Abstract
Purpose
Glaucoma, frequently associated with elevated intraocular pressure (IOP), is characterized by progressive retinal ganglion cell (RGC) death and vision loss. Brain-derived neurotrophic factor (BDNF) has been studied as a candidate for neuroprotection in rodent models of experimental glaucoma, yet it remains to be determined whether BDNF exerts long-term protection for subtype RGCs and vision against chronic IOP elevation.
Methods
We induced modest and sustained IOP elevation by laser illumination and microbead injection in mice. Using a tamoxifen-induced Cre recombinase system, BDNF was upregulated in the mouse retina when sustained IOP elevation was induced. We then examined whether overexpression of BDNF protected RGCs and vision during the period of ocular hypertension. Given that BDNF modulates axon growth and dendritic formation in a subtype-dependent manner, we tested whether BDNF protects RGC dendritic structure against the hypertensive insult also in a subtype-dependent manner.
Results
Sustained IOP elevation was induced and lasted up to 6 months. Overexpression of BDNF delayed progressive RGC and axon loss in hypertensive eyes. Brain-derived neurotrophic factor overexpression also helped to preserve acuity against the chronic hypertensive insult. We classified RGCs into ON and ON–OFF subtypes based on their dendritic lamination pattern in the inner plexiform layer and found that BDNF prevented ON–RGC dendritic degeneration in mice with sustained ocular hypertension.
Conclusions
Our data demonstrated that BDNF can protect the dendritic fields of ON RGCs and reduce RGC and vision loss in mice with sustained ocular hypertension.
Keywords: retinal ganglion cells (RGCs), brain-derived neurotrophic factor (BDNF), neuroprotection, visual acuity, experimental glaucoma
Brain-derived neurotrophic factor (BDNF), which is known to regulate neuronal survival and function in the central nervous system,1,2 plays an important role in the treatment of neurodegenerative diseases.3–5 Glaucoma is a group of neurodegenerative diseases characterized by a loss of retinal ganglion cells (RGCs), optic nerve defects, and atrophy.6,7 Many studies suggest that BDNF promotes RGC health in animal models of experimental glaucoma (reviewed in Refs. 8–10) and that retinal BDNF signaling is affected in rodents with experimental glaucoma11–14 and in glaucoma patients.12,15,16 Administration of exogenous BDNF following optic nerve injury or a glaucomatous insult slows RGC loss (e.g., Refs. 17–23, but see Refs. 23, 24). The inconsistent outcomes may be due to issues such as drug specificity, protein sustainability, and the efficiency of the method of delivery.10 In addition, different rodent models of experimental glaucoma produce different patterns and degrees of RGC loss,25,26 which has made it difficult to determine whether BDNF exerts long- or short-term neuroprotection for different types of glaucomatous insult.
Here, we induced modest IOP elevation using a combination of laser illumination and microbead injection and showed that the elevation lasts up to 6 months.27–29 Using the tamoxifen-induced Cre system, we upregulated BDNF in the mouse retina at the moment that ocular hypertension (OHT) was induced. We then investigated whether BDNF protects RGCs and vision during this extended period of OHT. Recent studies have begun to reveal that different types of RGCs are susceptible to a glaucomatous insult differentially, with dendritic alternation in the OFF sublamina of the inner plexiform layer (IPL) occurring as early as 1 week post IOP elevation.26,30 At 2 to 4 weeks post IOP elevation, OFF–transient RGCs exhibit significant dendritic and functional degeneration.31 At 6 to 8 weeks post IOP elevation, ON RGCs have smaller dendritic trees and their visual response properties affected.28,29 Given that RGC death in glaucoma is preceded by changes in dendritic morphology and that BDNF promotes RGC dendritic branching during development,32,33 we hypothesized that and tested whether BDNF would preserve RGC dendritic structure in mice with experimental glaucoma, leading to an improvement in RGC survival.
Materials and Methods
Animals
The strain of BDNFstop transgenic mice was a generous gift from Qiang Chang at the University of Wisconsin-Madison.34 Thy-1-CreERT(T2) transgenic mice (line A; The Jackson Laboratory, Bar Harbor, ME, USA), in which yellow fluorescent protein (YFP) is expressed in the retina and the brain, were bred with BDNFstop mice to generate double-transgenic, Thy-1-CreERT2::BDNFstop mice. Tamoxifen (Sigma-Aldrich Corp., St. Louis, MO, USA) was prepared in ethanol at 10 mg/mL. One aliquot of tamoxifen was added with one-fourth volume of sunflower seed oil (Sigma-Aldrich Corp.), rapidly spun, and vacuum dried.35 Tamoxifen was then administered to 2- to 3-month-old mice of either sex by one or two intraperitoneal injections (100 mg/kg body weight). Wild-type (WT) and Thy-1-YFP transgenic mice (line H; The Jackson Laboratory) with the C57BL/6 background were used as additional controls. All animal procedures were approved by the Northwestern University Institutional Animal Care and Use Committee and were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and National Institutes of Health guidelines.
Induction of Ocular Hypertension
Intraocular pressure (IOP) elevation was induced by laser photocoagulation and injection of microbeads into mouse eyes.27–29 We induced OHT in right eyes and used the left eyes of the same animals either untreated or injected with saline (1 μL; Molecular Toxicology, Boone, NC, USA) as controls. The IOP of the right eye was measured (TonoLab; Colonial Medical Supply, Franconia, NH, USA) every 2 weeks for the first month and every 2 to 4 weeks thereafter until the mice were killed for experiments.27–29,36 The IOP of the left eye of the same animals was measured at the same time to serve as baseline control. Mice with IOP of the right eye constantly more than 50% above baseline (i.e., left eye) were considered ocular hypertensive (OHT).28,29 The optomotor test was performed with vertical gratings moving horizontally at a fixed speed of 12 °/s.27–29,36 The visual acuity of both eyes was measured as described previously.27–29,36
Immunohistochemistry, Western Blot, and Axon Counting
Flat-mount retinas and sections were prepared for immunostaining as described previously.37,38 The primary antibodies used in this study included rabbit anti-GFP (1:1000; Invitrogen A-6455, Carlsbad, CA, USA), mouse anti-Brn-3a (1:400; EMD Millipore MAB1585, Billerica, MA, USA), goat anti-Brn-3b (1:1000; Santa Cruz Biotechnology sc-6026; Santa Cruz, CA, USA), mouse anti-tyrosine hydroxylase (TH; 1:400, EMD Millipore MAB318), mouse anti-SMI-32 (1:1000; Covance, Princeton, NJ, USA), rabbit anti-PKC (protein kinase C) (1:1000, EMD Millipore), and rabbit anti-calretinin (1:1000, EMD Millipore). Alexa Fluor–conjugated secondary antibodies were used (1:1000; Life Technologies, Eugene, OR, USA), and images were captured with a Zeiss Pascal confocal microscope (Zeiss, Thornwood, NY, USA). Retinal protein samples were prepared for Western blot.32,39 Antibodies used for Western blots included rabbit anti-BDNF (1:400; Santa Cruz Biotechnology sc-546; and EMD Millipore AB1779SP) and mouse anti-Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2000, EMD Millipore MAB374).
