Abstract
Background
Coagulation abnormalities contribute to poor outcomes in critically ill patients. In trauma patients exposed to a hot environment, a systemic inflammatory response syndrome, elevated body temperature, and reduced central blood volume occur in parallel with changes in hemostasis and endothelial damage. The objective of this study was to evaluate whether experimentally elevated body temperature and reduced central blood volume (CBV) per se affects hemostasis and endothelial activation.
Methods
Eleven healthy volunteers were subjected to heat stress, sufficient to elevate core temperature, and progressive reductions in CBV by lower body negative pressure (LBNP). Changes in hemostasis were evaluated by whole blood haemostatic assays, standard hematologic tests and by plasma biomarkers of coagulation and endothelial activation/disruption.
Results
Elevated body temperature and decreased CBV resulted in coagulation activation evidenced by shortened activated partial tromboplastin time (−9% [IQR −7; −4]), thrombelastography: reduced reaction time (−15% [−24; −4]) and increased maximum amplitude (+4% (2; 6)), all P < 0.05. Increased fibrinolysis was documented by elevation of D-dimer (+53% (12; 59), P = 0.016). Plasma adrenaline and noradrenaline increased 198% (83; 346) and 234% (174; 363) respectively (P = 0.006 and P = 0.003).
Conclusions
This experiment revealed emerging hypercoagulability in response to elevated body temperature and decreased CBV, whereas no effect on the endothelium was observed. We hypothesize that elevated body temperature and reduced CBV contributes to hypercoagulability, possibly due to moderate sympathetic activation, in critically ill patients and speculate that normalization of body temperature and CBV may attenuate this hypercoagulable response.
Keywords: Catecholamines, Central blood volume, Hyperthermia, Hypovolemia, LBNP, Sepsis, SIRS, TEG, Trauma
1. Introduction
Disturbances in coagulation are seen as complications to various clinical conditions contributing to the morbidity, and in severe cases, the mortality of patients [1]. Infection, systemic inflammatory syndrome (SIRS) [2] and trauma in a hot environment [3,4] can all reduce the central blood volume (CBV) [5]. Only sparse information exists on the effects of elevated body temperature and reduced CBV isolated from concurrent influence of endotoxins in severe infections [1], or tissue injury in various trauma [4].
SIRS is a non-specific host reaction that manifests in response to various insults such as infection, trauma, and surgery [6], and is defined by two or more of the following symptoms: an elevated or decreased core temperature, increased heart rate, increased respiration rate or decreased arterial CO2 content, and an elevated or decreased leucocyte count. SIRS caused by infection is by definition sepsis. During the time course of SIRS and sepsis, core temperature can increase dramatically initially, but then decrease below normal when the body can no longer meet the metabolic demands associated with fever. Increases in vascular conductance and capillary leakage during severe sepsis can lead to shock and the endothelial damage that accompanies SIRS and sepsis may lead to disturbed hemostasis reflecting an imbalance between pro-coagulant and fibrinolytic mechanisms [1]. Signs of an activated coagulation system in SIRS include increased thrombin formation, D-dimer levels, and markers of endothelial damage [7]. Disruption or dysfunction of the endothelium and the associated glycocalyx contributes to the disorders of the coagulation system and bridges the pathways of inflammation and coagulation [1,8]. The anticoagulant pathways: tissue factor pathway inhibitor, anti-thrombin, and thrombomodulin/protein C, are all linked to the endothelium and/or glycocalyx and conditions with profound systemic inflammation are often associated with disturbances in these pathways [1]. As these pathologic changes, i.e. elevations in body temperature, decreased central blood volume and inflammation often occur simultaneously, it is difficult to determine cause and effect. A model capable of inducing elevated body temperature and central hypovolemia without concurrent presence of endotoxin/infection or profound tissue injury could provide new insight to the pathophysiology of coagulation disturbances in SIRS.
In the present study, elevated body temperature and decreased CBV were induced successively in healthy volunteers by whole-body heating combined with lower body negative pressure (LBNP) to investigate their distinct impact on the hemostatic system. Applied as separate interventions, both heat stress and LBNP have been shown to lower CBV [9,10] and lead to an activation of coagulation [11,12]. Furthermore, sympathetic activity increases during heat stress [13] and LBNP [14], and catecholamines are expected to modulate the coagulation system [15].
The purpose of this study was to investigate hemostasis as evaluated by whole blood hemostatic assays together with plasma markers of coagulation and endothelial activation/disruption, as well as plasma concentrations of catechol-amines, in response to elevated core temperature and decreased central blood volume. We hypothesized that combined heat stress and LBNP would provoke activation of the coagulation system and concurrent counteractive activity of the endothelium [16].
2. Methods
This was an experimental study in healthy volunteers. Eleven healthy males provided written informed consent to participate in the study that was approved by the ethical committee of Capital Region, Denmark (protocol number H-1-2010-040) and conducted in accordance with the Declaration of Helsinki. Subjects had a median age of 23 y (IQR 22–25), a height of 179 cm (179–183), and weighed 79 kg (73–83). No subject was taking any medication 2 wk prior to the experiments and had not shown symptoms of orthostatic intolerance prior to the experiment. Sample size was based on experiences from a previous study of coagulation during LBNP [12]. Results from the study, focusing on the effect of heat stress and LBNP on cardiac output and systemic vascular resistance, have previously been published [17].
