Abstract
Purpose
Ascorbic acid (AsA) is an important antioxidant in the eye. Ascorbic acid is usually transported by sodium-dependent AsA transporters (SVCTs), and dehydroascorbic acid (DHA) by glucose transporters (GLUTs). This study investigates these AsA-related transporters in human compared with mouse eyes.
Methods
Five pairs of human donor eyes and 15 pairs of mouse eyes were collected. Immunofluorescence and in situ hybridization were performed to detect SVCTs and GLUTs expression in the ciliary epithelium, retina, and lens epithelial cells (LECs). These tissues were isolated with laser microdissection followed by extraction of total RNA. Quantitative PCR (qPCR) was performed to examine the mRNA level of SVCTs and GLUTs in human and mouse ocular tissues.
Results
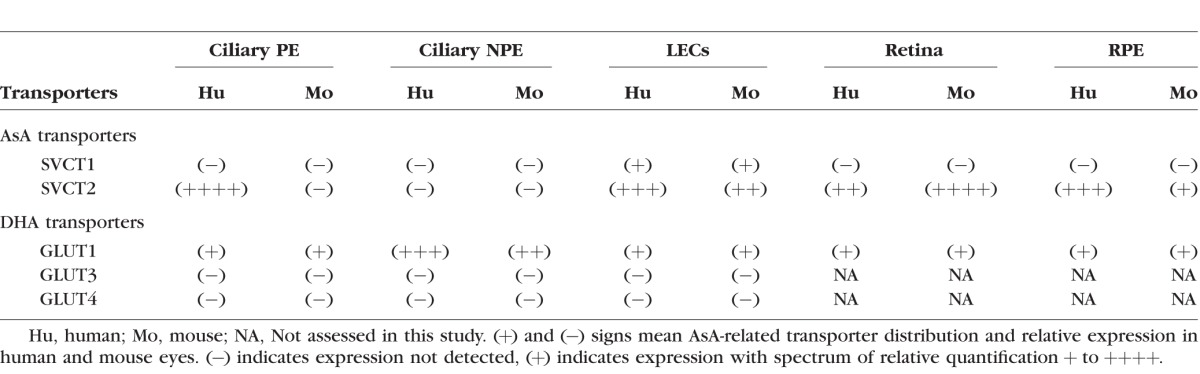
Immunofluorescence and in situ hybridization showed SVCT2 and GLUT1 expression in human ciliary epithelium with varied distributions. Sodium-dependent AsA transporter 2 is expressed only in the pigmented epithelium (PE), and GLUT1 is predominately expressed in the nonpigmented epithelium (NPE). However, SVCT2 was not identified in mouse ciliary epithelium, whereas GLUT1 expressed in both PE and NPE. Laser microdissection and qPCR revealed high levels of SVCT2 mRNA in human RPE cells and murine neural retina. Sodium-dependent AsA transporter 1 mRNA could be detected only in human and murine LECs. Glucose transporter 3 and GLUT4 mRNA could not be detected in either the human or mouse ciliary processes or in the lens epithelium.
Conclusions
These fundamental findings indicate AsA transporter expression in eyes of humans is significantly different compared with mice. This may explain why human aqueous and vitreous humors contain higher AsA levels compared with other animals.
Keywords: ascorbic acid, transporters, SVCT2, GLUT1
Ascorbic acid (AsA) is essential for many critical enzymatic reactions that maintain prosthetic metal ions in their reduced forms and protects tissues from oxidative damage by scavenging free radicals.1–3 Numerous studies have reported that AsA in the aqueous humor protects the cornea, lens, and other intraocular tissues against oxidative damage.4–8 Conversely, the oxidized byproduct of AsA is dehydroascorbic acid (DHA), which can be reduced back to AsA intracellularly by several mechanisms.9–11 Ascorbic acid is specifically transported by sodium-dependent vitamin C transporters (SVCTs) encoded by the SLC23 family, which consists of SVCT1 and SVCT2.3,4,12–14 Meanwhile, DHA can be transported via facilitated diffusion with the assistance of members of the glucose transporter (GLUT) family (GLUT1, GLUT3, and GLUT4).15–17
It is known that AsA biosynthesis capabilities differ among species. All animal cells are strictly dependent on the presence of functional vitamin C transporters, which determine the distribution of this molecule between extra- and intracellular fluids.11,12 As a diurnal species, humans are susceptible to light-induced eye injury.18,19 Consequently, increased concentrations of antioxidants, such as AsA, are required to protect human eyes from oxidative stress and damage.4,8,14,18,20 Ascorbic acid is found in very high concentrations in various human ocular tissues, which may exceed plasma concentrations of AsA by as much as 20- to 70-fold.8,21
The ciliary body provides the aqueous humor with nutrients and antioxidants, including AsA.22 However, little is known about the AsA transport pathways used by the ciliary epithelium or other ocular tissues. A few animal studies have reported that the ciliary body actively concentrates AsA from the plasma, via SVCT2 located in the pigmented epithelial (PE) cell or uptake of DHA via GLUT1 located in both the PE and nonpigmented epithelial (NPE) layers followed by DHA recycling and ultimately secretion of AsA into the eye.3,23,24 There is a paucity of consistent information of human AsA transport mechanisms. For example, we reported previously that AsA levels were higher in vitreous25 as compared with aqueous humor at a ratio of 3:2 in human eyes (Siegfried, et al. IOVS 2015;56:ARVO E-Abstract 4414), whereas the ratio of AsA levels in other species is inversed,20,25 with higher levels in the aqueous humor. The high concentration of AsA in human vitreous (≈2 mM) provides protection for both the lens and retina from oxidative damage by consuming oxygen diffusing across from the retina.25,26 However, the detailed mechanisms for this concentration gradient and the source of the intraocular AsA remain unclear, as well as differences in metabolism and transport among different species.
To clarify these fundamental questions, we chose a representative nocturnal animal, the mouse, which is capable of self-synthesizing AsA; and a diurnal animal, the human, which is incapable of self-synthesizing AsA, as our research subjects. We designed this study to detect and compare expression and distribution of SVCTs and GLUTs in various ocular tissues: ciliary processes, RPE, neural retina, and lens epithelial cells (LECs).
Materials and Methods
Acquisition of Donor Eyes
All procedures conformed to the provisions of the Declaration of Helsinki for the use of human tissue in research and were approved by the Washington University Human Subjects Institutional Review Board. Five pairs of healthy adult donor eyes (age range 50–65 years) were obtained from Mid-America Transplant Services (St. Louis, MO, USA). To obtain fresh tissues for staining and RNA extraction, we restricted the globes in the present study to those received less than 48 hours postmortem. The previous medical and ocular histories of all donors were assessed to exclude donors with any eye disease. An ophthalmologist examined every globe before use and confirmed all eyes lacked pathology. Systemic diseases, such as diabetes, cancer, or other chronic diseases, that may affect nutritional uptake of AsA were also excluded.
