19 Walcott et al.19 demonstrated the important role of NKCC1 in fluid secretion by using the whole LG in an in situ experimental set-up they developed. However, until now there is no direct evidence regarding the functional role of NKCC1 in isolated LG duct epithelium. Therefore, we aimed to investigate the activity of NKCC1 in isolated rabbit LG duct segments.
Part of the results in this study have been reported in abstract form previously (Tóth-Molnár E, et al. IOVS 2015;56:ARVO E-Abstract 2482).
Methods
Animals
Adult male New Zealand white rabbits weighing 2 to 2.5 kg (Devai Farm, Kondoros, Hungary) were used throughout the studies. The animals were narcotized with a mixture of ketamine (10 mg/kg) and xylazine (3 mg/kg) and were euthanized with an overdose of pentobarbital (80 mg/kg). All experiments were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol has been approved by the Ethical Committee for the Protection of Animals in Research of the University of Szeged, Szeged, Hungary and conformed to the Directive 2010/63/EU of the European Parliament.
Solutions and Chemicals for Isolation, Culture, and Perfusion of LG Ducts
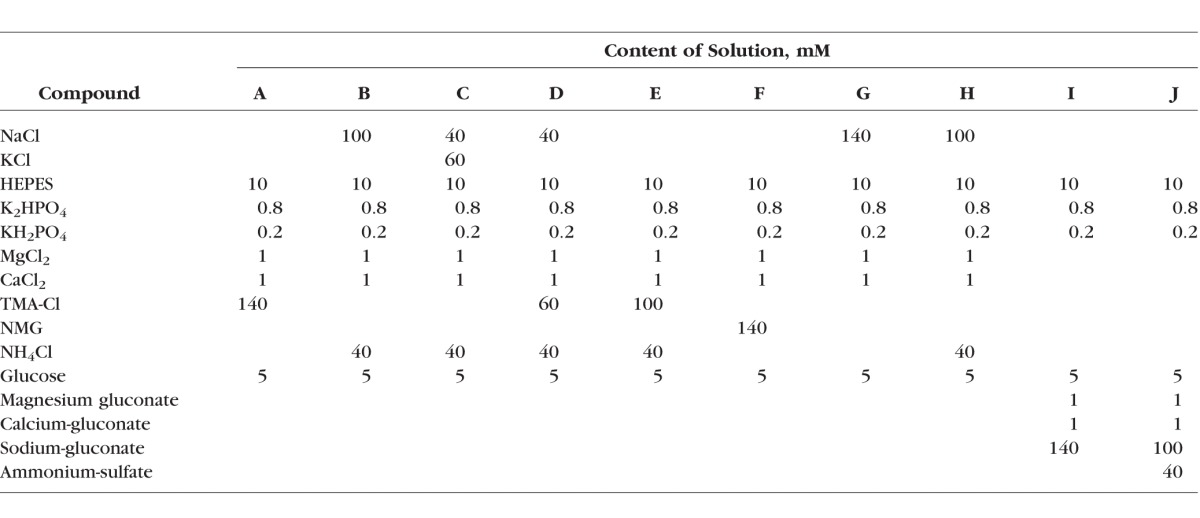
Isolation solution contained Dulbecco's modified eagle medium (DMEM) supplemented with 100 U/mL collagenase (Worthington, Lakewood, NJ, USA) and 1 mg/mL BSA. Storage solution contained DMEM and 3% (wt/vol) BSA. Culture solution contained McCoy's 5A tissue culture medium, 10% (vol/vol) fetal calf serum (FCS), and 2 mM glutamine. Media supplements (DMEM, McCoy, FCS, glutamine, and BSA) were purchased from Sigma-Aldrich (Budapest, Hungary). The composition of solutions used in our studies are summarized in the Table.
Table.
Solutions Used in the Experiments and Their Compositions
Carbachol (carbamoylcholine chloride), forskolin, bumetanide, phorbol 12-myristate 13-acetate (PMA), calcium ionophore A23187, 8-bromoadenosine-3-5-cyclic monophosphate (8-bromo cAMP), and vasoactive intestinal peptide (VIP) were purchased from Sigma-Aldrich. 2.7-bis-(2-carboxyethyl)-5-(and-6-)carboxyfluorescein-acetoxymethylester (BCECF-AM) was purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA).
Isolation and Culture of LG Duct Segments
Rabbit LG interlobular ducts were isolated as described previously by our laboratory.20 In brief, LG was dissected and transferred to a sterile flat-bottom glass flask containing storage solution (+4°C). Isolation solution was injected into the LG tissue and the pieces were transferred to a glass flask containing 2 mL of isolation solution for incubation in a shaking water bath at 37°C. Isolation solution was removed after incubating for 25 minutes and 5 mL of fresh storage solution (+4°C) was added to the flask. Lacrimal gland tissues then were transferred onto a glass microscope slide, and interlobular ducts were micro-dissected under a stereomicroscope and then transferred to the culture solution in a Petri dish. Ducts then were cultured overnight in a 37°C incubator gassed with 5% CO2/95% O2.