2.1. Instrumentation and measurements
Subjects were dressed in a water-perfused tube-lined suit to allow for control of skin temperature, then placed in a supine position, instrumented for monitoring and blood sampling, and positioned with the lower body in the LBNP chamber as previously published [17]. Core temperature was measured via right heart catheter (Arteria pulmonalis). Referred blood values are arterial.
2.2. Experimental protocol
Following instrumentation, the subject rested quietly in the supine position while thermoneutral water (34°C) circulated through the suit. After a 10 min steady state rest period, the subjects were exposed to passive whole-body heat stress by perfusion of the suit with 49°C water until core temperature had increased by 1.5°C or as tolerated by the subjects (median increase: 1.3°C). Once this objective was achieved, the water temperature was slightly reduced to attenuate further increases in core temperature. At this point, mild decrease in CBV was induced by application of 15 mmHg LBNP for ~6 min. More severe LBNP was then imposed by elevating the negative pressure to ~40 mmHg and held at this level until signs or symptoms of presyncope (e.g., sudden onset of nausea, light-headedness, bradycardia, and/or hypotension) were identified, at which time LBNP was terminated. During the ensuing 15 min recovery period, the suit was perfused with cold water to return the subject towards normal core temperature. The time from initiation of heat stress until termination of LBNP was 51 (51–53) min, including a total duration of LBNP of 7 (6–8) min. Blood samples were obtained while subjects were normothermic, heat stressed prior to the onset of LBNP, immediately following the termination of LBNP, and 15 min after the end of LBNP. LBNP has previously been shown to produce drastic changes in CBV, and a LBNP ≥40 mmHg can displace upwards of 1000 mL fluid from the circulation to the pelvis and lower extremities. This corresponds to a severe hemorrhage with loss of 20% or greater of total blood volume [18].
2.3. SIRS severity scoring
SIRS is diagnosed when two or more of the following four criteria are present [6]: (1) systemic temperature >38° or < 35° C; (2) heart rate >90 beats min−1; (3) respiratory rate >20 breaths*min−1 or PaCO2 <4.3 kPa (32 mmHg); (4) leukocytes > 12 or <4 109*L−1 or >10% immature neutrophils.
2.4. Thrombelastography (TEG)
Whole blood viscoelastic hemostatic assays (VHA) provide insight to the hemostatic process of secondary hemostasis, from initiation of clot formation to amplification and propagation as well as the fibrinolytic activity [19]. Blood samples for TEG coagulation analysis were drawn into tubes containing citrate (nine volumes of blood into one volume of 0.129 M citrate; Vacutainer System; BD Biosciences, Plymouth, UK). The following tests were performed: citrated kaolin (CK), citrated kaolin with heparinase (CKH), and functional fibrinogen (FF) in concordance with instructions by the manufacturer. CK and CKH are activated through the internal pathway and FF through the external pathway by tissue factor. CKH contains substances capable of inhibiting the anticoagulant effect of heparin and, accordingly, any differences in results obtained from CK and CKH could reflect an influence of heparin in the patient; for this study, no heparin was used and a heparin effect would be of endogenous origin. FF reflects the enzymatic cascade in coagulation, particularly fibrin polymerization, since platelets are inhibited by blocking of the GpIIb/IIIa fibrinogen receptors. Samples were analyzed within 30 to 60 min after retrieval. The hemostatic process was recorded by a TEG coagulation analyzer (5000 series; Haemonetics Corp, Braintree, MA) and the variables determined include: R (min), Angle (degrees), the maximal amplitude (MA; mm), and lysis (Ly30; %). All samples were analyzed at 37°C regardless of core temperature, to monitor persistent changes in hemostasis in the subjects and not changes in enzyme kinetics due to temperature alterations in the TEG cup.
2.5. Electronic platelet aggregometry (multiplate)
Platelet aggregometry addresses the primary hemostasis i.e., aggregation of platelets after specific agonist activation. The electronic platelet aggregometer measures the increase in electrical impedance (i.e., the resistance to current) between two electrodes suspended in whole blood while platelets adhere to their surfaces upon activation [20]. Blood samples for multiplate analysis were drawn into tubes containing heparin Vacutainer system and analyzed by impedance aggregometry using a multiple platelet function analyzer (Multiplate Analyzer, Software v. 2.02.11; Dynabyte GmbH, Munich, Germany) according to the manufacturer’s recommendations within 2 h after retrieval. Platelet aggregation was determined in response to test reagents (Dynabyte GmbH). The increase in impedance by the attachment of platelets onto the Multiplate sensors is transformed to arbitrary aggregation units (AU) and plotted against time. For each applied platelet agonist, the area under the aggregation curve (AUC; AU × min or U [1 U = 10 AU × min]) was recorded after 6 min of analysis.