The optic nerves were prepared and imaged with a Zeiss inverted microscope for axon counting.28 The axon numbers per image were counted manually by two observers using ImageJ (National Institutes of Health, Bethesda, MD, USA), averaged, and normalized to the total area of the optic nerve.28
Dendritic Structure Analysis
Retinal ganglion cells were classified into ON and ON–OFF types based on their patterns of dendritic lamination: ON RGCs having their dendrites laminated within the inner half of the IPL, and ON–OFF RGCs having their dendrites laminated in the ON and OFF sublaminae of the IPL.28,32 OFF RGCs were not analyzed due to their low rate of sampling.28,32 Z-stack images of YFP-expressing RGCs were projected onto a two-dimensional plane to measure the field size of RGC arbors.28
Full-Field Electroretinogram and Optical Coherence Tomography (OCT)
Electroretinogram (ERG) recordings were performed using the Micron III platform (Phoenix Research Laboratories, Pleasanton, CA, USA).29 Full-field ERGs were obtained for flashing white light of a range of intensities (−2.0, −0.97, 0.51, 2.13, and 3.53 log cd × s/m2). The light pulse was 10 ms in duration and the stimulus was repeated five times. The electrical signals from the retinas were band-pass filtered between 0.5 Hz and 2 kHz over a 300-ms period and analyzed using customized programs in MATLAB (Mathworks, Natick, MA, USA).29
The OCT system was custom built by the Zhang lab at Northwestern University.29,40 After the three-dimensional image of a mouse retina was taken, the fundus image was obtained through the mean intensity projection of z-stacked images.40,41 Five to 10 cross-sectional images were obtained from each retina. The thickness of the nerve fiber layer (NFL) and ganglion cell layer (GCL), and the total thickness, which included retina, pigment epithelium, and choroid, was measured manually in National Institutes of Health ImageJ.29
Statistical Analyses
Results were expressed as the mean ± standard error (SEM). Student's t-test was performed to compare paired samples, the Kolmogorov-Smirnov (K-S) test to compare the distributions of two samples, 1-way ANOVA to compare multiple samples, and 2-way ANOVA to compare two independent categorical variables.
Results
Retinal BDNF Was Upregulated in a Spatially and Temporally Controlled Manner
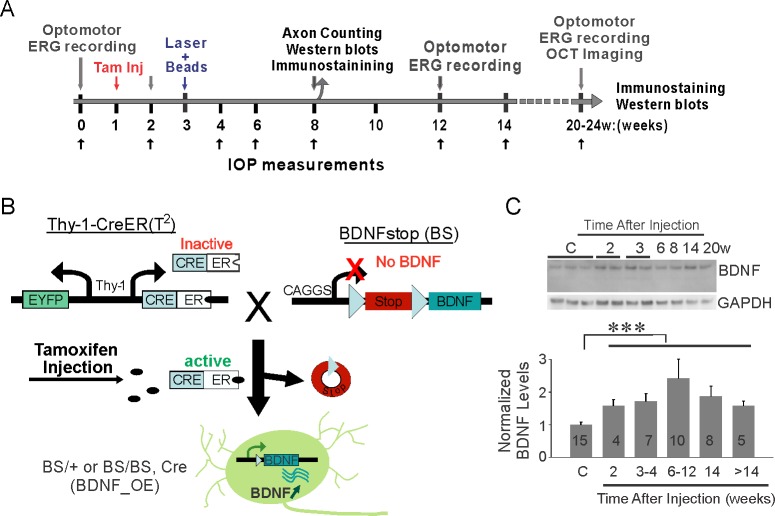
We induced modest and sustained IOP elevation and then determined whether BDNF protects RGCs and visual function (see schematic experimental design in Fig. 1A). We generated conditional BDNF–overexpression mice using the tamoxifen-induced Cre recombinase system (Fig. 1B). In brief, BDNFstop mice (labeled BS/BS or BS/+) carry a stop cassette flanked by loxP inserted before the BDNF coding region, and the Thy-1-CreERT(T2) transgenic mice have YFP and CreER recombinase driven by the Thy-1 promoter (labeled Cre, Fig. 1B). The two lines of mice were crossed (Thy-1-CreERT2::BDNFstop mice), and the transcription of BDNF was upregulated by one or two intraperitoneal injection(s) of tamoxifen (labeled BDNF_OE, Fig. 1B).
Figure 1.
Experimental design to examine BDNF neuroprotection of RGCs and vision following chronic ocular hypertension. (A) Schematic time line of experiments. (B) Generation of double transgenic line overexpressing BDNF in a spatially and temporally controlled manner. Thy-1-CreERT(T2) transgenic mice (labeled Cre) were crossed with BDNFstop mice (labeled BS). One or two intraperitoneal injections of tamoxifen (tam) activated Cre recombinase, which turned on the transcription of BDNF in Thy-1-CreERT2::BDNFstop mice (labeled BDNF_OE). (C) Western blot analysis showed the steady increase of retinal BDNF levels following the tamoxifen injections. Sample numbers are labeled. ***P < 0.001 in Student's t-test.
We quantified retinal BDNF levels at different time points following the administration of tamoxifen (Fig. 1C). Because Thy-1-CreERT(T2) BDNFstop mice injected with tamoxifen and Thy-1-CreERT2::BDNFstop mice without tamoxifen injection exhibited no significant difference in retinal BDNF levels (n = 5 in each group, P = 0.16 in 1-way ANOVA), we combined them into one control group (n = 15 in total). Compared to observations in the control group, BDNF was upregulated as early as 2 weeks post tamoxifen injection (n = 4, P = 0.045 in Student's t-test, Fig. 1C). Brain-derived neurotrophic factor was 71 ± 18% higher at 3 to 4 weeks, and remained higher than in controls over the following 4 months (n = 5–10 in each group, P < 0.001 in Student's t-test, Fig. 1C).
We also confirmed that BDNF overexpression did not affect retinal morphology (Supplementary Fig. S1). Retinal sections stained by DAPI (4′,6-diamidino-2-phenylindole) showed that the overall retinal structure was unchanged by tamoxifen injection or BDNF overexpression (Supplementary Fig. S1A). Retinal sections immunostained with antibodies against calretinin and PKC, markers for amacrine and bipolar cells, also suggested that the inner retina was largely normal (Supplementary Fig. S1B). Together our data demonstrated that retinal BDNF was upregulated by tamoxifen administration, providing us with the genetic tool to examine whether overexpression of BDNF prevents RGC and vision loss when OHT is induced.
