Abstract
This study tested the hypothesis that hyperthermia attenuates the increase in cerebral perfusion during cognitive activation. Mean middle cerebral artery blood velocity (MCAVmean) served as an index of cerebral perfusion, while the nBack test (a test of working memory) was the cognitive task. Hyperthermia was characterized by elevations (P < 0.001) in skin (by 5.0 ± 0.8°C) and intestinal temperatures (by 1.3 ± 0.1°C) and reductions (P < 0.020) in mean arterial pressure (by 11 ± 10 mmHg), end-tidal CO2 tension (by 3 ± 6 mmHg) and MCAVmean (by 10 ± 9 cm s−1). Hyperthermia had no influence on nBack test performance (mean difference from normothermia to hyperthermia, −1 ± 11%; P = 0.276) or, counter to the hypothesis, the increase in MCAVmean during nBack testing (mean difference from normothermia to hyperthermia: 0 ± 16 cm s−1; P = 0.608). These findings indicate that the capacity to increase cerebral perfusion during cognitive activation is unaffected by hyperthermia.
Introduction
Upon the initiation of a cognitive task, a cascade of cerebral events unfolds. This cascade commences with increases in neural and metabolic activity that are ultimately accommodated by increases in cerebral blood flow (Girouard & Iadecola, 2006; Rosengarten et al. 2012). This ‘coupling’ between cognitive activation and cerebral perfusion enables the cognitive task to be suitably accomplished. Interestingly, however, the capacity to increase cerebral perfusion during cognitive activation is modulated by changes in baseline (i.e. before cognitive activation) blood flow. For instance, chronic suppression of resting cerebral perfusion (by 10–20%) is accompanied by an impaired increase in cerebral perfusion during cognitive activation in healthy older individuals (Sorond et al. 2008) and in patients with essential hypotension (Duschek & Schandry, 2004). Acute reductions in brain blood flow, induced via hypocapnia, likewise attenuate the increase in perfusion during a subsequent cognitive task (Szabo et al. 2011). On the contrary, however, acute elevations in resting perfusion, whether induced via hypercapnia (Rosengarten et al. 2003), acetazolamide (Yonai et al. 2010) or exercise (Willie et al. 2011b), have little impact on the increase in cerebral perfusion in response to such tasks. Thus, it is clear that acute or chronic reductions in cerebral perfusion deleteriously affect the capacity to increase perfusion during cognitive activation.
Hyperthermia, i.e. elevations in both mean skin and internal temperatures, reduces resting cerebral perfusion by 10–30% (Wilson et al. 2006; Brothers et al. 2009; Nelson et al. 2011). This decrease is, at least partly, a result of hypocapnia occurring concomitant to hyperthermia-induced hyperventilation (Fujii et al. 2008; Brothers et al. 2009; Ross et al. 2012). Given that hyperthermia is characterized by reductions in cerebral perfusion and hypocapnia, and that hypocapnia, alone, attenuates increases in cerebral perfusion during cognitive stimulation (Szabo et al. 2011), it seems likely that the capacity to increase cerebral perfusion during cognitive activation is impaired by hyperthermia. Therefore, the purpose of this study was to test the hypothesis that hyperthermia attenuates increases in cerebral perfusion during a cognitive task.
Methods
Subjects
Twenty-one healthy, physically active volunteers (13 men) participated in this study. The subject characteristics were as follows (means ± SD): age, 29 ± 7 years; height, 175 ± 13 cm; weight, 70.4 ± 15.3 kg; and body surface area, 1.9 ± 0.3 m2. Nineteen of the subjects identified themselves as being right handed (Oldfield, 1971). All subjects were non-smokers, not taking medications, free of any known cardiovascular, metabolic, neurological or psychological diseases and had normal or corrected-to-normal vision. Each subject was fully informed of the experimental procedures and possible risks before giving informed written consent. This protocol was approved by the Institutional Review Boards at the University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital of Dallas, and all procedures conformed to the standards set by the Declaration of Helsinki. Subjects arrived at the laboratory euhydrated (confirmed via a urine specific gravity <1.025) and having refrained from strenuous exercise, alcohol and caffeine for a period of 24 h.
Instrumentation and measurements
Approximately 90 min prior to experimental testing, each subject swallowed a telemetry pill (HQ Inc., Palmetto, FL, USA) for the measurement of intestinal temperature. Mean skin temperature was measured as the weighted average of six thermocouples attached to the skin. Body temperature was controlled via a water-perfused, tube-lined suit (Med-Eng, Ottawa, Ontario, Canada) that covered the entire body except the head, hands, one forearm and the feet. Heart rate was continually recorded from an electrocardiogram (HP Patient Monitor; Agilent, Santa Clara, CA, USA) interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Beat-to-beat arterial pressure was continuously measured via the Penaz method (Finometer Pro; FMS, Amsterdam, The Netherlands), which was confirmed intermittently via auscultation of the brachial artery by electrosphygmomanometry (Tango+; SunTech, Raleigh, NC, USA). Throughout the protocol, the subjects wore a nose-clip and breathed through a mouthpiece. Expired gases were measured for the partial pressure of end-tidal carbon dioxide (PET,CO2 ; VitalCap Capnograph Monitor; Oridion, Needham, MA, USA), which served as a surrogate for arterial carbon dioxide tension (Brothers et al. 2011).
