Abstract
Hippocampal volume has been found to be smaller in individuals with stress-related disorders, but it remains unclear whether smaller volume is a consequence of stress or rather a vulnerability factor. Here, we examined this issue by relating stress levels to hippocampal volumes in healthy participants examined every 5 years in a longitudinal population-based study. Based on scores of 25- to 60-year–old participants on the perceived stress questionnaire, we defined moderately to high (n = 35) and low (n = 76) stress groups. The groups were re-examined after 5 years (at the 6th study wave). Historical data on subjective stress were available up to 10 years prior to Wave 5. At the first MRI session, the moderately to high stress group had a significantly smaller hippocampal volume, as measured by FreeSurfer (version 5.3), compared with the low-stress group. At follow-up, group differences in stress levels and hippocampal volume remained unchanged. In retrospective analyses of subjective stress, the observed group difference in stress was found to be stable. The long-term stability of group differences in perceived stress and hippocampal volume suggests that a small hippocampal volume may be a vulnerability factor for stress-related disorders.
Keywords: healthy individuals, hippocampal volume, magnetic resonance imaging, stress, susceptibility
Introduction
The hippocampus is considered sensitive to chronic or traumatic stressful experiences (O'Doherty et al. 2015). Numerous experimental findings from animal studies have shown that chronic stress can lead to a reduction in hippocampal volumes (Sapolsky 1990; Uno et al. 1994; Magarinos and Mcewen 1995; Magarinos et al. 1996). Structural changes have been related to inhibition of neurogenesis as well as to shrinkage of dendrites in CA3 and the dentate gyrus, and loss of spiny synapses in CA1 neurons via circulating adrenal steroids and glutamatergic activity (McEwen 1999; McEwen et al. 2016).
In humans, stress-related structural changes in hippocampal subfields were reported in a study of individuals with post-traumatic stress disorder (PTSD) that used high-resolution magnetic resonance imaging (MRI) and found volumetric diminution in CA3 and the dentate gyrus (Wang et al. 2010). The results from several other studies (Lupien et al. 1998; Starkman et al. 1999; Felmingham et al. 2009; Chao et al. 2014) support the acquisition hypothesis; that stress induces changes in human hippocampus. However, it has been debated whether a smaller hippocampal region must reflect a consequence of trauma/chronic stress, or rather could represent vulnerability for developing stress-related disorders (Bremner 2001). To resolve this issue, longitudinal studies have been called for (Gianaros et al. 2007). In a prospective study, reports of chronic life stress over a 20-year period and their relationship with hippocampal gray matter volume in healthy postmenstrual women were studied (Gianaros et al. 2007). It was found that chronic life stress predicted lower hippocampal volume. However, longitudinal assessment of regional brain volumes was not conducted, so the authors could not exclude the possibility that changes in hippocampal volume preceded individual differences in stress perception over time (Gianaros et al. 2007). There are indeed some study results indicating that a small hippocampal volume might precede pathological stress reactions (Gilbertson et al. 2002; Kremen et al. 2012; van Rooij et al. 2015). In a study of identical twin pairs discordant for trauma exposure, it was found that the twin who developed PTSD symptoms as well as his identical twin (not exposed to trauma) had lower hippocampal volume compared with the trauma-exposed twin without PTSD symptoms (Gilbertson et al. 2002). Thus, rather than being a consequence of a traumatic or stressful experience, reduced hippocampal volume may be a vulnerability factor for developing stress-related disorders.
In the present study, we evaluated whether individuals with moderately to high perceived stress levels had smaller hippocampal volumes than individuals experiencing lower levels of stress. Furthermore, we related perceived stress levels to retrospective reports on stress as well as to changes in hippocampal volume over time. Although prospective assessment of chronic life stress has been reported (Gianaros et al. 2007), to the best of our knowledge, no prior study evaluated changes in perceived stress and hippocampal volume over a period of several years. Based on findings of cumulative depression and PTSD-related hippocampal volume loss (Sheline et al. 1999; Videbech and Ravnkilde 2004; Felmingham et al. 2009; Chao et al. 2014), we predicted that group differences in hippocampal volume would be magnified over time if driven by high perceived stress over time, whereas no magnification of volume differences over time would be more consistent with stable individual differences.
Materials and Methods
Study Population
A nonclinical population-based sample was used for the longitudinal study of various parameters of health, memory, and aging (Nilsson et al. 1997). The present study is based on four time points of the Betula study: 1998–2000 (T3), 2003–2005 (T4), 2008–2010 (T5), and 2012–2014 (T6). At each time point, participants underwent a health examination, blood sampling, and answered several questionnaires. In addition, at T5 and T6 the participants underwent an MRI examination (for further descriptions of the Betula study, see Nilsson et al. 1997). The Regional Ethical Vetting Board at Umea University approved this study (approval no. 97–173 and 08–132M). Written informed consent was obtained from all participants.