2.6. Enzyme-linked immunosorbent assay (ELISA)
Blood samples for ELISA analysis were drawn into a tube containing EDTA (Vacutainer system) and were immediately suspended into ice water and spun (2000 g for 10 min) within 60 min and then stored at −80°C until analysis. Analysis was performed using commercially available kits: catecholamines (2-CAT ELISA, Labor Diagnostika Nord GmbH & Co. KG, Nordhorn, D; lower level of detection (LLD) Noradrenaline: 44 pg*mL−1, Adrenalin: 11 pg*mL−1), Syndecan-1 (sCD138 ELISA Kit, Diaclone SAS, Besancon, F; LLD <2.56 ng*mL−1), soluble thrombomodulin (CD141 ELISA KIT, DIACLONE SAS, Besancon, F; LLD <0.380 ng*mL−1), Protein C (Protein C ELISA Kit, Helena Laboratories, Beaumont, TX; LLD 5% relative to reference plasma), D-dimer (IMUCLONE D-Dimer ELISA, American Diagnostica Inc, Stamford, CT; LLD 2–4 ng*mL−1).
2.7. Hematology
Hematocrit, platelet, and leukocyte counts were measured using XE-2100 (Sysmex Corporation, Kobe, Japan); C-reactive protein was determined with a “Modular P-Module” (Roche, Basel, Switzerland); fibrinogen concentration (Clauss method), activated partial tromboplastin time (aPTT) and international normalized ratio were determined utilizing the ACL TOP (Beckman Coulter, Brea, CA); aPTT was initiated using Hemosil aPTT-SP liquid, local reference value 23–35 s.
2.8. Statistics
Statistical analysis was performed using SPSS 17 (SPSS Inc, Chicago, IL). Sample data were tested for normal distribution with Shapiro–Wilkinson test and found largely not to be normally distributed; consequently, all data were expressed as medians and IQR. Friedman nonparametric repeated measures test was used to evaluate for variance and followed, if significant, by Wilcoxon signed rank post hoc test. A level of P < 0.05 was considered statistical significant.
3. Results
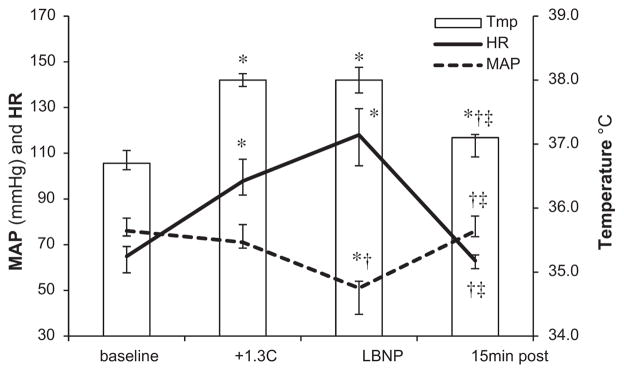
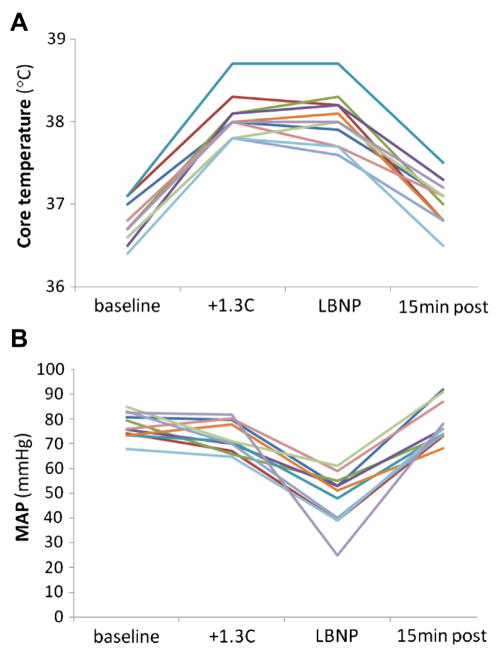
Vital signs: heart rate (HR), mean arterial blood pressure (MAP), and temperature are depicted in Figure 1. HR increased in response to heat stress (P = 0.003), and remained elevated during the decrease in CBV by LBNP (versus baseline P = 0.004 and versus heat stress P = 0.075), which also caused a decrease in MAP (versus baseline and heat stress P = 0.003 for both). Raw values for core temperature (Arteria pulmonalis) and MAP (Arteria brachialis) are shown in Figure 2. During the recovery period, as the subjects were actively cooled to approximate normothermia, HR and MAP returned to baseline values (P = 0.959 and P = 0.574, respectively). Five of the eleven subjects fulfilled three of the four SIRS criteria (HR >90 beats*min−1, PaCO2 <4.3 kPa, temperature >38°C), and another four met two criteria during the experiment (HR >90, PaCO2 <4.3). Therefore, nine of the 11 subjects were classified as fulfilling SIRS criteria.
Fig. 1.