Chronic Ocular Hypertension Downregulated Retinal BDNF
Ocular hypertension was induced in the right eyes with the left eyes of the same animals left as normal-tension controls (see details in Methods). The mean IOP of right eyes of both BDNF_OE mice and control mice was constantly elevated for more than 3 months (Fig. 2A). At 3 to 5 months post laser illumination and microbead injection, the mean IOP of right eyes of control mice was 26.6 ± 5.2, significantly higher than in their left eyes (14.8 ± 0.6, n = 84, Fig. 2A). Similarly, IOP was also elevated in BDNF_OE mice (26.8 ± 4.1 in right eyes versus 14.6 ± 0.6 in left eyes, n = 22, Fig. 2A).
Figure 2.
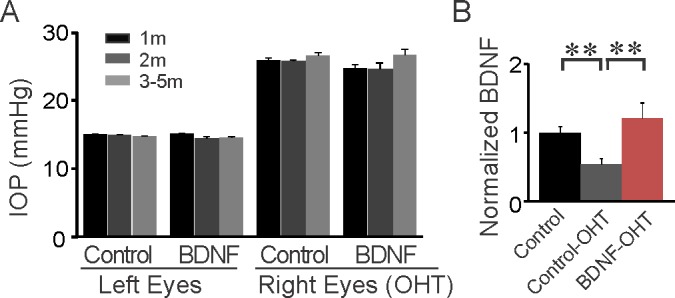
Chronic ocular hypertension (OHT) was induced in both BDNF_ overexpression (OE) and control mice. (A) Laser illumination and microbead injection was performed in the right eyes (OHT) while the untreated left eyes of the same mice were used as controls. IOP was measured at 1 month (m), 2 months, and 3 to 5 months post surgery. (B) Retinal BDNF level in OHT eyes was reduced in control mice but remained upregulated in BDNF_OE mice. **P < 0.01 in Student's t-test.
Next we used Western blot analysis to determine if retinal BDNF changed following sustained IOP elevation (Fig. 2B). We found that it was 46 ± 9% lower in the control group 2 months after IOP elevation (n = 4, P < 0.01 in Student's t-test, Fig. 2B). By contrast, retinal BDNF for the right eyes (OHT) in BDNF_OE mice (n = 7) was 125 ± 22% higher than for the right eyes of controls (P < 0.01 in Student's t-test, Fig. 2B). In other words, the downregulation of endogenous BDNF was compensated for by the genetic manipulation to overexpress BDNF in the mouse retina.
Overexpression of BDNF Delayed the Progressive Vision Loss Normally Seen in Mice With Chronic Ocular Hypertension
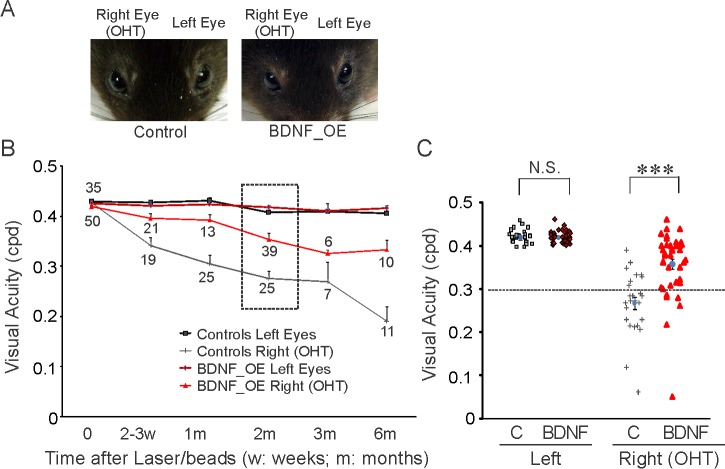
We measured mouse visual acuity by the optomotor test (Fig. 3, Supplementary Fig. S2), which permits one to test each eye of a mouse separately by reversing the direction of motion of the stimulus.42 Mice with no ocular abnormality or other physical signs of damage to the eyes formed our sample of experimental subjects for the optomotor test (Fig. 3A). Compared to the unmanipulated left eyes, the visual acuity of the right eyes decreased with time after induction of OHT (Figs. 3B, 3C). At 2 months post IOP elevation, the mean acuity of the right eyes (OHT) was 0.28 ± 0.08 cyc/deg (n = 25) and at 6 months was 0.19 ± 0.10 cyc/deg (n = 11), while their left eyes remained unchanged (2 months: 0.41 ± 0.06 cyc/deg, 6 months: 0.41 ± 0.03 cyc/deg, Figs. 3B, 3C). We also confirmed that different genetic backgrounds and the tamoxifen injection did not affect visual acuity (Supplementary Fig. S2). The mean acuity of Thy-1-CreERT2::BDNFstop mice was 0.44 ± 0.02 cyc/deg (n = 16), comparable to that in WT C57BL/6 mice (0.44 ± 0.02 cyc/deg, n = 21, Supplementary Fig. S2). Tamoxifen injection itself also did not affect acuity (n = 18); neither did overexpression of BDNF (n = 29, P = 0.65, 1-way ANOVA test, Supplementary Fig. S2).
Figure 3.
Overexpression of BDNF prevented the progressive loss of visual acuity for hypertensive eyes. (A) Photos of a control and a BDNF_OE mouse. Right eyes had induced ocular hypertension and left eyes were used as controls. (B) The mean visual acuity of right eyes decreased with time following IOP elevation, while that of the left eyes did not change. BDNF overexpression reduced the progressive loss of visual acuity (bright red line). Sample numbers are given for each data point, and a 2-way ANOVA test was performed (see Results). (C) Scatter plot of visual acuity of left and right eyes of individual mice in the dashed-line box in (B). Dotted line at 0.3 cyc/deg. NS, not significant; ***P < 0.001 in Student's t-test.