Participants were included and excluded as indicated in Table 1. First, we identified participants with available data of the perceived stress questionnaire (PSQ) at T5 (n = 1141, Subgroup A). Then, we only included participants from Subgroup A with available MRI data at T5 and T6 (n = 218; Subgroup B). According to normative data reported by Bergdahl and Bergdahl (2002), <5% of individuals over 60 years old perceive moderately to high stress levels (defined as PSQ >0.34). This was in line with the present study, in which only 11% of the participants over 60 years of age perceived moderately to high stress levels. There was a significant difference in PSQ scores between individuals >60 (n = 107) and ≤60 (n = 111) years [mean PSQ index for those over 60 = 0.17; mean PSQ index for those under 60 = 0.28; t(216) = 6.21 P < 0.001]. Individuals >60 years of age were therefore excluded from further analyses, leaving 111 participants (age 25–60) for inclusion (Subgroup C). Finally, in order to evaluate early markers of perceived stress, we included participants from Subgroup C with available data at T3 and T4 (n = 67; Subgroup D) (Table 1). Thus, only data for Subgroups C and D are presented in the result section.
Table 1.
Overview of the inclusion/exclusion process. Data from Subgroups C and D were used for the analyses in the present study
| Subgroup | n | F/M% | Age T5 M (SD) | T5 PSQ index M (SD) | Description of subgroups |
|---|---|---|---|---|---|
| A.T5 and PSQ | 1141 | 54/46 | 63.2 (14,5) | 0.22 (0.14) | Entire cohort |
| B.T5, T6, PSQ and MRI | 218 | 47/53 | 60.2 (12.5) | 0.23 (0.15) | Participants with available PSQ MRI data at T5 and T6, before age exclusion |
| C.T5, T6, PSQ and MRI ≤60 | 111 | 43/57 | 50.8 (10.6) | 0.28 (0.16) | Participants included after excluding those older than 60 years at T5 |
| D.T5, T6, PSQ and MRI ≤60 with stress data at T3 and T4 | 67 | 40/60 | 57.5 (2.5) | 0.26 (0.15) | Participants with early markers of perceived stress at T3 and T4 |
PSQ, perceived stress questionnaire; T, time point; MRI, magnetic resonance imaging; F, female; M, male.
Assessment of Background Variables
A health questionnaire was used for background variables such as sex, age, and years of education and were completed at home before the testing period by the participants. Episodic memory was measured by a composite of 5 tasks; the score can range from 0 to 76 with higher scores indicating better episodic memory. The procedure is fully described in Nilsson et al. (1997). A Self-Report Depression Scale (CES) was used to evaluate depression (Cronbach’s α 0.85–0.90). The total score ranges from 0 to 60, and a CES-D score ≥16 is considered to be of clinical relevance (Radloff 1977). To evaluate sleeping problems, the Karolinska Sleep Questionnaire (KSQ) was used. In a population study of adults, three KSQ dimensions were identified: poor sleep quality M = 1.58 (SD = 1.03), nonrestorative sleep M = 1.47 (SD = 1.04), and sleep apnea M = 0.74 (SD = 0.88) (Cronbach's α= 0.73–0.87) (Nordin et al. 2013). Background variables are presented in Table 2.
Table 2.
Characteristics of participants in the low versus moderately to high PSQ groups
| Low PSQ index <0.34 at T5 |
Moderately to high PSQ index >0.34 at T5 |
P | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Age | 76 | 51.5 | 10.4 | 35 | 49.3 | 11.0 | 0.31a |
| Sex (female/male) | (31/45) | (17/18) | 0.54b | ||||
| Education (year) | 76 | 14.1 | 3.3 | 35 | 14.8 | 2.9 | 0.16a |
| EMC | 76 | 43.8 | 8.2 | 35 | 45.4 | 7.6 | 0.33c |
| BMI | 76 | 26.0 | 3.3 | 35 | 26.6 | 4.5 | 0.93a |
| CES | 76 | 6.50 | 5.43 | 35 | 13.0 | 7.06 | <0.001c |
| Poor sleep quality | 76 | 1.28 | 0.75 | 35 | 2.03 | 0.96 | 0.00c |
| Poor restorative sleep | 76 | 1.06 | 0.68 | 35 | 1.94 | 1.06 | <0.001c |
| Sleep apnea | 76 | 0.82 | 1.10 | 35 | 0.90 | 1.27 | 0.91c |
| PSQ index T4 | 48 | 0.24 | 0.12 | 19 | 0.41 | 0.16 | <0.001a |
| PSQ index T5 | 76 | 0.20 | 0.09 | 35 | 0.47 | 0.10 | <0.001a |
| PSQ index T6 | 76 | 0.21 | 0.11 | 35 | 0.40 | 0.13 | <0.001a |
EMC, episodic memory composite; BMI, body mass index; CES, Self-Report Depression scale; poor sleep quality, poor restorative sleep and sleep apnea index from KSQ, Karolinska Sleep questionnaire; PSQ, perceived stress questionnaire. Variables are displayed as mean (SD).