nBack test
The nBack test was the cognitive stimulus. This test is a standardized test of working memory (Braver et al. 1997; Owen et al. 2005), can be graded in difficulty (Braver et al. 1997; Ocon et al. 2012; Stewart et al. 2012) and is relatively short, minimizing internal (intestinal) body temperature elevations during test administration. The nBack test was administered using commercially available software (NTT Systems Inc., Toronto, Ontario, Canada) in a manner that has been described in detail previously (Braver et al. 1997; Ocon et al. 2012; Stewart et al. 2012). During the test, subjects monitored a series of letters and were instructed to respond whenever a stimulus was presented that matched the one presented ‘n’ trials previously, where ‘n’ is the specified ‘nBack level’ (Owen et al. 2005). This test consisted of four levels, i.e. 1, 2, 3 and 4 Back, with 1 Back being the easiest condition and 4 Back being the most challenging. The test was completed five times, the first three being practice sessions (avoiding a potential learning effect; Stewart et al. 2012 and see Results of present study) and the final two were experimental. The order of the nBack level (i.e. 1, 2, 3 or 4 Back) was randomized, with 20 s between each level. The stimulus duration was 0.5 s and the interstimulus duration 2.5 s. The total duration of each nBack level was 68–75 s, which was dependent upon the nBack level (1 Back being the shortest and 4 Back the longest). There were 10 ‘targets’ in each nBack level, and all inputs were made with the right hand. nBack test responses were made by pressing the spacebar on a keyboard (Black Widow; Razer, San Diego, CA, USA) placed to the right side of the body. The same keyboard was used in all trials, which recorded responses with a 1 ms response latency. nBack performance data are reported as the percentage of correct responses (i.e. percentage correct = number of correct responses/total number of possible responses × 100).
Cerebral perfusion
Cerebral blood flow was quantified using transcranial Doppler (Duschek & Schandry, 2003; Willie et al. 2011a) from bilateral insonation of the middle cerebral arteries. Transcranial Doppler provides a valid and reliable index of time-dependent changes in cerebral blood velocity (Duschek & Schandry, 2003; Willie et al. 2011a). The middle cerebral artery was chosen because it supplies ~80% of the blood flow to each hemisphere (Stroobant & Vingerhoets, 2000), and given its responsiveness to tests of working memory (Cupini et al. 1996; Sorond et al. 2011; Ocon et al. 2012; Stewart et al. 2012) and hyperthermia (Wilson et al. 2006; Fujii et al. 2008; Brothers et al. 2009; Nelson et al. 2011). Middle cerebral artery blood velocity (MCAV ) was measured by adjusting a 2 MHz Doppler probe (Multi-flow; DWL Elekronishche Systeme, Singen, Germany) over the temporal windows, using search techniques previously described (Aaslid et al. 1982; Willie et al. 2011a). Once optimal signals were identified, the probes were secured in place using a headband. Peak systolic, diastolic and (integrated) mean blood velocities were extracted over each cardiac cycle. Mean middle cerebral artery blood velocities (MCAVmean) were used in the analysis, given that this value is highly correlated with the volume of blood flowing through a vessel (Duschek & Schandry, 2003). The MCAVmean did not lateralize at any time (lowest P value = 0.331), and therefore the data presented represent average responses from both temporal windows (e.g. Stewart et al. 2012).
Experimental protocol
Following instrumentation, subjects rested quietly in the supine position while normothermic water (34°C) perfused the suit. During this time, the subjects underwent three practice tests (~20 min in total duration). This was followed by 5 min of quiet rest, with eyes open, with the last 60 s recorded as normothermic baseline. This resting period transitioned into normothermic nBack testing. The nBack test was presented on a standard computer monitor suspended a comfortable distance overhead, allowing the subjects to remain supine throughout the experiment. During all nBack testing, the laboratory was quiet and the lights were dimmed. Following completion of the normothermic nBack test battery, the subjects underwent whole-body passive heat stress by perfusing 49°C water through the suit. This heat stress continued until intestinal temperature was elevated by ~1.3°C. Upon the attainment of this hyperthermia, a 5 min period of quiet rest was undertaken, with eyes open, during the last 60 s of which hyperthermic baseline data were collected. This baseline period transitioned into hyperthermic nBack testing, which was followed by whole-body cooling and the restoration of normothermia. The subjects were not allowed to drink at any time during the experimental procedures.
Data analysis
Thermal and haemodynamic data were sampled at 50 Hz via a data acquisition system (Biopac System, Santa Barbara, CA, USA). During each nBack level, MCAVmean data were averaged in 1 s bins, starting 5 s immediately preceding each nBack level. These data were analysed using three distinct, but commonly employed, approaches. First, changes in cerebral perfusion during cognitive activation were calculated as the percentage change in MCAVmean (ΔMCAVmean) from baseline, as done previously (e.g. Sorond et al. 2008; Matteis et al. 2009; Sorond et al. 2011; Szabo et al. 2011; Ocon et al. 2012). Baseline was defined as the average MCAVmean during the 5 s period preceding each nBack level (Szabo et al. 2011). These data were analysed through 65 s for each nBack level. Second, given the expected (and confirmed; see Results) time-dependent increases in MCAVmean during each nBack level, area-under-the-curve (AUC) analysis was also employed to discern the extent of the changes occurring over time (Pruessner et al. 2003). Third, cerebral blood velocity dynamics during cognitive activation were evaluated by analysing the peak MCAVmean response to each nBack level and the time at which this peak occurred (Szabo et al. 2011; Willie et al. 2011b). These analyses were also undertaken for mean arterial pressure (MAP) and PET,CO2, because these factors can independently modulate cerebral blood velocity during cognitive activation (Moody et al. 2005; Panerai et al. 2005).
Statistical analysis
Differences between the normothermic and hyperthermic baseline periods were identified using Student’s paired t tests. All other data were analysed using repeated measures ANOVA. These data were assessed for approximation to a normal distribution and sphericity, and no corrections were necessary. When the ANOVAs revealed a significant F test, post hoc pairwise comparisons were made, incorporating a Bonferroni adjustment. A priori linear relationships between dependent variables were evaluated using Pearson product–moment correlation analysis. To confirm that a learning effect from repeated nBack testing was minimized, within-subject coefficients of variation were calculated to identify nBack performance variability between the nBack practice trials and experimental nBack testing. Data were analysed using SigmaPlot (v12; Systat Software Inc., Chicago, IL, USA) with a priori statistical significance set at P < 0.05. All data are reported as means ± SD.