Sample Collection and Slide Preparation
We obtained five pairs of healthy human globes and 15 pairs of adult mouse (C57BL/6, wild-type) globes. One globe from each pair was used to prepare paraffin slides and the other was frozen for laser microdissection. The globes were injected with 0.5 mL 10% formaldehyde via the vitreous chamber first and then the whole globe was fixed in 10% formaldehyde solution for 24 hours to maintain the integrity of the ciliary body. Sections of 5 μm embedded in paraffin were prepared as slides for immunofluorescence staining and fluorescence in situ hybridization (FISH). The fellow eyes were embedded in Optimal Cutting Temperature (OCT) compound, flash frozen on dry ice for 10 to 15 minutes, and then stored at −80°C for future use. The frozen eye tissues were prepared for laser microdissection. Anterior lens capsules were excised and the central region was used for RNA extraction.
Immunofluorescence Staining
Before immunofluorescence staining, deparaffinized slides were treated with 3% H2O2 in PBS to inactivate endogenous peroxidase activity. Antigen retrieval was performed in Vector antigen unmasking solution (pH 6.0, H-3300; Vector Laboratories, Inc., Burlingame, CA, USA) by placing the slides in a pressure cooker for 5 minutes. After cooling, sections were blocked with 5% goat serum/3% BSA/0.1% Triton X-100 in PBS at room temperature for 1 hour and incubated with primary antibody at 4°C overnight. Then the slides were incubated for 1 hour at room temperature with Alexa-Fluor-labeled secondary antibodies (Molecular Probes, Eugene, OR, USA). The primary antibodies included anti-SVCT2 antibody (1:100; sc-30114; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-GLUT1 antibody (1:100; ab40084; Abcam, Cambridge, UK). Fluorescent images were captured using the Olympus BX51 with Spot camera (Olympus, Melville, NY, USA) or Zeiss 510 confocal microscope (Carl Zeiss, Thornburgh, NY, USA).
Fluorescence In Situ Hybridization on Paraffin Sections
Fluorescence in situ hybridization was performed on paraffin sections using in situ hybridization tissue assay (Quantigene ViewRNA; Affymetrix, Santa Clara, CA, USA) according to the manufacturer's instructions. For paraffin sections, FISH conditions were optimized to include a 5-minute boiling and 5-minute protease treatment (Protease QF, Affymetrix Quantigene ISH kit, QVT0050; 1:200; Affymetrix). Oligonucleotide probes (Type 1 probe set) were designed as follows: RefSeq RNA ID, Affymetrix Probe ID (Affymetrix): human SLC23A2 (NM_005116), mouse slc23a2 (NM_018824), human SLC2A1 (NM_006516), and mouse slc2a1 (NM_011400).
Laser Microdissection
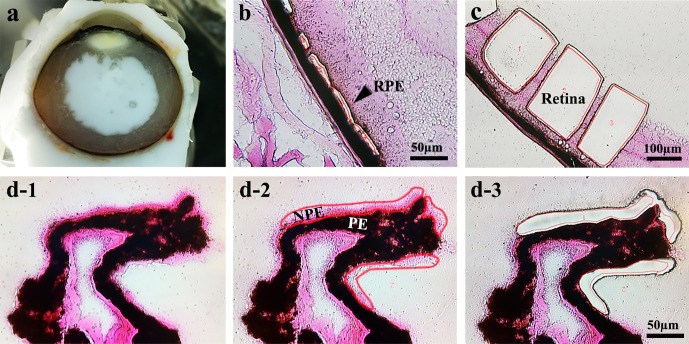
Human and mouse eye tissues embedded in OCT and stored at −80°C were used for laser dissection (Fig. 1a). Frozen sections of 10 μm were transferred to glass polyethylene naphthalate (PEN) foil slides (Leica Microsystems, Wetzlar, Germany). To avoid separations of the foil and slides, the slides were dipped in 70% ethanol at 4°C for 1 minute, washed in RNAase-free water twice for 30 seconds, rinsed in 95% ethanol, and then stained in Eosin Y. Stained samples were washed in 95% ethanol and dehydrated in 100% ethanol and xylene. After drying, the PE and NPE of the ciliary body, neural retina, and RPE were carefully identified and outlined, then collected by laser microdissection using the Leica LMD 6000 Laser microdissection system (Figs. 1b–d).
Figure 1.
Laser microdissection images of collected tissues from human and mouse eyes. (a) Fresh frozen section of human globe. (b) Human RPE tissue. (c) Human neural retinal tissue. (d1) Human ciliary process staining with Eosin Y, NPE (pink); (d2) NPE is selected in red; (d3) following laser microdissection of NPE.
Total RNA Extraction and Amplification
Approximately 50 ng total RNA was extracted from the tissue using the RNeasy Microkit (Qiagen, Hilden, Germany); 10 ng total RNA was reverse transcribed and amplified to produce 3 to 5 μg cDNA using the WT-Ovation Pico RNA Amplification System (NuGEN Technologies, San Carlos, CA, USA). The amplified cDNA samples were used for the quantitative PCR (qPCR) analyses and RT-PCR.
Quantitative PCR and RT-PCR
The qPCR analysis was performed using SYBR Green JumpStart Taq ReadyMix (s4438; Sigma Chemicals, St. Louis, MO, USA) and the Eco Real-Time PCR System (Illumina, Inc., San Diego, CA, USA) according to the manufacturers' protocols. Quantified values for each gene of interest were normalized against the input determined by the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Actb, NM_007393). Primer pairs are listed in the Table. The melting temperature for the hSLC23A1 (hSVCT1), hSLC23A2 (hSVCT2), hRPE65, hTYRP1, mslc23a1 (mSVCT1), mslc23a2 (mSVCT2), hGAPDH, and mGAPDH primers was 55°C, and for the hSLC2A1 (hGLUT1) and mslc2a1 (mGLUT1) primers was 59°C. Each assay was performed in triplicate. For all qPCR experiments, three independent biological samples were analyzed.
Table.
Distribution of AsA-Related Transporters in Human and Mouse Eyes
Polymerase chain reaction was performed to detect GLUT3 and GLUT4 in human and mouse ciliary processes. For amplification, 1 μL cDNA aliquot was added to a 20-μL mix containing 10 μL 2×PCR Master Mix, 2 μL primers, and 7 μL ddH2O. The mix was incubated at 95°C for 2 minutes, then run for 35 cycles at 95°C for 15 seconds, 55°C for 15 seconds, and 68°C for 15 seconds. Polymerase chain reaction products were separated using 3% agarose gel electrophoresis and visualized using ethidium bromide. Primer sequences are listed in the Supplementary Table S1.
Statistical Analysis
All results are expressed as mean ± SD. Student's t-test was performed to calculate statistical significance. Any difference between mean values is considered statistically significant for P ≤ 0.05.