Importantly, we found that overexpression of BDNF reduced vision loss following sustained IOP elevation (Figs. 3B, 3C). Before OHT was induced in the right eyes, the baseline acuity of BDNF_OE mice was 0.42 ± 0.02 cyc/deg (n = 35), comparable to that of their left eyes (0.43 ± 0.02 cyc/deg, n = 35). At 1 month post IOP elevation, the mean acuity of right eyes was 0.39 ± 0.04 cyc/deg (n = 13), significantly higher than in the OHT eyes from the control group (0.31 ± 0.09 cyc/deg, P < 0.001, 2-way ANOVA, Tukey's posttest, Fig. 3B). At 2 months post IOP elevation, the acuity of right OHT eyes from BDNF_OE mice was reduced 17% (0.35 ± 0.08 cyc/deg of right eyes versus 0.42 ± 0.02 cyc/deg of left eyes, n = 39), while the mean acuity of OHT eyes in control mice fell by 35% (n = 25, Figs. 3B, 3C). More than 50% of the OHT eyes of control mice (17 out of 25 mice) had acuities below 0.30 cyc/deg, while only 15% of the OHT eyes from BDNF_OE mice (6 out of 39 mice) showed similarly low acuities (Fig. 3C). By 6 months following chronic IOP elevation, the mean acuity of OHT eyes of control mice had decreased from 0.43 ± 0.01 to 0.19 ± 0.03 cyc/deg (n = 11), whereas the mean acuity of OHT eyes of BDNF_OE mice decreased only from 0.42 ± 0.01 to 0.33 ± 0.02 cyc/deg (n = 10, P < 0.001, 2-way ANOVA, Tukey's posttest, Fig. 3B). In other words, even though overexpression of BDNF did not eliminate a reduction in visual acuity, it helped to preserve acuity in mice with OHT.
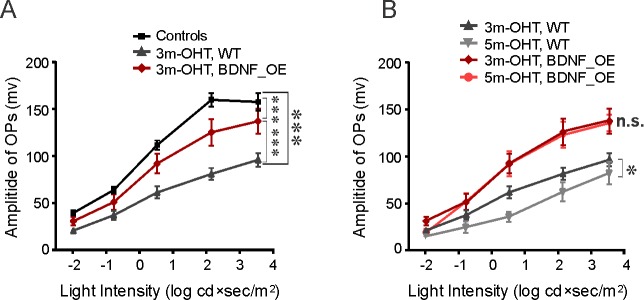
We also investigated whether BDNF protected retinal function using full-field ERG recordings. The amplitudes of the oscillatory potentials (OPs), which reflect inner retinal function, were measured for control and BDNF_OE mice with and without OHT (Fig. 4). At 3 months following IOP elevation, the mean amplitude of OPs was significantly reduced in the right OHT eyes (n = 12) of control mice (P < 0.001, 2-way ANOVA, Tukey's posttest, Fig. 4A). The amplitudes of OPs continued to decrease at 5 months post IOP elevation (n = 5, P < 0.05, 2-way ANOVA, Tukey's posttest, Fig. 4B). Interestingly, we found that overexpression of BDNF in the mouse model also served to protect the retina from the functional changes seen in the ERG of the control mice. After 3 months of IOP elevation, the mean amplitude of OPs in BDNF_OE mice (n = 8) was significantly higher than that of control OHT mice (P < 0.001, 2-way ANOVA, Tukey's posttest, Fig. 4A). At 5 months post IOP elevation, BDNF_OE mice showed no further reduction in OP amplitude (n = 4, P = 0.95, 2-way ANOVA, Tukey's posttest, Fig. 4B). Together, our results suggest that BDNF overexpression prevents the progressive loss of inner retinal function following IOP elevation that was observed for control mice.
Figure 4.
BDNF protected the inner retinal function of mice with chronic ocular hypertension. (A) At 3 months, the mean amplitudes of OPs in OHT eyes had decreased by approximately a factor of 2 in control mice. For BDNF_OE mice (red line), the change was much less dramatic. (B) The mean amplitudes of OPs in control mice continued to decrease with time while it did not change for BDNF_OE mice. n = 5 to 28 mice in each group. *P < 0.05, ***P < 0.001 in 2-way ANOVA, Tukey's posttest.
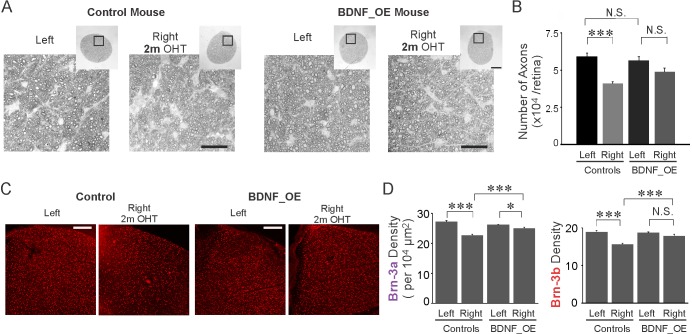
Overexpression of BDNF Reduced the Progressive RGC and Axon Loss Normally Observed Following Chronic Ocular Hypertension
We next examined whether overexpression of BDNF protected RGCs and their axons against the hypertensive insult (Figs. 5, 6). We quantified the number of axons at 2 months post IOP elevation (Figs. 5A, 5B). The optic nerve sections were prepared and the numbers of axons were compared between right (OHT) and left eyes of both control and BDNF_OE mice (Fig. 5A). In hypertensive eyes, the axonal loss was 30.7% (n = 6, P < 0.001, 1-way ANOVA, Tukey's posttest); by contrast, axonal loss was only 13.7% in BDNF_OE mice (n = 5, P = 0.30, Fig. 5B). Retinal ganglion cell density was also examined in flat-mounted retinas immunolabeled with two RGC markers, Brn-3a and Brn-3b (Figs. 5C, 5D). In control OHT retinas, an approximately 17.8% reduction in Brn-3b cell density was observed at 2 months post IOP elevation (n = 8, P < 0.001, 1-way ANOVA, Tukey's posttest). By contrast, only a 4.4% reduction in Brn-3b cell density was found in BDNF_OE mice (n = 16, P = 0.30, Fig. 5D). Similarly, a 16.7% reduction in Brn-3a density was found in control OHT retinas (n = 8, P < 0.001), while just a 4.7% reduction was seen for the BDNF_OE hypertensive eyes (n = 16, P = 0.03, 1-way ANOVA, Tukey's posttest, Fig. 5D). For both Brn-3a and Brn-3b cell types, the cell densities in OHT retinas of BDNF_OE mice were significantly higher than their counterparts in the control group (Brn-3a: P < 0.001, Brn-3b: P < 0.001, 1-way ANOVA, Tukey's posttest, Fig. 5D).
Figure 5.
BDNF overexpression reduced RGC and axon loss at 2 months post IOP elevation. (A) Cross sections of optic nerves stained by paraphenylenediamine from a control and a BDNF_OE mouse. (B) For control mice, the total number of axons decreased at 2 months post IOP elevation, while for BDNF_OE mice, the axon loss was not changed significantly. (C) Flat-mounted retinas immunostained by Brn-3b antibody from a control and a BDNF_OE mouse. (D) Brn-3a and Brn-3b densities were reduced post IOP elevation in control mice, while the loss of RGCs was reduced in BDNF_OE mice. Scale bars: 25 μm (A); 100 μm (inlets); 200 μm (C). *P < 0.05, ***P < 0.001 in 1-way ANOVA, Tukey's posttest.
