Abstract
To identify mechanotransductive signals for combating musculoskeletal deterioration, it is essential to determine the components and mechanisms critical to the anabolic processes of musculoskeletal tissues. It is hypothesized that the interaction between bone and muscle may depend on fluid exchange in these tissues by mechanical loading. It has been shown that intramedullary pressure (ImP) and low-level bone strain induced by muscle stimulation (MS) has the potential to mitigate bone loss induced by disuse osteopenia. Optimized MS signals, i.e., low-intensity and high frequency, may be critical in maintaining bone mass and mitigating muscle atrophy. The objectives for this review are to discuss the potential for MS to induce ImP and strains on bone, to regulate bone adaptation, and to identify optimized stimulation frequency in the loading regimen. The potential for MS to regulate blood and fluid flow will also be discussed. The results suggest that oscillatory MS regulates fluid dynamics with minimal mechanical strain in bone. The response was shown to be dependent on loading frequency, serving as a critical mediator in mitigating bone loss. A specific regimen of dynamic MS may be optimized in vivo to attenuate disuse osteopenia and serve as a biomechanical intervention in the clinical setting.
Keywords: Muscle Stimulation, Bone Fluid Flow, Intramedullary Pressure, Osteopenia, Loading Frequency
Introduction
A means of combating deterioration of the musculoskeletal system could involve harnessing bone’s sensitivity to mechanical signals, a key regulatory factor in attenuating, maintaining and recovering bone and muscle mass. This approach could be particularly useful in populations exposed to prolonged periods of disuse including astronauts exposed to long-term microgravity, bedrest patients and the elderly, all of which experience substantial physiological challenges to their musculoskeleton, including osteopenia and muscle atrophy1,2,37,43,51. Because bone is biomechanically linked to muscle and bones adapt to their mechanical environment, which includes muscle forces during development, it is assumed that muscle and bone grow in proportion to one another. Therefore, it is presumable that strong muscle would be associated with high bone mass as has been shown in individuals like professional tennis players. In several studies using GDF8 myostatin knockout mice, a member of the transforming growth factor (TGF)-beta superfamily of secreted growth and differentiation factors, knockout has approximately twice the skeletal muscle mass compared to normal mice, and indicates increased bone mass22,56,59. For example, the GDF8 knockout mice have approximately 10% greater cortical bone mineral contents (BMC) at the midshaft and over 20% greater cortical BMC at the metaphysis22,59. In various human studies, physical exercises seem to be most effective during rapid growth of the musculoskeletal system, the average gain in BMC and bone mineral density (BMD) in controlled trials being of the order of 2–5% per year. However, the net gain of BMD after exercise interventions among older people is conflicting, e.g., at a level of 1–3% per year18,83. While physical activity in older ages has shown to improve muscle strength and balance and to reduce the risk to fall and fractures29,30,83, the effects of vigorous exercises building bone mass is modest, considerably less than bisphosphonates, and largely dependant on the training protocols21,29,30,83. It was demonstrated that unloaded exercise such as swimming has no impact on bone mass, while walking or running has limited positive effects, though intensity could be high21. High impact loading, even a relatively small amount, appears to be effective for enhancing bone mass, although only limited data are available21,83,87. These may lead to several research questions regarding bone-muscle interactions and interrelations: how muscle and bone losses generated by functional disuse integrate in a physiologic system; can biomechanical intervention involving muscle mitigate bone loss; and what is the mechanism by which muscle dynamics attenuates bone loss and enhances fluid flow.
There is a great need to develop a clinically applicable intervention for the prevention of progressive muscle and bone loss associated with disuse osteoporosis and muscle atrophy. Studies of mechanical influences on tissue morphology have demonstrated that the removal of functional loads leads to a loss of bone mass12,73, whereas an increase of activity, such as exercise, results in the augmentation of muscle strength and BMD28,33. Nevertheless, the mechanism by which musculoskeletal tissues adapt to an environment of functional disuse remains a challenging mechanobiological problem, in which particularly importance is the mechanism by which muscle biomechanics affects mechanotransduction and bone quality or vice versa. Identifying regulatory components of the mechanical milieu may prove instrumental in devising a biomechanically based intervention for many serious clinical conditions including the prevention of osteopenia. Perhaps, by identifying key stimulation parameters required in muscle stimulation, i.e., frequency, we may generate beneficial adaptive responses and alleviate the consequence of bone loss. In this review, several viewpoints are discussed to address the idea that oscillatory muscle stimulation (MS) has the potential to mitigate and/or attenuate bone loss induced by disuse osteopenia, i.e., hindlimb suspension (HLS), using optimized mechanobiological signals. Thus, the objectives of this review are: (1) to evaluate the immediate effects on ImP and bone strain induced by dynamic muscle stimulation in response to a broad range of loading frequencies, (2) to characterize the regulatory role of dynamic MS at low and high frequencies on bone adaptation under conditions of functional disuse, e.g., HLS model, and 3) to discuss the potentials of MS generated blood flow in the musculoskeletal tissues.
Functional disuse induced bone loss and muscle atrophy
Disuse osteoporosis is a common skeletal disorder in patients subjected to prolonged immobility or bed-rest, e.g., fracture and spinal injury. In addition to bone loss, functional disuse and microgravity can cause muscle atrophy. These physiological changes generate additional health complications, including increased risk of falls and fracture, and poor long-term recovery. Analyses of spinal cord injury (SCI) patients showed a reduction in BMD in disused limbs7,11,16 and a higher incidence of fracture41,75,89,90. More than 1 year after SCI, 30% to 40% of demineralization was observed in the femoral neck, distal femur and proximal tibia11. It has been reported that SCI induced osteopenia/osteoporosis reaches the fracture threshold (BMD of 1 g/cm2) 1 to 5 years after the injury, with a fracture frequency of 5% to 34%23,24,41,85. These patients were also observed to have significant reductions in muscle mass48. Analyses from space missions of 4 to 12 months duration have shown that weightlessness can induce 1% to 2% BMD loss per month in the spine, hip, and the lower extremities42,50. The reduction in trabecular BMD in both hip and femur regions was greater than 2% per month, while there was only minimal decrease in cortical bone37. Similar results were observed in animal studies. Burr et al.8 showed an increase in bone turnover rate in cast-immobilized animals that received muscle stimulation, while significant bone loss was observed even for 17 days.
Lower extremity muscle volume was also altered by disuse. Exposure to a 6-month space mission resulted in a decrease in muscle volume of 10% in the quadriceps, 19% in the gastrocnemius and soleus44,45. Computed tomography measurements of the muscle cross sectional area (CSA) indicated a decrease of 10% in the gastrocnemius and 10–15% in the quadriceps after short-term missions46,58. Similar results were concluded after SCI, where patients suffered significant 21%, 28% and 39% reductions in CSA at the quadriceps femoris, soleus and gastrocnemius muscles, respectively19,76. In addition to the effects on whole muscle volume, muscle fiber characteristics were also modified due to inactivity70,72,100. There are two primary muscle fibers: slow (type I) fibers play an important role in maintaining body posture, while fast (type II) fibers are responsive during physical activity. Under disuse conditions, all fiber types were decreased in size, 16% for type I and 23–36% for type II72,82,100. The atrophied soleus muscles also underwent a shift from type I (−8% in fiber numbers) to type II fibers72,82,101.