aStudents t-test.
bχ2 test.
cMann–Whitney U test.
Assessment of Perceived Stress Levels
To measure general perceived stress, the PSQ was administered at T4, T5, and T6. The PSQ is a self-assessment-based instrument for recording subjective perceived stress and has been found to have high validity and reliability (Cronbach’s α > 0.90) (Levenstein et al. 1993; Bergdahl and Bergdahl 2002). The PSQ is a 30-item questionnaire and the items are scored from 1 to 4. A PSQ index, varying from 0 (lowest level of perceived stress) to 1 (highest level of perceived stress), was derived from the total score (Bergdahl and Bergdahl 2002). In addition, at T3, T4, T5, and T6 the participants rated how stressed they felt in general on a scale from 0 (not stressed) to 10 (very stressed). The scores on this stress scale have been shown to correlate with those from the PSQ scale (Ohman et al. 2007).
Image Acquisition and Processing
Structural brain imaging was done within 285 days from the self-rated scales at T5 and within 46 days at T6. Structural brain imaging was performed on a 3-T General Electric scanner with a 32-channel head coil. High-resolution T1-weighted images were collected with a 3D fast spoiled gradient echo sequence using the following parameters: 180 slices with 1 mm thickness, TR 8.2 ms, TE 3.2 ms, flip angle 12°, field of view 25 cm × 25 cm. For segmentation and parcellation of cortical and subcortical structures the FreeSurfer version 5.3 tool was used http://surfer.nmr.mgh.harvard.edu/. In brief, this processing includes motion correction and normalization of the structural T1 weighted images. A hybrid surface deformation procedure removes nonbrain tissue and transforms the images to Talairach space. Tesselation of gray and white matter and intensity gradients places the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to other tissues. For further description, see Dale et al. (1999). Images were automatically processed with the longitudinal stream in FreeSurfer (Reuter et al. 2012) and brain volumes were adjusted for total intracranial volume (Raz et al. 2005).
Statistical Analysis
The Kolmogorov–Smirnov test was used to test for normality and outliers were assessed by histograms and boxplots. Three outliers were identified for hippocampal volume and excluded. Group differences in demographic and clinical data were examined with Student's t-test, the Mann–Whitney U, and the χ2 test. To explore main and interaction effects, mixed ANOVAs were conducted. A two-tailed α level <0.05 was considered as significant. Multiple comparisons were corrected using the Bonferroni–Holm method (Holm 1979). The statistical analyses were performed with SPSS software (Version 22.0, SPSS, Inc., Chicago, IL, USA).
Results
Identifying Moderately to High and Low-Stress Groups
Participants' PSQ scores at T5 were dichotomized based on the Bergdahl and Bergdahl's (2002) cutoff, with low stress defined as a PSQ index <0.34 and moderately to high stress as a PSQ index >0.34. Based on their PSQ ratings at the 5th test wave, at the time for the first MRI session, the participants (Subsample C in Table 1) were divided into a low and a moderately to high perceived stress group.
There were no group differences in self-reported stress-related diseases, such as heart [χ2(1, n = 111) = 0.47, P = 0.492], stroke [χ2(1, n = 111) = 2.16, P = 0.141], diabetes [χ2(1, n = 111) = 0.31, P = 0.577], tumor [χ2(1, n = 111) = 0.31, P = 0.57], or psychiatric disease [χ2(1, n = 111) = 2.15, P = 0.142]. The participants mainly reported two different kinds of psychiatric diseases: exhaustion syndrome and depression. There were no significant differences in reported exhaustion syndrome [χ2(1, n = 111) = 0.02, P = 0.584] or depression [χ2(1, n = 111) = 0.3.83, P = 0.064] between the low and moderately to high stress groups. There was no significant difference in use of antidepressants [χ2(1, n = 111) = 2.27, P = 0.132], and antihypertensive medicine [χ2(1, n = 111) = 0.001, P = 0.974] between the two groups. Additional participants’ characteristics are given in Table 2.
