Abstract
BACKGROUND
Cues paired with drug administration trigger relapse to drug-seeking by inducing conditioned drug craving and withdrawal. Because drug cues hinder abstinence in addicts, therapies that reduce responsiveness to drug cues might facilitate rehabilitation. Extinction is a means of reducing conditioned responses and involves exposure to the conditioned stimulus (CS) in the absence of the unconditioned stimulus (US) with which it was paired previously. We examined conditioned withdrawal extinction using naloxone-induced conditioned place aversion (CPA) in morphine-dependent rats.
METHOD
Morphine-dependent rats were trained to associate an environment with naloxone-precipitated withdrawal. Subsequently they received extinction training in which they were confined in the previously naloxone-paired environment in the absence of acute withdrawal. In some rats, the NMDA receptor partial agonist D-cycloserine (DCS) was administered prior to extinction training.
RESULTS
Morphine withdrawal-induced CPA persists in the absence of extinction training. Administration of DCS prior to extinction training facilitates extinction.
CONCLUSIONS
DCS facilitates extinction of morphine withdrawal-associated place aversion. This effect is qualitatively similar to the effect of DCS on extinction of conditioned fear, raising the possibility of common neural mechanisms. This work extends our understanding of drug cue responsivity and provides a rationale for the development of extinction-based treatments for addiction.
Introduction
Cues paired with drug administration trigger relapse to drug-seeking behavior by inducing conditioned drug craving and withdrawal (1). Because drug cues represent a barrier to long-term abstinence in recovering addicts, there is interest in therapies that reduce responsiveness to them. Extinction is a means of reducing Pavlovian conditioned responses and involves exposure to the conditioned stimulus (CS) in the absence of the unconditioned stimulus (US) with which it was paired previously. In animal models including cue-induced reinstatement of drug seeking and conditioned place preference, drug craving elicited by drug-paired cues can be extinguished by presenting the cues repeatedly without drug administration. The neurobiological mechanisms involved in extinction of conditioned drug craving appear similar to those underlying extinction of conditioned fear (2–4).
In contrast, there currently is little information about extinction of conditioned withdrawal. Conditioned withdrawal can be studied using the conditioned place aversion (CPA) paradigm. In this model, rats are allowed to briefly explore a two- or three-chambered apparatus. Each rat then is confined in one of the chambers during drug withdrawal, which in opiate-dependent animals can be precipitated by an opiate receptor antagonist such as naloxone (5). When such rats are later allowed to explore the entire apparatus, they tend to avoid the previously withdrawal-paired context. Conditioned withdrawal is defined operationally as the difference in time spent in the withdrawal-paired chamber vs. the opposite chamber, which typically is paired with a neutral treatment (saline).
Here we describe experiments designed to examine extinction of CPA established by precipitated opiate withdrawal. We show that CPAs can be extinguished by confining the animals in the formerly naloxone-paired environment in the absence of withdrawal. This reduced avoidance response is due to extinction rather than forgetting, because rats that do not receive extinction training continue to exhibit persistent place aversions. We also show that the NMDA receptor partial agonist D-cycloserine (DCS) facilitates extinction of withdrawal-induced CPAs.
Methods
Experiments were conducted according to National Institutes of Health policies. Male Sprague-Dawley rats (250–275 g; Charles River Laboratories, Raleigh, NC) were trained to acquire CPA in 3-chambered place conditioning boxes (Med Associates, St. Albans, VT) with equally-sized black and white compartments separated by a smaller gray compartment (6) (Table S1 in Supplement 1). Briefly, each rat was given a pre-training (screening) test during which it freely explored the entire apparatus for 30 min. Rats with a strong initial preference (≥60% of the session time) for any chamber were not tested further. Twenty-four hrs later rats were implanted with subcutaneous pellets under isofluorane anesthesia. Some rats were made dependent on morphine by implantation of two 75-mg morphine pellets (National Institute on Drug Abuse [NIDA], Bethesda, MD) (5). These pellets slow-release morphine continuously for a period of 14 d (7). Control rats were implanted with two placebo (blank) pellets (NIDA). Three days after implantation, rats underwent conditioning in which they were confined in either the black or white compartment for 1 hr immediately following subcutaneous (SC) injection of 0.9% saline (1 ml/kg); 3 hrs later they were confined in the opposite compartment for 1 hr immediately following SC injection of naloxone (15 μg/kg; Sigma-Aldrich, St. Louis, MO). This dose elicits motivational symptoms of withdrawal without somatic symptoms (8). Compartment assignment was counterbalanced across rats and unbiased relative to the inherent preferences determined in the pre-training test (6). Post-training tests were identical to the pre-training test (no drugs or injections).