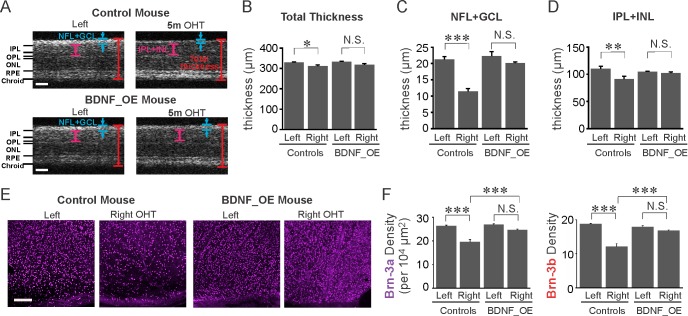
Figure 6.
BDNF overexpression reduced the progressive loss of RGCs at 5 months post IOP elevation. (A) Cross sections of retinas obtained by OCT imaging. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium. (B–D) At 5 months post IOP elevation, the thickness of all layers (B), the NFL+GCL (C), and the IPL+INL (D) was decreased significantly in control mice, respectively, while no significant reduction was observed in BDNF_OE mice (B–D). (E) Flat-mounted retinas immunostained by Brn-3a antibody. Scale bar: 200 μm. (F) BDNF overexpression reduced RGC loss at 5 months post IOP elevation. NS, not significant; *P < 0.05, **P < 0.01, ***P < 0.001 in 1-way ANOVA, Tukey's posttest.
To determine whether BDNF exhibited a long-term protective effect on RGCs and their axons, we performed OCT imaging to examine the overall morphologic changes of the mouse retina at 5 months following sustained IOP elevation (Figs. 6A–D). The total thickness and the thickness of NFL+GCL and IPL+INL were measured (Fig. 6A). Compared to the left eyes (n = 5), the total thickness was reduced in right hypertensive eyes in control mice (n = 3, P = 0.02, 1-way ANOVA, Tukey's posttest, Fig. 6B). The reduction of total thickness of OHT eyes was mainly due to a decrease of the thickness of the NFL+GCL, which was approximately 45% thinner compared to left eyes (P < 0.001, 1-way ANOVA, Tukey's posttest, Fig. 6C), a result similar to previous reports.29 In BDNF_OE mice, the thickness of the NFL+GCL of the OHT eyes was no different from that of their left eyes (n = 5, P = 0.44, 1-way ANOVA, Tukey's posttest, Fig. 6C). For the control group, the thickness of the inner retina (IPL+INL) was also reduced in the right eyes following IOP elevation (P < 0.01, 1-way ANOVA, Tukey's posttest), while no significant change was seen in BDNF_OE mice (P = 0.93, Fig. 6D). Note that the total thickness of the left eyes was similar for BDNF_OE (n = 5) and control mice (n = 5, P = 0.99, Fig. 6B), confirming once more that overexpression of BDNF does not affect the overall morphology of the retina.
We examined RGC densities after OCT imaging. We observed an approximately 25.7% reduction of Brn-3a cell density in OHT eyes (n = 5, P < 0.001, 1-way ANOVA, Tukey's posttest, Figs. 6E, 6F). A sizable decrease was found in the Brn-3b cell density too (35.0% decrease, n = 5, P < 0.001). In BDNF_OE mice, OHT resulted in only an 8.7% reduction in Brn-3a density (n = 5) and a 6.3% drop in Brn-3b density (n = 5), differences which were clearly much less than for OHT eyes of control mice (Brn-3a: P < 0.001, Brn-3b: P < 0.001, 1-way ANOVA, Tukey's posttest, Fig. 6F) and which were also found to be statistically insignificant from the control left eyes of the same animals (Brn-3a: P = 0.07, Brn-3b: P = 0.38, 1-way ANOVA, Tukey's posttest, Fig. 6F). These results strongly support the notion that overexpression of BDNF significantly reduces RGC loss following sustained IOP elevation.
Dendritic Atrophy in RGCs Was Rescued in BDNF_OE Mice
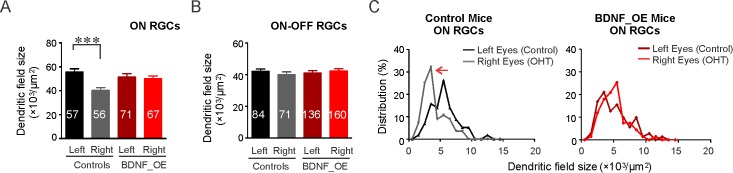
We next investigated whether overexpression of BDNF protects RGC dendritic structure against the hypertensive insult. The dual promoter in Thy-1-CreERT(T2) transgenic mice, which also drives the YFP reporter gene, made it possible to investigate the dendritic structural changes of RGCs when BDNF is overexpressed. Retinal ganglion cells were classified into ON and ON–OFF types based on their laminar pattern of dendritic stratification: ON RGCs having their dendrites laminated within the inner half of the IPL, and ON–OFF RGCs having their dendrites laminated in both ON and OFF sublamina in the IPL (Supplementary Fig. S3, also see Methods). Ocular hypertension induced dendritic degeneration for ON RGCs: The mean size of dendritic coverage in right eyes was 40.2 ± 5.4 × 103 μm2 (56 cells), which is approximately 27.4% smaller than for the control left eyes (55.4 ± 7.3 × 103 μm2, 57 cells, Fig. 7A). The cumulative distribution curve of ON RGC dendritic field sizes showed that more cells had smaller dendritic fields (P < 0.01 in the K-S test, Supplementary Fig. S3). By contrast, the mean dendritic field area of ON RGCs in BDNF_OE mice was 50.0 ± 6.1 × 103 μm2 (67 cells), comparable to that of their left eyes (51.4 ± 6.1 × 103 μm2, 71 cells, Fig. 7A; P = 0.42 in K-S test, Supplementary Fig. S3). We also plotted the distribution of dendritic field sizes for ON cells (Fig. 7C). In OHT eyes of control mice, the distribution of dendritic field sizes for ON cells shifted leftward (gray line, Fig. 7C), suggesting a decrease in dendritic field size for all types of ON cells following IOP elevation. By contrast, no significant shrinkage for ON RGCs was found in BDNF_OE mice (Fig. 7C), supporting the contention that BDNF overexpression maintains dendritic field size, counteracting the destructive effects induced by OHT and, as noted above, promoting RGC survival.
Figure 7.
BDNF protects the dendritic structure of ON RGCs against sustained ocular hypertension. (A, B) The mean dendritic field sizes of ON and ON–OFF cells from control and BDNF_OE mice. Numbers of RGCs studied are indicated. (C) The distribution of dendritic field sizes (left) of ON RGCs from control mice was shifted to the left (red arrow), whereas no shift was found in BDNF_OE OHT mice. ***P < 0.001 in K-S test.