Clinical muscle stimulation has been examined extensively in SCI patients to strengthen skeletal muscle and alleviate muscle atrophy with promising outcomes20,47,71,78. A few physical training studies further investigated this electrical stimulation technique to determine it’s effect on osteopenia. These studies showed mixed results with respect to bone density data13–15,20,97. Using dual energy X-ray absorptiometry (DXA), BeDell et al. found no change in BMD of the lumbar spine and femoral neck regions after functional electrical stimulation-induced cycling exercise, while Mohr et al. showed a 10% increase in BMD in the proximal tibia following 12 months of similar training4,5,53. In a 24-week study of SCI patients in whom 25 Hz electrical stimulation was applied to the quadriceps muscles daily, Belanger and colleagues reported a 28% recovery of BMD in the distal femur and proximal tibia, along with increased muscle strength5. A number of reported animal studies also indicated that muscle stimulation can not only enhance muscle mass, but bone mineral density as well3,84.
Dynamic muscle stimulation as a means to enhance fluid flow in bone
It is hypothesized that skeletal muscle contraction can increase blood flow within musculoskeletal tissues and generate bone strain within the physiological range52,88. Therefore, functional muscle stimulation at the physiological level may generate sufficient mechanobiologic signals (i.e., fluid pressure and blood flow in bone) to trigger musculoskeletal adaptation36,67,80,79,86. Dynamic stimulation frequencies, which alter intramedullary pressure (ImP) and bone strain simultaneously or independently maybe the key determinants responsible for mechanotransductive signals in bone36,67. It is widely accepted that dynamic mechanical stimulation through various loading frequencies plays a critical role in the skeletal adaptive response and further enhances cellular level perfusion91,93. Previous in vivo studies have demonstrated that the rate of the osteogenic response in bone tissue increases at higher frequencies66,74,99. Vibration studies have shown that low-level mechanical signals at 45 Hz has lower osteoclastic activity and enhance the rate of trabecular bone formation in the tibia of either growing animals or functional disused animals17,96. Stimulation of the knee region at 15 Hz increased the cortical mineralizing surface and apposition rate in the femur99. Alternatively, by inducing 20 Hz of fluid pressure into the marrow cavity of turkey ulna, the formation of periosteal and endosteal new bone was augmented at the cortex66.
Exercise plays an important role in regulating fluid flow in the microcirculation within muscular tissues. Various mechanisms that are thought to cause blood flow to rise during rhythmic exercise25–27,38,49. Mechanisms including the muscle pump, substances released by skeletal muscle, substances transported by blood, and factors released by nerves have been postulated to contribute to the rise in muscle blood flow during exercise. Additionally, the factors that initiate the dilation may not be those which sustain it. Although there is normally a close relationship between contractile activity, metabolic rate, and muscle blood flow, this relationship can be disrupted under a variety of circumstances and the active skeletal muscle over perfused. The mechanism of muscle pump as a driving source for capillary filtration and bone interstitial fluid flow is proposed as a coupling factor between muscle contraction and fluid flow through bone. Pressure waves from muscle pump contractions, aided by increased blood pressure during exercise and coupled with temporary occlusion of arteries and veins leading to and from bone, increase hydraulic pressure in cortical bone capillaries, thus amplifying capillary filtration61,94,95. Previous studies have demonstrated that increased venous pressure can promote the formation of periosteal new bone in growing dogs31. The results of paired comparisons, involving experimental and control tibia, showed an increase in venous pressure, and an increase in periosteal new bone formation on the side of increased venous pressure. The data suggest that an increase in venous pressure results in an increase in passage of fluid from capillary to bone matrix. Increased extravascular perfusion could be a factor in increasing periosteal bone formation. In a rat tail suspension model, applying constant venous ligation at femurs increased intramedullary pressure relative to sham-operated control femurs (27.8 mmHg vs. 16.4 mmHg, p<0.05), suggesting venous ligation increased interstitial fluid flow proportional to the pressure drop across the bone6. BMC increased significantly in the venous-ligated femurs relative to control limbs (115.9±15.6% vs. 103.8±13.2%, p<0.001) for a period of 19 days. Trabecular density was significantly higher in the femurs with venous ligation (351±12 g/cm3 vs. 329±11 g/cm3, p<0.05). These results suggest that fluid flow can directly influence bone adaptation independent of mechanical loading, and venous pressure is directly related to ImP and interstitial fluid flow. This implies that an adaptive response can be initiated and accelerated by altering venous pressure by a muscular pump effect, which could serve as a potential noninvasive countermeasure. Understanding the mechanisms responsible for the dynamic patterns of muscle pump, i.e., frequency, rest-insertion, pressure magnitude and duration, can provide new insight into the mechanisms which govern exercise hyperemia and its relation to bone fluid flow.
Frequency dependant marrow pressure and bone strain generated by muscle stimulation
A recent study has revealed that induced marrow fluid pressure and bone strain by muscle stimulation were dependant on dynamic loading parameters and optimized at certain loading frequencies67. Adult Sprague Dawley retired breeder rats with a mean body weight of 387g±41g (Taconic, NY) were used to measure the relationships between ImP, bone strain, and induced-muscle contraction. Rats were anesthetized using standard isoflurane inhalation. A microcardiovascular pressure transducer (Millar Instruments, SPR-524, Houston, TX) was inserted into the femoral marrow cavity, guided via a 16-guage catheter. A single element strain gauge (120Ω, factor 2.06, Kenkyojo Co., Tokyo) was firmly attached onto the lateral surface of the same femur at the mid-diaphyseal region, with minimal disruption to the quadriceps, for measuring bone strains during the loading.
Two disposable needle-sized electrodes (L-type guage #3, Seirin, Weymouth, MA) were inserted into the quadriceps, about ~5 mm anterior to the femur. The electrodes were then connected to a 100MHz arbitrary waveform generator (Model 395, Wavetek) which applied MS at various frequencies (1, 2.5, 5, 10, 15 20, 30, 40, 50, 60 and 100 Hz) to induce MS. Stimulation was induced at 2V with 1ms square pulse for one second, followed by a rest of 4 seconds. Three signals (ImP, bone strain, load feedback) were collected simultaneously using a strain gauge amplifier (National Instruments) with a 160 Hz low-pass filter and A/D conversion at 1000 Hz with 16-bit resolution.
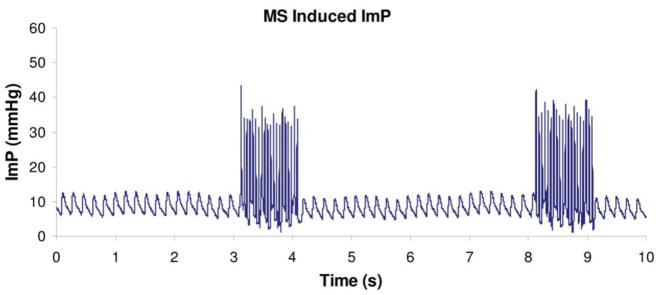
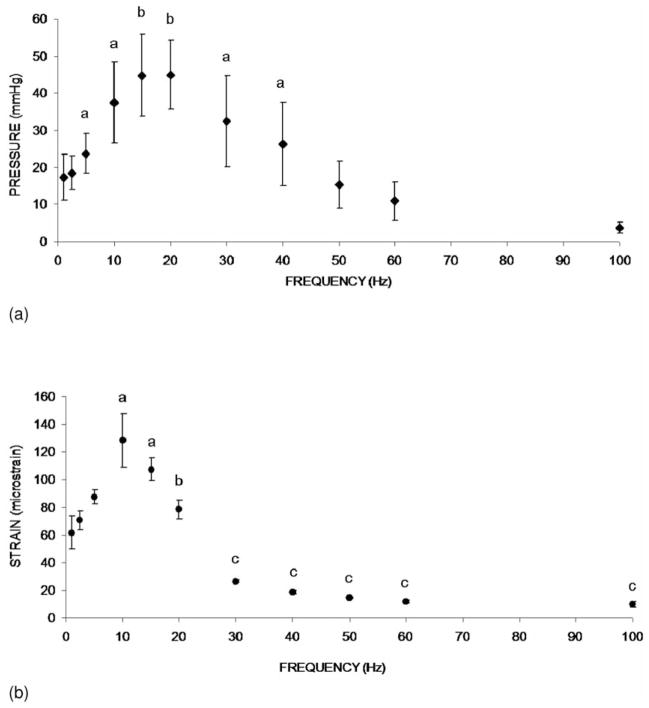
Oscillatory MS was shown to have a significant effect in increasing ImP. A representative ImP profile induced by MS at 20 Hz was shown in Figure 1. Normal heart beat generated approximately 5 mmHg of ImP in the femur at a frequency of 5.37±0.35 Hz. The ImP value (peak-peak) was increased significantly by dynamic MS at 5, 10, 15, 20, 30, and 40 Hz (p<0.05 for 5, 10, 30, and 40 Hz, p<0.01 for 15 and 20 Hz). The response trend of the ImP against frequency was nonlinear; the ImP reached a maximum value of 45±9.3 mmHg (peak-peak) at 20 Hz (Figure 2a), although there was no significant difference between 10, 20, and 30 Hz. The MS generated ImP in the marrow cavity with values of 17.4±6.2, 24±5.4, 37.5±11.0, 26.3±11.1, and 3.7±1.5 mmHg at frequencies of 1, 5, 10, 40, and 100 Hz, respectively.