In Experiment 1, rats were implanted with morphine pellets or blank pellets. Extinction training began 24 hrs after the post-training test and consisted of 30 min confinements in the previously saline- and naloxone-paired boxes. On each confinement day the rats were exposed to each chamber once, with 3 hrs between exposures; the order of exposure was counterbalanced across rats and reversed relative to the preceding session. Saline was administered immediately prior to each confinement. Three confinement days were conducted at 48 hr intervals, and 24 hrs after each confinement day was a 30 min post-training (extinction) test. In Experiment 2, all rats were implanted with morphine pellets and given a single post-training test either 1 d or 7 d after training. In Experiment 3, all rats were implanted with morphine pellets and exposed to an abbreviated extinction training and test protocol consisting of one confinement day and one extinction test. D-cycloserine (15 mg/kg; Sigma-Aldrich) was administered IP immediately prior to confinement in the previously naloxone-paired box, whereas saline was administered IP immediately prior to confinement in the opposite box.
Data are expressed as change scores (seconds in the naloxone-paired box minus seconds in the saline-paired box) for a post-training or extinction test minus the aversion score for the same animal in the pre-training test. Because rats that did not develop a CPA cannot (by definition) undergo CPA extinction, rats not exhibiting a strong aversion (≤-300 sec in the post-training test) were excluded from the parts of Experiments 1 and 3 where extinction was quantified. Data were analyzed with t-tests or two-way ANOVA (Treatment x Test Day) with Test Day as a repeated measure, followed by simple main effects tests or post hoc Scheffé comparisons.
Results
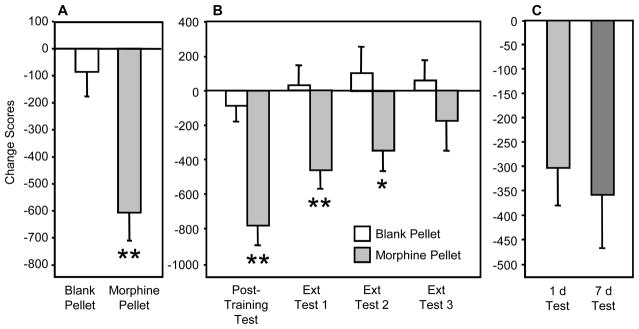
In Experiment 1, rats with morphine pellets (n=20) expressed strong CPAs in the post-training test, whereas rats with blank pellets (n=20) did not (t(38)=3.76; p<0.01) (Fig. 1A). Fourteen rats in the morphine group met the criterion for inclusion in the extinction study (Fig. 1B). Changes in time spent in withdrawal-associated environments depended on main effects of Treatment (F(1,32)=12.23, p<0.01) and Test Day (F(1,32)=5.28, p<0.01). Simple main effects tests indicated that morphine-dependent rats spent less time in the naloxone-associated environment than did controls during the post-training test (F(1,32)=21.83, p<0.01), extinction test 1 (F(1,32)=8.93, p<0.01), and extinction test 2 (F(1,32)=4.73, p<0.05). Group differences were not significant by extinction test 3.
Figure 1.
Extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. A. Rats implanted with morphine pellets exhibit conditioned place aversion (CPA) to an environment previously paired with a low dose of naloxone (15 μg/kg), whereas animals implanted with blank (placebo) pellets do not. B. The magnitude of CPA in morphine-dependent rats declines following repeated confinements in the previously naloxone-paired environment in the absence of acute withdrawal. C. Morphine-dependent rats not exposed to the previously naloxone-paired environment in the absence of withdrawal exhibit persistent CPA. *p< .05, ** indicates p< .01 vs. blank pellet, Scheffé test.
In Experiment 2, rats were tested for the first time either 1 d (n=19) or 7 d (n=17) after training (corresponding to the post-training test and third extinction test time points in Experiment 1). The CPAs in the 1 d and 7 d test groups did not differ (Fig. 1C), indicating no spontaneous decrease in CPA over this period of time.
