Abstract
Patient: Male, 61
Final Diagnosis: AITL in Klinefelter syndrome
Symptoms: —
Medication: —
Clinical Procedure: Chemotherapy
Specialty: Hematology
Objective:
Rare disease
Background:
Although patients with Klinefelter syndrome have elevated risk and incidence rates for several solid cancers, reports on the incidence of hematological malignancies have been equivocal.
Case Report:
We report a patient diagnosed with angioimmunoblastic T-cell lymphoma in whom Klinefelter syndrome was newly detected. Moreover, we discuss the development of a variety of lymphomas in patients with Klinefelter syndrome.
Conclusions:
This is the first case describing angioimmunoblastic T-cell lymphoma in a patient with Klinefelter syndrome who was treated with chemotherapy.
MeSH Keywords: Hematologic Neoplasms; Klinefelter Syndrome; Lymphoma, T-Cell
Background
Klinefelter syndrome is the most common sex-linked chromosomal abnormality causing primary hypogonadism, occurring in approximately 1 in 1000 live male births; more than 3000 affected males are born yearly [1]. The most common karyotype is 47,XXY, but other variants (including mosaicism of more than 2 X chromosomes) are also observed [2]. The major clinical manifestations of Klinefelter syndrome include tall stature, gynecomastia, small testes, a small penis, and infertility. In addition to these clinical characteristics and rare manifestations [3,4], Klinefelter syndrome patients have an increased risk of several malignancies, especially male breast cancer [5] and extragonadal germ cell tumors, primarily localized in the mediastinum [6]. However, hematological malignancies, such as leukemia and lymphoma, are not frequent in Klinefelter syndrome patients [7–10]. Moreover, data on the incidence of hematological malignancies is limited. In this case report, we describe a patient diagnosed with angioimmunoblastic T-cell lymphoma in whom Klinefelter syndrome was newly detected through physical examination and cytogenetic analyses. To demonstrate the relationship between Klinefelter syndrome and lymphoma, we review previous cases in which lymphoma developed in patients with Klinefelter syndrome.
Case Report
A 61-year-old man initially presented with a palpable neck mass and weight loss in February 2013. He had no relevant family history and had never been married. A physical examination revealed multiple enlarged lymph nodes in the neck, and in axillar and inguinal areas.
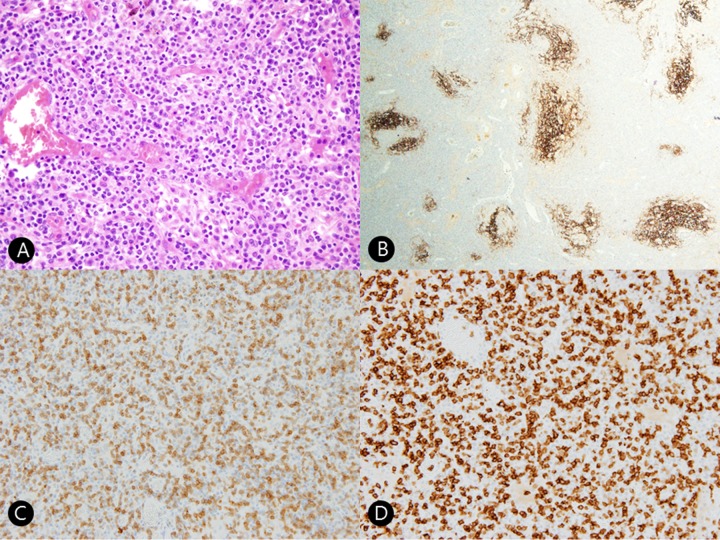
The patient was suspected to have a lymphoproliferative disease because multiple homogeneous lymph node enlargements were observed on computed tomography (CT) (Figure 1A, 1B) and positron emission tomography (PET)-CT scans (Figure 1C). He underwent the excisional biopsy of a right submandibular lymph node showing medium-to-large-sized atypical lymphoid cells infiltration with proliferative high endothelial venules. Immunohistochemical staining showed tumor cells positive for CD3, CD4, PD-1, and negative for CD20. CD21 and clusterin stains showed arborizing follicular dendritic cells. Therefore, these histologic findings and immunohistochemical staining result are compatible with angioimmunoblastic T-cell lymphoma (Figure 2).