Vital signs HR, MAP, and pulmonary artery temperature (tmp) in 11 healthy subjects: at baseline, after a 1.3°C increase in central temperature (+1.3°C), at the end of maximum LBNP, and after a 15 min cooling period (15 min post). Median values with IQR are displayed. *P < 0.05 versus baseline; †P <0.05 versus heat stress; ‡P <0.05 versus LBNP (Wilcoxon signed rank post hoc test).
Fig. 2.
Raw values (A), core temperature (B) MAP. Individual data from 11 healthy subjects: at baseline, after a 1.3°C increase in central temperature (+1.3°C), at the end of maximum LBNP, and after a 15 min cooling period (15 min post).
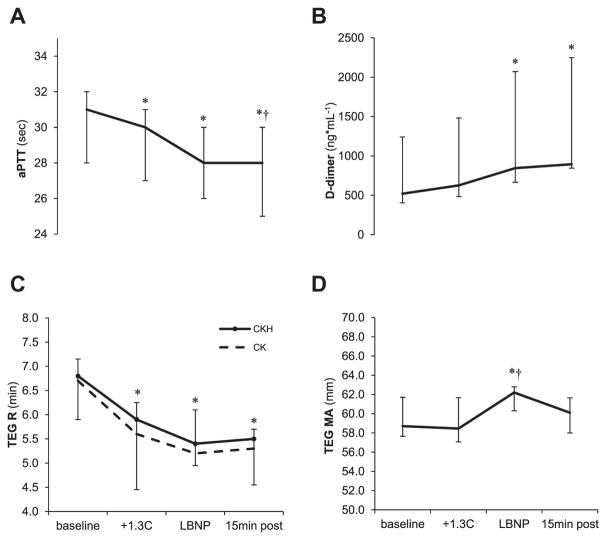
Blood variables are listed in Table (top). aPTT decreased during both heat stress (P = 0.04) and LBNP (versus baseline P = 0.003) (Fig. 3A), corresponding to a decrease of 9% (4–7) during LBNP versus baseline levels. Hematocrit increased during heat stress and remained elevated during LBNP and recovery (P < 0.001) with elevations corresponding to relative increases of 2% (2–4), 5% (2–7), and 2% (1–4), respectively versus baseline. Platelet and leukocyte count increased during both heat stress (both P = 0.003) and LBNP (both P = 0.003) and returned in the recovery phase to the levels observed during heat stress (platelets: +8% (4–12), +17% (16–20), +6% (3–6), respectively, versus baseline) (leukocytes: +15% (9–23), +43% (25–47), +17% (12–22), respectively, versus baseline). D-dimer increased during LBNP (versus baseline P = 0.016) and remained elevated during the recovery (Fig. 3B). The endothelial derived biomarkers syndecan-1 and soluble thrombomodulin and the anticoagulant protein C, did not change significantly during the experiment. Plasma adrenaline increased during LBNP (versus baseline P = 0.006 and versus heat stress P = 0.026) and noradrenaline increased during heat stress (P = 0.006) with a further increase during LBNP (versus baseline P = 0.003 and versus heat stress P = 0.026); reaching levels of 198% (83; 346) and 234% (174; 363), respectively, of baseline values during LBNP.
Table.
Blood values and functional hemostatic assays.
| Measurement | Baseline | Heat stress | LBNP | Recovery | P1 |
|---|---|---|---|---|---|
| PaO2 (kPa) | 13.7 (13.1–14.1) | 15.6 (15.1–15.9)* | 16.6 (15.7–18.4)* | 12.9 (11.8–14.2)†,‡ | P = 0.009 |
| PaCO2 (kPa) | 5.4 (5.2–5.5) | 4.6 (3.6–4.8)* | 3.6 (3.3–3.9)*,† | 5.2 (4.7–5.5)*,‡ | P = 0.001 |
| Hematocrit (ratio) | 0.41 (0.41–0.43) | 0.42 (0.41–0.45)* | 0.43 (0.43–0.44)* | 0.42 (0.41–0.44)*,‡ | P < 0.001 |
| Platelet count (109* L−1) | 214 (201–255) | 231 (221–277)* | 248 (241–298)*,† | 225 (211–272)*,‡ | P < 0.001 |
| Leukocytes (109* L−1) | 4.7 (4.5–5.6) | 5.4 (5.3–6.0)* | 6.5 (5.7–7.9)*,† | 5.3 (5.0–6.4)*,‡ | P < 0.001 |
| aPTT (s) | 31 (28–32) | 30 (27–31)* | 28 (26–30)* | 28 (25–30)*,† | P < 0.001 |
| INR | 1.2 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) | P = 0.083 |
| Fibrinogen (g* L−1) | 2.2 (2.2–2.3) | 2.4 (2.2–2.5) | 2.4 (2.3–2.4) | 2.4 (2.2–2.4) | P = 0.799 |
| FII, FVII, FX (relative conc.) | 0.69 (0.60–0.80) | 0.73 (0.63–0.82)* | 0.73 (0.63–0.81)* | 0.72 (0.62–0.77)†,‡ | P < 0.001 |
| C-reactive protein (mg* L−1) | ≥1 | ≥1 | ≥1 | ≥1 | NS |