Perceived Stress Over Time
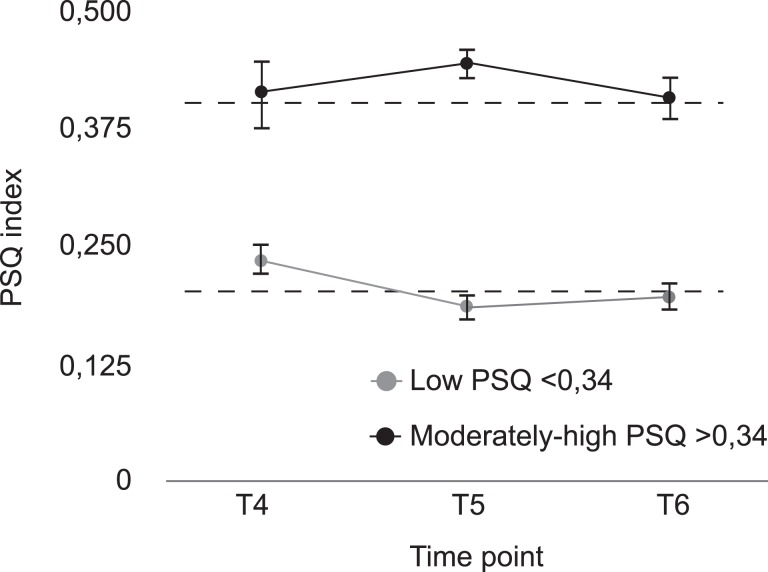
Figure 1 shows PSQ scores for the two groups measured 5 years before and 5 years after the point in time when the groups were defined (T5). As can be seen, the group separation was constant across this time period, with significant differences at both T4 [t(65) = −5.03, P ≤ 0.001], T5 [t(65) = −10.91, P ≤ 0.001] and T6 [t(65) = −7.63, P ≤ 0.001]. A mixed 2 × 3 within–between ANOVA showed a significant main effect of PSQ group [F1,65 = 90.12, P < 0.001, partial η2 = 0.58], no main effect of time [F2,130 = 0.82 P = 0.444, partial η2 = 0.02], and an interaction between PSQ group and time [F2,130 = 4.05, P = 0.022, partial η2 = 0.11]. The latter interaction reflected some dynamic changes over time, but as shown in Figure 1, the group difference in PSQ ratings remained present over a decade.
Figure 1.
Stability in group differences in PSQ levels over a decade. The moderately to high PSQ group rated PSQ levels significant higher than the low PSQ group at all three test waves. The groups showed stable PSQ levels (moderately to high PSQ group >0.34 and low PSQ group <0.34) across time points. The dashed lines reflect the mean over time points for each group.
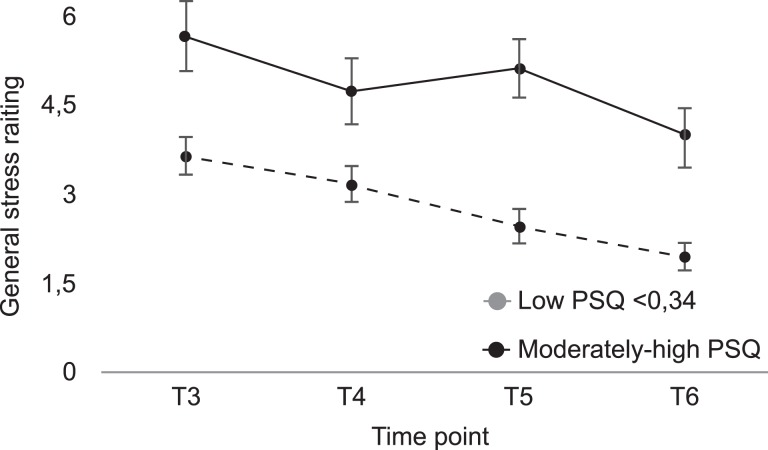
Stability in perceived stress was further underscored by the stress ratings over 15 years (Fig. 2). Stress levels were rated on a scale ranging from 0 to 10 at T3–T6, allowing markers of perceived stress to be examined over a decade prior to MRI at T5. The perceived general stress ratings correlated significantly with PSQ ratings (n = 67; T4 r = 0.71, P < 0.001; T5 r = 0.53, P < 0.001; T6 r = 0.61, P < 0.001). The same trend observed for the PSQ levels could be seen in the general stress ratings, such that the moderately to high PSQ group constantly rated higher stress levels compared with the low PSQ group. Note that the final stress rating for the moderately to high group was higher than that for the low-stress group 15 years earlier. A Mann–Whitney U test revealed differences in perceived general stress ratings between the two PSQ groups at T3–T6; after Bonferroni–Holm multiple corrections the differences were significant at all time points (T3, U = 244, P = 0.003; T4, U = 281, P = 0.014; T5, U = 194, P < 0.001; T6, U = 228, P = 0.001).