Results
nBack practice
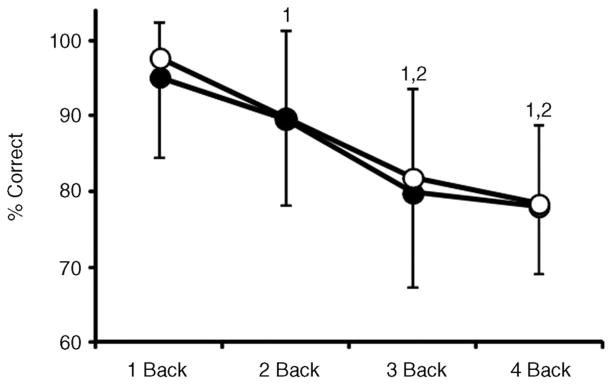
The practice nBack testing was effective in minimizing a learning effect. For all levels of the nBack assessment, nBack performance was lowest (P = 0.027) during the first practice trial, but improved thereafter such that there were no differences between nBack performance in the second (P = 0.236) and third practice trials (P = 0.998) relative to the normothermic experimental nBack testing. Within-subject coefficients of variation in nBack performance for all levels of nBack were similar between the second and third (final) nBack practice tests (P = 0.998) and between the third nBack practice test and the normothermic experimental nBack test (P = 0.998). Mean within-subject coefficients of variation between the third nBack practice test and the normothermic experimental nBack test for each nBack level were as follows: 1 Back, 4 ± 8%; 2 Back, 7 ± 7%; 3 Back, 11 ± 9%; and 4 Back, 7 ± 5%.
Baseline thermal and haemodynamic data
Hyperthermia was characterized by ~5.0°C elevation (P < 0.001) in mean skin temperature, a ~1.3°C increase (P < 0.001) in intestinal temperature, reductions (P < 0.020) in right and left diastolic MCAV and MCAVmean, MAP and PET,CO2, and elevated (P <0.001) heart rates. Systolic MCAV was preserved (P = 0.380) during hyperthermia (Table 1).
Table 1.
Normothermic and hyperthermic baselines
| Parameter | Normothermia | Hyperthermia |
|---|---|---|
| Mean skin temperature (°C) | 34.4 ± 0.5 | 39.4 ± 1.0* |
| Intestinal temperature (°C) | 36.9 ± 0.3 | 38.3 ± 0.3* |
| Right MCAV (cm s−1) | ||
| Peak systolic | 97 ± 12 | 97 ± 20 |
| End diastolic | 52 ± 7 | 43 ± 10* |
| Mean | 70 ± 10 | 61 ± 14* |
| Left MCAV (cm s−1) | ||
| Peak systolic | 94 ± 16 | 90 ± 25 |
| End diastolic | 50 ± 9 | 41 ± 13* |
| Mean | 68 ± 11 | 58 ± 17* |
| MAP (mmHg) | 76 ± 11 | 66 ± 7* |
| PET,CO2 (mmHg) | 38 ± 3 | 34 ± 5* |
| Heart rate (beats min−1) | 56 ± 9 | 90 ± 14* |
Values are means ± SD. Abbreviations: MAP, mean arterial blood pressure; MCAV, middle cerebral artery blood velocity; and PET,CO2, partial pressure of end-tidal carbon dioxide.
Significantly different from normothermia (P < 0.020).
nBack test responses
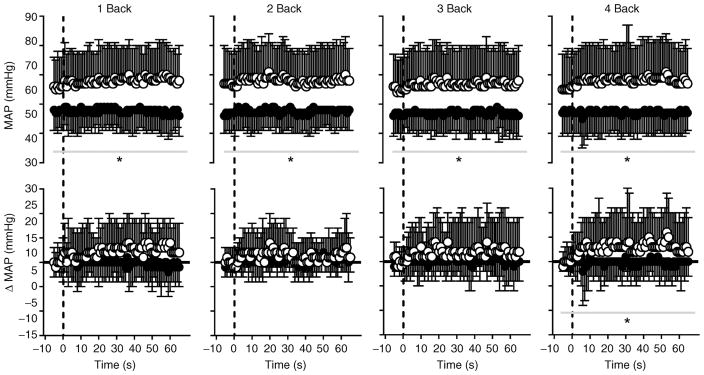
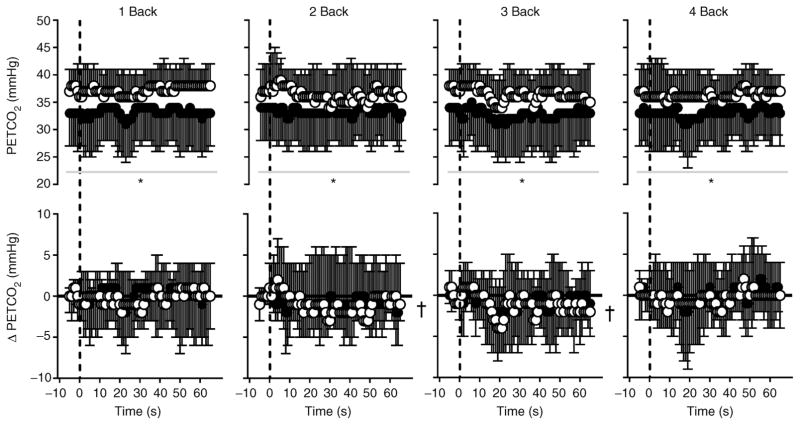
nBack performance decreased (P < 0.001) with increasing nBack level. However, there was no effect (P = 0.276) of body temperature on test performance (Fig. 1). Mean within-subject coefficients of variation between the normothermic and hyperthermic experimental nBack testing were as follows: 1 Back, 4 ± 8%; 2 Back, 5 ± 7%; 3 Back, 11 ± 11%; and 4 Back, 8 ± 6%; these values were similar (P = 0.998) to those occurring between the final nBack practice test and the normothermic experimental nBack test (see above section ‘nBack Practice’). Increases in intestinal temperature (P = 0.201), mean skin temperature (P = 0.639) and heart rate (P = 0.840) remained stable (from baseline) during nBack testing. Both MAP (Fig. 2) and PET,CO2 (Fig. 3) were reduced (P < 0.010) by hyperthermia. Irrespective of thermal condition (P = 0.381), changes in MAP remained stable (P ≥ 0.051) throughout nBack testing, except during 4 Back (P = 0.014; Fig. 2). Changes in PET,CO2 during nBack testing were also similar (P ≥ 0.793) between thermal conditions (Fig. 3). Area-under-the-curve analysis indicated that ΔMAP did not differentiate (P = 0.996) between nBack levels, but ΔMAP was generally greater during normothermia (Table 2). Area-under-the-curve analysis for ΔPET,CO2 indicated the responses were quite variable (Table 2), and therefore changes over time during each nBack level were negligible and thus were not differentially affected by body temperature (P = 0.864) or nBack level (P = 0.242; Table 2). This AUC analysis corroborates the MAP and PET,CO2 time series analysis (Figs 2 and 3) and collectively indicates that changes in MAP and PET,CO2 during nBack testing were minimally affected by nBack levels or thermal conditions.