Figure 1.
Intramedullary pressure generated by dynamic electrical muscle stimulation at 20Hz. Stimulation was applied for 1 seconds followed by 4 seconds rest. Normal heart beat generated approximately 4 mmHg of ImP (mean) in the femur. Muscle contraction increased ImP to 45 mmHg in a rat femoral model. (Courtesy of J Biomechanics 2009;42:140–5).
Figure 2.
(a). Graphs show mean SD values from the ImP measurement. ImP in femur increased significantly with electrical frequency at 5, 10, 15, 20, 30, and 40 Hz. In the loading spectrum from 1 to 100 Hz, stimulation at 1 Hz generated an ImP of 18 mmHg. A maximum ImP of 45 mmHg was measured at 20 Hz, which was 2.5 folds higher than 1 Hz. ap<0.05 vs. baseline ImP; bp<0.01 vs. baseline ImP. (Courtesy of J Biomechanics 2009;42:140–5). (b). Graphs show mean SD values from the bone surface strain measurement. Dynamic muscle stimulation applied at various frequencies significantly increased bone strain. In the loading spectrum from 1 to 100 Hz, stimulation at 1 Hz produced a strain of 62 με. Peak strain of 128 was recorded at 10 Hz stimulation. The strain magnitude was reduced by >75% of the peak strain for stimulation frequencies greater than 30 Hz. ap<0.01 vs. 1, 2.5, and 5 Hz; bp<0.01 vs. 10 Hz; cp<0.001 vs. stimulation 20 Hz and below. (Courtesy of J Biomechanics 2009;42:140–5).
The response of matrix strain to the MS frequency also was nonlinear (Figure 2b). The MS generated femoral matrix strains of 61.8±6.2, 87.5±5.1, 128.4±19.2, 78.3±6.8, 18.7±1.3, and 10.1±1.8 at frequencies of 1, 5, 10, 20, 40, and 100 Hz, respectively. While the ImP trend indicated that the peak ImP value was observed at 20 Hz, the maximum matrix strain was measured at 10 Hz. Bone strain induced by MS at 10 Hz was significantly different (p<0.01) compared to all other stimulations with the exception of 15 Hz. In addition, the strains generated by MS above 30 Hz were significantly lower than those values loaded at and below 20 Hz (p<0.005), in which matrix strains, when loaded above 30 Hz, decreased by more than 75% of the peak strain measured at 10 Hz. For frequencies from 40 Hz to 100 Hz, MS induced matrix strain were less than 20 με.
These results suggest that muscle force alone, if applied at a low rate, such as resistant weigh lifting with high intensity, would not be able to generate sufficient strain and fluid pressure in bone. MS with relatively high rate and small magnitude, however, can trigger significant fluid pressure in the skeleton.
Dynamic muscle stimulation induced attenuation of bone loss
These findings were verified in an in vivo experiment under functional disuse conditions36. Fifty-six 6-months-old female Sprague-Dawley retired breeder rats (Taconic, NY) were used to investigate the effects of frequency-dependent dynamic muscle stimulation (MS) on skeletal adaptation in a disuse environment. Animals were randomly assigned to seven groups with n=8 per group: (1) baseline control, (2) age-matched control, (3) HLS, (4) HLS+1 Hz MS, (5) HLS+20 Hz MS, (6) HLS+50 Hz MS, and (7) HLS+100 Hz MS. Functional disuse was induced by a HLS, setup modified from Morey-Holton and Globus54,55. An approximately 30 head-down tilt was set to prevent contact of the animal’s hindlimbs with the cage bottom. The body weight of each animal was measured three times per week throughout the study.
For the four experimental groups, dynamic MS was applied in conjunction with HLS for 4 weeks. For the daily stimulation, animals were anesthetized and remained suspended on a counter-top with the experimental set-up. Muscle contraction was induced with two disposable needle-size electrodes (L-type guage #3, Seirin, Weymouth, MA) to transmit a 1 ms square pulse with various stimulation frequencies (1 Hz, 20 Hz, 50 Hz and 100 Hz) for 10 minutes per day, 5 days per week, for a total of 4 weeks. A rest-insertion period (2 seconds contraction followed by 8 seconds rest) was added in the MS regimen to avoid muscle fatigue. Using a high resolution μCT scanner (μCT-40, SCANCO Medical AG, Bassersdorf, Switzerland), the distal portion of the femur was scanned with a spatial resolution of 15 μm. Three consecutive 750 μm regions of trabecular bone (M1, M2 and M3) were analyzed in the distal metaphysis, immediately proximal to the growth plate (Figure 3). M1 is the section closest to the diaphysis, M2 is the middle section between M1 and M3, and M3 is the section closest to the growth plate. One 750 μm region of trabecular bone was also analyzed in the distal epiphysis of each femur (Figure 3). Values for bone volume fraction (BV/TV, given as %), connectivity density (Conn.D, 1/mm3), structural model index (SMI), trabecular number (Tb.N, 1/mm), thickness (Tb.Th, mm) and separation (Tb.Sp, mm) were evaluated for each region35,54,57. After scanning with μCT, histomorphometric measurements were made by tracing calcein labels in the trabecular bone at both metaphyseal and epiphyseal regions, for analyses of bone volume fraction (BV/TV – Histo, %), mineralizing surface/bone surface (MS/BS, %), mineral apposition rate (MAR, μm/day), and bone formation rate (BFR/BS, μm3/μm2/yr)62,63,66.
Figure 3.
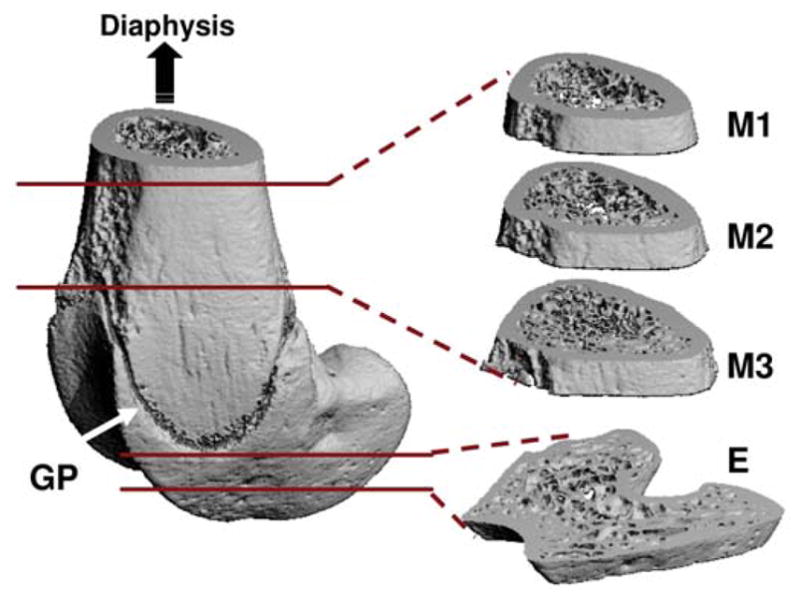
Trabecular bone at three metaphyseal sections and one epiphyseal region of the distal femurs was evaluated using microcomputed tomography. GP=growth plate (arrow); M=metaphysis; E=epiphysis. (Courtesy of BONE 2008;43:1093–1100).