Figure 2.
Rated stress levels over a 15-year period for the low and moderately to high PSQ groups as defined at T5. The moderately to high group constantly rated general stress significantly higher at all time points compared with the low PSQ group.
Differences in Hippocampal Volume Between Moderately to High- and Low-Stress Groups
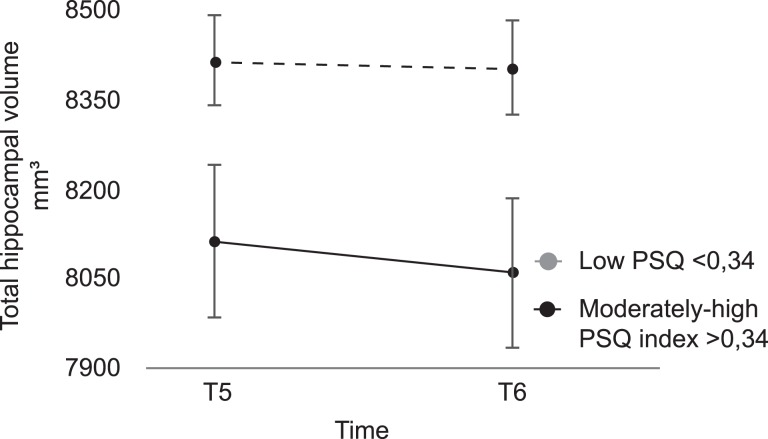
Individuals with moderately to high perceived stress levels at T5 showed significantly smaller total [t(109) = 2.09, P = 0.039] hippocampal volumes compared with individuals with low perceived stress levels. Corresponding cross-sectional differences in hippocampal volumes were observed between moderately to high and low PSQ groups at T6, [tot HCV, t(109) = 2.32, P = 0.022].
A 2 × 2 (group by time) mixed between–within ANOVA was used to investigate changes in total hippocampal volume over time in the moderately to high and low PSQ groups (Fig. 3). The results showed a significant main effect of group [F1,109 = 4.94, P = 0.028 partial η2 = 0.04], but no main effect of time [F1,109 = 3.35, P = 0.07, partial η2 = 0.03] and critically no interaction between group and time [F1,109 = 1.26, P = 0.264, partial η2 = 0.01]. Thus, there was a group difference in total hippocampal volume but no group difference in volume change over time.
Figure 3.
The moderately to high PSQ group had significant smaller total hippocampal volume at both T5 and T6 compared with the low PSQ group. However, no significant changes in total hippocampal volume over a 5-year period in either group could be found.
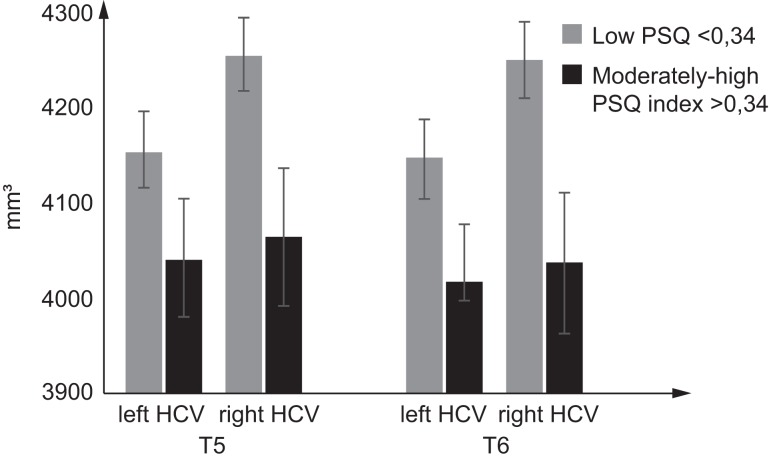
We examined if there were any differences between change in right and left hippocampal volume over time, as some studies indicate more marked effects for the right than the left hippocampus (Videbech and Ravnkilde 2004). For right hippocampal volume, a significant main effect of group [F1,109 = 6.72, P = 0.011, partial η2 = 0.06] was seen along with no main effect of time [F1,109 = 2.23, P = 0.138, partial η2 = 0.02] or interaction effect [F1,109 = 1.08, P = 0.302, partial η2 = 0.01]. In the left hippocampus a similar but nonsignificant trend was seen as for the right hippocampus; main effect of group [F1,109 = 2.60, P = 0.11, partial η2 = 0.02], time [F1,109= 2.34, P = 0.129, partial η2 = 0.02], and interaction [F1,109 = 0.65), P = 0.442, partial η2 = 0.006]. A three-way ANOVA was run to examine the effect of low and moderately to high PSQ and time across the left and right hippocampal volume (Fig. 4). The three-way interaction was nonsignificant [F1,327 = 0.013, P = 0.911].