For ON–OFF RGCs, no significant change in dendritic field size was observed in control mice (mean of left: 41.9 ± 1.6 × 103 μm2, 84 cells, right: 39.9 ± 1.8 × 103 μm2, 71 cells, P = 0.49 in K-S test, Fig. 7B, Supplementary Fig. S3), consistent with our previous findings.28 Similarly, no significant change was found in BDNF_OE mice (mean of left: 41.1 ± 1.5 × 103 μm2, 136 cells, right: 42.7 ± 1.3 × 103 μm2, 160 cells, P = 0.46 in K-S test, Fig. 7B, Supplementary Fig. S3).
Discussion
Long-Term Neurotrophic Protection in Mice With Chronic IOP Elevation
Neurotrophic protection of RGCs has been studied in different rodent models of experimental glaucoma and acute optic nerve damage.8–10 Acute or chronic OHT is often induced by surgical procedures or by genetic manipulation in rodents. For example, Ren and his colleagues22 induced a transient IOP elevation to 130 mm Hg for 45 minutes in mice, and BDNF-mediated neuroprotection was studied over the following months. Sappington and her colleagues43 injected polystyrene beads into mouse eyes, which elicited a ∼30% elevation in IOP for a couple of weeks; and long-term IOP elevation can be achieved by repeated injections of microbeads.43 In this study, we induced a modest increase of IOP (∼50% elevation) by one surgical procedure and showed that the IOP elevation lasted up to 6 months (also see Refs. 28, 29).
In this study, we produced transgenic mice to upregulate the retinal BDNF level continuously (Figs. 1, 2), providing an excellent system in which to investigate how a modest continuous dose of BDNF might affect the slow progressive RGC and vision loss often seen in glaucoma patients. Brain-derived neurotrophic factor is produced locally by inner retinal neurons such as RGCs and amacrine cells, as well as retinal glial cells.32 Brain-derived neurotrophic factor can also be retrogradely transported through axons from higher brain centers.32,44 Glaucomatous insults could reduce the retinal BDNF produced locally as well as blocking the retrograde transport of BDNF.13,14 Application of recombinant BDNF to the eye has been shown to reduce RGC loss following optic nerve injury in cats, and application of BDNF to both the eye and visual cortex produced more protection of RGCs than treatment of the eye alone,21 suggesting that both sources of BDNF play a role in neuroprotection, the cortical application presumably inhibiting subcortical visual neuron loss that could affect BDNF delivery from the brain to RGCs.
The Thy-1-CreERT(T2) line used in this study differs from the Thy-1-YFP (H) line used in our previous studies28,32 in that the Thy-1-YFP (H) line has only a very small number of RGCs labeled, while the Thy-1-CreERT(T2) line has both RGCs and amacrine cells labeled. So, BDNF upregulation through tamoxifen-induced Cre recombinase under the Thy-1 promoter will occur in RGCs and amacrine cells. We found that overall retinal BDNF is reduced in control mice by chronic IOP elevation, but that BDNF overexpression can return this neurotrophin to its normal level and compensate for the deleterious effects on RGCs and vision of OHT-induced loss of retinal BDNF (Fig. 2).
We are unable to say specifically how BDNF acts to accomplish this at the cellular level. Some studies support the idea that BDNF synthesized by RGCs and displaced amacrine cells rescues neighboring neurons through a paracrine mechanism,22,45 though we cannot rule out the possibility of an autocrine mechanism.46,47 Della Santina and his colleagues31 suggested that the changes of RGC light response properties preceded dendritic structural rearrangements, which were largely attributed to a loss of synapses. Given that the vasculature, glial processes, and synapses interact closely,48,49 El-Danaf and Huberman30 further suggested that loss of capillary support could induce dendritic and synaptic changes at an early stage of experimental glaucoma. How BDNF protects the synaptic function and structural integrity of RGCs against the hypertensive insult remains to be fully investigated.
One of the important questions of clinical relevance is whether BDNF provides long-term protection for RGCs and vision in glaucoma. Comparison of BDNF-mediated neuroprotection between different animal studies is not straightforward due to the variability of the length and degree of IOP elevation used by different investigators, with the result that the same molecular and cellular mechanisms may not be involved in all studies.25,26,50,51 For example, in a rat model of laser-induced experimental glaucoma, RGC loss was significantly reduced by BDNF transfected with adeno-associated viral (AAV) vector at 4 weeks post laser illumination to the trabecular meshwork.19 However, another study suggested that BDNF alone made no significant improvement in axon survival in rats with laser-induced glaucoma. Ren and his colleagues22 showed that the major parameters of the visual evoked potential (VEP) were protected by BDNF at 9 weeks following a transient IOP spike; moreover, visual acuity and contrast sensitivity were well maintained for up to 1 year.22 We found that a mild sustained elevation of IOP produced a gradual decrease of visual acuity, but the loss in acuity was less when BDNF was overexpressed (Fig. 3). We also found that the amplitude of OPs was significantly reduced in mice with sustained IOP elevation (Fig. 4), consistent with previous studies,29,52,53 but not generally seen with primate models or in human glaucoma.54 The OP has been classified into early, intermediate, and late components especially in clinical studies.55 The amplitude of OP we measured was the difference between the maximum positive- and negative-valued peaks from the filtered waveform (see the inset of Fig. 2M, Ref. 29)—and we presume therefore that it corresponds roughly to the intermediate component, which is believed to originate from the inner retina. Taken together, all these results support the notion that BDNF exerts long-term protection on RGCs and vision, providing reason to believe that therapies that upregulate BDNF or engage its signaling pathway should help to preserve vision in glaucoma.
Subtype-Dependent BDNF Protection in Glaucoma
During retinal development, BDNF exhibits various actions on RGC dendritic morphology, which in turn have profound effects on the functional pathways of the visual system.32,37,56 Given that BDNF promotes dendritic growth for ON RGCs during postnatal development,32,37 we tested whether BDNF might prevent the dendritic degeneration for ON RGCs in experimental glaucoma (Fig. 7). Our previous studies have shown that OHT can induce significant degeneration of the dendritic trees of ON RGCs and weaken their visual responses.28,29 Here we show that overexpression of BDNF prevents the degeneration of ON RGC dendrites in mice with sustained IOP elevation (Fig. 7). While we have segregated RGCs into just three broad categories, it is now known that there are more than 20 types of RGC, each having a unique dendritic morphology and pattern of response to visual stimuli.57,58 The technical challenge of classifying RGCs physiologically makes the study of type-dependent RGC degeneration in eye diseases difficult. One encouraging future direction would be to use transgenic mouse lines with specific RGC subtypes labeled to study RGC subtype loss at a fine granularity as others have done.30,59 For example, ON RGCs include ON direction-selective (DS) and nondirection-selective cells.57,60,61 Transgenic lines such as Hoxd10-GFP, which had three motion directions for ON DS cells labeled,62 would help immensely in the investigation of degeneration and BDNF protection of these specific RGC subtypes in experimental glaucoma.