Figure 1.
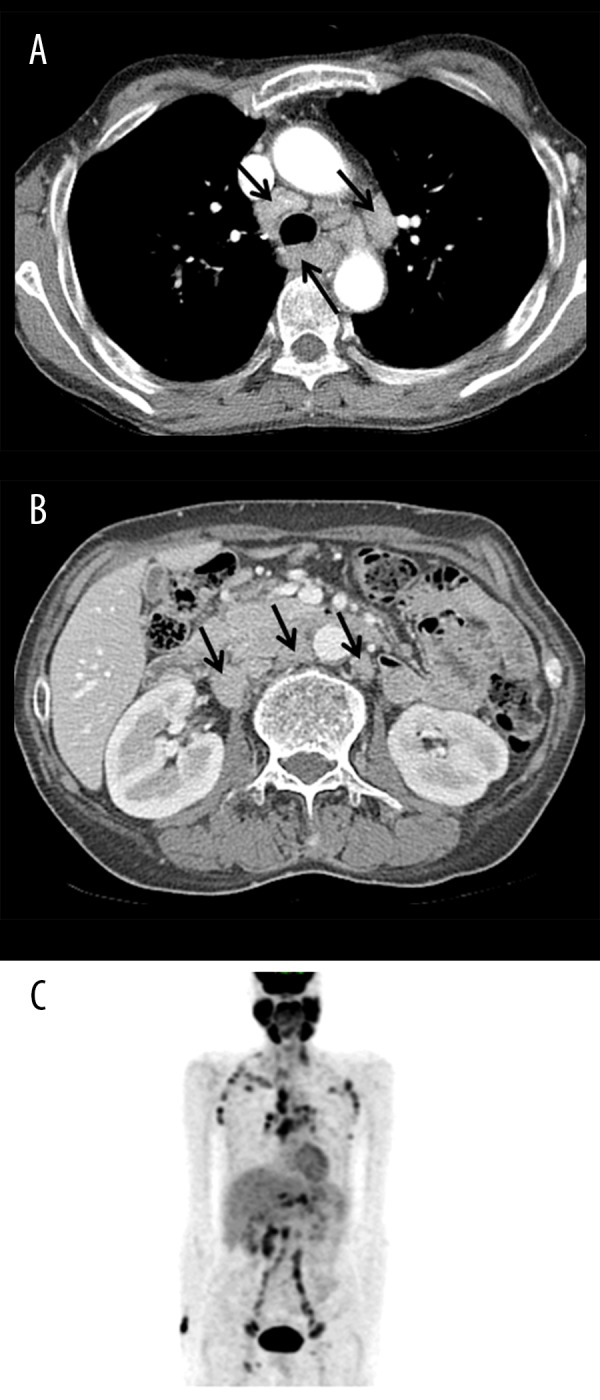
(A, B) An initial contrast-enhanced computed tomography (CT) scan revealed multiple homogeneous lymph node enlargements in the retrotracheal, paratracheal, and aortopulmonary window, and the retrocaval, aortocaval, and paraaortic lymph nodes (arrow) without solid-organ involvement, which are suggestive of lymphoma. (C) Initial 18F-fluorodeoxyglucose positron emission tomography/CT showed multiple hypermetabolic lymph nodes in the bilateral cervical, axillary, mediastinal, hilar, interlobar, retroperitoneal, iliac, and inguinal areas, which were suggestive of lymphoma. Additionally, the oral cavity and major salivary glands had diffusely increased metabolism.
Figure 2.