Body weights were not significantly different between groups at the beginning of the study, with an average of 320g±47g. Age-matched control animals were able to maintain a steady body weight throughout the study, with only a −0.15% difference between the start and end date. Animals subjected to 4-week functional disuse lost a significant amount of body mass. These weight reductions were similar in HLS and HLS+MS groups, with −10% for HLS (p<0.05), −8% for 1 Hz (p=0.07), −9% for 20 Hz (p<0.05), −11% for 50 Hz (p<0.01) and −8% for (p=0.09).
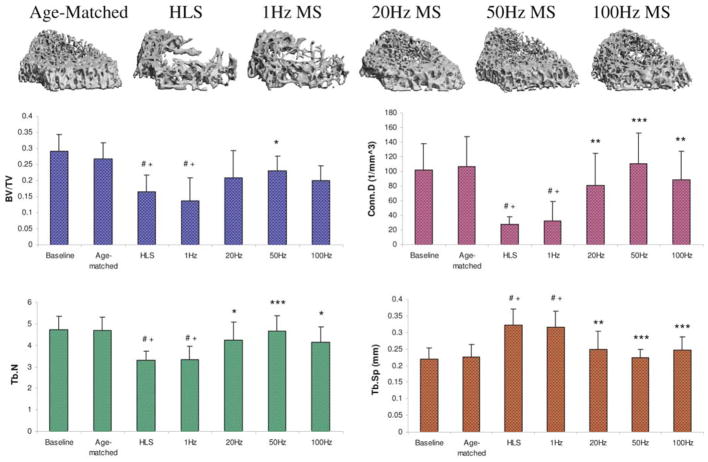
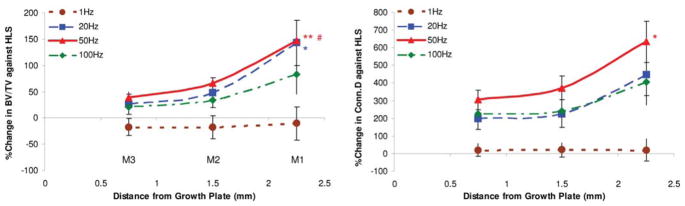
Trabecular bone structure changes by MS stimulation seem to be sensitive to the fluid pressure magnitude experienced by the tissue, where a larger response occurred at the region near the marrow cavity, and attenuated at the region near the growth plate. For example, M1 is the distal metaphyseal region 1.5mm above the growth plate. The lack of weight-bearing activity for 4 weeks significantly reduced trabecular bone quantity and quality, demonstrated by a 70% decreases in BV/TV, an 86% decrease in Conn.D, a 28% decrease in Tb.N, a 57% increases in SMI, and a 43% increase in Tb.Sp compared with baseline (p<0.001). Similar results were observed when compared with age-matched control (p<0.001); decreases in BV/TV (66%), Conn.D (86%) and Tb.N (26%), as well as increases in SMI (39%), and Tb.Sp (39%) were observed. Trabecular BV/TV in electrically stimulated animals, with the exception of 1 Hz, was significantly greater than that of disused bone. Animals with MS at 20 Hz and 50 Hz showed an increase in BV/TV by 143% (p<0.05) and 147% (p<0.01), respectively. Stimulation at 100 Hz showed an 86% increase in BV/TV, but this change was not statistically different from the HLS group. The other outcome measures of Conn.D, Tb.N and Tb.Sp were also significantly affected by MS at 20 Hz, 50 Hz and 100 Hz frequencies. There were up to 600% and 38% increases for Conn.D and Tb.N, and up to a 36% decrease for trabecular separation (20 Hz p<0.01, 50 Hz p<0.001 and 100 Hz p<0.05). SMI and Tb.Th were not affected by the stimulus, regardless of its frequency. The animals subjected to 4 weeks of 1 Hz MS showed the same level of bone loss and structural deterioration as did the HLS animals without MS, and were significant differences compared to stimulation at higher frequencies. M3, the distal metaphyseal portion directly above the growth plate, is a region with the most abundant trabecular network with 0.3±0.05 BV/TV and 4.72±0.64 Tb.N (Figure 4). Disuse induced a 38% bone loss, 75% decrease in Conn.D, 30% reduction in Tb.N and 43% more spacing within this region. Similar to the results reported for the M2 portion, 50 Hz MS resulted in the greatest preventive effects against disuse osteopenia, with increased BV/TV (40%; p<0.05), Conn.D (305%; p<0.001), and Tb.N (41%; p<0.001), and reduced Tb.Sp (31%; p<0.001). While BV/TV was not significantly altered by MS at 20 Hz (+26%) and 100 Hz (+20%), trabecular qualities, Conn.D, Tb.N and Tb.Sp, were improved (up to 226%, 28% and 24% respectively, p<0.001). Like the other metaphyseal regions, SMI and Tb.Th were not affected by the stimulation. These regional changes were summarizes in Figure 5. With the exception of 1 Hz, stimulation frequencies at 20 Hz, 50 Hz, and 100 Hz had greater effects on the trabecular bone 2.25 cm away from the growth plate, closer to the diaphysis. With MS at 50 Hz, the percent changes at M1 were significantly different from those measured at M2 and M3 for BV/TV (both, p<0.001) and from those measured at M3 for Conn.D only (p<0.05). Also, BV/TV inhibition at M1 was significantly higher (p<0.05) than that of M3 with 20 Hz MS. Although following a trend similar to that of the above indices, at 50 Hz and 20 Hz MS, the percent changes of the μCT measurements were not statistically significant between the three metaphyseal regions.
Figure 4.
Representative 3D μCT images of trabecular bone in the M3 region (750 μm, immediately above the growth plate). Graphs show mean + SD values for bone volume fraction (BV/TV, %), connectivity density (Conn.D, 1/mm3), trabecular number (Tb.N, 1/mm), and separation (Tb.Sp, mm) at the M3 region. Only 50 Hz MS demonstrated significant preventive effects for all indices against 4-week HLS. #p<0.001 vs. baseline; +p<0.001 vs. age-matched; *p<0.05 vs. HLS & 1 Hz MS; **p<0.01 vs. HLS & 1 Hz MS; ***p<0.001 vs. HLS & 1 Hz MS. (Courtesy of BONE 2008;43:1093–100).
Figure 5.
Graphs show the percentage differences between HLS and MS experimental groups SD values in all three metaphyseal regions for bone volume fraction (BV/TV) and connectivity density (Conn.D). For MS with mid to high stimulation frequencies, the levels of effectiveness on trabecular bone against functional disuse alone were always greatest at M1 and least at M3. **p<0.001 vs. M3; *p<0.05 vs. M3; #p<0.001 vs. M2. (Courtesy of BONE 2008;43:1093–1100).