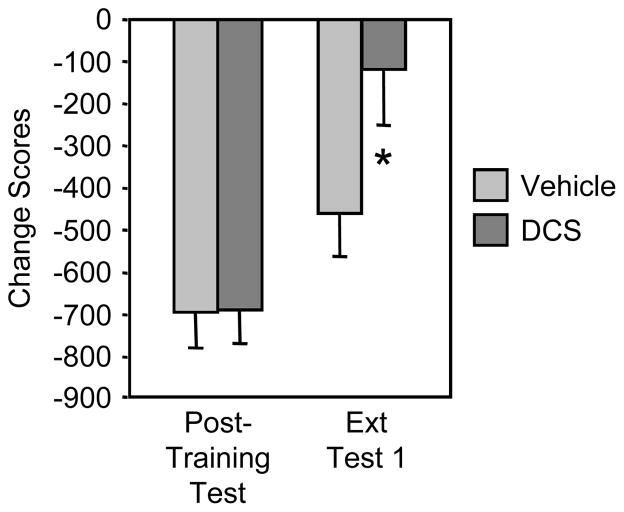
In Experiment 3, 31 morphine pellet-implanted rats met the criterion for inclusion in the extinction study. The change in time spent in the withdrawal-associated environment depended on an interaction of Treatment and Test Day (F(1,29)=4.74, p<0.05) (Fig. 2). DCS prior to confinement in the previously naloxone-paired box facilitated CPA extinction: there was a significant reduction in the CPA between the post-training test and the first extinction test in the group treated with DCS (n=16) (Scheffé test, p<0.01), whereas there was no difference after saline treatment (n=15).
Figure 2.
D-cycloserine administered prior to confinement in the previously naloxone-paired box facilitates CPA extinction. *p<.05 vs. vehicle, Scheffé test.
Discussion
Naloxone-induced CPA in morphine-dependent rats is diminished following exposure to the previously naloxone-paired environment in the absence of acute withdrawal. CPA persists for at least 7 days unless the rats receive additional exposures to the environment without withdrawal, indicating that the effect is due to extinction and not forgetting. Administration of DCS immediately prior to extinction training dramatically increases the rate of extinction of the CPA. Our data suggest that extinction of conditioned drug withdrawal involves mechanisms similar to those involved in other types of extinction (e.g., extinction of conditioned fear) (9). Common approaches might treat a wide spectrum of disorders in which conditioning contributes to maladaptive behavior.
Avoidance responses are difficult to extinguish unless exposure to the CS is forced (10). To ensure that the rats would be exposed to the aversion-paired CS, we conducted extinction training by confining them in the contexts and testing them in separate sessions rather than simply testing them repeatedly. This approach guarantees equivalent CS exposure in all rats and enables control over extinction rates through titration of the number and duration of exposures.
DCS facilitates extinction of conditioned fear (11,12), cocaine conditioned place preference (13,14), and ethanol seeking maintained by response-contingent delivery of ethanol-paired cues (15). DCS also enhances cue exposure therapy in patients with anxiety-related disorders (16–18). CPA extinction thus seems similar to other types of extinction in terms of sensitivity to DCS. The putative mechanism of DCS is enhanced NMDA receptor function, which facilitates new learning (19). Our findings highlight the possibility that a variety of experience-dependent maladaptive behaviors involve common neural substrates, and may provide an explanation for co-morbidity of psychiatric conditions such as stress/anxiety disorders and addiction (20).
Research on extinction of drug-paired cues has focused on conditioned drug craving, even though conditioned withdrawal contributes to relapse (1). Considering interest in the use of extinction protocols in clinical settings to reduce responsiveness to drug cues in recovering addicts, it is important to understand the behavioral features and neurobiological mechanisms of reward-like and aversive-like conditioned responses that are established by drugs of abuse.
Since DCS is known to be safe in humans (16), our data provide a rationale for studies in addicts. Regardless of whether DCS is useful for diminishing relapse, a more thorough understanding of the mechanisms of extinction of conditioned withdrawal might facilitate the development of improved treatments for addictive disorders.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse (DA012736 to W.A.C.).
Footnotes
Conflict of Interest Notification Statement:
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- 3.Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89:504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlezon WA. Place conditioning to study drug reward and aversion. In: Pan ZZ, editor. Opioid Research: Methods and Protocols. Humana Press, Inc; Totowa, NJ: 2003. pp. 243–249. [DOI] [PubMed] [Google Scholar]
- 7.Gold LH, Stinus L, Inturrisi CE, Koob GF. Prolonged tolerance, dependence and abstinence following subcutaneous morphine pellet implantation in the rat. Eur J Pharmacol. 1994;253:45–51. doi: 10.1016/0014-2999(94)90755-2. [DOI] [PubMed] [Google Scholar]
- 8.Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- 9.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 10.Baum M. Rapid extinction of an avoidance response following a period of response prevention in the avoidance apparatus. Psychol Rep. 1966;18:59–64. doi: 10.2466/pr0.1966.18.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of conditioned fear as assessed by freezing in rats. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 12.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 14.Botreau F, Paolone G, Stewart J. D-cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- 16.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 20.Brown PJ, Wolfe J. Substance abuse and post-traumatic stress disorder comorbidity. Drug Alcohol Depend. 1994;35:51–59. doi: 10.1016/0376-8716(94)90110-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.