Intracellular pH Measurement
After overnight culture, LG duct segments were carefully transferred to a coverslip (24 mm) pretreated with diluted (dilution ratio 1:9) poly-l-lysine (Sigma-Aldrich). The coverslip formed the base of a perfusion chamber mounted on an inverted microscope (Olympus; Olympus Ltd, Budapest, Hungary). Ducts were bathed in standard HEPES solution at 37°C and loaded with the pH-sensitive fluorescent dye BCECF-AM (2 μM) for 25 minutes. Thereafter, the ducts were superfused continuously with solutions at a rate of 4 to 5 mL/min. Intracellular pH was measured using an imaging system (Cell; Olympus; Olympus Ltd). Four to six regions of interest (ROI) of 5 to 10 cells each in an intact duct were excited at 490 and 440 nm, respectively, and the 490/440 fluorescence emission ratio then was measured at 535 nm. One intracellular pH measurement per second was recorded.
Measurement of NKCC1 Activity
Ammonium (NH4+) pulse technique was used to measure the activity rate of NKCC1. It was determined by the rate of intracellular acidification caused by NH4+ entry into the cells via this transport mechanism on abrupt application of NH4Cl as described by Shumaker et al.13 and Heitzmann et al.16 The theoretical background of this technique is the competition between NH4+ and K+ uptake as NKCC1 can accept NH4+ at its K+ binding site. The fluorescence ratio of BCECF-loaded duct cells was measured as a function of time. An increase in fluorescence ratio corresponds to the elevation of intracellular pH. The addition of NH4Cl resulted in a four-phase curve representing the four-phasic alterations in cytosolic pH: (1) rapid initial alkalinization caused by NH3 entry into the cell, followed by (2) a slower decline in pH representing NH4+ uptake of the cell via NKCC1 (among other potentially contributing channels), (3) a rapid acidification after NH4Cl administration, and finally (4) the last phase represents the recovery of pH determined by hydrogen (H+) extrusion and bicarbonate (HCO3−) import. The kinetics of the second phase acidification is of particular interest with respect to NKCC1 activity, as this phase is associated with influx of NH4+, a substrate for NKCC1. Therefore, NKCC1 activity was determined as bumetanide-sensitive part of the second phase acidification representing bumetanide-dependent NH4+ entry into the cell. The slope of the second phase acidification was characterized by calculating the initial rates of recovery from alkalosis (dpH/dt) over the first 60 seconds.
Statistics
A mixed ANOVA model was used for statistics, by using SPSS 22 software (IBM, Armonk, NY, USA). The effect of “bumetanide” was taken into account as a fixed effect. The effect of the individual “duct” and the “duct and effect of bumetanide” interaction (we assumed that the value of the effect of bumetanide depends on the individual duct) were taken into account as random effects in the model. P < 0.05 was considered as significant.
Results
Functional Involvement of NKCC1 in LG Duct Cells
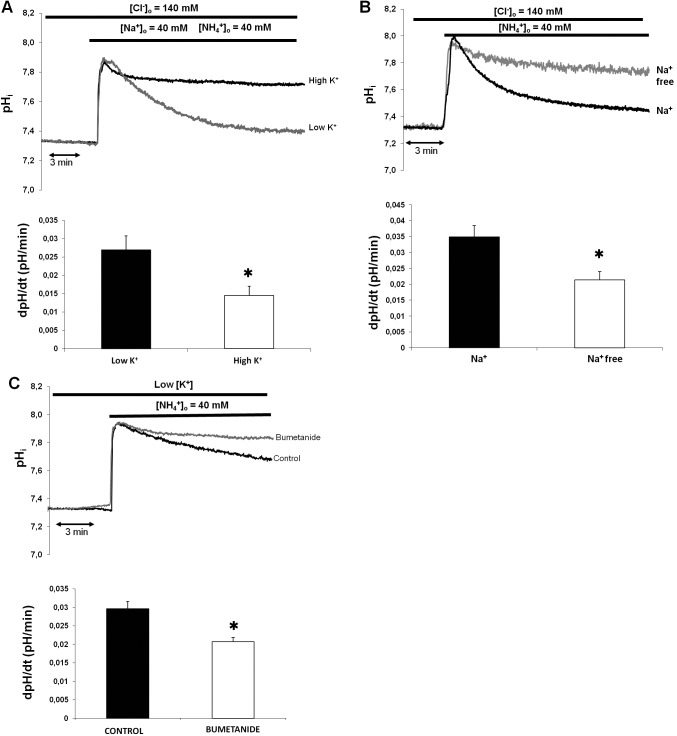
In general, NKCC1 can be characterized functionally during NH4+ pulse as bumetanide-sensitive, Na+- and K+-dependent NH4+entry into the cells. In the first series of experiments we tested the hypothesis that NKCC1 can transport NH4+ instead of K+ with a resultant change in intracellular pH. Ammonium-induced acidification was reduced in the presence of high K+ concentration in the superfusate (solutions A, C, and D, Table), indicating competition between K+ and NH4+ (low K+, 0.027 ± 0.002 pH unit/60 seconds; high K+, 0.014 ± 0.002 pH unit/60 seconds; P = 0.008; Fig. 1A). To determine whether