While trabecular bone responded to MS showing structural property changes, the epiphyseal trabecular bone was not significantly affected by the 4-week HLS. The percentage changes were minor versus the metaphyseal regions, with −5% BV/TV, −44% Conn.D, −7% Tb.N, and +4% Tb.Sp. In this region, MS did not induce any measurable effect on bone volume and trabecular integrity at any stimulation frequency. All stimulated values were comparable to age-matched and HLS animals, with up to 10% greater in BV/TV, 8% greater in Tb.N, and a 9% reduction in Tb.Sp. These changes were not statistically significant.
The μCT data were correlated with the histomorphometry analyses. In the metaphyseal trabecular bone, BV/TV measured by the 2-D histomorphometric method, was 43% lower in HLS group than in age-matched controls (p<0.001). Animals subjected to MS also experienced 22–29% bone loss (p<0.01). The result was correlated with the BV/TV values from the CT analysis of the M2 region, giving an R2 value of 0.84 (p<0.05). In other bone formation indices, HLS animals also showed significant decline in MS/BS (76%, p<0.001), MAR (80%, p<0.001) and BFR/BS (92%, p<0.001) (Table 1). Disuse had an insignificant effect on the trabecular BV/TV (−10%) at the epiphyseal region, similar to the results of the μCT analysis. Bone formation indices were reduced due to HLS (52% for MS/BS, 147% for MAR and 59% for BFR/BS), and daily MS failed to prevent such reduction of bone formation activity.
Table 1.
Distal femur metaphysis histomorphometry.
| Control | HLS | 1 Hz | 20 Hz | 50 Hz | 100 Hz | |
|---|---|---|---|---|---|---|
| BV/TV - Histo (%) | 41.2±7.7 | 23.3±4.3** | 24.0±8.1** | 29.6±3.9* | 32.1±4.3*# | 29.1±3.5* |
| MS/BS (%) | 9.15±5.5 | 2.11±1.0** | 1.38±1.3** | 2.37±1.7** | 3.94±2.8** | 2.21±1.7** |
| MAR (μm/day) | 1.77±0.5 | 0.36±0.3** | 0.38±0.5** | 0.48±0.3** | 0.65±0.4* | 0.57±0.4** |
| BFR/BS (μm3/μm2/yr) | 46.9±21 | 3.67±5.1** | 3.15±2.6** | 6.63±7.8** | 10.3±8.5* | 6.56±7.1* |
Values are mean±SE. BV/TV, bone volume/tissue volume; MS/BS, mineralized surface/bone surface; MAR, mineral apposition rate; BFR/BS, bone formation rate/bone surface.
p<0.01 vs. age-matched;
p<0.001 vs. age-matched;
p=0.07 vs. HLS. (Courtesy of BONE 2008;43:1093–100).
These data imply that MS, applied at a high frequency with low magnitude and for a short duration, is able to mitigate bone loss induced by the functional disuse. There was, however, no evidence to suggest that such loading would enhance overall new bone formation, e.g., the total bone mass was less than age-match animals. Further studies related to cellular activities, e.g., osteoclast and osteoblast, and linked either bone resorption and bone formation, may be necessary to further explore the balance of resorption and formation in such functional disuse model.
Muscle stimulation enhanced fracture healing
In addition to the role of dynamic muscle stimulation in mitigating osteopenia and muscle atrophy, it has been shown that MS can enhance bone fracture healing. Taking into account that MS can increase blood flow and ImP in the muscle and marrow cavity10,67,77 and that blood flow has a close relationship to the fracture healing, it is likely that applying MS may result in an enhancement of fracture healing. Using a rabbit model with a 3mm tibial transverse osteotomy, Park and Silva have shown that fracture treated with MS showed 31% higher mineral content and 27% larger callus area than control osteotomies at eight weeks64. In addition to the morphometric changes, the maximum torque, torsional stiffness, angular displacement at maximum torque, and energy required to failure of specimens in the study group were 62%, 29%, 34.6%, and 124% higher, respectively, than those in the control group at eight weeks64. The results suggested that the use of MS can enhance callus mineralization and biomechanical strength in the callus region. This may, at least partially, be the result of MS enhanced blood circulation. Using a bone chamber, Winet and his group observed that muscle contractions directly increased bone blood flow rates by 130%, but uncoupled from mechanical loading, while heart rates and blood pressure did not significantly increase due to the MS treatment9. Thus enhanced fluid flow by MS may directly involve increase fluid flow in callus and trigger anabolic response under such acute conditions, e.g., fracture healing.
Age-dependant acceptance of mechanical signals for maintaining bone and muscle health
It should be noted that the MS signals mentioned above were in the physiological range, e.g., low magnitude, high frequency and short daily duration. Age-related patterns of musculoskeletal sensitivities involve peak bone mass during growth, a plateau in adulthood, and bone loss during aging. The decline in bone mass and structural integrity results in increased risk of fractures, particularly in post-menopausal women. Muscle exercise training seems to be most effective for bone during rapid growth, e.g., among young athletes, the average gain in BMC and BMD in controlled trials being of the order of 2–5% per year. Under similar exercise interventions the net gain of BMD, including both bone formation and resorption, e.g., only among elderly is modest, at a level of 1–3% per year, but it is not clear whether positive effects can be maintained over a longer time83. In general high intensity exercises may not be practical in elderly population. In addition, old bone does not respond to mechanical stimuli in the same way as in young bone. In a recent study, Kumar et al.34 examined how myofibrillar protein synthesis (MPS) and muscle anabolic signaling were affected by resistance exercise in two groups (25 each) of healthy, young (24 +/−6 years) and old (70 +/− 5 years) men with identical body mass indices (24 +/− 2 kg m−2). In each group, there was a sigmoidal dose-response relationship between MPS at 1–2 h post-exercise and exercise intensity, which was blunted (P<0.05) in the older men. The phosphorylation predicted the rate of MPS at 1–2 h post-exercise in the young but not in the old. The results suggest that older men show anabolic resistance of signaling and MPS to resistance exercise. To design non-pharmaceutical MS intervention for treating osteoporosis shall consider the age-dependant effects in terms of dosage, duration and frequency. These considerations is perhaps true for other similar musculoskeletal tissues and diseases, e.g., cartilage and osteoarthritis.
Discussion
The animal study results have indicated that dynamic muscle stimulation generates fluid pressure in bone with simultaneously low-level bone strain. MS adjacent to the rat femur induces a peak ImP at 20–30 Hz. The increase in bone fluid pressure by dynamic MS suggests that hypertension in the skeletal nutrient vessels may increase ImP and regulate fluid flow in bone95. Similarly, bone strain was highest at approximately 10 Hz. Stimulus-induced bone fluid and matrix deformation are dependent on stimulation frequency. It appears that the oscillatory MS stimulates relatively high fluid pressure at the frequency range between 20 Hz to 50 Hz in the tested frequencies up to 100 Hz. In such optimized loading rate (e.g., 20–50 Hz), relatively high ImP and relatively low bone strain were observed in response to the MS loading, which may be critical to regulating fluid flow, and adaptation in bone as a functional loading frequency dependant manner. It is also noted that both ImP and strain at 10 and 20 Hz are higher than the value at lower frequency, i.e., 1 Hz, and at very high frequency, i.e., 100 Hz. There is a significant difference in bone strain between 10 Hz and 20 Hz, but no significant difference in ImP between 10 Hz and 20 Hz. At an optimal frequency, MS can produce high fluid pressure gradients within the femoral marrow cavity and a high strain value in bone. It is possible that loading generated matrix strain and fluid pressure in bone may have combined effects in adaptation if loaded at relatively high frequencies.