Results
Expression Profiling of SVCT2 in Human and Mouse Ciliary Processes
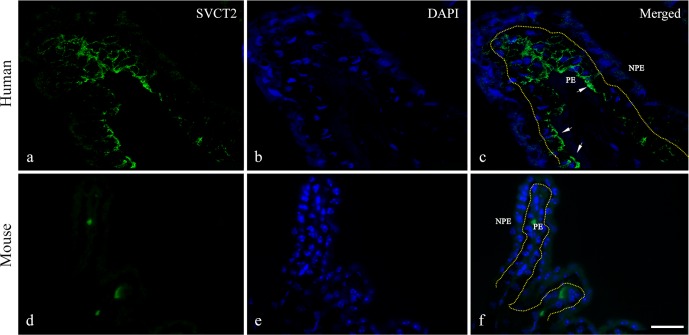
We used immunofluorescence staining and in situ hybridization to detect SVCT2 as well as mRNA levels in both human and mouse ciliary processes. In Figure 2, immunofluorescence staining of SVCT2 was observed in human ciliary processes and its distribution was limited to the PE layer. When merged with nuclear staining, SVCT2 was mainly localized at the basolateral membrane of the PE cells facing the ciliary stromal microvasculature (Figs. 2a–c); however, in the mouse ciliary processes, SVCT2 protein expression was not found in either the PE or NPE layers.
Figure 2.
(a–c) Immunofluorescence staining of SVCT2 in human and mouse ciliary processes. Sodium-dependent vitamin C transporter 2 expression was limited to the PE layer (green, arrows) of the human ciliary process. (d–f) Sodium-dependent vitamin C transporter 2 was not identified in the mouse ciliary process. Scale bar: 25 μm.
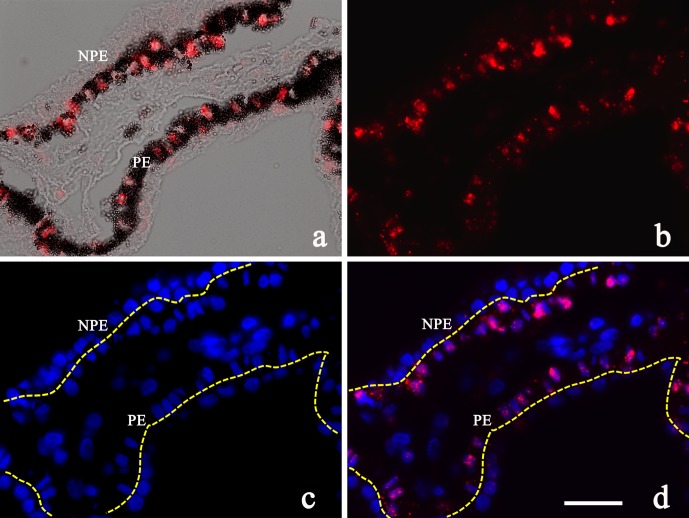
In situ hybridization showed similar results compared with protein expression (Fig. 3). The SLC23A2 probe only bound to human PE cells surrounding the nuclei but not in the NPE layer (Figs. 3a–d). Expression of SLC23A2 was not identified in either the PE or NPE in the mouse ciliary process (data not shown).
Figure 3.
(a–d) SLC23A2 (SVCT2) in situ hybridization in human ciliary processes. SVCT2 mRNA (SLC23A2) was detected in the PE layer of human ciliary processes surrounding the nucleus (red). Scale bar: 25 μm.
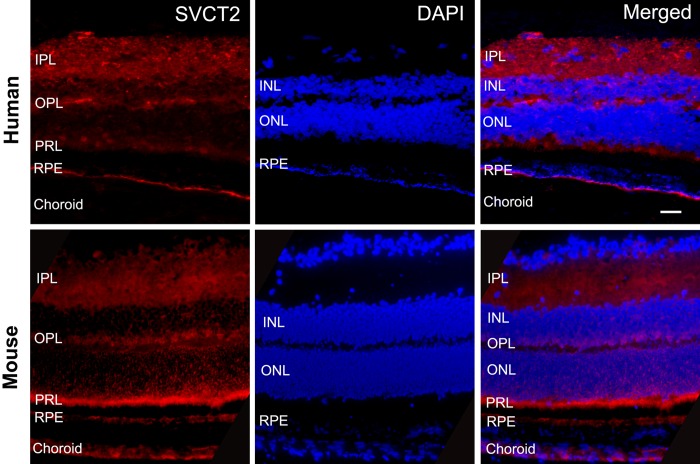
Distribution of SVCT2 in Human and Mouse Retina
We used immunofluorescence to identify protein expression of SVCT2 in human and mouse retina. The paraffin sections using whole globes were prepared and stained with anti-SVCT2 antibody. In Figure 4, the unstained space between the RPE and neural retina designates the region of rods and cones (photoreceptor layer [PRL]). In human retina, prominent staining was observed on the basal membrane of the RPE, adjacent to the choroid. Expression of SVCT2 also was detected in several layers of the neural retina. When SVCT2 staining was merged with nuclear staining, we found that SVCT2 was weakly expressed at the inner segments of PRL, with more intense expression at the outer plexiform (OPL) and inner plexiform layers (IPL). In mice, SVCT2 was observed in similar retinal locations as the human retina, but SVCT2 staining was most intense at the base of segments of the PRL, with less staining in the OPL and IPL. Interestingly, SVCT2 expression in mouse RPE was dispersed and significantly weaker as compared with the PRL.
Figure 4.
In human retina (top row), prominent staining was observed on the basal membrane of the RPE. Sodium-dependent vitamin C transporter 2 expression was also detected in several layers of the neural retina and weakly expressed at the inner segments of PRL, with more intense expression at the OPL and IPL. In mice (bottom row), SVCT2 was most intense at the base of segments of PRL, with less staining in the OPL and IPL. Sodium-dependent vitamin C transporter 2 expression in mouse RPE was dispersed and significantly weaker as compared with its PRL. Scale bar: 25 μm.
Distribution of SVCT2 in Human and Mouse Lens Epithelial Cells
Expression of SVCT2 was detected in both human and mouse LECs using immunofluorescence staining. There is no clear difference in these SVCT2 distribution patterns (see Supplementary Material for SVCT2, Supplementary Fig. S1).