NH4+ transport in duct cells occurs via Na+-dependent pathway, the NH4+ pulse was repeated with tetramethylammonium-chloride, instead of NaCl in the superfusate (solutions A, B, and E, Table). Ammonium-induced cell acidification was reduced significantly in the absence of Na+ (Na+, 0.034 ± 0.002 pH unit/60 seconds; Na+ free, 0.021 ± 0.002 pH unit/60 seconds; P = 0.012; Fig. 1B). To test the existence of a bumetanide-sensitive basolateral transport system (indicative for the presence of NKCC1), standard NH4+ pulse was administered in the presence and absence of bumetanide (solutions A and B, Table). In the presence of bumetanide (100 μM), NH4+-induced cell acidification was significantly reduced in duct cells (control, 0.029 ± 0.002 pH unit/60 seconds; bumetanide, 0.018 ± 0.002 pH unit/60 seconds; P = 0.004; Fig. 1C). Overall, these results confirmed the existence of a Na+-dependent, bumetanide-sensitive pathway in the duct cells where K+ transport is in competition with NH4+ suggesting the functional involvement of NKCC1.
Figure 1.
Functional involvement of NKCC1 in isolated rabbit LG ducts. (A) Ammonium-induced acidification during NH4− pulse is reduced by high K+ content of the medium, indicating competition between NH4+ and K+ transport. (B) Ammonium transport process is Na+ dependent. (C) Ammonium transport is decreased by bumetanide. (A–C) Top: Representative curves of the experiments. Bottom: Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds. Data were obtained from six ducts isolated from three different animals in each series. *P < 0.05.
Influence of Repeated NH4+ Pulse on the Slope of Second Phase Acidification
To determine whether repeated ammonium pulse administration itself could influence the slope of the second phase, three consecutive pulses were added to the same duct segment (solutions G and H, Table). The slope of the second phase was stable during repeated pulses; thus, the “fatigue” effect of the repeated base administration should not be taken into consideration during calculation of NKCC1 activity (pulse 1, 0.08 ± 0.006 pH unit/60 seconds; pulse 2, 0.08 ± 0.005 pH unit/60 seconds; pulse 3, 0.078 ± 0.006 pH unit/60 seconds).
Basal Activity of NKCC1
To determine whether basal activity of NKCC1 affects the measurements, the slope of second phase acidification was determined and compared during NH4+ pulse in the presence and absence of bumetanide (solutions G and H, Table). Basal activity of NKCC1 was negligible and statistically not significant (0.009 ± 0.006 pH unit/60 seconds; P = 0.320).
Activation of NKCC1 by Low Cytosolic Cl−
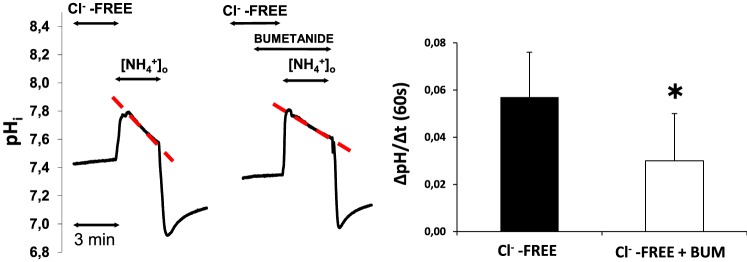
Isolated LG duct segments were preincubated in Cl−-free solution for 20 minutes, then NH4+ pulse was performed in the presence and absence of bumetanide in these experiments (solutions I and J, Table). The results are summarized in Figure 2. The bumetanide-inhibited component of the second-phase pH alteration during ammonium pulse represents the activation of NKCC1 by low cytosolic Cl−. Low cytosolic Cl− increased the activity of NKCC1, the increase was statistically significant (0.026 ± 0.009 pH unit/60 seconds; P = 0.023).
Figure 2.
The effects of low cytosolic Cl− concentration on NKCC1 activity. Ducts were superfused with Cl− free medium and NH4+ pulse was administered in the absence and presence of bumetanide (bum, 100 μM). Initial uptake rate of NH4+ was calculated and compared. Left: Representative curves from our experiments. Right: Summary of data from left. Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds is shown. Data were obtained from nine ducts isolated from four different animals. *P < 0.05.