The data strongly indicated that dynamic muscle stimulation was able to inhibit bone loss and trabecular architectural deterioration caused by a lack of daily weight-bearing activities. In the current study we investigated the role of stimulation frequency on bone’s skeletal adaptive responses. The optimized frequency, which resulted in a strong adaptive response in the disuse osteopenia, was in the range of 50 Hz. Because the strain level generated at 50 Hz by MS was relatively low, e.g., approximately 10 με, bone fluid flow mechanisms may be uncoupled to strain signals and the driving force behind bone’s adaptive response9. The degree of effectiveness of MS in attenuating bone loss varied in different regions of the distal femur. Such spatial response may also depend on the fluid pressure generated in the local region in a dose dependant manner. While low-frequency MS was unsuccessful in preventing osteopenia, mid-frequency MS applied to the quadriceps was able to maintain trabecular bone mass.
These result were consistent with previous in vivo results in which mechanical loading at frequencies between 20 and 50 Hz were shown to be anti-catabolic to bone17,66,69,98. This sensitivity was even more apparent in trabecular bone, perhaps due to the increased surface area in the trabecular network, which exposes it to rapid changes in fluid pressure68. For example, trabecular osteoblast surface in the tibia was increased by 26% when a MS protocol at 10 Hz was applied for 3 weeks98. Likewise, whole body vibration at 45 Hz increased the rate of formation of the growing skeleton by 30%96.
Both ImP and matrix strain have indicated a nonlinear response in the MS spectrum between 1 Hz and 100 Hz, though peaked differently at 20 Hz (ImP) and 10 Hz (strain). While no obvious muscle fatigue was observed, perhaps due to the rest period during the stimulation, the mechanism behind such a nonlinear response is not clear. From a material property point of view, the viscoeastic characteristics of both muscle and bone could quickly dampen the response at high frequencies through the MS loading. But, due to the difference in densities and viscosities between muscle and bone, MS induced ImP and matrix strain could result in different frequency responses or optimized/resonant patterns for different tissues against the loading. In addition, mechanotransduction of MS through different connective tissues may attenuate the high frequency components of the response in bone, e.g., via the connective pathway from muscle, tendon to bone, thus resulting in peak strain and peak ImP at varied frequencies. Future research on such complex interrelations between muscle kinematics, bone fluid flow, and matrix strain is necessary to further elucidate the mechanism.
The interrelationship between muscle dynamics and bone adaptation via induced ImP and strain was presented through MS with variation in the frequency of the stimulus. One limitation of this approach was that frequency-induced variations in ImP and in matrix strain could not be separated. However, it is important to note that both 1 Hz and 20 Hz MS generated about 60 to 70 of surface strain at the mid-diaphysis of the rat femur. Still, the ImP induced by 20 Hz MS was 2.5-fold greater than the ImP induced at 1 Hz. In a previous study, continuous electrical stimulation of muscles inserting on the tibia at 30 Hz produced a compressive strain of 350 to 500 and demonstrated preventive effects against disuse osteopenia52. Yet, the stimulation could only maintain a small fraction of BMD and did not enhance bone tissue quality. Nevertheless, the MS induced bone strain at 20 Hz cannot be ignored. Perhaps, both dynamic fluid pressure and matrix strain regulated the inhibition of bone loss in this experiment. A more extensive spectrum of electrical frequencies for MS, e.g., 1–100 Hz, could further elucidate these interactions.
Even in the absence of bone matrix strain, previous data has shown that ImP alone can induce bone adaptation66. Using a turkey ulna osteotomy model, disuse alone resulted in a 5.7% loss of cortical bone. Direct fluid loading at 20 Hz for 4 weeks increased cortical bone mass by 18% by enhancing the formation of bone at both periosteal and endosteal surfaces66. Transcortical fluid pressure gradient and total bone formation were strongly, positively correlated. Strong evidence suggests that interstitial fluid flow in bone interacts strongly with external muscular activities via various mechanisms81,88. According to a muscle pump hypothesis, an arteriovenous pressure gradient enhances muscle perfusion39,40. This process may in turn increase the hydraulic pressure in skeletal nutrient vessels and amplify the capillary filtration in bone tissue39,61,95.
As a clinical application, MS on spinal cord-injured patients can cause partial reversal of disuse osteopenia and recovery of muscular strength5. Other in vivo studies have also reported positive effects of using muscle stimulation to inhibit muscle atrophy. Immobilization studies using MS at 50 to 100 Hz have been shown to minimize the reduction of the cross-sectional area of muscle fibers and to restore mechanical properties32,65. Previous data showed that stimulation of distal nerve stumps had similar action potential response between normal and innervated muscle60. Although the response of ImP and bone mass by MS under such periphery nerve block conditions still remains unknown, MS could serve as a mitigating agent to retain bone mass under chronic nerve damage conditions, e.g., spinal cord injury. Taken together with the results from our current study, dynamic MS may be applied as both a skeletal therapy and a muscular therapy to prevent osteopenia and sarcopenia.
Summary
Functional disuse has been shown to affect both muscle and bone. In addition, the close interrelationship between bone and muscle may be harnessed in such a way that muscle stimulation can act as a mechanobiological mediator in regulating musculoskeletal adaptation, particularly under disuse conditions. Dynamic muscle stimulation could serve as a non-invasive method of generating low-level bone strain and ImP as a function of stimulation frequency. However, selection of protocols of stimulations would alter the outcomes significantly. The increase in ImP may ultimately enhance interstitial fluid flow and mechanotransduction in bone. Furthermore, dynamic muscle contraction, if applied at an optimal frequency, has been shown to have preventive potential in osteopenia and in a functional disuse environment as a biomechanically based intervention for preventing and/or treating osteoporosis and muscle atrophy.
Acknowledgments
This work is kindly supported by The National Institute of Health (R01 AR52379 and R01, AR49286, YXQ), the US Army Medical Research and Materiel Command, and the National Space Biomedical Research Institute through NASA contract NCC 9–58 (TD00405 and SMST01603).
Footnotes
The authors have no conflict of interest.
