Abstract
Background
Mutations in PNPLA2, a gene encoding adipose triglyceride lipase, lead to neutral lipid storage disease with myopathy.
Objective
To report the clinical and molecular features of a case of neutral lipid storage disease with myopathy resulting from a novel mutation in PNPLA2.
Design
Case report.
Setting
University hospital.
Patient
A 65-year-old man with progressive muscle weakness and high serum creatine kinase levels.
Intervention
Direct sequencing of the PNPLA2 gene.
Results
Identification of a novel homozygous mutation in the patient’s PNPLA2 gene confirmed the suspected diagnosis of neutral lipid storage disease with myopathy.
Conclusion
Screening of the PNPLA2 gene should be considered for patients presenting with high levels of creatine kinase, progressive muscle weakness, and systemic lipid accumulation. The presence of Jordans anomaly can be a strong diagnostic clue.
Neutral lipid storage diseases (NLSDs) are autosomal recessive disorders characterized by marked accumulation of lipid droplets in the cells of most tissues.1–3 Two forms of NLSD are currently recognized, both attributed in the last decade to primary defects in triglyceride catabolism: NLSD with ichthyosis (also known as Chanarin-Dorfman syndrome), which is caused by mutations in CGI-58,4 and NLSD with myopathy (NLSDM), which is caused by mutations in PNPLA2, the gene encoding adipose triglyceride lipase (ATGL).5 Despite extensive accumulation of lipid droplets in all tissues, symptoms of NLSDM are limited to myopathy, sometimes with cardiomyopathy and hepatomegaly, without any involvement of the central or peripheral nervous system.6
Patients typically present in middle age with limb weakness, moderate to severe elevation of creatine kinase levels, and extensive lipid accumulation in most cells.6 A key diagnostic feature common to both NLSD with ichthyosis and NLSDM is the appearance of large neutral lipid droplets in granulocytes (Jordans anomaly); however, NLSDM is easily distinguished by the lack of severe skin involvement that is characteristic of NLSD with ichthyosis. There is some variability in age of onset, but myopathy has not been reported before the third decade. However, an apparently healthy 18-year-old woman was serendipitously diagnosed with NLSDM after she was found to have an unexplained high level of creatine kinase and muscle biopsy showed massive lipid storage, which usually indicates lipid accumulation over an extended period of time before muscle weakness develops.7
REPORT OF A CASE
A 65-year-old man had a 30-year history of progressive muscle weakness beginning in distal leg muscles, with difficulty climbing stairs and standing from a seated position. His previous medical history had been unremarkable, and there was no family history of neuromuscular disease. On initial neurological evaluation at age 41 years, the patient was found to have proximal and distal leg weakness, with marked atrophy of distal leg muscles and a waddling gait. Electromyography showed “prolonged pseudomyotonic discharges in the left tibialis anterior,” which were interpreted as a sign of myopathy. The results of nerve conduction studies were normal. A muscle biopsy of the left vastus lateralis revealed multiple neutral lipid vacuoles in more than 50% of the fibers. Based on these findings, the patient was diagnosed with a lipid storage myopathy. Subsequent investigations showed normal carnitine levels and normal activity of carnitine palmitoyltransferase II
At age 51 years, his muscles and plasma carnitine levels were normal. The result of a urine lipid profile, as determined by chromatography, was normal. His urine organic acid levels were grossly normal, with a slightly low level of β-hydroxybutyrate at 0.01 mg/dL (normal range, 0.1–1.0 mg/dL) and slightly elevated level of alanine at 3.53 mg/dL (normal range, 1.34–3.20 mg/dL; to convert to micromoles per liter, multiply by 112.2). Serum lipid and acyl-carnitine profiles, as well as venous lactate, pyruvate, and acetoacetate levels, were normal. The results of β-oxidation pathway enzyme activities measured in a second muscle biopsy were normal.
The patient’s condition continued to worsen. He began using a cane at age 57 years. Since age 60 years, he has experienced hand weakness resulting in difficulty with fasteners and writing. By the time he was evaluated at Columbia University Medical Center, New York, New York, in 2010, he was able to climb stairs only with handrail support and to walk by supporting himself against the walls. Outside of his home, he uses a wheelchair. In spite of these difficulties, he has been able to continue working full-time as an engineer. He has no brain or peripheral nerve symptoms, and there is no clinical or laboratory evidence of cardiomyopathy.