Activation of NKCC1 by Hyperosmolarity
To investigate the role of hyperosmotic environment in the activation of NKCC1, ducts were preincubated with bath solution of 390 mOsm/L in the absence and presence of bumetanide (solution G+100 mM mannitol and solution H, Table). Hyperosmotic challenge, that is, elevation of bath osmolarity from 290 to 390 mOsm increased the activity of NKCC1 significantly (0.024 ± 0.007 pH unit/60 seconds; P = 0.025) indicating that hyperosmolarity has an important role in the activation of NKCC1 (Fig. 3).
Figure 3.
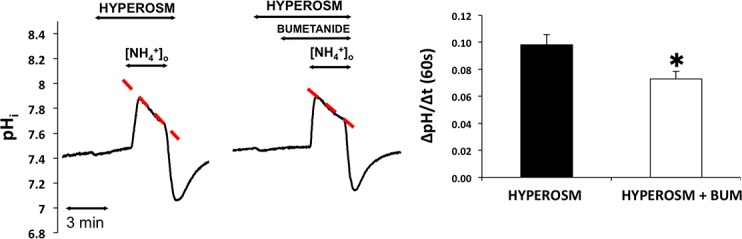
Effect of hyperosmolarity on NKCC1 activity. Ducts were superfused with hyperosmotic medium (390 mOsm) and NH4+ pulse was administered in the absence and presence of bumetanide (bum). Initial uptake rate of NH4+ was calculated and compared. Hyperosmotic challenge enhanced the activity of NKCC1. Left representative curves of the experiments. Summary of data is shown at the right. Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds is shown. Data were obtained from five ducts isolated from three different animals. *P < 0.05.
Effects of Carbachol, PMA, and Ca2+ Ionophore A23187 in the Activation of NKCC1
Acetylcholine analogue carbachol was used to investigate the effect of cholinergic agonists in the activation of NKCC1. Isolated ducts were superfused with HEPES-Tris-phosphate medium, and NH4+ pulse was administered in the absence and presence of bumetanide after preincubation with carbachol (100 μM, 5 minutes, solutions G and H, Table). Results are summarized in Figure 4A. The slope of second-phase acidification was increased compared to the control as a result of cholinergic stimulation. Bumetanide treatment (100 μM) did not change the slope of second phase acidification during NH4+ pulse, indicating that carbachol had no significant effect on NKCC1 activity (0.006 ± 0.006 pH unit/60 seconds; P = 0.388).
Figure 4.
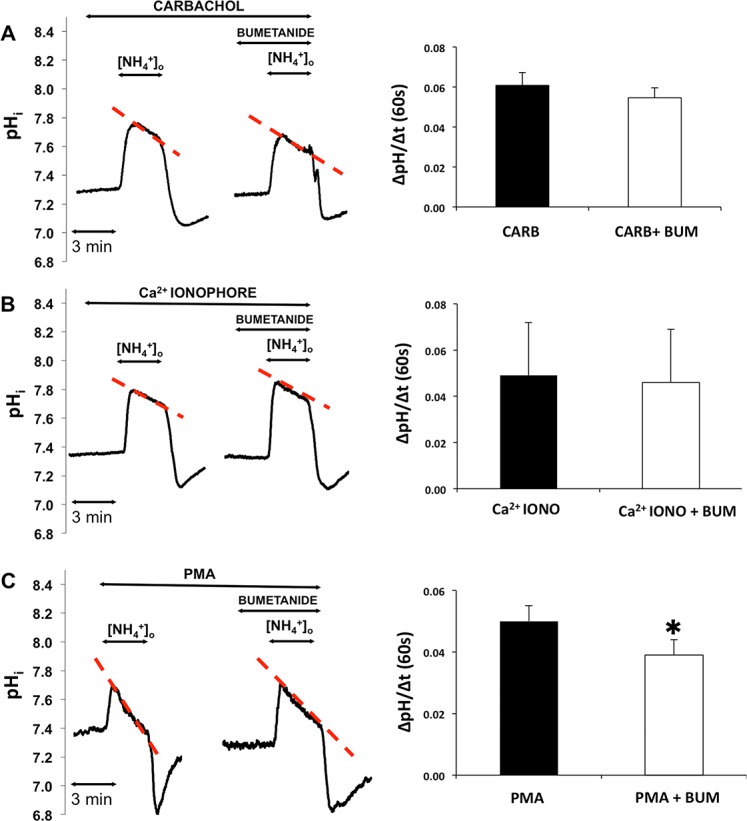
Effect of cholinergic signaling pathway on NKCC1 activity. Ducts were superfused either with (A) carbachol (carb, 100 μM), (B) Ca2+ ionophore A23187 (iono, 1 μM), or (C) PMA (100 nM) and ammonium pulse was administered in the absence and presence of bumetanide (bum, 100 μM). Initial uptake rate of NH4+was calculated and compared. Left: Representative curves of the experiments. Summary of data is shown at the right. Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds is shown. Data were obtained from seven ducts isolated from three different animals in each group of experiments. *P < 0.05.