References
- 1.Akima H, Katayama K, Sato K, Ishida K, Masuda K, Takada H, Watanabe Y, Iwase S. Intensive cycle training with artificial gravity maintains muscle size during bed rest. Aviat Space Environ Med. 2005;76:923–9. [PubMed] [Google Scholar]
- 2.Akima H, Kawakami Y, Kubo K, Sekiguchi C, Ohshima H, Miyamoto A, Fukunaga T. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc. 2000;32:1743–7. doi: 10.1097/00005768-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J Musculoskelet Neuronal Interact. 2006;6:217–25. [PubMed] [Google Scholar]
- 4.BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil. 1996;75:29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–8. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- 6.Bergula AP, Huang W, Frangos JA. Femoral vein ligation increases bone mass in the hindlimb suspended rat. Bone. 1999;24:171–7. doi: 10.1016/s8756-3282(98)00165-3. [DOI] [PubMed] [Google Scholar]
- 7.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 8.Burr DB, Frederickson RG, Pavlinch C, Sickles M, Burkart S. Intracast muscle stimulation prevents bone and cartilage deterioration in cast-immobilized rabbits. Clin Orthop. 1984:264–78. [PubMed] [Google Scholar]
- 9.Caulkins C, Ebramzadeh E, Winet H. Skeletal muscle contractions uncoupled from gravitational loading directly increase cortical bone blood flow rates in vivo. J Orthop Res. 2008:11–8. doi: 10.1002/jor.20780. [DOI] [PubMed] [Google Scholar]
- 10.Clemente FR, Barron KW. Transcutaneous neuromuscular electrical stimulation effect on the degree of microvascular perfusion in autonomically denervated rat skeletal muscle. Arch Phys Med Rehabil. 1996;77:155–60. doi: 10.1016/s0003-9993(96)90160-4. [DOI] [PubMed] [Google Scholar]
- 11.Dauty M, Perrouin VB, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–9. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson CL, Hulley SB, Vogel JM, Hattner RS, Bayers JH, McMillan DE. Effect of prolonged bed rest on bone mineral. Metabolism. 1970;19:1071–84. doi: 10.1016/0026-0495(70)90032-6. [DOI] [PubMed] [Google Scholar]
- 13.Dudley-Javoroski S, Littmann AE, Iguchi M, Shields RK. Doublet stimulation protocol to minimize musculoskeletal stress during paralyzed quadriceps muscle testing. J Appl Physiol. 2008;104:1574–82. doi: 10.1152/japplphysiol.00892.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley-Javoroski S, Shields RK. Dose estimation and surveillance of mechanical loading interventions for bone loss after spinal cord injury. Phys Ther. 2008;88:387–96. doi: 10.2522/ptj.20070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008;45:283–96. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am. 2001;83-A:1195–1200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–40. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 18.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–9. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 20.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, Ivy JL. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19:614–622. doi: 10.1016/j.jelekin.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JA. Exercise and bone mass in adults. Sports Med. 2009;39:439–68. doi: 10.2165/00007256-200939060-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hamrick MW. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:388–91. doi: 10.1002/ar.a.10044. [DOI] [PubMed] [Google Scholar]
- 23.Heaney RP. Pathophysiology of osteoporosis. Endocrinol Metab Clin North Am. 1998;27:255–65. doi: 10.1016/s0889-8529(05)70004-9. [DOI] [PubMed] [Google Scholar]
- 24.Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–139. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- 25.Joyner MJ, Lennon RL, Wedel DJ, Rose SH, Shepherd JT. Blood flow to contracting human muscles: influence of increased sympathetic activity. J Appl Physiol. 1990;68:1453–7. doi: 10.1152/jappl.1990.68.4.1453. [DOI] [PubMed] [Google Scholar]
- 26.Joyner MJ, Proctor DN. Muscle blood flow during exercise: the limits of reductionism. Med Sci Sports Exerc. 1999;31:1036–40. doi: 10.1097/00005768-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Wieling W. Increased muscle perfusion reduces muscle sympathetic nerve activity during handgripping. J Appl Physiol. 1993;75:2450–5. doi: 10.1152/jappl.1993.75.6.2450. [DOI] [PubMed] [Google Scholar]
- 28.Judex S, Zernicke RF. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol. 2000;88:2183–91. doi: 10.1152/jappl.2000.88.6.2183. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson MK. Physical activity, skeletal health and fractures in a long term perspective. J Musculoskelet Neuronal Interact. 2004;4:12–21. [PubMed] [Google Scholar]
- 30.Karlsson MK, Nordqvist A, Karlsson C. Physical activity, muscle function, falls and fractures. Food Nutr Res. 2008:52. doi: 10.3402/fnr.v52i0.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly PJ, Bronk JT. Venous pressure and bone formation. Microvasc Res. 1990;39:364–75. doi: 10.1016/0026-2862(90)90049-w. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Roy RR, Zhong H, Suzuki H, Ambartsumyan L, Haddad F, Baldwin KM, Edgerton VR. Electromechanical stimulation ameliorates inactivity-induced adaptations in the medial gastrocnemius of adult rats. J Appl Physiol. 2007;103:195–205. doi: 10.1152/japplphysiol.01427.2006. [DOI] [PubMed] [Google Scholar]
- 33.Krolner B, Toft B, Pors NS, Tondevold E. Physical exercise as prophylaxis against involutional vertebral bone loss: a controlled trial. Clin Sci (Lond) 1983;64:541–6. doi: 10.1042/cs0640541. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hilde-brandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laib A, Kumer JL, Majumdar S, Lane NE. The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int. 2001;12:936–941. doi: 10.1007/s001980170022. [DOI] [PubMed] [Google Scholar]
- 36.Lam H, Qin YX. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 38.Larina IM, Tcheglova IA, Shenkman BS, Nemirovskaya TL. Muscle atrophy and hormonal regulation in women in 120 day bed rest. J Gravit Physiol. 1997;4:121–2. [PubMed] [Google Scholar]
- 39.Laughlin MH. The muscle pump: what question do we want to answer? J Appl Physiol. 2005;99:774. doi: 10.1152/japplphysiol.00578.2005. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin MH, Joyner M. Closer to the edge? Contractions, pressures, waterfalls and blood flow to contracting skeletal muscle. J Appl Physiol. 2003;94:3–5. doi: 10.1152/japplphysiol.00829.2002. [DOI] [PubMed] [Google Scholar]
- 41.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–14. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc A. Summary of research issues in human studies. Bone. 1998;22:117S–8S. [PubMed] [Google Scholar]
- 43.LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after space-flight. J Appl Physiol. 2000;89:2158–64. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- 44.LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after space-flight. J Appl Physiol. 2000;89:2158–64. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- 45.LeBlanc A, Rowe R, Evans H, West S, Shackelford L, Schneider V. Muscle atrophy during long duration bed rest. Int J Sports Med. 1997;18(Suppl 4):S283–S285. doi: 10.1055/s-2007-972726. [DOI] [PubMed] [Google Scholar]
- 46.LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med. 1995;66:1151–1154. [PubMed] [Google Scholar]
- 47.Lim PA, Tow AM. Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singapore. 2007;36:49–57. [PubMed] [Google Scholar]
- 48.Macdonald JH, Evans SF, Davie MW, Sharp CA. Muscle mass deficits are associated with bone mineral density in men with idiopathic vertebral fracture. Osteoporos Int. 2007;18:1371–1378. doi: 10.1007/s00198-006-0223-x. [DOI] [PubMed] [Google Scholar]
- 49.Mayet-Sornay MH, Hoppeler H, Shenkman BS, Desplanches D. Structural changes in arm muscles after microgravity. J Gravit Physiol. 2000;7:S43–S44. [PubMed] [Google Scholar]
- 50.McCarthy I, Goodship A, Herzog R, Oganov V, Stussi E, Vahlensieck M. Investigation of bone changes in microgravity during long and short duration space flight: comparison of techniques. Eur J Clin Invest. 2000;30:1044–54. doi: 10.1046/j.1365-2362.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy ID. Fluid shifts due to microgravity and their effects on bone: a review of current knowledge. Ann Biomed Eng. 2005;33:95–103. doi: 10.1007/s10439-005-8967-6. [DOI] [PubMed] [Google Scholar]
- 52.Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys. 2005;27:285–93. doi: 10.1016/j.medengphy.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Mohr T. Electric stimulation in muscle training of the lower extremities in persons with spinal cord injuries. Ugeskr Laeger. 2000;162:2190–4. [PubMed] [Google Scholar]