| Adrenaline (pg* mL−1) | 31.2 (22.0–55.6) | 55.3 (29.5–66.5) | 87.5 (59.8–159.4)*,† | 54.0 (37.5–77.8)‡ | P = 0.043 |
| Noradrenaline (pg* mL−1) | 65.8 (54.2–89.6) | 144.8 (110.5–196.7)* | 238.8 (168.2–264.0)*,† | 124.3 (93.2–170,4)* | P < 0.001 |
| Protein C (relative conc.) | 60.7 (56.4–64.5) | 61.1 (48.6–72.3) | 60.2 (51.2–66.7) | 60.0 (46.7–67.7) | P = 0.896 |
| sTM (ng* mL−1) | 1.35 (0.87–1.63) | 1.32 (1.04–1.42) | 0.85 (0.80–1.05) | 0.95 (0.84–1.19) | P = 0.315 |
| Syndecan-1 (ng* mL−1) | 18.1 (15.9–21.3) | 17.4 (12.9–21.7) | 17.4 (12.3–20.8) | 17.4 (12.7–23.0) | P = 0.158 |
| D-dimer (ng* mL−1) | 521 (404–1241) | 627 (484–1483) | 845 (666–2071)* | 895 (845–2248)* | P = 0.010 |
| Functional hemostatic assays: | |||||
| TEG: | |||||
| CK R (min) | 6.7 (5.4–7.1) | 5.6 (4.4–6.2) | 5.2 (4.9–5.6) | 5.3 (5.1–5.8) | P = 0.153 |
| CK angle (°) | 61.2 (58.9–63.6) | 61.1 (59.9–64.7) | 64.1 (61.1–65.8) | 63.4 (61.4–66.0) | P = 0.421 |
| CK MA (mm) | 58.7 (57.7–61.7) | 58.5 (57.1–61.7) | 62.2 (60.3–62.8)*,† | 60.1 (58.0–61.7) | P = 0.015 |
| CK Ly30 (%) | 1.2 (0.4–1.5) | 1.2 (0.4–1.9) | 1.1 (0.5–2.0) | 0.4 (0.2–0.8) | P = 0.095 |
| CKH R (min) | 6.8 (5.9–7.2) | 5.9 (4.5–6.3)* | 5.4 (5.0–6.1)* | 5.5 (4.6–5.7)* | P = 0.040 |
| FF MA (mm) | 15.4 (14.5–16.7) | 16.4 (15.1–17.0) | 16.0 (15.0–16.5) | 16.3 (14.6–17.2) | P = 0.110 |
| Multiplate: | |||||
| TRAP (AUC) | 95.0 (88.5–110.0) | 98.9 (82.1–108.9) | 103.0 (92.7–122.5) | 95.7 (88.1–109.6) | P = 0.315 |
| ADP (AUC) | 71.6 (59.1–77.0) | 62.6 (55.3–69.1) | 72.6 (57.7–85.1) | 66.1 (56.2–69.3) | P = 0.066 |
| COL (AUC) | 73.1 (66.2–91.6) | 74.0 (68.5–80.6) | 75.7 (70.7–83.5) | 74.9 (70.9–82.0) | P = 0.591 |
| ASPI (AUC) | 81.3 (62.7–99.8) | 70.8 (64.4–79.4) | 82.2 (63.6–87.5) | 78.6 (68.5–87.7) | P = 0.178 |
FII, coagulation factor II; FVII, coagulation factor VII; FX, coagulation factor X; Ly30, clot lysis 30 min after MA; TRAP, thrombin-receptor activating peptide; ADP, adenosine 5′-diphosphate; COL, collagen; ASPI, arachidonic acid.
Data are shown as median (IQR).
Friedman, repeated measures.
P < 0.05 versus base line.
P < 0.05 versus heat stress.
P < 0.05 versus LBNP (Wilcoxon signed rank post hoc test).
Fig. 3.
Coagulation tests (A) aPTT, (B) D-dimer, (C) TEG CKR, (D) TEG MA in 11 healthy subjects: at baseline, after a 1.3°C increase in internal temperature (+1.3°C), at the end of maximum (LBNP), and after a 15 min of cooling (15 min post). TEG, thrombelastograpy; R, reaction time; CK, citrated kaolin TEG assay; CKH, citrated kaolin with heparinase TEG assay; MA, maximum amplitude. Median values with IQR. *P < 0.05 versus baseline; †P < 0.05 versus heat stress (Wilcoxon signed rank post hoc test).
Results for functional hemostatic assays are depicted in Table (bottom) and Figure 3C and D. TEG R (CKH only) decreased during heat stress and LBNP (baseline, versus heat stress P = 0.01; versus LBNP P = 0.02) and MA increased during LBNP (versus baseline P = 0.041 and versus heat stress P = 0.022), whereas no significant effect of neither heat stress nor subsequent LBNP was found for Ly30 or TEG FF parameters. Multiplate revealed no changes in platelet aggregation (AUC) at any of the time points investigated.