Figure 4.
The moderately to high PSQ group had significant smaller right hippocampal volume at both T5 and T6 compared with the low PSQ group. No significant changes in left hippocampal volume were found, although the trend was similar to that for the right hippocampus. *Significant group differences (P < 0.05). The graphs represent mean values ± SEM.
Discussion
In this article, we identified individuals who experienced low or moderately to high subjective stress. Our longitudinal data revealed that individuals in the moderately to high PSQ group over a 15-year period constantly rated higher stress levels compared with individuals in the low PSQ group. Individuals in the moderately to high PSQ group had significantly smaller hippocampal volumes compared with those reporting low levels of stress. This finding is in line with results from numerous empirical studies and meta-analyses of hippocampal volumes in individuals with depression and PTSD (Gilbertson et al. 2002; Videbech and Ravnkilde 2004; Kitayama et al. 2005; Smith 2005; Dedovic et al. 2010; Kremen et al. 2012; O'Doherty et al. 2015; van Rooij et al. 2015).
Critically, although the hippocampal volumes were smaller in the moderately to high PSQ group compared with the low PSQ group, there were no significant group differences in change over 5 years. To our knowledge, this is the first study to relate perceived stress data over 15 years to data on hippocampal volume change over a 4- to 5-year period. In an earlier prospective study by Gianaros et al. (2007) perceived stress scores from 50 women were collected over a 20-year period. The result was similar to our finding, in that individuals with higher perceived stress scores showed a smaller (right) hippocampal volume than people perceiving low levels of stress. However, as only one structural MRI session was performed, strong conclusions on the causal relationship between stress and hippocampal reduction could not be made (Gianaros et al. 2007).
The findings of several past human studies indicate that a smaller hippocampal volume could be a vulnerability to PTSD and depression rather than an effect (Gilbertson et al. 2002; Dedovic et al. 2010; Kremen et al. 2012; van Rooij et al. 2015). In this study, we predicted that group differences in hippocampal volume would be magnified over time if driven by high perceived stress over time, whereas no magnification of volume differences over time would be more consistent with stable individual differences. The lack of cumulative stress-related hippocampal volume loss thus argues against the “acquisition” hypothesis; that stress induces reduced hippocampal volume. However, it cannot be completely ruled out that a stress-induced reduction of the hippocampus had occurred very early, prior to the first MRI session. Studies investigating childhood PTSD have not found volumetric differences in hippocampus (De Bellis et al. 2001, 2002; Veer et al. 2015), but there is some evidence for impaired development of the hippocampus during childhood mistreatment (Dannlowski et al. 2012; Teicher et al. 2012; Keding and Herringa 2015). Traumatic or stressful events in childhood may impair the development of the hippocampus, which later in life can be a vulnerability factor. Genetic factors could also have contributed to individual differences in hippocampal volume (Baune et al. 2012; Dannlowski et al. 2015; Hibar et al. 2015). The study population in this study was 60 years old or younger at the first MRI session, in a range of 25–60 years, and at the first included retrospective test wave (T3 in Fig. 2) the age range was 35–45. There is evidence for marked between-person differences in hippocampal volume in this age segment (Lupien et al. 2007), likely reflecting both the influence of genetic factors or/and early life events (Rabl et al. 2014; Hibar et al. 2015; Keding and Herringa 2015).
We can only speculate why a small hippocampal volume might contribute to increased vulnerability to stress. A smaller hippocampal volume has been associated with negative memory bias in healthy populations and may influence how individuals deal with new challenges and stressors (McEwen and Gianaros 2011; Gerritsen et al. 2012). Moreover, a smaller hippocampus may less efficiently influence the hypothalamic–pituitary–adrenal (HPA) axis (Buchanan et al. 2004; Clow et al. 2010), thereby making individuals more vulnerable to different stressors as the regulation of stress functions less effectively. A similar hippocampal volume account has been offered to explain individual differences in learning of cognitive maps (Schinazi et al. 2013).