Although a small sample size prohibited us from investigating OFF RGCs, it is possible that they would be protected by BDNF. Our previous study on the functional degeneration of RGCs using multielectrode array (MEA) recording of WT retinas showed that overall the OFF cells were susceptible to the sustained IOP elevation.29 Della Santina and his colleagues31 further demonstrated that OFF transient, but not OFF sustained, had smaller dendritic field and receptive field (RF) sizes. Given the small sample of OFF RGCs we had here, the best animal model in which to investigate the effect of BDNF on OFF RGC survival in experimental glaucoma would be a transgenic line in which a specific type of OFF RGCs is labeled. Interestingly, ON–OFF RGCs do not seem to be adversely affected by a hypertensive insult (Fig. 7). Because visual acuity declines (Fig. 3) while ON–OFF RGCs are unaffected, preservation of ON and perhaps OFF RGCs is likely important for preserving visual acuity.
Neurotrophins can activate survival signals as well as suppress the intrinsic apoptotic process via two different receptors: the Trk receptor family and the p75 neurotrophin receptor (p75NTR).1,2 Brain-derived neurotrophic factor may also interact with other factors to promote RGC survival in experimental glaucoma.10 The combination of nerve growth factor (NGF), or a selective TrkA agonist, with a p75 inhibitor has been shown to produce strong RGC protection following optic nerve transection.63 However, another study found that the ciliary-derived neurotrophic factor (CNTF), but not CNTF+BDNF, exerted a protective effect on RGCs in rats with experimental glaucoma.24 How far differences in experimental methods and time points for recording protection can account for the inconsistent results remains to be determined. It is likely that BDNF promotes RGC survival but does not alone produce complete rescue of RGCs in animal models of experimental glaucoma. Our results suggest that some RGC subtypes are more vulnerable than others in glaucoma as the contrasting outcomes for ON and ON–OFF RGCs reported here demonstrate. It may also be that some subtypes of RGCs are more amenable to BDNF protection than others. This study should be considered as just a step toward examining cell type–dependent protection of RGCs and visual function by BDNF against the chronic insult of OHT; and, importantly, it is noted that our animal model offers a system in which to test the effectiveness of any signaling molecules or combinations thereof for protection from sustained OHT-induced RGC and vision loss.
Supplementary Material
Acknowledgments
The authors thank Yuzhi Jia for helping with the preparation of optic nerve samples, Genn Suyeoka for mouse husbandry, and Zhen Puyang for helping with ERG recordings. The authors also thank the Biological Imaging Facilities (BIF) at Northwestern University for confocal microscopy and the Northwestern University Atomic and Nanoscale Characterization Experimental Center (NUANCE) for preparation of optic nerve sections.
Supported by National Institutes of Health Grants R01EY026286 (XL) and R01EY01995 (HFZ), Research to Prevent Blindness (Department of Ophthalmology), Northwestern Memorial Foundation (XL), BrightFocus Foundation (XL, LF), a Midwest Eye Banks Research grant (XL), and the Illinois Society for the Prevention of Blindness (HC).
Disclosure: L. Feng, None; H. Chen, None; J. Yi, None; J.B. Troy, None; H.F. Zhang, None; X. Liu, None
References
- 1. Huang EJ,, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001; 24: 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang EJ,, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003; 72: 609–642. [DOI] [PubMed] [Google Scholar]
- 3. Nagahara AH,, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011; 10: 209–219. [DOI] [PubMed] [Google Scholar]
- 4. Lu B,, Nagappan G,, Guan X,, Nathan PJ,, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013; 14: 401–416. [DOI] [PubMed] [Google Scholar]
- 5. Gupta VK,, You Y,, Gupta VB,, Klistorner A,, Graham SL. TrkB receptor signalling: implications in neurodegenerative psychiatric and proliferative disorders. Int J Mol Sci. 2013; 14: 10122–10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quigley HA,, Addicks EM,, Green WR,, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981; 99: 635–649. [DOI] [PubMed] [Google Scholar]
- 7. Nickells RW,, Howell GR,, Soto I,, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012; 35: 153–179. [DOI] [PubMed] [Google Scholar]
- 8. Chader GJ. Advances in glaucoma treatment and management: neurotrophic agents. Invest Ophthalmol Vis Sci. 2012; 53: 2501–2505. [DOI] [PubMed] [Google Scholar]
- 9. Johnson TV,, Bull ND,, Martin KR. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res. 2011; 93: 196–203. [DOI] [PubMed] [Google Scholar]
- 10. Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 2011; 22: 78–86. [DOI] [PubMed] [Google Scholar]
- 11. Guo Y,, Johnson E,, Cepurna W,, Jia L,, Dyck J,, Morrison JC. Does elevated intraocular pressure reduce retinal TRKB-mediated survival signaling in experimental glaucoma? Exp Eye Res. 2009; 89: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta V,, You Y,, Li J,, et al. BDNF impairment is associated with age-related changes in the inner retina and exacerbates experimental glaucoma. Biochim Biophys Acta. 2014; 1842: 1567–1578. [DOI] [PubMed] [Google Scholar]
- 13. Pease ME,, McKinnon SJ,, Quigley HA,, Kerrigan-Baumrind LA,, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000; 41: 764–774. [PubMed] [Google Scholar]
- 14. Quigley HA,, McKinnon SJ,, Zack DJ,, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000; 41: 3460–3466. [PubMed] [Google Scholar]
- 15. Lambert W,, Agarwal R,, Howe W,, Clark AF,, Wordinger RJ. Neurotrophin and neurotrophin receptor expression by cells of the human lamina cribrosa. Invest Ophthalmol Vis Sci. 2001; 42: 2315–2323. [PubMed] [Google Scholar]
- 16. Knox DL,, Eagle RC,, Jr,, Green WR. Optic nerve hydropic axonal degeneration and blocked retrograde axoplasmic transport: histopathologic features in human high-pressure secondary glaucoma. Arch Ophthalmol. 2007; 125: 347–353. [DOI] [PubMed] [Google Scholar]
- 17. Mansour-Robaey S,, Clarke DB,, Wang YC,, Bray GM,, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994; 91: 1632–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Polo A,, Aigner LJ,, Dunn RJ,, Bray GM,, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998; 95: 3978–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin KR,, Quigley HA,, Zack DJ,, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003; 44: 4357–4365. [DOI] [PubMed] [Google Scholar]
- 20. Parrilla-Reverter G,, Agudo M,, Sobrado-Calvo P,, Salinas-Navarro M,, Villegas-Perez MP,, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009; 89: 32–41. [DOI] [PubMed] [Google Scholar]
- 21. Weber AJ,, Viswanathan S,, Ramanathan C,, Harman CD. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Invest Ophthalmol Vis Sci. 2010; 51: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren R,, Li Y,, Liu Z,, Liu K,, He S. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2012; 53: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 23. Ko ML,, Hu DN,, Ritch R,, Sharma SC,, Chen CF. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001; 305: 139–142. [DOI] [PubMed] [Google Scholar]