4. Discussion
The main finding of the study was that elevated body temperature and decreased central blood volume, by LBNP, induced an activation of the sympathetic, coagulation, and fibrinolytic systems, whereas no effect on the endothelium was observed. Nine of 11 subjects fulfilled the SIRS criteria, indicating profound physiologic stress. In essence, the coagulation tests indicated a relative hypercoagulability and corresponding increase in fibrinolysis in response to increased core temperature and decreased central blood volume.
The impact of heat stress, as illustrated by increased circulating catecholamine levels, was sufficient to provoke both activation of the cardiovascular system with an increase in HR and activation of the coagulation system as evidenced by a decrease in both TEG R and aPTT. Furthermore, addition of LBNP, provoking a decrease in CBV, resulted in a decrease in MAP and further activated the coagulation system documented by an increase in clot strength (MA). Increased fibrinolysis could be identified by increased D-dimer during LBNP, whereas TEG Ly30 did not change, probably reflecting relatively low sensitivity of TEG [21]. The decrease in aPTT was larger than that reported during LBNP in normothermic subjects [12]. This difference in coagulation activation could reflect different stress levels since plasma adrenaline levels reached higher levels during combined heat and LBNP than LBNP alone [14]. The dose-dependency in coagulation activation in relation to sympathetic activation was further illustrated by the increase in D-dimer levels. Hence, there was an increase in D-dimer only after the combination of heat and LBNP, which is in accordance with a study reporting that LBNP alone does not cause increased fibrinolytic activity as evaluated by D-dimer [12]. The modest increase in hematocrit, probably reflecting a reduction of plasma volume that occurred during this experiment [22], could partially be responsible for the concomitant increases in platelets and leukocytes, although the increase in hematocrit was relatively smaller. Also, this apparent hemoconcentration might have modulated factors not measured during this experiment that could have affected the observed relative hypercoagulability.
Investigations of hemostasis during hyperthermia have revealed a similar activation of coagulation [11] and fibrinolysis [23] as in the present experiment but there exists no clear description of the mechanism responsible for these changes on hemostasis. It has, however, been considered that platelet activation in response to increased temperature with a secondary activation of the coagulation factors or changes in endothelial function could be responsible for the alterations in coagulation [11]. Similarly, LBNP activates coagulation [12]. Common to hyperthermia [13], LBNP [14], and here the combination of both, is an increase in sympathetic activity, which results in increasing levels of catecholamines in the systemic circulation.
The influence of physiological stress on coagulation has been studied during for example exercise and various pathological conditions. Results from healthy individuals performing a marathon revealed activation of coagulation with decreased clotting time and increased clot strength as evaluated by VHA, an increase in thrombin generation and D-dimer levels, and a reduction in aPTT [24]. Similarly, major visceral surgery has been associated with development of a hypercoagulable state that correlated with development of thromboembolic complications postoperatively [25]. Also, some patients with sepsis demonstrate TEG hypercoagulability secondary to coagulation activation [26].
Remarkably, no significant effect of combined heat stress and LBNP was found when evaluating platelet aggregation with Multiplate despite the finding of increased clot strength (TEG MA). Thus, the increase in clot strength could be an effect of increased numbers of circulating platelets rather than increased platelet reactivity. Regarding the increase in platelet numbers, this could reflect an effect of increased concentration of epinephrine, which reportedly recruits the splenic pool of platelets [27,28].
The importance and activation of the neurohumoral system in critical illness, including trauma and sepsis [29], and its dose-dependent effects on the vascular system [29,30], including the endothelium [31], are well established. Syndecan-1, a constituent of the glycocalyx, is increased in non-surviving trauma patients [32], and in patients with ST-segment elevation myocardial infarction (STEMI) in shock compared with non-shocked STEMI patients [33], indicating severe derangement of this structure that correlates with levels of circulating adrenaline and noradrenaline. In the present study, no significant change in endothelial markers, including syndecan-1, was observed secondary to heat and LBNP indicating that the “stressor” provided in the present experiment and/or the catecholamine surge secondary to this were together insufficient to derange the vasculature. Studies of high-dose endotoxin infusion in healthy volunteers to model systemic inflammation have demonstrated an increase in core temperature and an acute hypercoagulability with indications of endothelial cell activation [34]. In a similar study of low-dose endotoxin infusion, we observed an induction of SIRS, hypercoagulability by TEG, and reduced platelet aggregation by Multiplate that was paralleled by increases in circulating tissue plasminogen activator [35]. Considering that hyperthermia is associated with hypercoagulability both in the present study, where no infection exists, and in the endotoxin study, it is tempting to consider hyperthermia as a contributor to the hypercoagulability that is seen in response to SIRS and sepsis [1]. Although we did not observe any changes in the measured biomarkers related to endothelial activation/damage, we consider it plausible that the observed changes in the present study were driven by a partial activation of the vascular endothelium, possibly by increased sympathetic activity, and consequent activation of hemostatic processes including a relative hypercoagulability and profibrinolytic state [15,16,36]. Activation of the endothelium has been described to include the release of both pro-coagulant and anticoagulant factors from endothelial cells during in vivo experiments of catecholamine infusion [15].