To further elucidate the role of cholinergic cellular signaling pathways in the activation of NKCC1 in rabbit LG ducts, the effects of Ca2+ ionophore A23187 and protein kinase C (PKC) activator PMA was investigated. Preincubation with Ca2+ ionophore A23187 (3 min, 1 μM) was followed by NH4+ pulse administration with and without bumetanide in the superfusate. Calcium ionophore A23187 did not result in activation of the cotransporter (0.002 ± 0.002 pH unit/60 seconds; P = 0.226, Fig. 4B). In the next series of experiments, effect of PMA was tested. Ammonium pulse was administered to the ducts in the absence and presence of bumetanide after preincubation with PMA (3 minutes, 100 nM, solutions G and H, Table). Bumetanide treatment slightly but significantly reduced the slope of second phase acidification (0.011 ± 0.001 pH unit/60 seconds; P = 0.0007, Fig. 4C).
Activation of NKCC1 by VIP
Parasympathetic nerves release VIP in addition to the cholinergic agonist acetylcholine; thus, in the next series of experiments we investigated the effect of VIP in the activation of NKCC1. Isolated ducts were superfused with HEPES-Tris-phosphate medium and NH4+ pulse then was administered in the absence and presence of bumetanide after preincubation with VIP (5 minutes, 200 nM, solutions G and H, Table). The results are summarized in Figure 5. Vasoactive intestinal peptide treatment increased the rate of second phase acidification, which was reduced by bumetanide (100 μM). The bumetanide-sensitive part of the second phase acidification was 0.051 ± 0.009 pH unit/60 seconds (P = 0.033) indicating the VIP-induced marked increase in NKCC1 activity.
Figure 5.
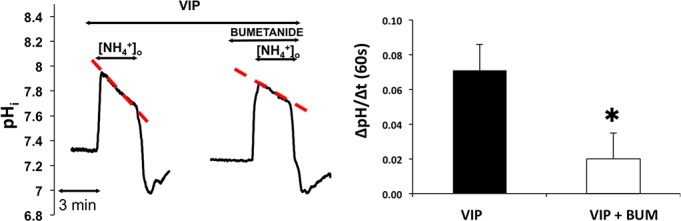
Effect of VIP on NKCC1 activity. Ducts were superfused with VIP (200 nM) and NH4+ pulse was administered in the absence and presence of bumetanide (bum, 100 μM). Initial uptake rate of NH4+was calculated and compared. Left: Representative curves of the experiments. Summary of data is shown at the right. Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds is shown. Data were obtained from five ducts isolated from three different animals. *P < 0.05.
Activation of NKCC1 by Forskolin and Cell Permeable cAMP Analogue 8-Bromo cAMP
Next, we studied the role of elevated intracellular cAMP level in the activation of NKCC1. Isolated ducts were superfused with HEPES-Tris-phosphate medium and NH4+ pulse then was administered after a 5-minute incubation with forskolin (10 μM) in the absence and presence of bumetanide (solutions G and H, Table). The results are summarized in Figure 6A. Forskolin increased the rate of second phase acidification, which was reduced by bumetanide (100 μM). The bumetanide-sensitive part of the second phase acidification was 0.024 ± 0.008 pH unit/60 seconds (P = 0.045), indicating the forskolin-induced significant increase in NKCC1 activity.
Figure 6.
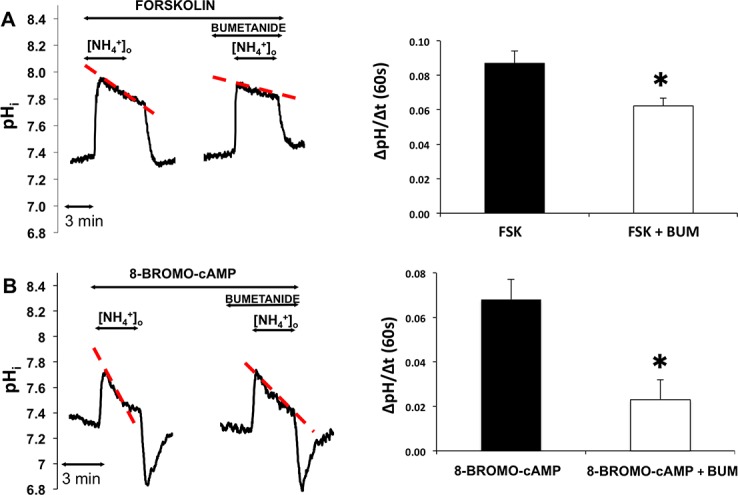
Effects of forskolin and cell permeable cAMP analogue 8-bromo cAMP on NKCC1 activity. Ducts were superfused either with forskolin (FSK, 10 μM, [A]) or 8-bromo cAMP (100 μM, [B]) and ammonium pulse was administered in the absence and presence of bumetanide (bum, 100 μM). Initial uptake rate of NH4+was calculated and compared. Left: Representative curves of the experiments. Summary of data is shown at the right. Initial rate of recovery from alkalosis (dpH/dt) over the first 60 seconds is shown. Data were obtained from five ducts isolated from three different animals in both groups of experiments. *P < 0.05.