Expression and Distribution of GLUT1 in Human and Mouse Ciliary Processes
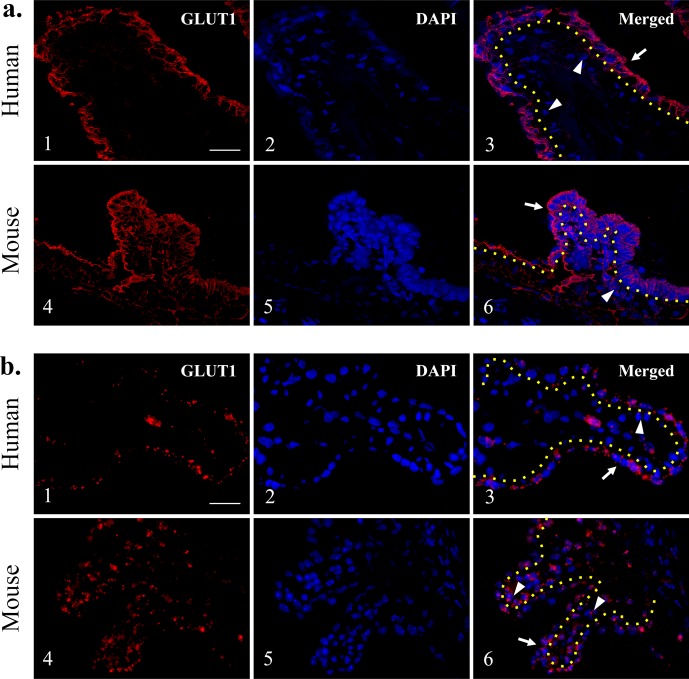
We performed immunofluorescence staining for GLUT1 in human and mouse ciliary processes as well as SLC2A1 (GLUT1) in situ hybridization (Fig. 5). In humans, GLUT1 fluorescence was identified predominantly in the NPE layer (Figs. 5a1–3, red). In addition, its location was at the basolateral membrane of the NPE cells facing the aqueous and vitreous humors (Figs. 5a1–3, arrow). In mice, GLUT1 expression was shown at both the NPE and PE layers, appearing to be greater in the NPE compared with the PE (Figs. 5a4–6). Murine GLUT1 expression in NPE was localized mainly at the basolateral membrane of the cells facing the aqueous and vitreous humors, whereas GLUT1 expression in the PE was mainly localized at the basolateral membrane of PE cells facing the ciliary vasculature (Figs. 5a4–6, arrow). In situ hybridization showed similar results compared with the immunofluorescence staining (Figs. 5b4–6). In human ciliary processes, the SLC2A1 probe predominantly bound to nonpigmented cells and surrounded the nuclei (Figs. 5b1–3, arrow). Minimal staining was observed around the pigmented cells (Figs. 5b1–3, arrowhead); however, in the mouse ciliary process, the probe bound to both PE and NPE cells, demonstrated by several red dots on both PE and NPE cells with comparable distributions (Figs. 5b4–6, arrow and arrowheads).
Figure 5.
Glucose transporter 1 immunofluorescence staining in human and mouse ciliary processes. Glucose transporter 1 expression was found predominantly at the NPE layer of human ciliary processes (a1–3), whereas mouse GLUT1 expression was present in both NPE and PE layers (a4–6). (b) Glucose transporter 1 in situ hybridization in human and mouse ciliary processes. The GLUT1 expression appears to be limited in the NPE as compared with the PE in humans (b1–3). Both NPE and PE layers reveal GLUT1 expression in the mouse ciliary processes (b4–6). Yellow dotted lines: separate PE and NPE; arrows: NPE; arrowheads: PE. Scale bar: 25 μm.
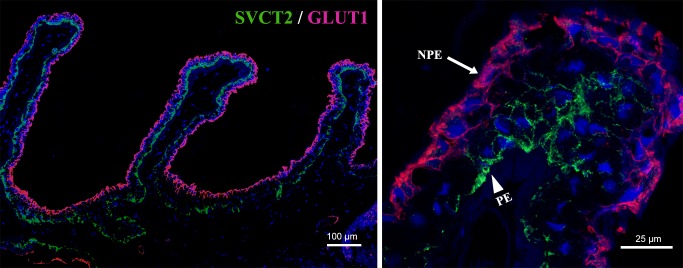
Colocalization of SVCT2 and GLUT1 in Human and Mouse Ciliary Processes
Figure 6 provides more details about the relationship between SVCT2 and GLUT1. In human ciliary processes, SVCT2 expression was limited to PE layers (green), whereas GLUT1 was predominantly located in the nonpigmented layer (red). The enlarged merged image (Fig. 6a1) clearly shows the details about the relationship between SVCT2 and GLUT1. In mice, SVCT2 was not found in the PE and NPE layers, whereas GLUT1 was observed in both PE and NPE layers (Figs. 2f, 5b). The merged image shows there was only GLUT1 expression predominantly located at NPE layer at higher magnification in mouse ciliary process (data not shown).
Figure 6.
Colocalization of immunofluorescence staining for SVCT2 (PE in green indicated by arrowhead) and GLUT1 (NPE in red indicated by arrow) in human ciliary processes. Sodium-dependent vitamin C transporter 2 and GLUT1 showed clear differences in localization in human ciliary processes. Scale bars: 100 μm and 25 μm.
Separation and Identification of Ocular Tissues
After identifying both SVCT2 and GLUT1 expression and distribution in a variety of ocular tissues, laser microdissection was used to collect various ocular tissues from human and mouse eyes for quantification of mRNA for SVCT2 and GLUT1 (Fig. 1). To identify the purity of tissue obtained by this technique, following total RNA extraction and amplification, two tissue-specific primers were initially selected to ensure specificity of the distinct tissues. Tyrosine-related protein 1 (TYRP1), a melanocyte-specific gene product, can be used as a marker for distinguishing PE with NPE. Quantitative PCR was performed and the results showed that the signals of TYRP1 could be detected only in mRNA extracted from human and mouse PE, whereas it could not be detected in NPE (see Supplementary Fig. S2). Retinal pigment epithelium–specific 65-kDa protein (RPE65) is specifically expressed in the RPE; thus, application of its gene primer differentiates RPE from neural retina. Quantitative PCR results showed a positive RPE65 signal in the mRNA extracted from human and mouse RPE, yet RPE65 signal was negative in mRNA extracted from human and mouse neural retina (see Supplementary Fig. S2).
Quantification of SVCT2 mRNA in Human and Mouse Eyes
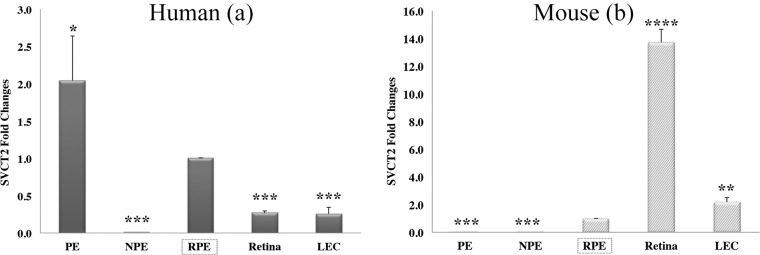
To confirm immunofluorescence and in situ hybridization staining results and further ascertain the expression profiling of AsA-related transporters, we performed qPCR to quantify the mRNA level of SVCT2 and GLUT1. First, we found SVCT2 mRNA expression in human PE but not NPE cells in the ciliary body (Fig. 3). Expression of SVCT2 mRNA was also noted in the retina as well as LECs in human. We could not detect SVCT2 mRNA in mouse ciliary body consistent with its immunofluorescence staining (data not shown). Quantitative PCR results also demonstrated that SVCT2 mRNA could be detected in PE cells in humans (Fig. 7a), whereas it could not be detected both in PE cells and NPE cells in mice (Fig. 7b). These results were consistent with the immunofluorescence and in situ hybridization staining results. Significant amplification of SVCT2 mRNA in mouse lungs confirmed the efficacy of SVCT2 primer (data not shown). In humans, the PE expressed the highest level of SVCT2 mRNA, followed by the RPE, neural retina, and LECs (Fig. 7a). In mice, SVCT2 signals could be detected in the RPE, neural retina, and LECs (Fig. 7d). We also found an interesting phenomenon regarding the differential expression of SVCT2 between human and mouse RPE and neural retina. Quantitative PCR testing depicted that SVCT2 mRNA expression in the human RPE exceeded 3-fold higher levels compared with human neural retina (P < 0.001). However, expression of SVCT2 mRNA in the mouse neural retina was more than 10 times higher than mouse RPE levels (P < 0.0001). Calculation of the ratio of SVCT2 expression in the RPE to its expression in neural retina in humans and mice indicates a significantly higher ratio in humans compared with the mouse (Supplementary Fig. S3), consistent with immunofluorescence staining (Fig. 4).