In trauma, focus on body temperature has been directed to hypothermia and the avoidance/correction by body and fluid heating. However, hyperthermia has a similar adverse impact on mortality as hypothermia [4] and could progress if a hyperthermic patient is kept warm or heated by default. The mechanism responsible for an increased mortality in hyperthermia has yet to be established. CBV decreases during hyperthermia because of redistribution of blood flow to cutaneous tissue [9]. Therefore, a hyperthermic person is likely to have a reduced CBV even before an injury. A subsequent hemorrhage will, accordingly, lead to a greater deficit in CBV relative to in a normothermic individual with a similar blood loss. The present study investigated this condition and found an alteration of hemostasis when the subject is first heated and then exposed to LBNP mimicking a central blood loss. This led to the described hypercoagulability in combination with increased fibrinolysis. The extent of fibrinolysis was not detectable by TEG, however, “occult fibrinolysis” (i.e., fibrinolysis undetectable by VHA), evident by increasing D-dimer, could be responsible for an increased risk of mortality in trauma.
Considering the increase in plasma catecholamines and activation of coagulation during heat stress and progressive reductions in CBV, the perspective of this study is that patients with clinical conditions associated with elevated body temperature and reduced CBV, both of which are common [2,5], could possibly benefit from optimization of CBV [37] and control of body temperature, which also have beneficial effects on vasopressor requirements in septic shock [38]. We suggest that the coagulation system of such patients should be monitored with both conventional and functional hemostatic assays in order to recognize and, if necessary, intervene on emerging hypercoagulable states. This should be investigated in prospective studies.
In hyperthermic trauma patients, acute hypercoagulability, as observed during this experiment, could serve to be protective against hemorrhage; however, such could not be evaluated during this experiment. What further remains to be investigated is whether hypercoagulability could evolve to a hypocoaguable state dominated by hyperfibrinolysis [39] and consumption of coagulation factors [40] as seen in trauma-associated coagulopathy.
4.1. Limitations
The present study has important limitations. Only presumed healthy males were evaluated so the results presented may not apply to females or patients with comorbidities. Also, by not evaluating the effect of heat or LBNP alone, and by not applying the interventions in random order, we cannot exclude the possibility that the changes seen when combining heat and LBNP only reflect the longer duration of heat stress (total duration of heat stress: 51 (51–53) min including 7 (6–8) min of LBNP), although reports have demonstrated an effect of LBNP alone on the hemostatic system limiting this possibility [12]. Owing to the low number of study subjects, there is a risk of introducing a Type II error that also should be taken into account.
5. Conclusions
In confirmation of our hypothesis, elevated core temperature and subsequent reduction of CBV resulted in activation of coagulation, indicating an emerging hypercoagulability, and caused an increase in fibrinolysis. This response is very similar to that observed in low-dose human experimental endotoxemia. Given the complications attributed to coagulation disturbances in trauma, SIRS, and sepsis, the hyperthermia and LBNP model appears to be useful in investigating the contribution of elevated body temperature and hypovolemia in the pathophysiology that cause these coagulation disturbances. We hypothesize that elevated body temperature and reduced CBV contribute to the hypercoagulability and hyperfibrinolysis observed in SIRS.
Acknowledgments
The authors declare no conflict of interest.
References
- 1.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 2.Niven DJ, Leger C, Stelfox HT, Laupland KB. Fever in the critically ill: a review of epidemiology, immunology, and management. J Intensive Care Med. 2012;27:290. doi: 10.1177/0885066611402463. [DOI] [PubMed] [Google Scholar]
- 3.Buller MJ, Wallis DC, Karis AJ, et al. Thermal-work Strain during Marine Rifle Squad Operations in Iraq (Summer 2008) U.S. Army Research Institute of Environmental Medicine; 2008. [Google Scholar]
- 4.Wade CE, Salinas J, Eastridge BJ, McManus JG, Holcomb JB. Admission hypo- or hyperthermia and survival after trauma in civilian and military environments. Int J Emerg Med. 2011;4:35. doi: 10.1186/1865-1380-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monnet X, Teboul J. Volume responsiveness. Curr Opin Crit Care. 2007;13:549. doi: 10.1097/MCC.0b013e3282ec68b2. [DOI] [PubMed] [Google Scholar]
- 6.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Fernandez N, Montes R, Purroy A, Rocha E. Hemostatic disturbances in patients with systemic inflammatory response syndrome (SIRS) and associated acute renal failure (ARF) Thromb Res. 2000;100:19. doi: 10.1016/s0049-3848(00)00306-6. [DOI] [PubMed] [Google Scholar]
- 8.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, Oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandall CG, Wilson TE, Marving J, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray RH, Thompson LJ, Bowers JA, Albright CD. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am Heart J. 1968;76:799. doi: 10.1016/0002-8703(68)90266-4. [DOI] [PubMed] [Google Scholar]