To further verify the effect of elevated cytosolic cAMP level in the activation of NKCC1, cell permeable cAMP analogue was used. Ammonium pulse was administered in the absence and presence of bumetanide after preincubation with 8-bromo cAMP (100 μM, 5 minutes). The bumetanide-sensitive part of the second phase acidification was 0.044 ± 0.007 pH unit/60 seconds (P = 0.011), representing a statistically significant increase in NKCC1 activity caused by cell-permeable cAMP analogue 8-bromo cAMP (Fig. 6B).
Discussion
Expression of NKCC1 in the duct cells of exocrine glands is remarkably species- and organ-specific. NKCC1 can be found in the basolateral membranes of rat and mouse pancreatic ducts, but not in the pancreatic and salivary gland duct cells of pig and guinea pig.21–23 Earlier we detected the expression of NKCC1 in the basolateral membranes of both acinar and duct cells from rabbit LG by immunfluorescence, with stronger staining observed in acinar cells.9 In the present study various factors that may influence the activity of NKCC1 in LG duct cells were investigated.
It is well established that low intracellular Cl− concentration can lead to the activation of NKCC1 in many cell types.14–16 Activation of NKCC1 by low intracellular Cl− level can result in enhanced Cl− entry into the cell through the basolateral membrane to restore cytosolic Cl− homeostasis. Similarly, to other investigated cells a significant increase in activity of NKCC1 was found in Cl−-depleted LG duct cells in our present study.
It is widely demonstrated that NKCC1 has a key role in volume regulation of cells, that is, cell shrinkage can be a potent signal of its activation. The precise molecular mechanism of how water transport is mediated by the cotransporter is unknown, but the consensus is that NKCC1 has a key role in this process. The ability of NKCC1 to couple water and ion transport may have a direct role in the secretory process in epithelial cells.24,25 Walcott et al.19 demonstrated the NKCC1-dependent manner of the regulatory volume increase in mouse LG acinar cells. In agreement with these previous findings hyperosmotic environment led to a marked increase of NKCC1 activity in rabbit LG duct cells.
We investigated the role of the parasympathetic pathway in the activation of NKCC1. We could not demonstrate a notable effect of the cholinergic agonist carbachol in the activation of NKCC1. This finding is in agreement with our previous results, that is, cholinergic stimulation of isolated rabbit LG duct segments resulted in a very weak fluid secretory response.11 To further characterize the role of the cholinergic cellular signaling pathways, potential effects of a Ca2+-ionophore (A23187) and PKC activator (PMA) were measured. Elevation of cytosolic Ca2+ level with Ca2+ ionophore did not cause activation of NKCC1 in our experiments. In contrast, direct stimulation of PKC with its potent activator PMA resulted in a significant increase of NKCC1 activity, even though the rate of activation of the cotransporter was very weak. This contradiction between carbachol effect (no activation of NKCC1) and PMA effect (activation of NKCC1) might be explained by the weaker extent of activation of PKC during cholinergic effect compared to the direct and robust activation of the enzyme by PMA. Lack of effect of carbachol on NKCC1 also might be explained by the rapid cholinergic-evoked internalization of the cotransporter.26 This later effect could be a possible explanation by which carbachol and forskolin influence the activity of the cotransporter in different ways.26,27 This theory is in agreement with our results, and explains, at least in part, the different fluid secretory patterns of Ca2+- and cAMP-mediated mechanisms.
Besides acetylcholine, parasympathetic nerves also release VIP. Earlier reports demonstrated the presence of VIP receptors on acinar and duct cells of LG. Thus, we investigated the effects of VIP in the activation of NKCC1.28 This transmitter acts predominantly through elevation of cytosolic cAMP level, the minority of its action thought to be mediated by Ca2+ signaling. We could demonstrate a considerable increase of NKCC1 activity evoked by VIP stimulation. Further studies are needed to investigate the effect of VIP on ductal fluid secretion.