Figure 1. nBack performance expressed as a percentage of correct responses (% correct) across levels of nBack (mean ± SD) in normothermia (open circles) and hyperthermia (filled circles).
‘1’ and ‘2’ indicate significant difference (P < 0.004) from 1 Back and 2 Back tests, respectively.
Figure 2. Mean arterial pressure (MAP) expressed as absolute values (top panels) and as the change from baseline (bottom panels; ΔMAP) over time during 1 Back, 2 Back, 3 Back and 4 Back tests (means ± SD) in normothermia (open circles) and hyperthermia (filled circles).
The vertical dashed line indicates the start of each nBack test; the horizontal continuous black line (in ΔMAP graphs) indicates zero (or no change). *Significantly different (P ≤ 0.014) from normothermia. Owing to the large number of potential comparisons, pairwise comparisons were not conducted in this analysis.
Figure 3. End-tidal carbon dioxide tension (PET,CO2) expressed as absolute values (top panels) and as the change from baseline (bottom panels; ΔPET,CO2) over time during 1 Back, 2 Back, 3 Back and 4 Back tests (means ± SD) in normothermia (open circles) and hyperthermia (filled circles).
The vertical dashed line indicates the start of each nBack test; the horizontal continuous black line (in ΔPET,CO2 graphs) indicates zero (or no change). *Significantly different (P ≤ 0.019) from normothermia; and †significant (P ≤ 0.040) main effect of time. Owing to the large number of potential comparisons, pairwise comparisons were not conducted in this analysis.
Table 2.
Area-under-the-curve analysis
| Parameter | 1 Back
|
2 Back
|
3 Back
|
4 Back
|
||||
|---|---|---|---|---|---|---|---|---|
| Normothermia | Hyperthermia | Normothermia | Hyperthermia | Normothermia | Hyperthermia | Normothermia | Hyperthermia | |
| MCAVmean AUC (a.u.) | 4562 ± 655 | 3916 ± 981* | 4678 ± 681 | 3956 ± 927* | 4661±649 | 3935 ± 913* | 4713 ± 668 | 4000 ± 962* |
| ΔMCAVmean AUC (a.u.) | 348 ± 533 | 356 ± 584 | 348 ± 552 | 230 ± 476 | 348 ± 845 | 513 ± 570 | 507 ± 585 | 427 ± 564 |
| MAP AUC (a.u.) | 5152 ± 691 | 4469 ± 429* | 5148 ± 743 | 4454 ± 410* | 5067 ± 771 | 4420 ± 419* | 5161 ± 779 | 4431 ± 469* |
| ΔMAP AUC (a.u.) | 217 ± 334 | 20 ± 293* | 104 ± 227 | 82 ± 108 | 160 ± 497 | 49 ± 210 | 193 ± 323 | 19 ± 204* |
| PET,CO2 AUC (a.u.) | 2452 ± 199 | 2189 ± 384* | 2392 ± 199 | 2195 ± 350* | 2399 ± 193 | 2165 ± 383* | 2387 ± 206 | 2189 ± 344* |
| ΔPET,CO2 AUC (a.u.) | −18 ± 317 | 65 ± 787 | −58 ± 861 | −138 ± 363 | −191 ± 387 | −161 ± 465 | 2 ± 615 | 30 ± 508 |
Values are means ± SD. Abbreviations: a.u., arbitrary units; AUC, area under the curve; MAP, mean arterial pressure; ΔMAP, percentage change in mean arterial pressure; MCAVmean, mean middle cerebral artery blood velocity; ΔMCAVmean, percentage change in mean middle cerebral artery blood velocity; PET,CO2, partial pressure of end-tidal CO2; and ΔPET,CO2, percentage change in the partial pressure of end-tidal CO2.
Significantly different from normothermia (P < 0.01).
Cerebral blood velocity during nBack testing
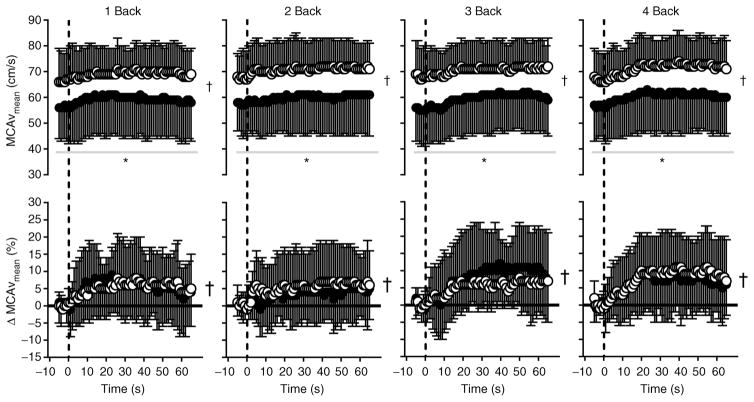
Given that heat stress itself decreased MCAVmean (Table 1), MCAVmean was lower (P < 0.001) throughout all nBack levels during hyperthermia (Fig. 4). Notably, the magnitude of the increase in MCAVmean (ΔMCAVmean) during nBack testing was similar (P = 0.608) between thermal conditions (Fig. 4). This finding was confirmed when evaluated using the AUC analysis (Table 2), such that there was no effect of nBack level (P = 0.419) or body temperature (P = 0.947) on the magnitude of the increase in MCAVmean (Table 2). The peak MCAVmean response to each nBack level was higher (P < 0.001) during normothermia, but the time at which this peak occurred (P = 0.745) and the relative change (i.e. ΔMCAVmean) from baseline (P = 0.180) were independent of changes in body temperature (Table 3).