Figure 7.
Quantitation of SVCT2 mRNA levels by qPCR in different ocular tissues of humans (a) and mice (b). Both human and mouse RPE (red) are used as references to compare with other ocular tissues. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Quantification of GLUT1 mRNA in Human and Mouse Ciliary Processes
To confirm GLUT1 immunofluorescence and in situ hybridization staining results, we also performed qPCR to measure GLUT1 mRNA levels in human and mouse pigmented and nonpigmented cells. In humans, ciliary nonpigmented cells expressed a significantly higher level of GLUT1 than the pigmented layer of ciliary epithelium. Moreover, GLUT1 mRNA in human NPE cells was 30 times higher than the PE cells (Fig. 8). In mice, ciliary nonpigmented cells also exhibit significantly higher GLUT1 expression than the ciliary pigmented cells. However, GLUT1 mRNA in mouse NPE cells was only seven times higher than the PE cells (Fig. 8).
Figure 8.
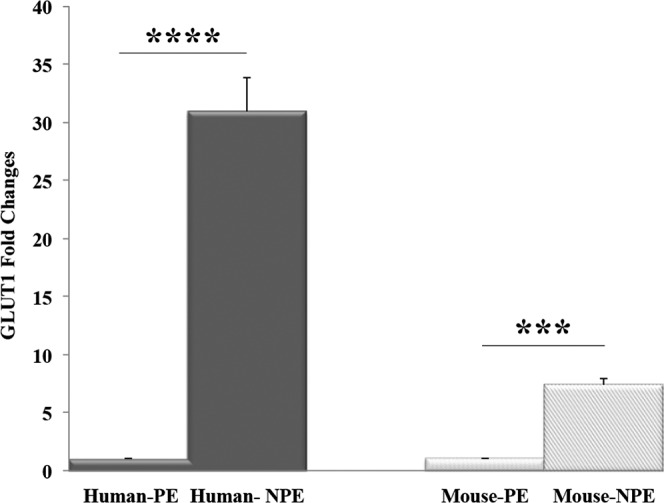
Quantitation of GLUT1 mRNA level in human and mouse ciliary processes. Quantitative PCR showed results in accordance with immunofluorescence staining and in situ hybridization results: GLUT1 was expressed predominantly in the NPE of humans (dark), whereas in the mouse (light) it was expressed at both NPE and PE ciliary processes. ***P < 0.001; ****P < 0.0001.
Detection of Other AsA-Related Transporters in Human and Mouse Eyes
We performed qPCR to assess mRNA levels of another ascorbate transporter, sodium-dependent vitamin C transporter-1 (SVCT1), in human and mouse ocular tissues. In both human and mouse LECs, SVCT1 mRNA could be detected at very low levels. In mouse neural retina, a weak signal was also detected. However, other ocular tissues did not express any measurable SVCT1 (data not shown). Moreover, GLUT3 and GLUT4 expression was also investigated in human and mouse ciliary processes and lens epithelium using RT-PCR. However, we could not detect any GLUT3 or GLUT4 in either the human or mouse ciliary processes as well as in the lens epithelium.
The Table summarizes the findings of AsA-related transporter distribution and relative expression in human and mouse eyes in our current study.
Discussion
Herrmann and Hickman27 first reported the presence of AsA in corneal epithelium in 1948. The metabolism and function of AsA in human and animal eyes have been extensively studied by many researchers in subsequent years.6,7,14,28–30 In 1987, Kern and Zolot31 first found the evidence of an AsA transport system in the lens, then Tsukaguchi et al.3 reported expression of SVCT2 in animal eyes by in situ hybridization, expanding the horizons of AsA research in the eye. Our group discovered extremely high levels of AsA in human vitreous, even higher than its concentration in aqueous humor (Siegfried, et al. IOVS 2015;56:ARVO E-Abstract 4414).25 The high levels of AsA in human vitreous may play an important role in the protection of ocular tissues, as others have reported. It protects tissue from oxidative damage from excess molecular oxygen and aids to maintain the hypoxic intraocular environment.25,26,32
In this study, we chose the human and mouse as research subjects. The human is a typical diurnal species requiring high levels of AsA to protect ocular tissues from oxidative damage caused by UV irradiation and other harmful factors.2,4,6,7,11,14,21,29 However, AsA is not synthesized endogenously by humans and must be obtained exogenously, mandating an efficient transport system to maintain high levels of AsA in human eyes. Unlike humans, the mouse is a typical nocturnal animal and able to self-synthesize AsA.33 Sufficient endogenous AsA supply with a lower requisite concentration predicates an alternate transport system in mouse eyes compared with human eyes. Therefore, these contrasts between human and mouse eyes provide optimal species for our research of AsA transporters.
The innermost portion of the structure of the ciliary body is occupied by the ciliary processes, which produce aqueous humor. The ciliary process is the most important tissue involved in the transport of AsA.8,14,23,34 Therefore, we first focused on the ciliary processes in the human and mouse eyes to study the transport of AsA. In previously published studies related to AsA transporters in ciliary processes, many studies used the intact tissues or ciliary epithelial isolates and most were performed as in vitro experiments or on animals due to limitation of technique.23,34–37 The ciliary process consists of two monolayers of epithelial cells: the PE cells representing the anterior continuation of the retinal pigmented epithelium, and the nonpigmented ciliary epithelial cells facing the posterior chamber and AH and is the continuation of the neural retina. The ciliary processes have increased lateral interdigitations and increased gap junctions and cellular organelles.38 There is little information about how AsA/DHA transport through these two layers and no information about AsA-related transporters in normal human eyes.