- 24. Pease ME,, Zack DJ,, Berlinicke C,, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson TV,, Tomarev SI. Rodent models of glaucoma. Brain Res Bull. 2010; 81: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puyang Z,, Chen H,, Liu X. Subtype-dependent morphological and functional degeneration of retinal ganglion cells in mouse models of experimental glaucoma. J Nat Sci. 2015; 1: e103. [PMC free article] [PubMed] [Google Scholar]
- 27. Feng L,, Chen H,, Suyeoka G,, Liu X. A laser-induced mouse model of chronic ocular hypertension to characterize visual defects. J Vis Exp. 2013; 78:50440. [DOI] [PMC free article] [PubMed]
- 28. Feng L,, Zhao Y,, Yoshida M,, et al. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Invest Ophthalmol Vis Sci. 2013; 54: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H,, Yan Z,, Liu M,, et al. Progressive degeneration of retinal and superior collicular functions in mice with sustained ocular hypertension. Invest Ophthalmol Vis Sci. 2015; 56: 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El-Danaf RN,, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015; 35: 2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Della Santina L,, Inman DM,, Lupien CB,, Horner PJ,, Wong RO. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci. 2013; 33: 17444–17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X,, Grishanin RN,, Tolwani RJ,, et al. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007; 27: 7256–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leung CK,, Weinreb RN,, Li ZW,, et al. Long-term in vivo imaging and measurement of dendritic shrinkage of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011; 52: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 34. Chang Q,, Khare G,, Dani V,, Nelson S,, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006; 49: 341–348. [DOI] [PubMed] [Google Scholar]
- 35. Badea TC,, Wang Y,, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003; 23: 2314–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rangarajan KV,, Lawhn-Heath C,, Feng L,, Kim TS,, Cang J,, Liu X. Detection of visual deficits in aging DBA/2J mice by two behavioral assays. Curr Eye Res. 2011; 36: 481–491. [DOI] [PubMed] [Google Scholar]
- 37. Liu X,, Robinson ML,, Schreiber AM,, et al. Regulation of neonatal development of retinal ganglion cell dendrites by neurotrophin-3 overexpression. J Comp Neurol. 2009; 514: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida M,, Feng L,, Grimbert F,, et al. Overexpression of neurotrophin-3 stimulates a second wave of dopaminergic amacrine cell genesis after birth in the mouse retina. J Neurosci. 2011; 31: 12663–12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang BS,, Feng L,, Liu M,, Liu X,, Cang J. Environmental enrichment rescues binocular matching of orientation preference in mice that have a precocious critical period. Neuron. 2013; 80: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yi J,, Wei Q,, Liu W,, Backman V,, Zhang HF. Visible-light optical coherence tomography for retinal oximetry. Opt Lett. 2013; 38: 1796–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang Q,, Cho KS,, Chen H,, et al. Microbead-induced ocular hypertensive mouse model for screening and testing of aqueous production suppressants for glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Douglas RM,, Alam NM,, Silver BD,, McGill TJ,, Tschetter WW,, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005; 22: 677–684. [DOI] [PubMed] [Google Scholar]
- 43. Sappington RM,, Carlson BJ,, Crish SD,, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010; 51: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lom B,, Cogen J,, Sanchez AL,, Vu T,, Cohen-Cory S. Local and target-derived brain-derived neurotrophic factor exert opposing effects on the dendritic arborization of retinal ganglion cells in vivo. J Neurosci. 2002; 22: 7639–7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baumgartner BJ,, Shine HD. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci. 1997; 17: 6504–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen-Cory S,, Escandon E,, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Dev Biol. 1996; 179: 102–115. [DOI] [PubMed] [Google Scholar]
- 47. Ghosh A,, Carnahan J,, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994; 263: 1618–1623. [DOI] [PubMed] [Google Scholar]
- 48. Pournaras CJ,, Rungger-Brandle E,, Riva CE,, Hardarson SH,, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 49. Puyang Z,, Feng L,, Chen H,, Liang P,, Troy JB,, Liu X. Retinal ganglion cell loss is delayed following optic nerve crush in NLRP3 knockout mice. Sci Rep. 2016; 6: 20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Almasieh M,, Wilson AM,, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 51. Calkins DJ,, Horner PJ. The cell and molecular biology of glaucoma: axonopathy and the brain. Invest Ophthalmol Vis Sci. 2012; 53: 2482–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bayer AU,, Danias J,, Brodie S,, et al. Electroretinographic abnormalities in a rat glaucoma model with chronic elevated intraocular pressure. Exp Eye Res. 2001; 72: 667–677. [DOI] [PubMed] [Google Scholar]
- 53. Grozdanic SD,, Betts DM,, Sakaguchi DS,, Allbaugh RA,, Kwon YH,, Kardon RH. Laser-induced mouse model of chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2003; 44: 4337–4346. [DOI] [PubMed] [Google Scholar]
- 54. Colotto A,, Falsini B,, Salgarello T,, Iarossi G,, Galan ME,, Scullica L. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41: 2205–2211. [PubMed] [Google Scholar]
- 55. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998; 17: 485–521. [DOI] [PubMed] [Google Scholar]
- 56. Cohen-Cory S,, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature. 1995; 378: 192–196. [DOI] [PubMed] [Google Scholar]
- 57. Sun W,, Li N,, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002; 451: 115–126. [DOI] [PubMed] [Google Scholar]
- 58. Volgyi B,, Chheda S,, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009; 512: 664–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duan X,, Qiao M,, Bei F,, Kim IJ,, He Z,, Sanes JR. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron. 2015; 85: 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen H,, Liu X,, Tian N. Subtype-dependent postnatal development of direction- and orientation-selective retinal ganglion cells in mice. J Neurophysiol. 2014; 112: 2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu H,, Tian N. Pathway-specific maturation, visual deprivation, and development of retinal pathway. Neuroscientist. 2004; 10: 337–346. [DOI] [PubMed] [Google Scholar]
- 62. Dhande OS,, Estevez ME,, Quattrochi LE,, et al. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. J Neurosci. 2013; 33: 17797–17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lebrun-Julien F,, Morquette B,, Douillette A,, Saragovi HU,, Di Polo A. Inhibition of p75(NTR) in glia potentiates TrkA-mediated survival of injured retinal ganglion cells. Mol Cell Neurosci. 2009; 40: 410–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.