In our present study we found that forskolin stimulation (i.e., elevation of cytosolic cAMP level) resulted in a marked increase in NKCC1 activity in isolated LG duct segments. Similarly, cell permeable cAMP analogue 8-bromo cAMP also resulted is a significant elevation of NKCC1 activity. These results are consistent with our previous findings where the potent fluid secretory effect of forskolin could be blocked completely by the NKCC1 inhibitor bumetanide and suggest a decisive role of cAMP-dependent mechanisms and NKCC1 in ductal fluid secretion.11
In conclusion, our results demonstrated the functional presence of NKCC1 in rabbit LG duct cells, providing further support that this transporter can be the main route of basolateral Cl− uptake. We found that low cytosolic Cl− level caused a significant increase in the activation of NKCC1. Hyperosmolarity of bath media, which results in cell shrinkage, proved to be a potent activator of NKCC1. NKCC1 also could be activated by elevated cytosolic cAMP level, VIP treatment, and, in a considerably smaller extent, by direct activation of PKC (Fig. 7).
Figure 7.
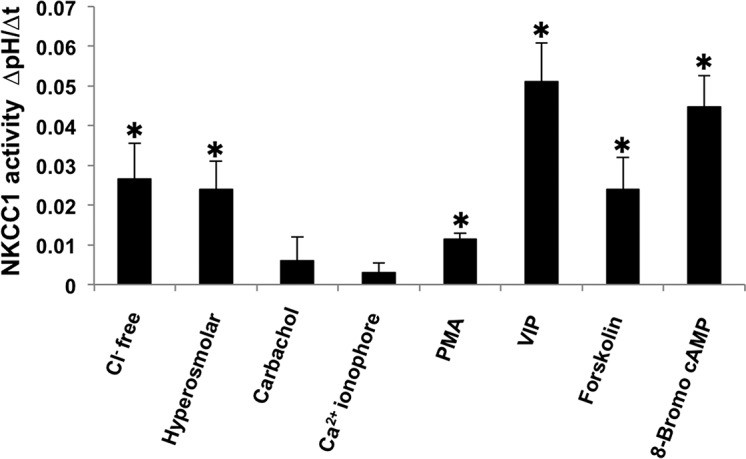
Activity of NKCC1 evoked by low intracellular Cl− level, hyperosmolar environment and various secretagogues. Activity of NKCC1 was calculated from the rates of recovery from alkalosis over the first 60 seconds (dpH/dt) during ammonium pulse. *P < 0.05.
More detailed understanding of LG function is essential to develop novel approaches in the treatment of dry eye disease, which is an increasing health care problem in the industrialized countries.29 Unfortunately, duct cells have been understudied for many years compared to acinar cells, although recent advances clearly indicated that these duct cells have critical and indispensable roles in LG production. Further studies certainly are needed to investigate the role of apical transport processes and the interaction between basolateral and apical transport mechanisms underlying ductal fluid and electrolyte secretion. These future results may further facilitate our understanding of lacrimal gland function and may contribute to the development of targeted pharmacologic interventions in case of dry eye disease.
Acknowledgments
Supported by Grants NKFIH NN 115611 (ETM), NEI/NIH EY017731 (CD), The Webb Foundation Grant (CD), and LP2014-10/2014 Momentum Gant of the Hungarian Academy of Sciences (PH).
Disclosure: E. Vizvári, None; M. Katona, None; P. Orvos, None; O. Berczeli, None; A. Facskó, None; F. Rárosi, None; V. Venglovecz, None; Z. Rakonczay Jr, None; P. Hegyi, None; C. Ding, None; E. Tóth-Molnár, None
References
- 1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 2. Parod RJ,, Leslie BA,, Putney JW,, Jr. Muscarinic and alpha-adrenergic stimulation of Na and Ca uptake by dispersed lacrimal cells. Am J Physiol. 1980; 239: G99–G105. [DOI] [PubMed] [Google Scholar]
- 3. Petersen OH. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986; 251: G1–G13. [DOI] [PubMed] [Google Scholar]
- 4. Alexander JH,, vanLennep EW,, Young JA. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization technique. Pflugers Arch. 1972. ; 337: 299–309. [DOI] [PubMed] [Google Scholar]
- 5. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009. ; 28: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mircheff AK. Control of lacrimal gland function: water and electrolyte secretion and fluid modification. : DM Albert,, Jakobiec FA, Principles and Practice in Ophthalmology. Philadelphia: WB Saunders; 1994: 466–472. [Google Scholar]
- 7. Dartt DA,, Moller M,, Poulsen JH. Lacrimal gland electrolyte and water secretion in the rabbit: localization and role of (Na++ K+)-acivated ATP-ase. J Physiol. 1981. ; 321: 557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ubels JL,, Hoffman HM,, Srikanth S,, Resau JH,, Webb CP. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: evidence for K+ secretion by the duct cells. Invest Ophthalmol Vis Sci. 2006. ; 47: 1876–1885. [DOI] [PubMed] [Google Scholar]