In this study, we found the AsA-related transporters in human and murine isolates of pigmented and nonpigmented cells are quite different. We revealed that SVCT2, one of the most important AsA active transporters, is expressed in the human ciliary epithelium with the unique distribution within the PE only. Furthermore, SVCT2 located in human pigmented ciliary epithelial cells was mainly distributed around the cellular basal membranes facing the ciliary vessels, whereas human nonpigmented cells did not express SVCT2. Although a few studies reported SVCT2 expression in the ciliary epithelium of rabbit eyes,3,39,40 as well as indirect evidence in an in vitro study of bovine ciliary epithelium,39 this is the first time, to our knowledge, clearly indicating the differences of SVCT2 distribution in human ciliary epithelium as compared with GLUT1 (Figs. 2, 3, 5, 6). Lack of SVCT2 expression in both the PE and NPE of the mouse ciliary process is interesting and consistent with the prior studies on rats.3 In contrast to these nocturnal species, SVCT2 was abundantly expressed in the human PE adjacent to the ciliary process vasculature, indicating that humans rely on SVCT2 to actively transport AsA from plasma into the aqueous humor. This SVCT2 distribution pattern corresponds with known sites of high levels of AsA in human eyes. The lack of SVCT2 expression in the mouse ciliary body indicates that the mouse does not rely on SVCT2 transporters and lower levels of AsA may provide sufficient antioxidant protection in this nocturnal species as previously reported.21
The present study also identified expression of GLUT1, the major transporter of DHA and glucose molecule10,11,15,41 in both human and murine ciliary processes. Interestingly, GLUT1 was abundantly expressed in human NPE, and absent from PE. It is distributed adjacent to the aqueous humor and vitreous, implicating its important function for these fluids. In humans, DHA is mainly produced in the aqueous or vitreous humor and it is well known to be associated with antioxidant defense in the eye. Glucose transporter 1 distribution at NPE may result in rapid uptake of DHA, followed by DHA recycling back to AsA.11 Because the glucose level in the blood is higher than aqueous humor, the purpose for GLUT1 in NPE is presumably not for glucose transport from aqueous humor to ciliary body. Figure 6 clearly shows the different distributions of SVCT2 and GLUT1, reflecting the unique function of each transporter in the human eye.
In mouse eyes, alternative techniques have identified GLUT1 in both PE and NPE, and we identified a relatively high expression in NPE compared with PE (Figs. 5, 8), in contrast to other reports.10 Because we have confirmed this finding by three methods (immunofluorescence staining from the protein level, in situ hybridization from mRNA level, and qPCR), we believe this finding is reliable. The core function of GLUT1 may be to remove DHA from aqueous humor, although other functions are still unclear. Several studies have previously confirmed GLUT3 in the retina,42–45 and in this study, we examined GLUT3 and GLUT4 only in the ciliary process (PE and NPE) and lens epithelium (Table). We could not detect either GLUT3 or GLUT4 in both PE and NPE of the ciliary processes of both human and mouse eyes. We detected GLUT1, but not GLUT3 and GLUT4, in both human and mouse lens epithelium. This result is consistent with a previous report by Merriman-Smith et al.46 detecting GLUT3 expression in the lens fiber layers but not in the lens epithelium.
In the present study, we applied laser microdissection techniques of ocular tissues for the first time, precisely isolating single cell layers of PE and NPE (Fig. 1). This aids in further understanding the differences between these cells at a molecular level as well as their unique functions. Importantly, we can confirm that this laser microdissection successfully separates PE and NPE accurately (Supplementary Fig. S2).
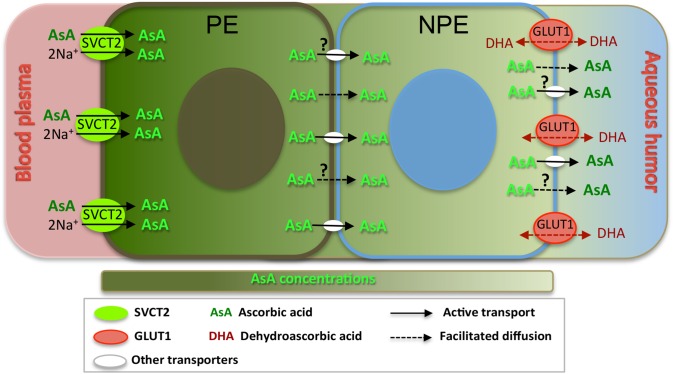
Based on these findings, together with previous studies on Na+ dependent AsA transport39 and the presence of tight junctions in the ciliary epithelium located only between the NPE cells,47 we hypothesize the following model of AsA-DHA transport and recycling pathways in human ciliary epithelium and ocular fluids (Fig. 9). Sodium-dependent vitamin C transporter 2, located in PE cells plays a major role to transport AsA in blood plasma to accumulate high concentration in the PE. According to a previous in vitro study on bovine eyes using 14C ascorbate detected by thin-layer chromatography, AsA in PE side could be approximately 40 times higher concentration as compared with the culture medium.39 Because AsA molecule size is relatively small, such high AsA concentration in PE may be a result of facilitated diffusion or possibly other yet unidentified active transporters through bilayer membranes to NPE, finally delivered to the aqueous humor creates a high AsA gradient. This may explain why AsA concentrations are more than 20 times higher in aqueous and vitreous humors compared with plasma. However, it remains unclear how these high levels of AsA in PE cross into NPE and ultimately into the anterior chamber. Further studies are required to answer these important questions. Ascorbic acid transporters in mice (nocturnal animals) are quite different compared with humans (diurnal species). The lack of SVCT2 in the ciliary process and abundance within the retina indicates that the retina is the more likely source of AsA in the mouse eye. Glucose transporter 1 may play an important role for recycling DHA to AsA in the anterior eye segment in mice; consequently, leading to a much lower level of AsA in aqueous humors in mice/rats as compared with diurnal species, but still rendering sufficient antioxidant protection in the eyes of nocturnal species.8,20,29,48,49
Figure 9.
Possible mechanism for AsA transport in human ciliary epithelium. Sodium-dependent vitamin C transporter 2, located at PE adjacent to ciliary stroma microvasculature, transports AsA from low concentration in blood plasma to accumulate high concentration in the PE. Then through facilitated diffusion or other unidentified active transporters, AsA may cross through bilayer membranes to NPE, and finally delivered to aqueous humor. Meanwhile, the DHA in the aqueous humor could be transported by GLUT1, which is located adjacent to aqueous humor side, quickly recycling in the ciliary epithelium.
Meanwhile, the AsA in the aqueous humor and vitreous humor converts to its oxidized state (DHA) as it plays an important role as an antioxidant in the ocular tissues. In aqueous humor, the transport of accumulated DHA may be facilitated by GLUT1 into the ciliary epithelial cells and immediately transformed to AsA again before diffusing back into the aqueous humor and vitreous humor.
As noted in Figure 4, SVCT2 immunofluorescence staining results showed the strongest signals in human RPE, compared with the strongest signal in the photoreceptor layer near the outer nuclear layer in the mouse. The ratio of SVCT2 mRNA level in human RPE compared with neural retina was 10:3, whereas its ratio in the mouse was 1:14. Therefore, this suggests that in humans, AsA transport from choroidal vasculature by SVCT2 in RPE may be an important source of AsA for the neural retina. On the other hand, human retinal tissues are rich with vasculature as well as SVCT2, but relatively less than murine retina. For the mouse, transport of AsA by retinal SVCT2 could be more important than RPE. Our data provide a basis for a proposal of a new hypothesis that SVCT2 expression in human RPE may be an important source of AsA for the retina. Therefore, human RPE damage may interrupt active transport of AsA potentially altering the antioxidant-oxidant balance and ocular oxygen environment in the entire eye.