The participants in this study represented a normal sample from a longitudinal population-based study, and were thus not included based on a history of diseases or other conditions. We found no differences between the two groups in stress-related physical and mental diseases or in the use of antidepressant and antihypertensive medicine. However, individuals in the moderately to high PSQ group rated significantly higher depression levels measured by the CES-D. Although reduced hippocampal volume is one of the most replicated findings in patients with major depressive disorder (MDD) (Videbech and Ravnkilde 2004; McKinnon et al. 2009), the mean CES-D score for individuals in the moderately to high group was 13. CES-D scores under 16 have proved to be of minor clinical relevance (Radloff 1997). Nevertheless, stress-related diseases have high comorbidity with depression and there is some indication that preexisting MDD can make individuals more vulnerable to stress-related disorders such as PTSD and, conversely, the presence of PTSD may increase the risk for developing MDD (O'Donnell et al. 2004). The moderately to high PSQ group also reported poorer sleep quality and restorative sleep. Stress has been associated with both depression and sleeping problems in the normal population (Bergdahl and Bergdahl 2002).
The main results on hippocampal volume reflected the total (left + right) hippocampus. In additional analyses of the left and the right hippocampus separately, we found some support for a stronger difference between stress groups in the right hippocampus. This is in line with some previous findings in chronic life stress (Gianaros et al. 2007) and in studies of depressed patients (Mathias et al. 2016). However, qualitatively (Fig. 3) the pattern was similar for the left and the right hippocampus, and the formal analysis of laterality effects did not reach significance.
Limitations of the study include the fact that we did not control for the number of critical life events, which could have influenced the results (Rabl et al. 2014). However, the number of life events might not be as important as how individuals experience different events (Sundstrom et al. 2014). There were no significant differences in reported psychiatric diseases between low and moderately to high PSQ groups. However, the information on diseases was based on the participants' subjective reports and we cannot be sure that they reported correct diseases and we cannot exclude that participants had psychiatric diseases that they did not report. We chose not to analyze subregions of the hippocampus. Segmentation of hippocampal subfields is a challenge for both the Freesurfer automatic segmentation tool and for manual hippocampus segmentation protocols (Wisse et al. 2014; Yushkevich et al. 2015). However, further analyses of hippocampal subfields such as the dentate gyrus, CA1, and CA3 may contribute additional information.
In conclusion, to our knowledge, this is the first study to examine perceived stress levels over a fifteen-year period in relation to hippocampal volumes over a 5-year period. Our findings demonstrate that long-term stability in perceived stress was associated with smaller hippocampal volume, but no cumulative stress-related hippocampal volume loss. These findings suggest that a smaller hippocampal volume in younger and middle age might be a vulnerability factor that contributes to why some perceive events as more stressful than others, and ultimately why some individuals develop stress-related disorders.
Funding
This work was supported by Knut and Alice Wallenberg Foundation. Funding to pay the Open Access publication charges for this article was provided by Knut and Alice Wallenberg Foundation.
Notes
Conflict of Interest: None declared.
References
- Baune BT, Konrad C, Grotegerd D, Suslow T, Ohrmann P, Bauer J, Arolt V, Heindel W, Domschke K, Schoning S et al. . 2012. Tumor necrosis factor gene variation predicts hippocampus volume in healthy individuals. Biol Psychiatry. 72(8):655–662. [DOI] [PubMed] [Google Scholar]
- Bergdahl J, Bergdahl M. 2002. Perceived stress in adults: prevalence and association of depression, anxiety and medication in a Swedish population. Stress Health. 18(5):235–241. [Google Scholar]
- Bremner JD. 2001. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder. Hippocampus. 11(2):75–81; Discussion 82–4. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. 2004. Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry. 56(9):651–656. [DOI] [PubMed] [Google Scholar]
- Chao LL, Yaffe K, Samuelson K, Neylan TC. 2014. Hippocampal volume is inversely related to PTSD duration. Psychiatry Res Neuroimaging. 222(3):119–123. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. 2010. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 35(1):97–103. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 9(2):179–194. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Grabe HJ, Wittfeld K, Klaus J, Konrad C, Grotegerd D, Redlich R, Suslow T, Opel N, Ohrmann P et al. . 2015. Multimodal imaging of a tescalcin (TESC)-regulating polymorphism (rs7294919)-specific effects on hippocampal gray matter structure. Mol Psychiatry. 20(3):398–404. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J et al. . 2012. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 71(4):286–293. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. 2001. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 50(4):305–309. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. 2002. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 52(11):1066–1078. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC. 2010. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biol Psychiatry. 68(9):847–853. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA. 2009. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 20(16):1402–1406. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Rijpkema M, van Oostrom I, Buitelaar J, Franke B, Fernandez G, Tendolkar I. 2012. Amygdala to hippocampal volume ratio is associated with negative memory bias in healthy subjects. Psychol Med. 42(2):335–343. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. 2007. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 35(2):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 5(11):1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, Toro R, Wittfeld K, Abramovic L, Andersson M et al. . 2015. Common genetic variants influence human subcortical brain structures. Nature. 520(7546):U224–U216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand J Stat. 6(2):65–70. [Google Scholar]