- 11.Strother SV, Bull JM, Branham SA. Activation of coagulation during therapeutic whole body hyperthermia. Thromb Res. 1986;43:353. doi: 10.1016/0049-3848(86)90155-6. [DOI] [PubMed] [Google Scholar]
- 12.Zaar M, Johansson PI, Nielsen LB, et al. Early activation of the coagulation system during lower body negative pressure. Clin Physiol Funct Imaging. 2009;29:427. doi: 10.1111/j.1475-097X.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMorris T, Swain J, Smith M, et al. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol. 2006;61:204. doi: 10.1016/j.ijpsycho.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Franke WD, Johnson CP, Steinkamp JA, Wang R, Halliwill JR. Cardiovascular and autonomic responses to lower body negative pressure: do not explain gender differences in orthostatic tolerance. Clin Auton Res. 2003;13:36. doi: 10.1007/s10286-003-0066-x. [DOI] [PubMed] [Google Scholar]
- 15.von KR, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65:357. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 16.Johansson PI, Ostrowski SR. Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses. 2010;75:564. doi: 10.1016/j.mehy.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Ganio MS, Overgaard M, Seifert T, et al. Effect of heat stress on cardiac output and systemic vascular conductance during simulated hemorrhage to presyncope in young men. Am J Physiol Heart Circ Physiol. 2012;302:H1756. doi: 10.1152/ajpheart.00941.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol. 2004;96:1249. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- 19.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3:135. doi: 10.1016/0160-5402(80)90024-8. [DOI] [PubMed] [Google Scholar]
- 21.Genet GF, Ostrowski SR, Sorensen AM, Johansson PI. Detection of tPA-induced hyperfibrinolysis in whole blood by RapidTEG, KaolinTEG, and functional fibrinogenTEG in healthy individuals. Clin Appl Thromb Hemost. 2012;18:638. doi: 10.1177/1076029611434527. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen SE, Weder AB, Egan B, Neubig R, Zweifler AJ, Julius S. Effect of circulating epinephrine on platelet function and hematocrit. Hypertension. 1995;25:1096. doi: 10.1161/01.hyp.25.5.1096. [DOI] [PubMed] [Google Scholar]
- 23.Dodman B, Cunliffe WJ, Roberts BE, Buchan CW. Effects of changes in temperature (local and central) on plasma fibrinolytic activity. J Clin Pathol. 1973;26:248. doi: 10.1136/jcp.26.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumann G, Fries D, Griesmacher A, et al. Blood coagulation activation and fibrinolysis during a downhill marathon run. Blood Coagul Fibrinolysis. 2007;18:435. doi: 10.1097/MBC.0b013e328136c19b. [DOI] [PubMed] [Google Scholar]
- 25.McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576. doi: 10.1213/01.ANE.0000155290.86795.12. [DOI] [PubMed] [Google Scholar]
- 26.Gonano C, Sitzwohl C, Meitner E, Weinstabl C, Kettner SC. Four-day antithrombin therapy does not seem to attenuate hypercoagulability in patients suffering from sepsis. Crit Care. 2006;10:R160. doi: 10.1186/cc5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misselwitz B, Bachli EB, Kaiser P, Fehr J, Goede JS. Diagnosis of hypersplenism with the epinephrine stimulation test—23 years of experience at a tertiary care hospital. Swiss Med Weekly. 2012;141:w13324. doi: 10.57187/smw.2012.13324. [DOI] [PubMed] [Google Scholar]
- 28.Schaffner A, Augustiny N, Otto RC, Fehr J. The hypersplenic spleen. A contractile reservoir of granulocytes and platelets. Arch Intern Med. 1985;145:651. doi: 10.1001/archinte.145.4.651. [DOI] [PubMed] [Google Scholar]
- 29.Cryer PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980;303:436. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- 30.Chernow B, Rainey TG, Lake CR. Endogenous and exogenous catecholamines in critical care medicine. Crit Care Med. 1982;10:409. doi: 10.1097/00003246-198206000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Makhmudov RM, Mamedov Y, Dolgov VV, Repin VS. Catecholamine-mediated injury to endothelium in rabbit perfused aorta: a quantitative analysis by scanning electron microscopy. Cor Vasa. 1985;27:456. [PubMed] [Google Scholar]
- 32.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care. 2013;17:R32. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowski SR, Berg RM, Windelov NA, et al. Discrepant fibrinolytic response in plasma and whole blood during experimental endotoxemia in healthy volunteers. PLoS One. 2013;8:e59368. doi: 10.1371/journal.pone.0059368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 37.Bundgaard-Nielsen M, Wilson TE, Seifert T, Secher NH, Crandall CG. Effect of volume loading on the Frank–Starling relation during reductions in central blood volume in heat-stressed humans. J Physiol. 2010;588(Pt 17):3333. doi: 10.1113/jphysiol.2010.191981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schortgen F, Clabault K, Katsahian S, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012;185:1088. doi: 10.1164/rccm.201110-1820OC. [DOI] [PubMed] [Google Scholar]
- 39.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 40.Shaz BH, Winkler AM, James AB, Hillyer CD, MacLeod JB. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011;70:1401. doi: 10.1097/TA.0b013e31821266e0. [DOI] [PMC free article] [PubMed] [Google Scholar]