Previous studies of SVCT2 expression identified this transporter in human and mouse retina and RPE.50,51 We found that its distribution patterns are somewhat different (Fig. 4). In human RPE, SVCT2 expression was oriented to the choroid vasculature, indicating the main source of AsA from the plasma with delivery to the retina. Other layers of retina also express SVCT2, and may explain why human vitreous contains higher levels of AsA, even higher than aqueous humor.25 Thus, both the ciliary process and the retina may serve as a source for AsA in the vitreous gel. Sodium-dependent vitamin C transporter 2 expression was dispersed and weak at the level of the RPE, and increased in the photoreceptor layer (Fig. 4), requiring further experimentation to understand this finding.
Lens epithelial cells also play a crucial role in antioxidant protection of the lens and transport of nutrients from the aqueous humor. We detected SVCT2 expression in both human and mouse LECs, confirming the results reported by other researchers.31,52 Additionally, SVCT1 and SVCT2 were expressed by qPCR in both human and mouse LECs, although SVCT1 expression was minimal (data not shown). To the best of our knowledge, no previous research has reported the expression of SVCT1 in the eye other than its presence in exocrine cells of the lacrimal gland as reported by Tsukaguchi et al.3 We further observe the presence of SVCT1 in lens epithelium in the current study; however, the exact functional significance of SVCT1 in ocular tissues awaits further investigation.
In summary, we provide the initial reports of two AsA-related transporters, SVCT2 and GLUT1, in human and mouse eyes at the level of mRNA and in situ protein expression, confirmed with qPCR of precisely identified tissue specimens via our laser microdissection technique. Understanding basic characteristics of AsA transporters as well as DHA is important, aiding to clarify mechanisms of antioxidant protection of the ocular tissues and maintenance of the healthy intraocular environment. Our discoveries of differences in the distributions of the active AsA transporters between diurnal and nocturnal species provide important information about this antioxidant protection. Moreover, differential SVCT2 distribution in human RPE and neural retina provide a potential explanation for the source of the high AsA levels and low oxygen tension in human vitreous. This result highlights the importance of the normal function of human RPE in maintaining AsA levels and the overall oxidant-antioxidant status of ocular tissues.
Supplementary Material
Acknowledgments
The authors thank Mid-America Transplant Services for the donation of eye tissues in this study.
Supported by National Natural Science Foundation of China Grant 81370997; Study Abroad Fund of Tangdu Hospital; National Eye Institute NEI-EY021515 (CS), NEI-EY 50080 (DCB), NEI-EY 77487 (DCB), and Core Grant NEI-EY02687 (WU); and an unrestricted grant from Research to Prevent Blindness, Inc.
Disclosure: N. Ma, None; C. Siegfried, None; M. Kubota, None; J. Huang, None; Y. Liu, None; M. Liu, None; B. Dana, None; A. Huang, None; D. Beebe, None; H. Yan, None; Y.-B. Shui, None
References
- 1. Rose RC,, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993; 7: 1135–1142. [PubMed] [Google Scholar]
- 2. Englard S,, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986; 6: 365–406. [DOI] [PubMed] [Google Scholar]
- 3. Tsukaguchi H,, Tokui T,, Mackenzie B,, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999; 399: 70–75. [DOI] [PubMed] [Google Scholar]
- 4. Brubaker RF,, Bourne WM,, Bachman LA,, McLaren JW. Ascorbic acid content of human corneal epithelium. Invest Ophthalmol Vis Sci. 2000; 41: 1681–1683. [PubMed] [Google Scholar]
- 5. Talluri RS,, Katragadda S,, Pal D,, Mitra AK. Mechanism of L-ascorbic acid uptake by rabbit corneal epithelial cells: evidence for the involvement of sodium-dependent vitamin C transporter 2. Curr Eye Res. 2006; 31: 481–489. [DOI] [PubMed] [Google Scholar]
- 6. Anderson EI,, Spector A. Oxidation-reduction reactions involving ascorbic acid and the hexosemonophosphate shunt in corneal epithelium. Invest Ophthalmol. 1971; 10: 41–53. [PubMed] [Google Scholar]
- 7. Varma SD,, Kumar S,, Richards RD. Light-induced damage to ocular lens cation pump: prevention by vitamin C. Proc Natl Acad Sci U S A. 1979; 76: 3504–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delamere NA. Ascorbic acid and the eye. Subcell Biochem. 1996; 25: 313–329. [DOI] [PubMed] [Google Scholar]
- 9. Wells WW,, Xu DP. Dehydroascorbate reduction. J Bioenerg Biomembr. 1994; 26: 369–377. [DOI] [PubMed] [Google Scholar]
- 10. Takata K,, Kasahara T,, Kasahara M,, Ezaki O,, Hirano H. Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci. 1991; 32: 1659–1666. [PubMed] [Google Scholar]
- 11. Umapathy A,, Donaldson P,, Lim J. Antioxidant delivery pathways in the anterior eye. Biomed Res Int. 2013; 2013: 207250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Savini I,, Rossi A,, Pierro C,, Avigliano L,, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008; 34: 347–355. [DOI] [PubMed] [Google Scholar]
- 13. Subramanian VS,, Marchant JS,, Boulware MJ,, Said HMA. C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J Biol Chem. 2004; 279: 27719–27728. [DOI] [PubMed] [Google Scholar]
- 14. Rose RC,, Bode AM. Ocular ascorbate transport and metabolism. Comp Biochem Physiol A Comp Physiol. 1991; 100: 273–285. [DOI] [PubMed] [Google Scholar]
- 15. Rumsey SC,, Kwon O,, Xu GW,, Burant CF,, Simpson I,, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem. 1997; 272: 18982–18989. [DOI] [PubMed] [Google Scholar]
- 16. Rumsey SC,, Daruwala R,, Al-Hasani H,, Zarnowski MJ,, Simpson IA,, Levine M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J Biol Chem. 2000; 275: 28246–28253. [DOI] [PubMed] [Google Scholar]
- 17. Montel-Hagen A,, Kinet S,, Manel N,, et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008; 132: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 18. Lien EL,, Hammond BR. Nutritional influences on visual development and function. Prog Retin Eye Res. 2011; 30: 188–203. [DOI] [PubMed] [Google Scholar]
- 19. Wenzel A,, Grimm C,, Samardzija M,, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005; 24: 275–306. [DOI] [PubMed] [Google Scholar]