- Keding TJ, Herringa RJ. 2015. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 40(3):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. 2005. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 88(1):79–86. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Koenen KC, Afari N, Lyons MJ. 2012. Twin studies of posttraumatic stress disorder: Differentiating vulnerability factors from sequelae. Neuropharmacology. 62(2):647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Luzi C, Andreoli A. 1993. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 37(1):19–32. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1(1):69–73. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Evans A, Lord C, Miles J, Pruessner M, Pike B, Pruessner JC. 2007. Hippocampal volume is as variable in young as in older adults: Implications for the notion of hippocampal atrophy in humans. Neuroimage. 34(2):479–485. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Mcewen BS. 1995. Stress-induced atrophy of apical dendrites of hippocampal Ca3c neurons - involvement of glucocorticoid secretion and excitatory amino-acid receptors. Neuroscience. 69(1):89–98. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. 1996. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 16(10):3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias SR, Knowles EE, Kent JW Jr, McKay DR, Curran JE, de Almeida MA, Dyer TD, Goring HH, Olvera RL, Duggirala R et al. . 2016. Recurrent major depression and right hippocampal volume: a bivariate linkage and association study. Hum Brain Mapp. 37(1):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 1999. Stress and hippocampal plasticity. Annu Rev Neurosci. 22:105–122. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. 2011. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 62:431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. 2016. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 41(1):3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. 2009. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Nilsson L-G, BÄCkman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Karlsson S, Widing M, Winblad B. 1997. The Betula prospective cohort study: Memory, health, and aging. Aging Neuropsychol Cogn. 4(1):1–32. [Google Scholar]
- Nordin M, Åkerstedt T, Nordin S. 2013. Psychometric evaluation and normative data for the Karolinska Sleep Questionnaire. Sleep Biol Rhythms. 11(4):216–226. [Google Scholar]
- O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. 2015. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 232(1):1–33. [DOI] [PubMed] [Google Scholar]
- O'Donnell ML, Creamer M, Pattison P. 2004. Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am J Psychiatry. 161(8):1390–1396. [DOI] [PubMed] [Google Scholar]
- Ohman L, Bergdahl J, Nyberg L, Nilsson LG. 2007. Longitudinal analysis of the relation between moderate long-term stress and health. Stress and Health. 23(2):131–138. [Google Scholar]
- Rabl U, Meyer BM, Diers K, Bartova L, Berger A, Mandorfer D, Popovic A, Scharinger C, Huemer J, Kalcher K et al. . 2014. Additive gene-environment effects on hippocampal structure in healthy humans. J Neurosci. 34(30):9917–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Measur. 1(3):385–401. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15(11):1676–1689. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. 2012. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 61(4):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. 1990. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 86:13–23. [DOI] [PubMed] [Google Scholar]
- Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. 2013. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 23(6):515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. 1999. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 19(12):5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. 2005. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 15(6):798–807. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. 1999. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 46(12):1595–1602. [DOI] [PubMed] [Google Scholar]
- Sundstrom A, Ronnlund M, Adolfsson R, Nilsson LG. 2014. Stressful life events are not associated with the development of dementia. Int Psychogeriatr. 26(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 109(9):E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, Dejesus O, Holden J. 1994. Neurotoxicity of glucocorticoids in the Primate Brain. Hormones Behav. 28(4):336–348. [DOI] [PubMed] [Google Scholar]
- van Rooij SJ, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E. 2015. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med. 45(13):2737–2748. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SA. 2015. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. 233(3):436–442. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. 2004. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 161(11):1957–1966. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N. 2010. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 67(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LE, Biessels GJ, Geerlings MI. 2014. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 6:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RS, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Bocchetta M, Burggren AC, Carr VA, Chakravarty MM et al. . 2015. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 111:526–541. [DOI] [PMC free article] [PubMed] [Google Scholar]