Figure 4. Mean middle cerebral artery blood velocity (MCAVmean) expressed as absolute values (top panels) and as the percentage change from baseline (bottom panels; ΔMCAVmean) over time during 1 Back, 2 Back, 3 Back and 4 Back tests (means ± SD) in normothermia (open circles) and hyperthermia (filled circles).
The vertical dashed line indicates the start of each nBack test; the horizontal continuous black line (in ΔMCAVmean graphs) indicates zero (or no change). *Significantly different (P < 0.001) from normothermia; and †significant (P < 0.001) main effect of time. Owing to the large number of potential comparisons, pairwise comparisons were not conducted in this analysis.
Table 3.
Activation–flow coupling dynamics of MCAVmean
| Parameter | Normothermia
|
Hyperthermia
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 Back | 2 Back | 3 Back | 4 Back | 1 Back | 2 Back | 3 Back | 4 Back | |
| Baseline (cm s−1) | 66 ± 12 | 68 ± 10 | 68 ± 12 | 67 ± 11 | 56 ± 13* | 58 ± 12* | 56 ± 13* | 57 ± 13* |
| Peak (cm s−1) | 77 ± 12† | 80 ± 12† | 78 ± 11† | 79 ± 12† | 65 ± 16*† | 67 ± 16*† | 67 ± 14*† | 67 ± 16*† |
| Change (%) | 18 ± 11 | 19 ± 11 | 16 ± 15 | 19 ± 11 | 17 ± 11 | 15 ± 7 | 22 ± 14 | 17 ± 11 |
| Time (s) | 31 ± 18 | 28 ± 18 | 31 ± 19 | 38 ± 15 | 28 ± 19 | 33 ± 22 | 36 ± 19 | 29 ± 20 |
Values are means ± SD. Abbreviations: MCAVmean, mean middle cerebral artery blood velocity; Baseline, average MCAVmean during 5 s prior to initiation of nBack; Peak, highest MCAVmean during nBack; Change, Peak as a percentage change from Baseline; and Time, time at which Peak occurred.
Significantly different from normothermia (P < 0.001);
significantly different from Baseline (P < 0.001).
Correlation analysis
No significant relationships between ΔMCAVmean AUC and nBack performance were observed when evaluated during normothermia (r2 = 0.0025, P = 0.653), hyperthermia (r2 = 0.0018, P = 0.699) or combined normothermia and hyperthermia (r2 = 0.0000, P = 0.937). There was a very weak but significant relationship between ΔMCAVmean AUC and ΔMAP AUC during hyperthermia (r2 = 0.0600, P = 0.034), but not during normothermia (r2 = 0.0185, P = 0.242), while ΔMCAVmean AUC and ΔPET,CO2 AUC were found to be correlated weakly during both normothermia (r2 = 0.0462, P = 0.049) and hyperthermia (r2 = 0.2510, P < 0.001).
Discussion
The novel finding of this study is that the increase in cerebral perfusion during cognitive activation is well preserved during hyperthermia; therefore, we reject our hypothesis. These data specifically demonstrate that hyperthermia-induced reductions in MCAVmean, MAP and PET,CO2 (Table 1 and Figs 2 and 3) do not alter subsequent increases in MCAVmean during graded nBack tests. This conclusion was found to be consistent when expressed as: (i) average changes occurring over time (Fig. 4); (ii) the accumulated magnitude of these time-dependent changes, i.e. AUC (Table 2); or (iii) the dynamics of the peak responses (Table 3). Collectively, these findings indicate that the capacity to increase cerebral perfusion in response to a cognitive task involving processes of working memory is unaffected by increases in mean skin and internal (intestinal) body temperatures at the levels achieved in the present study.
Cognitive activation and cerebral blood velocity during hyperthermia
Chronic reductions in baseline cerebral perfusion (i.e. by 10–20%) impair subsequent increases in cerebral perfusion during a cognitive challenge in healthy older individuals (Sorond et al. 2008) and in patients with essential hypotension (Duschek & Schandry, 2004). Based upon those observations, we hypothesized that a reduction in (baseline) cerebral perfusion by ~14% during hyperthermia (Table 1) would attenuate subsequent increases in cerebral perfusion during a test of working memory. On the contrary, such coupling was remarkably preserved (Fig. 4 and Tables 2 and 3), despite additional hyperthermia-induced reductions in MAP and PET,CO2 (Table 1 and Figs 2 and 3). Importantly, hyperthermia had little influence on ΔPET,CO2 and Δ MAP during nBack testing (Table 2 and Figs 2 and 3). As such, changes in MAP during nBack testing accounted for no more than 6% of the variance associated with cognitive-induced changes in MCAVmean. From these findings, we are confident that the observed increases in MCAVmean were due to reductions in cerebral vascular resistance (as opposed to increases in perfusion pressure; i.e. Poiseuille’s Law), the former of which is likely to have occurred secondary to an increase in metabolic and neuronal demand. Importantly, these data indicate that the magnitude of the change in cerebral vascular resistance during the nBack procedures is independent of increases in body temperature. This observation is consistent with previous findings from our laboratory demonstrating that hyperthermia has little effect on dynamic cerebral autoregulation (Low et al. 2009) or cerebrovascular responsiveness to carbon dioxide (Low et al. 2008), which are factors that are proposed to be mediated by the same physiological mechanisms as those governing changes in cerebral haemodynamics during cognitive activation (Rosengarten et al. 2001).