(A) Histopathologic findings revealed intermediate-sized neoplastic T-cells with clear cytoplasm and proliferation of high endothelial venules (hematoxylin and eosin ×100). (B) Immunohistochemical staining for CD21 revealed arborizing follicular dendritic cells in a low-power view (CD21 immunostain ×40). (C) Tumor cells are positive for CD3 (×200). (D) Tumor cells are positive for CD4 (×200).
Because the patient was tall and thin (height: 180.6 cm, body weight: 58.3 kg, and body mass index: 17.87 kg/m2) and had a small penis and testes on physical examination, we suspected that he had gonadal dysfunction, similar to that associated with Klinefelter syndrome. As expected, the patient was diagnosed with Klinefelter syndrome (47,XXY/46,XX mosaicism) based on chromosomal analysis of his bone marrow (Figure 3). We regarded the body weight loss (14 kg loss during 3 months, 72 kg –> 58 kg) as B symptom. With respect to IPI (Internal Prognostic Index) score, the patient was in the ‘high risk’ category of (IPI score 4 point; age >60 years, serum lactate dehydrogenase concentration above normal, Ann Arbor stage III or IV, number of extranodal disease sites >1).
Figure 3.
Cytogenetic analysis of a bone marrow specimen showed 46,XX(A)/47,XXY(B) mosaicism (arrow indicates the additional X chromosome), which indicates a diagnosis of Klinefelter syndrome.
After diagnosis, he received 6 cycles of CHOEP (cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone); he showed an effective response to chemotherapy and achieved a complete response (Figure 4). The complete remission state has been maintained for 2 years and he has been receiving regular check-ups every 3 months at an outpatient clinic.
Figure 4.

(A, B) After 6 cycles of CHOEP chemotherapy, the multiple enlarged lymph nodes disappeared or decreased on a post-chemotherapy contrast-enhanced computed tomography (CT) scan. (C) Multiple lymphoma lesions disappeared or decreased on post-chemotherapy 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT. However, focal mildly hypermetabolic lymph nodes remained in the bilateral mediastinal, hilar, and interlobar areas and were assessed as reactive nodes rather than residual lymphoma lesions. The 18F-FDG uptakes of the oral cavity and major salivary glands were normal when compared with the initial 18F-FDG PET/CT.
Discussion
There is a strong suspicion that patients with constitutional chromosome abnormalities may be more susceptible to malignancies [11]. Klinefelter syndrome patients also have an elevated risk of several cancers, such as male breast cancer and extragonadal germ cell tumors, that are primarily localized in the mediastinum [5,6]. Altered endogenous hormones, such as elevated estrogen-to-testosterone ratio and elevated gonadotropin level, have been the primary focus for explaining the observed increase in male breast cancer and extragonadal germ cell tumors among Klinefelter syndrome patients [11,12], but hematological malignancies are not frequent in Klinefelter syndrome. The current case study joins a handful of previously documented cases of lymphoproliferative disease in patients with Klinefelter syndrome (Table 1), indicating that Klinefelter syndrome may, in fact, be associated with an increased incidence of and mortality from lymphoproliferative disease [11]. Swerdlow et al. suggested that this relationship is due to the over-activation of an oncogene on the X chromosome that evokes escape of X inactivation, and that the risk of lymphoma was especially high in males who have multiple X chromosomes [13]. There are no available data on whether immunologic problems develop more frequently in patients with Klinefelter syndrome than in those with normal karyotype; however, several reports suggested that the prevalence of autoimmune diseases, including systemic lupus erythematosus, may be elevated in males with Klinefelter syndrome [14–16].
Table 1.