- 9. Ding C,, Parsa L,, Nandoskar P,, Zhao P,, Wu K,, Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 2010. ; 51: 2960–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu M,, Ding C. CFTR-mediated Cl− transport in the acinar and duct cells of rabbit lacrimal gland. Curr Eye Res. 2012; 37: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katona M,, Vizvári E,, Németh L,, et al. Experimental evidence of fluid secretion of lacrimal gland duct epithelium. Invest Ophthalmol Vis Sci. 2014. ; 55: 4360–4367. [DOI] [PubMed] [Google Scholar]
- 12. Evans RL,, Park K,, Turner RJ,, et al. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J Biol Chem. 2000; 275: 26720–26726. [DOI] [PubMed] [Google Scholar]
- 13. Liedtke CM,, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-δ and ERK. Biochim Biophys Acta. 2002. ; 1589: 77–88. [DOI] [PubMed] [Google Scholar]
- 14. Shumaker H,, Soleimani M. CFTR upregulates the expression of the basolateral Na+-K+-2Cl− cotransporter in cultured pancreatic duct cells. Am J Physiol Cell Physiol. 1999. ; 277: C1100–C1110. [DOI] [PubMed] [Google Scholar]
- 15. Bachmann O,, Wüchner K,, Rossmann H,, et al. Expression and regulation of the Na+-K+-2Cl− cotransporter NKCC1 in the normal and CFTR-deficient murine colon. J Physiol. 2003. ; 549: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heitzmann D,, Warth R,, Bleich M,, Henger A,, Nitschke R,, Greger R. Regulation of the Na+2Cl−K+ cotransporter in isolated rat colonic crypts. Pflugers Arch-Eur J Physiol. 2000. ; 439: 378–384. [DOI] [PubMed] [Google Scholar]
- 17. Isenring P,, Jacoby SC,, Payne JA,, Forbush B,, III. Comparison of Na-K-Cl cotransporters. J Biol Chem. 1998. ; 273: 11295–11301. [DOI] [PubMed] [Google Scholar]
- 18. Haas M,, Forbush B,, III. The Na+-K+-2Cl− cotransporter of secretory epithelia. Ann Rev Physiol. 2000. ; 62: 515–534. [DOI] [PubMed] [Google Scholar]
- 19. Walcott B,, Birzgalis A,, Moore LC,, Brink PR. Fluid secretion and the Na+-K+-2Cl– cotransporter in mouse exorbital lacrimal gland. Am J Physiol Cell Physiol. 2005. ; 289: C860–C867. [DOI] [PubMed] [Google Scholar]
- 20. Tóth-Molnár E,, Venglovecz V,, Ozsvari B,, et al. New experimental method to study acid/base transporters and their regulation in lacrimal gland ductal epithelia. Invest Ophthalmol Vis Sci. 2007; 48: 3746–3755. [DOI] [PubMed] [Google Scholar]
- 21. Fernández-Salazar MP,, Pascua P,, Calvo JJ,, et al. Basolateral anion transport mechanisms underlying fluid secretion by mouse, rat and guinea-pig pancreatic ducts. J Physiol. 2004. ; 556: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grotmol T,, Buanes T,, Raeder MG. NN′-dicyclohexylcarbodimide (DCCD) reduces pancreatic NaHCO3 secretion without changing pancreatic tissue ATP levels. Acta Physiol Scand. 1986. ; 128: 547–554. [DOI] [PubMed] [Google Scholar]
- 23. Lee MG,, Ohana E,, Park HW,, Yang D,, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol Rev. 2012. ; 92: 39–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamann S,, Herrera-Perez JJ,, Bundgaard M,, Alvarez-Leefmans FJ,, Zeuthen T. Water permeability of Na+-K+-2Cl− cotransporters in mammalian epithelial cells. J Physiol. 2005; 568: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamann S,, Herrera-Perez JJ,, Zeuthen T,, Alvarez-Leefmans FJ. Cotransport of water by Na+-K+-2Cl− NKCC1 in mammalian epithelial cells. J Physiol. 2010. ; 588: 4089–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reynolds A,, Parris A,, Evans LA,, et al. Dynamic and different regulation of NKCC1 by calcium and cAMP in the native human colonic epithelium. J Physiol. 2007; 507–524. [DOI] [PMC free article] [PubMed]
- 27. Del Castillo IC,, Fedor-Chaiken M,, Song JC,, et al. Dynamic regulation of Na+-K+-2Cl- cotransporter surface expression by PKC in Cl- secretory epithelia. Am J Physiol Cell Physiol. 2005; 289: C1332–C1342. [DOI] [PubMed] [Google Scholar]
- 28. Hodges R,, Zoukhri D,, Sergheraert C,, Zieske JD,, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997. ; 38: 610–619. [PubMed] [Google Scholar]
- 29. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007; 5: 163–178. [DOI] [PubMed] [Google Scholar]