Hypocapnia independently impairs increases in cerebral perfusion during cognitive stimulation (Szabo et al. 2011). Therefore, our findings that hyperthermia, which was coupled with mild hypocapnia (Tables 1 and 2 and Fig. 3), did not alter cognitive-induced changes in MCAVmean (Fig. 4 and Tables 2 and 3) were unexpected. The rationale for these apparently contradictory findings is probably threefold. First, the magnitude of hypocapnia during hyperthermia in the present study was appreciably smaller than that imposed by Szabo et al. (2011; ~4 versus ~12 mmHg reductions). Second, the cognitive stimulus in the present study involved working memory, which is in contrast to the reading stimulus used by Szabo et al. (2011). Third, different cerebral arteries were assessed between these studies. Given its sensitivity to visual stimulation (Aaslid, 1987), Szabo et al. (2011) measured blood velocity in the posterior cerebral artery, while in the present study we assessed velocity in the middle cerebral artery because it is responsive to tests of working memory (Cupini et al. 1996; Sorond et al. 2008, 2011; Ocon et al. 2012; Stewart et al. 2012). To our knowledge, the effect of hypocapnia on increases in cerebral perfusion during cognitive activation in different cerebral vessels remains uncertain. However, given recent findings indicating regional variations in cerebral chemosensitivity (Sato et al. 2012; Willie et al. 2012), such differences appear likely. Notably, correlation analyses of the present data indicated that changes in PET,CO2 during cognitive activation were (very) weakly associated with changes in MCAVmean during both normothermia and hyperthermia. This corroborates previous work, indicating that changes in arterial carbon dioxide tension play no more than a minor role in modulating cerebral perfusion during cognitive activation (Moody et al. 2005; Panerai et al. 2005).
Cerebral perfusion and cognitive performance during hyperthermia
We anticipated that nBack performance would deteriorate during hyperthermia and that the magnitude of this deterioration would be related to the hypothesized attenuated increases in MCAVmean. This expectation was predicated on the previous findings indicating that the capability to increase cerebral perfusion during cognitive activation is positively related to task performance (Duschek & Schandry, 2006; Matteis et al. 2009; Sorond et al. 2011; Stewart et al. 2012) and that many aspects of cognitive function (e.g. attention, executive functioning and memory) are impaired during hyperthermia (Wilkinson et al. 1964; Hocking et al. 2001; McMorris et al. 2006; Racinais et al. 2008; Gaoua et al. 2011a,b, 2012a,b). However, we found no effect of hyperthermia on nBack performance (Fig. 1), and thus it is not surprising that there were no relationships between nBack performance and the increase in MCAVmean. The rationale for an absence of an effect of hyperthermia on nBack performance probably lies with the nBack protocol used in the present study. For instance, the impact of hyperthermia on cognitive function is largely task dependent (Hancock & Vasmatzidis, 2003). That is, those cognitive tests involving greater neuronal resources are impaired to a greater extent than less demanding tasks (Hocking et al. 2001; Gaoua, 2010, 2012b). The nBack test is a test of working memory (Owen et al. 2005), an aspect of cognition that demands a relatively large portion of the neuronal resources (Hocking et al. 2001). Thus, we anticipated that nBack test performance would be impaired by hyperthermia. Yet, despite the deterioration of nBack test performance with increasing nBack levels, hyperthermia did not affect test performance (Fig. 1). Although it is clear that the nBack test provided a challenging cognitive stimulus, it is speculated that the nBack protocol did not elicit a sufficient neuronal ‘overload’ necessary for hyperthermia-induced decrements in working memory performance (e.g. Gaoua et al. 2012b). Given that increases in cerebral neuronal activity are accurately reflected by increases in cerebral blood velocity (Zaletel et al. 2004; Rosengarten et al. 2006), our MCAVmean data appear to support this conjecture (Fig. 4 and Tables 2 and 3).
Methodological considerations
We used transcranial Doppler to quantify MCAV, given that this technique allows for convenient, continuous and non-invasive measurement of blood velocities in the cerebral arteries at a high temporal resolution (Stroobant & Vingerhoets, 2000; Duschek & Schandry, 2003; Willie et al. 2011a). It must be acknowledged that if the diameter of the insonated artery changes, blood velocity may not reflect changes in blood flow. Notably, however, the diameter of the middle cerebral artery is unaffected by moderate carbon dioxide and arterial pressure perturbations (Giller et al. 1993; Serrador et al. 2000).
A related consideration is the cerebral arteries that we chose to insonate. We monitored MCAV given its responsiveness to tests of working memory (Cupini et al. 1996); specifically, the nBack test (Sorond et al. 2011; Ocon et al. 2012; Stewart et al. 2012) and hyperthermia (Wilson et al. 2006; Fujii et al. 2008; Brothers et al. 2009; Nelson et al. 2011). However, it could be argued that the anterior cerebral artery is a more appropriate vessel to insonate because it provides more direct perfusion of the frontal lobes (Rosengarten et al. 2012), which are vital in the execution of tasks of working memory (Owen et al. 1990; Fletcher & Henson, 2001; Baldo & Shimamura, 2002). Relative to the middle cerebral artery, however, insonation of the anterior cerebral artery can be challenging due to its relatively small size (Willie et al. 2011a). Therefore, given that changes in MCAV during executive functioning tasks, which involve many aspects of working memory (Drag & Bieliauskas, 2010), reflect blood velocity changes in the anterior cerebral artery (Schuepbach et al. 2002, 2007), we deemed the insonation of the middle cerebral artery appropriate. Nevertheless, the possibility remains that hyperthermia modified the increase in perfusion during cognitive activation in other, non-insonated cerebral arteries.