Case reports of lymphoma associated with Klinefelter syndrome.
| Year | Age | Histology | Karyotype | Chemotherapy | Reference no. |
|---|---|---|---|---|---|
| 1965 | 53 y | Reticulum cell sarcoma | 48,XXXY/47,XXY/46,XY | [18] | |
| 1974 | 46 y | Low-grade lymphoma | 47,XXY/46,XY | [19] | |
| 1986 | 47 y | High-grade B-cell lymphoma | 47,XXY/46,XY | [20] | |
| 1987 | 10 y | Malignant histiocytosis | 47,XXY | [21] | |
| 1990 | 33 y | Immunoblastic B-cell lymphoma | 47,XXY | [22] | |
| 1994 | 40 mo | B-lymphoblastic lymphoma | 47,XXY | [23] | |
| 1997 | 81 y | Low-grade B-cell lymphoma | 47,XXY/46,XY | None | [14] |
| 2002 | 29 y | Anaplastic large-cell lymphoma | 47,XXY | Cyclophosphamide, Adriamycin, Oncovin, prednisone | [24] |
| 2002 | 31 y | Anaplastic Ki-1 lymphoma | 47,XXY | High-dose cyclophosphamide (followed by matched allogeneic bone marrow transplantation) | [24] |
| Current | 64 y | Angioimmunoblastic T-cell lymphoma | 47,XXY/46,XX | Cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone |
Klinefelter syndrome is a rare disorder. In addition, Klinefelter syndrome is severely underdiagnosed or diagnosed late in life. Roughly 25% of the expected number of cases are diagnosed, and the mean age at diagnosis is in the mid-30s. The reason for the underdiagnoses and late diagnoses is unknown, but the relatively mild phenotype is a tempting explanation [17]. Our patient also had mild phenotype (47,XXY/46,XX) and late diagnosis compared to the mean age. Because of its rarity and low diagnosis rate, there are few published studies or case reports about its association with lymphoma. Including our patient, we identified a total of 10 reported cases with malignant lymphoma in association with Klinefelter syndrome. Although the consensus is that associations between Klinefelter syndrome and hematological conditions are purely coincidental, the uncertainty can only be resolved through the collection of more data.
Conclusions
There is strong evidence that patients with constitutional chromosome abnormalities may be more susceptible to malignancy. Klinefelter syndrome patients have elevated risk of several cancers, but hematological malignancy has not been documented in Klinefelter syndrome patients until now. The development of angioimmunoblastic T-cell lymphoma in patients with Klinefelter syndrome is rare. Although evidence suggests that associations between Klinefelter syndrome and hematological conditions are purely coincidental, the uncertainty can only be resolved by more data.
References:
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. J Clin Endocrinol Metab. 2003;88(2):622–26. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 2.Paulsen CA, Gordon DL, Carpenter RW, et al. Klinefelter’s syndrome and its variants: A hormonal and chromosomal study. Recent Prog Horm Res. 1968;24:321–63. doi: 10.1016/b978-1-4831-9827-9.50013-7. [DOI] [PubMed] [Google Scholar]
- 3.Karagoz A, Dikbas O, Teker E, et al. Sinus node dysfunction requiring permanent pacemaker implantation in a young adult with Klinefelter syndrome. Am J Case Rep. 2015;16:136–39. doi: 10.12659/AJCR.893065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozata M, Yesilova Z, Saglam M, Tunca Y. A case of Klinefelter’s syndrome associated with unilateral renal aplasia. Med Sci Monit. 2000;6(5):1000–2. [PubMed] [Google Scholar]
- 5.Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter’s syndrome: Is there an association? Cancer J Clin. 1987;37(4):246–51. doi: 10.3322/canjclin.37.4.246. [DOI] [PubMed] [Google Scholar]
- 6.Nichols CR. Mediastinal germ cell tumors. Semin Thorac Cardiovasc Surg. 1992;4(1):45–50. [PubMed] [Google Scholar]