- 54.Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone. 1998;22:83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 55.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 56.Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–83. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller R, Koller B, Hildebrand T, Laib A, Gianolini S, Ruegsegger P. Resolution dependency of microstructural properties of cancellous bone based on three-dimensional mutomography. Technol Health Care. 1996;4:113–9. [PubMed] [Google Scholar]
- 58.Narici M, Kayser B, Barattini P, Cerretelli P. Effects of 17-day spaceflight on electrically evoked torque and cross-sectional area of the human triceps surae. Eur J Appl Physiol. 2003;90:275–82. doi: 10.1007/s00421-003-0955-7. [DOI] [PubMed] [Google Scholar]
- 59.Nicholson EK, Stock SR, Hamrick MW, Ravosa MJ. Bio-mineralization and adaptive plasticity of the temporomandibular joint in myostatin knockout mice. Arch Oral Biol. 2006;51:37–49. doi: 10.1016/j.archoralbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 60.O’Gara T, Urban W, Polishchuk D, Pierre-Louis A, Stewart M. Continuous stimulation of transected distal nerves fails to prolong action potential propagation. Clin Orthop Relat Res. 2006;447:209–13. doi: 10.1097/01.blo.0000203481.11797.0f. [DOI] [PubMed] [Google Scholar]
- 61.Otter MW, Qin YX, Rubin CT, McLeod KJ. Does bone perfusion/reperfusion initiate bone remodeling and the stress fracture syndrome? Med Hypotheses. 1999;53:363–8. doi: 10.1054/mehy.1998.0782. [DOI] [PubMed] [Google Scholar]
- 62.Parfitt AM. Bone histomorphometry: standardization of nomenclature, symbols and units. Summary of proposed system. Bone Miner. 1988;4:1–5. [PubMed] [Google Scholar]
- 63.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 64.Park SH, Silva M. Neuromuscular electrical stimulation enhances fracture healing: results of an animal model. J Orthop Res. 2004;22:382–7. doi: 10.1016/j.orthres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Qin L, Appell HJ, Chan KM, Maffulli N. Electrical stimulation prevents immobilization atrophy in skeletal muscle of rabbits. Arch Phys Med Rehabil. 1997;78:512–7. doi: 10.1016/s0003-9993(97)90166-0. [DOI] [PubMed] [Google Scholar]
- 66.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, are correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–37. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 67.Qin YX, Lam H. Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J Biomech. 2009;42:140–5. doi: 10.1016/j.jbiomech.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qin YX, Lin W, Rubin CT. Load-Induced Bone Fluid Flow Pathway as Definded by In-vivo Intramedullary Pressure and Streaming Potentials Measurements. Ann Biomed Eng. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 69.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–9. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 70.Riley DA, Slocum GR, Bain JL, Sedlak FR, Sowa TE, Mellender JW. Rat hindlimb unloading: soleus histochemistry, ultrastructure, and electromyography. J Appl Physiol. 1990;69:58–66. doi: 10.1152/jappl.1990.69.1.58. [DOI] [PubMed] [Google Scholar]
- 71.Rodgers MM, Glaser RM, Figoni SF, Hooker SP, Ezenwa BN, Collins SR, Mathews T, Suryaprasad AG, Gupta SC. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev. 1991;28:19–26. doi: 10.1682/jrrd.1991.10.0019. [DOI] [PubMed] [Google Scholar]
- 72.Roy RR, Pierotti DJ, Garfinkel A, Zhong H, Baldwin KM, Edgerton VR. Persistence of motor unit and muscle fiber types in the presence of inactivity. J Exp Biol. 2008;211:1041–19. doi: 10.1242/jeb.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubin CT, Lanyon LE. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res. 1987;5:300–10. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- 74.Rubin CT, Sommerfeldt DW, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6:848–58. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- 75.Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P. Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977–2001. J Bone Miner Res. 2009;24:1299–1307. doi: 10.1359/jbmr.090207. [DOI] [PubMed] [Google Scholar]
- 76.Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC, Bowden M, Behrman AL, Walter GA, Dudley GA, Vandenborne K. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil. 2006;87:772–8. doi: 10.1016/j.apmr.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 77.Sherry JE, Oehrlein KM, Hegge KS, Morgan BJ. Effect of burst-mode transcutaneous electrical nerve stimulation on peripheral vascular resistance. Phys Ther. 2001;81:1183–91. [PubMed] [Google Scholar]
- 78.Shields RK, Dudley-Javoroski S. Musculoskeletal adaptations in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair. 2007;21:169–79. doi: 10.1177/1545968306293447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shim SS. Bone and joint circulation. Physiological basis for clinical practice. Yonsei Med J. 1986;27:91–9. doi: 10.3349/ymj.1986.27.2.91. [DOI] [PubMed] [Google Scholar]
- 80.Shim SS, Hawk HE, Yu WY. The relationship between blood flow and marrow cavity pressure of bone. Surg Gynecol Obstet. 1972;135:353–60. [PubMed] [Google Scholar]
- 81.Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39:565–72. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, Phillips SM. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30:61–8. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- 83.Suominen H. Muscle training for bone strength. Aging Clin Exp Res. 2006;18:85–93. doi: 10.1007/BF03327422. [DOI] [PubMed] [Google Scholar]
- 84.Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated Resistance Training During Hindlimb Unloading Abolishes Disuse Bone Loss and Maintains Muscle Strength. J Bone Miner Res. 2009:8–4. doi: 10.1359/jbmr.090811. [DOI] [PubMed] [Google Scholar]
- 85.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Taylor PN, Tromans AM, Harris KR, Swain ID. Electrical stimulation of abdominal muscles for control of blood pressure and augmentation of cough in a C3/4 level tetraplegic. Spinal Cord. 2002;40:34–6. doi: 10.1038/sj.sc.3101250. [DOI] [PubMed] [Google Scholar]
- 87.Tolomio S, Ermolao A, Travain G, Zaccaria M. Short-term adapted physical activity program improves bone quality in osteopenic/osteoporotic postmenopausal women. J Phys Act Health. 2008;5:844–53. doi: 10.1123/jpah.5.6.844. [DOI] [PubMed] [Google Scholar]
- 88.Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol. 2005;98:72–6. doi: 10.1152/japplphysiol.00151.2004. [DOI] [PubMed] [Google Scholar]
- 89.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–6. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 90.Vestergaard P, Rejnmark L, Mosekilde L. Osteoarthritis and risk of fractures. Calcif Tissue Int. 2009;84:249–56. doi: 10.1007/s00223-009-9224-z. [DOI] [PubMed] [Google Scholar]
- 91.Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone’s interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L, Fritton SP, Cowin SC, Weinbaum S. Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J Biomech. 1999;32:663–72. doi: 10.1016/s0021-9290(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Fritton SP, Weinbaum S, Cowin SC. On bone adaptation due to venous stasis. J Biomech. 2003;36:1439–51. doi: 10.1016/s0021-9290(03)00241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winet H. The role of microvasculature in normal and perturbed bone healing as revealed by intravital microscopy. Bone. 1996;19:39S–57S. doi: 10.1016/s8756-3282(96)00133-0. [DOI] [PubMed] [Google Scholar]
- 95.Winet H. A bone fluid flow hypothesis for muscle pump-driven capillary filtration: II. Proposed role for exercise in erodible scaffold implant incorporation. Eur Cell Mater. 2003;6:1–10. doi: 10.22203/ecm.v006a01. [DOI] [PubMed] [Google Scholar]
- 96.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Yang YS, Koontz AM, Triolo RJ, Cooper RA, Boninger ML. Biomechanical analysis of functional electrical stimulation on trunk musculature during wheelchair propulsion. Neurorehabil Neural Repair. 2009;23:717–25. doi: 10.1177/1545968308331145. [DOI] [PubMed] [Google Scholar]
- 98.Zerath E, Canon F, Guezennec CY, Holy X, Renault S, Andre C. Electrical stimulation of leg muscles increases tibial trabecular bone formation in unloaded rats. J Appl Physiol. 1995;79:1889–94. doi: 10.1152/jappl.1995.79.6.1889. [DOI] [PubMed] [Google Scholar]
- 99.Zhang P, Tanaka SM, Sun Q, Turner CH, Yokota H. Frequency-dependent enhancement of bone formation in murine tibiae and femora with knee loading. J Bone Miner Metab. 2007;25:383–91. doi: 10.1007/s00774-007-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhong H, Roy RR, Siengthai B, Edgerton VR. Effects of inactivity on fiber size and myonuclear number in rat soleus muscle. J Appl Physiol. 2005;99:1494–9. doi: 10.1152/japplphysiol.00394.2005. [DOI] [PubMed] [Google Scholar]
- 101.Zhou MY, Klitgaard H, Saltin B, Roy RR, Edgerton VR, Gollnick PD. Myosin heavy chain isoforms of human muscle after short-term spaceflight. J Appl Physiol. 1995;78:1740–4. doi: 10.1152/jappl.1995.78.5.1740. [DOI] [PubMed] [Google Scholar]