Hyperthermia influences cerebral metabolic (Nunneley et al. 2002; Nybo et al. 2002) and neural responses (Dubois et al. 1980, 1981). To our knowledge, however, the effects of hyperthermia on metabolic and neural responses to a cognitive stimulus remain uncertain. Thus, in the present study we assumed that the magnitude of cerebral metabolic and neural demand at a given nBack level is unaffected by hyperthermia.
Perspectives and future directions
The findings of this study extend previous work from our laboratory indicating that cerebrovascular responsiveness is generally unaffected by hyperthermia. Specifically, dynamic cerebral autoregulation (Low et al. 2009), carbon dioxide reactivity (Low et al. 2008) and now cerebrovascular responsiveness to a cognitive stimulus (Fig. 4 and Tables 2 and 3) are all maintained irrespective of increases in body temperature and associated reductions in cerebral perfusion. It is notable, however, that the findings of this study pertain only to situations involving aspects of working memory, the performance of which was unaffected by hyperthermia.
Conclusions
The present study demonstrates that hyperthermia, and the accompanying moderate reductions in cerebral blood velocity, arterial pressure and end-tidal carbon dioxide tension, do not alter the capability to increase cerebral blood velocity during a cognitive challenge involving working memory. These findings confirm that hyperthermia has little influence on cerebrovascular reactivity.
New Findings.
-
What is the central question of this study?
Decreases in baseline cerebral perfusion impair the capacity to increase cerebral perfusion during cognitive activation. Hyperthermia reduces baseline cerebral perfusion, but it remains unknown whether hyperthermia also impairs the increase in cerebral perfusion during the execution of a cognitive task.
-
What is the main finding and its importance?
This study shows that hyperthermia does not alter the increase in cerebral perfusion during a cognitive task. These findings have implications for individuals who are frequently exposed to heat stress in concert with a cognitive challenge.
Acknowledgments
Funding
This study was supported by Award Numbers R01HL061388 and T32HL007360 from the National Institutes of Health, National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
We would like to thank the subjects for participating in our study and Jena Langlois RN and Naomi Kennedy RN for their technical assistance.
Footnotes
Competing interests
None declared.
References
- Aaslid R. Visually evoked dynamic blood flow response of the human cerebral circulation. Stroke. 1987;18:771–775. doi: 10.1161/01.str.18.4.771. [DOI] [PubMed] [Google Scholar]
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Frontal lobes and memory. In: Baddeley A, Wilson B, Kopelman M, editors. Handbook of Memory Disorders. John Wiley and Co; London: 2002. pp. 363–379. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Ganio MS, Hubing KA, Hastings JL, Crandall CG. End-tidal carbon dioxide tension reflects arterial carbon dioxide tension in the heat-stressed human with and without simulated hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2011;300:R978–R983. doi: 10.1152/ajpregu.00784.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol. 2009;587:3921–3927. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupini LM, Matteis M, Troisi E, Sabbadini M, Bernardi G, Caltagirone C, Silvestrini M. Bilateral simultaneous transcranial Doppler monitoring of flow velocity changes during visuospatial and verbal working memory tasks. Brain. 1996;119:1249–1253. doi: 10.1093/brain/119.4.1249. [DOI] [PubMed] [Google Scholar]
- Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Dubois M, Coppola R, Buchsbaum MS, Lees DE. Somatosensory evoked potentials during whole body hyperthermia in humans. Electroencephalogr Clin Neurophysiol. 1981;52:157–162. doi: 10.1016/0013-4694(81)90163-2. [DOI] [PubMed] [Google Scholar]
- Dubois M, Sato S, Lees DE, Bull JM, Smith R, White BG, Moore H, Macnamara TE. Electroencephalographic changes during whole body hyperthermia in humans. Electroencephalogr Clin Neurophysiol. 1980;50:486–495. doi: 10.1016/0013-4694(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Functional transcranial Doppler sonography as a tool in psychophysiological research. Psychophysiology. 2003;40:436–454. doi: 10.1111/1469-8986.00046. [DOI] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology. 2004;41:905–913. doi: 10.1111/j.1469-8986.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- Duschek S, Schandry R. Deficient adjustment of cerebral blood flow to cognitive activity due to chronically low blood pressure. Biol Psychol. 2006;72:311–317. doi: 10.1016/j.biopsycho.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fujii N, Honda Y, Hayashi K, Kondo N, Koga S, Nishiyasu T. Effects of chemoreflexes on hyperthermic hyperventilation and cerebral blood velocity in resting heated humans. Exp Physiol. 2008;93:994–1001. doi: 10.1113/expphysiol.2008.042143. [DOI] [PubMed] [Google Scholar]
- Gaoua N. Cognitive function in hot environments: a question of methodology. Scand J Med Sci Sports. 2010;20(Suppl 3):60–70. doi: 10.1111/j.1600-0838.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- Gaoua N, Grantham J, El Massioui F, Girard O, Racinais S. Cognitive decrements do not follow neuromuscular alterations during passive heat exposure. Int J Hyperthermia. 2011a;27:10–19. doi: 10.3109/02656736.2010.519371. [DOI] [PubMed] [Google Scholar]
- Gaoua N, Grantham J, Racinais S, El Massioui F. Sensory displeasure reduces complex cognitive performance in the heat. J Environ Psychol. 2012a;32:158–163. [Google Scholar]
- Gaoua N, Herrera CP, Racinais S, El Massioui F. Heat induces an overload during complex cognitive performance. Med Sci Sport Exerc. 2012b;44:S230. [Google Scholar]
- Gaoua N, Racinais S, Grantham J, El Massioui F. Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia. 2011b;27:1–9. doi: 10.3109/02656736.2010.516305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Vasmatzidis I. Effects of heat stress on cognitive performance: the current state of knowledge. Int J Hyperthermia. 2003;19:355–372. doi: 10.1080/0265673021000054630. [DOI] [PubMed] [Google Scholar]