- 7.Mamunes P, Lapidus PH, Abbott JA, Roath S. Acute leukaemia and Klinefelter’s syndrome. Lancet (London, England) 1961;2(7192):26–27. doi: 10.1016/s0140-6736(61)92712-x. [DOI] [PubMed] [Google Scholar]
- 8.Alimena G, Billstrom R, Casalone R, et al. Cytogenetic pattern in leukemic cells of patients with constitutional chromosome anomalies. Cancer Genet Cytogenet. 1985;16(3):207–18. doi: 10.1016/0165-4608(85)90047-0. [DOI] [PubMed] [Google Scholar]
- 9.Horsman DE, Pantzar JT, Dill FJ, Kalousek DK. Klinefelter’s syndrome and acute leukemia. Cancer Genet Cytogenet. 1987;26(2):375–76. doi: 10.1016/0165-4608(87)90073-2. [DOI] [PubMed] [Google Scholar]
- 10.Price WH, Clayton JF, Wilson J, et al. Causes of death in X chromatin positive males (Klinefelter’s syndrome) J Epidemiol Community Health. 1985;39(4):330–36. doi: 10.1136/jech.39.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasle H, Mellemgaard A, Nielsen J, Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71(2):416–20. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100(6):814–18. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow AJ, Schoemaker MJ, Higgins CD, et al. Cancer incidence and mortality in men with Klinefelter syndrome: A cohort study. J Natl Cancer Inst. 2005;97(16):1204–10. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys M, Lavery P, Morris C, Nevin N. Klinefelter syndrome and non-Hodgkin lymphoma. Cancer Genet Cytogenet. 1997;97(2):111–13. doi: 10.1016/s0165-4608(96)00386-x. [DOI] [PubMed] [Google Scholar]
- 15.Dillon S, Aggarwal R, Harding JW, et al. Klinefelter’s syndrome (47,XXY) among men with systemic lupus erythematosus. Acta Paediatr. 2011;100(6):819–23. doi: 10.1111/j.1651-2227.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawalha AH, Harley JB, Scofield RH. Autoimmunity and Klinefelter’s syndrome: When men have two X chromosomes. J Autoimmun. 2009;33(1):31–34. doi: 10.1016/j.jaut.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groth KA, Skakkebaek A, Host C, et al. Clinical review: Klinefelter syndrome – a clinical update. J Clin Endocrinol Metab. 2013;98(1):20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]
- 18.Macsween RN. reticulum-cell sarcoma and rheumatoid arthritis in a patient with XY/XXY/XXXY Klinefelter’s syndrome and normal intelligence. Lancet (London, England) 1965;1(7383):460–61. doi: 10.1016/s0140-6736(65)91591-6. [DOI] [PubMed] [Google Scholar]
- 19.Tsung SH, Heckman MG. Klinefelter syndrome, immunological disorders, and malignant neoplasm: Report of a case. Arch Pathol. 1974;98(5):351–54. [PubMed] [Google Scholar]
- 20.Becher R. Klinefelter’s syndrome and malignant lymphoma. Cancer Genet Cytogenet. 1986;21(3):271–73. doi: 10.1016/0165-4608(86)90008-7. [DOI] [PubMed] [Google Scholar]
- 21.Beasley SW, Tiedemann K, Howat A, et al. Precocious puberty associated with malignant thoracic teratoma and malignant histiocytosis in a child with Klinefelter’s syndrome. Med Pediatr Oncol. 1987;15(5):277–80. doi: 10.1002/mpo.2950150511. [DOI] [PubMed] [Google Scholar]
- 22.Liang R, Woo E, Ho F, et al. Klinefelter’s syndrome and primary central nervous system lymphoma. Med Pediatr Oncol. 1990;18(3):236–39. doi: 10.1002/mpo.2950180316. [DOI] [PubMed] [Google Scholar]
- 23.Attard-Montalto SP, Schuller I, Lastowska MA, et al. Non-Hodgkin’s lymphoma and Klinefelter syndrome. Pediatr Hematol Oncol. 1994;11(2):197–200. doi: 10.3109/08880019409141656. [DOI] [PubMed] [Google Scholar]
- 24.Keung YK, Buss D, Chauvenet A, Pettenati M. Hematologic malignancies and Klinefelter syndrome. a chance association? Cancer Genet Cytogenet. 2002;139(1):9–13. doi: 10.1016/s0165-4608(02)00626-x. [DOI] [PubMed] [Google Scholar]