- 20. DiMattio J. A comparative study of ascorbic acid entry into aqueous and vitreous humors of the rat and guinea pig. Invest Ophthalmol Vis Sci. 1989; 30: 2320–2331. [PubMed] [Google Scholar]
- 21. Reiss GR,, Werness PG,, Zollman PE,, Brubaker RF. Ascorbic acid levels in the aqueous humor of nocturnal and diurnal mammals. Arch Ophthalmol. 1986; 104: 753–755. [DOI] [PubMed] [Google Scholar]
- 22. Civan CWDM. Basis of chloride transport inciliary epithelium. J Membr Biol. 2004; 200: 1–13. [DOI] [PubMed] [Google Scholar]
- 23. Chu TC,, Candia OA. Active transport of ascorbate across the isolated rabbit ciliary epithelium. Invest Ophthalmol Vis Sci. 1988; 29: 594–599. [PubMed] [Google Scholar]
- 24. Socci RR,, Delamere NA. Characteristics of ascorbate transport in the rabbit iris-ciliary body. Exp Eye Res. 1988; 46: 853–861. [DOI] [PubMed] [Google Scholar]
- 25. Shui YB,, Holekamp NM,, Kramer BC,, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009; 127: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beebe DC,, Shui YB,, Siegfried CJ,, Holekamp NM,, Bai F. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Jpn J Ophthalmol. 2014; 58: 225–231. [DOI] [PubMed] [Google Scholar]
- 27. Herrmann H,, Hickman FH. Exploratory studies on corneal metabolism. Bull Johns Hopkins Hosp. 1948; 82: 225–250. [PubMed] [Google Scholar]
- 28. Woodford BJ,, Tso MO,, Lam KW. Reduced and oxidized ascorbates in guinea pig retina under normal and light-exposed conditions. Invest Ophthalmol Vis Sci. 1983; 24: 862–867. [PubMed] [Google Scholar]
- 29. Chandra DB,, Varma R,, Ahmad S,, Varma SD. Vitamin C in the human aqueous humor and cataracts. Int J Vitam Nutr Res. 1986; 56: 165–168. [PubMed] [Google Scholar]
- 30. Khatami M,, Roel LE,, Li W,, Rockey JH. Ascorbate regeneration in bovine ocular tissues by NADH-dependent semidehydroascorbate reductase. Exp Eye Res. 1986; 43: 167–175. [DOI] [PubMed] [Google Scholar]
- 31. Kern HL,, Zolot SL. Transport of vitamin C in the lens. Curr Eye Res. 1987; 6: 885–896. [DOI] [PubMed] [Google Scholar]
- 32. Beebe DC,, Holekamp NM,, Siegfried C,, Shui YB. Vitreoretinal influences on lens function and cataract. Philos Trans R Soc Lond B Biol Sci. 2011; 366: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Linster CL,, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007; 274: 1–22. [DOI] [PubMed] [Google Scholar]
- 34. Candia OA,, Shi XP,, Chu TC. Ascorbate-stimulated active Na+ transport in rabbit ciliary epithelium. Curr Eye Res. 1991; 10: 197–203. [DOI] [PubMed] [Google Scholar]
- 35. Mead A,, Sears J,, Sears M. Transepithelial transport of ascorbic acid by the isolated intact ciliary epithelial bilayer of the rabbit eye. J Ocul Pharmacol Ther. 1996; 12: 253–258. [DOI] [PubMed] [Google Scholar]
- 36. Delamere NA,, Coca-Prados M,, Aggarwal S. Studies on regulation of the ascorbic acid transporter in a cell line derived from rabbit non-pigmented ciliary epithelium. Biochim Biophys Acta. 1993; 1149: 102–108. [DOI] [PubMed] [Google Scholar]
- 37. Candia OA,, Alvarez LJ. Fluid transport phenomena in ocular epithelia. Prog Retin Eye Res. 2008; 27: 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hara K,, Lutjen-Drecoll E,, Prestele H,, Rohen JW. Structural differences between regions of the ciliary body in primates. Invest Ophthalmol Vis Sci. 1977; 16: 912–924. [PubMed] [Google Scholar]
- 39. Helbig H,, Korbmacher C,, Wohlfarth J,, Berweck S,, Kuhner D,, Wiederholt M. Electrogenic Na+-ascorbate cotransport in cultured bovine pigmented ciliary epithelial cells. Am J Physiol. 1989; 256: C44–C49. [DOI] [PubMed] [Google Scholar]
- 40. Kodama T,, Kabasawa I,, Tamura O,, Reddy VN. Dynamics of ascorbate in the aqueous humor and tissues surrounding ocular chambers. Ophthalmic Res. 1985; 17: 331–337. [DOI] [PubMed] [Google Scholar]
- 41. Wood IS,, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003; 89: 3–9. [DOI] [PubMed] [Google Scholar]
- 42. Badr GA,, Zhang JZ,, Tang J,, Kern TS,, Ismail-Beigi F. Glut1 and glut3 expression but not capillary density, is increased by cobalt chloride in rat cerebrum and retina. Brain Res Mol Brain Res. 1999; 64: 24–33. [DOI] [PubMed] [Google Scholar]
- 43. Watanabe T,, Nagamatsu S,, Matsushima S,, Kirino T,, Uchimura H. Colocalization of GLUT3 and choline acetyltransferase immunoreactivity in the rat retina. Biochem Biophys Res Commun. 1999; 256: 505–511. [DOI] [PubMed] [Google Scholar]
- 44. Badr GA,, Tang J,, Ismail-Beigi F,, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000; 49: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 45. Ban Y,, Rizzolo LJ. Regulation of glucose transporters during development of the retinal pigment epithelium. Brain Res Dev Brain Res. 2000; 121: 89–95. [DOI] [PubMed] [Google Scholar]
- 46. Merriman-Smith R,, Donaldson P,, Kistler J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest Ophthalmol Vis Sci. 1999; 40: 3224–3230. [PubMed] [Google Scholar]
- 47. Raviola G,, Raviola E. Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci. 1978; 17: 958–981. [PubMed] [Google Scholar]
- 48. Reddy VN,, Giblin FJ,, Lin LR,, Chakrapani B. The effect of aqueous humor ascorbate on ultraviolet-B-induced DNA damage in lens epithelium. Invest Ophthalmol Vis Sci. 1998; 39: 344–350. [PubMed] [Google Scholar]
- 49. Ringvold A,, Anderssen E,, Kjonniksen I. Ascorbate in the corneal epithelium of diurnal and nocturnal species. Invest Ophthalmol Vis Sci. 1998; 39: 2774–2777. [PubMed] [Google Scholar]
- 50. Wang Y,, Mackenzie B,, Tsukaguchi H,, Weremowicz S,, Morton CC,, Hediger MA. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem Biophys Res Commun. 2000; 267: 488–494. [DOI] [PubMed] [Google Scholar]
- 51. Khurana V,, Vadlapudi AD,, Vadlapatla RK,, Pal D,, Mitra AK. Functional characterization and molecular identification of vitamin C transporter (SVCT2) in human corneal epithelial (HCEC) and retinal pigment epithelial (D407) cells. Curr Eye Res. 2015; 40: 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kannan R,, Stolz A,, Ji Q,, Prasad PD,, Ganapathy V. Vitamin C transport in human lens epithelial cells: evidence for the presence of SVCT2. Exp Eye Res. 2001; 73: 159–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.