- Hocking C, Silberstein RB, Lau WM, Stough C, Roberts W. Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:719–734. doi: 10.1016/s1095-6433(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Cui J, Zhang R, Crandall CG. Dynamic cerebral autoregulation during passive heat stress in humans. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1598–R1605. doi: 10.1152/ajpregu.90900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol. 2008;104:976–981. doi: 10.1152/japplphysiol.01040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int J Psychophysiol. 2006;61:204–215. doi: 10.1016/j.ijpsycho.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Matteis M, Bivona U, Catani S, Pasqualetti P, Formisano R, Vernieri F, Troisi E, Caltagirone C, Silvestrini M. Functional transcranial Doppler assessment of cerebral blood flow velocities changes during attention tasks. Eur J Neurol. 2009;16:81–87. doi: 10.1111/j.1468-1331.2008.02351.x. [DOI] [PubMed] [Google Scholar]
- Moody M, Panerai RB, Eames PJ, Potter JF. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1581–R1588. doi: 10.1152/ajpregu.00837.2004. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Haykowsky MJ, Stickland MK, Altamirano-Diaz LA, Willie CK, Smith KJ, Petersen SR, Ainslie PN. Reductions in cerebral blood flow during passive heat stress in humans: partitioning the mechanisms. J Physiol. 2011;589:4053–4064. doi: 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunneley SA, Martin CC, Slauson JW, Hearon CM, Nickerson LDH, Mason PA. Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol. 2002;92:846–851. doi: 10.1152/japplphysiol.00072.2001. [DOI] [PubMed] [Google Scholar]
- Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci. 2012;122:227–238. doi: 10.1042/CS20110241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panerai RB, Moody M, Eames PJ, Potter JF. Dynamic cerebral autoregulation during brain activation paradigms. Am J Physiol Heart Circ Physiol. 2005;289:H1202–H1208. doi: 10.1152/ajpheart.00115.2005. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol. 2008;586:4751–4762. doi: 10.1113/jphysiol.2008.157420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten B, Deppe M, Kaps M, Klingelhofer J. Methodological aspects of functional transcranial Doppler sonography and recommendations for simultaneous EEG recording. Ultrasound Med Biol. 2012;38:989–996. doi: 10.1016/j.ultrasmedbio.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Huwendiek O, Kaps M. Neurovascular coupling and cerebral autoregulation can be described in terms of a control system. Ultrasound Med Biol. 2001;27:189–193. doi: 10.1016/s0301-5629(00)00332-x. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Molnar S, Trautmann J, Kaps M. Simultaneous VEP and transcranial Doppler ultrasound recordings to investigate activation-flow coupling in humans. Ultrasound Med Biol. 2006;32:1171–1180. doi: 10.1016/j.ultrasmedbio.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Rosengarten B, Spiller A, Aldinger C, Kaps M. Control system analysis of visually evoked blood flow regulation in humans under normocapnia and hypercapnia. Eur J Ultrasound. 2003;16:169–175. doi: 10.1016/s0929-8266(02)00070-8. [DOI] [PubMed] [Google Scholar]
- Ross EZ, Cotter JD, Wilson L, Fan JL, Lucas SJ, Ainslie PN. Cerebrovascular and corticomotor function during progressive passive hyperthermia in humans. J Appl Physiol. 2012;112:748–758. doi: 10.1152/japplphysiol.00988.2011. [DOI] [PubMed] [Google Scholar]
- Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol. 2012;590:3277–3290. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuepbach D, Boeker H, Duschek S, Hell D. Rapid cerebral hemodynamic modulation during mental planning and movement execution: evidence of time-locked relationship with complex behavior. Clin Neurophysiol. 2007;118:2254–2262. doi: 10.1016/j.clinph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Schuepbach D, Merlo MC, Goenner F, Staikov I, Mattle HP, Dierks T, Brenner HD. Cerebral hemodynamic response induced by the Tower of Hanoi puzzle and the Wisconsin Card Sorting test. Neuropsychologia. 2002;40:39–53. doi: 10.1016/s0028-3932(01)00074-4. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Messer ZR, Baugham IL, Terilli C, Ocon AJ. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1185–H1194. doi: 10.1152/ajpheart.00994.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant N, Vingerhoets G. Transcranial Doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: a review. Neuropsychol Rev. 2000;10:213–231. doi: 10.1023/a:1026412811036. [DOI] [PubMed] [Google Scholar]
- Szabo K, Lako E, Juhasz T, Rosengarten B, Csiba L, Olah L. Hypocapnia induced vasoconstriction significantly inhibits the neurovascular coupling in humans. J Neurol Sci. 2011;309:58–62. doi: 10.1016/j.jns.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT, Fox RH, Goldsmith FR, Hampton IF, Lewis HE. Psychological and physiological responses to raised body temperature. J Appl Physiol. 1964;19:287–291. doi: 10.1152/jappl.1964.19.2.287. [DOI] [PubMed] [Google Scholar]
- Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011a;196:221–237. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Willie CK, Cowan EC, Ainslie PN, Taylor CE, Smith KJ, Sin PY, Tzeng YC. Neurovascular coupling and distribution of cerebral blood flow during exercise. J Neurosci Methods. 2011b;198:270–273. doi: 10.1016/j.jneumeth.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;590:3261–3275. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–R1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonai Y, Boms N, Molnar S, Rosengarten B, Bornstein NM, Csiba L, Olah L. Acetazolamide-induced vasodilation does not inhibit the visually evoked flow response. J Cereb Blood Flow Metab. 2010;30:516–521. doi: 10.1038/jcbfm.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Rodi Z, Zvan B. The relationship between visually evoked cerebral blood flow velocity responses and visual-evoked potentials. Neuroimage. 2004;22:1784–1789. doi: 10.1016/j.neuroimage.2004.04.019. [DOI] [PubMed] [Google Scholar]