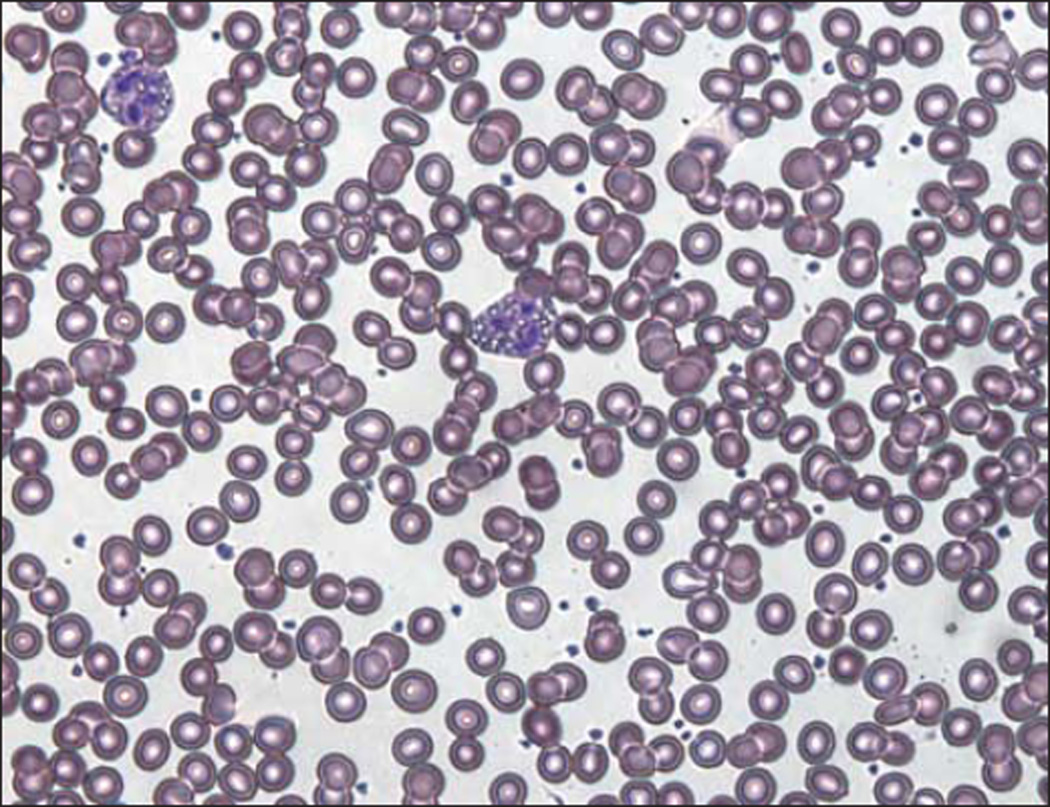
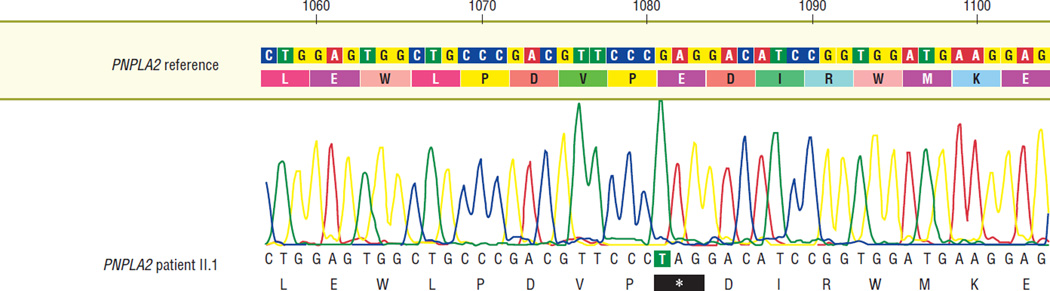
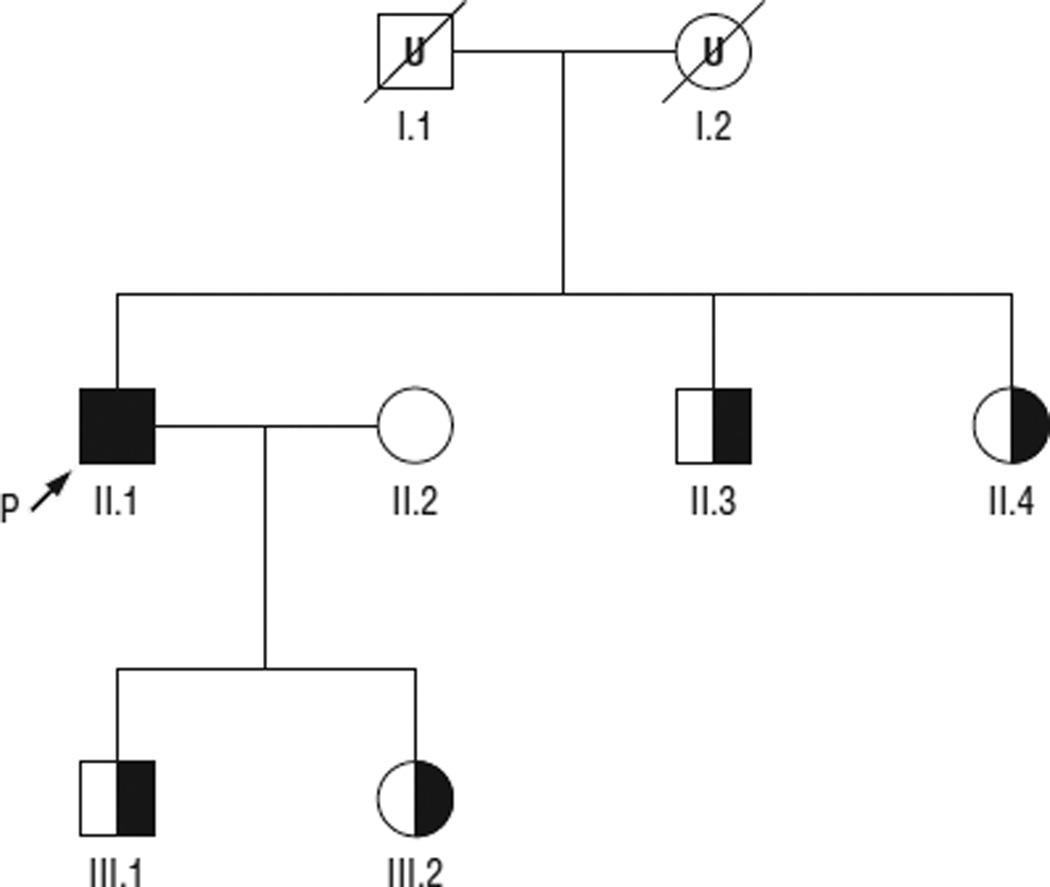
His serum creatine kinase level was 330 U/L (normal range, 51–295 U/L), and a Wright stain of a peripheral blood smear confirmed the presence of lipid droplets in the patient’s leukocytes (Figure 1). Sequencing of PNPLA2 revealed a homozygous nonsense mutation in exon 9 at c.1081G>T, with predicted protein defect p.E361X (Figure 2). The patient’s wife did not carry the mutation, but his 2 siblings and 2 children are all carriers (Figure 3), and the mutation was not found in 150 normal chromosomes.
Figure 1.
Vacuolization of granulocytes (Jordans anomaly) in a peripheral blood smear from the patient.
Figure 2.
Electropherogram showing the patient’s homozygous c.1081G>T mutation in exon 9 of the PNPLA2 gene.
Figure 3.
Pedigree of the family based on molecular data. Half-filled symbols are PNPLA2 mutation carriers, and the black symbol denotes homozygous mutation status of the proband (P). U indicates untested.
COMMENT
There are 2 particularly interesting aspects of this case. First, clinically, we have an example of a long-unsolved case whose underlying cause was recognized only after recent advances in the molecular biology of lipid metabolism. PNPLA2 was characterized as a triglyceride lipase gene in 2004,8 and its association with NLSDM was reported only in 2007.5 The establishment of a molecular diagnosis may prove especially important for this disorder because recent research on mammalian triglyceride metabolism suggests that β-agonists may be useful in the treatment of NLSDM by activating alternative pathways of triglyceride catabolism.6 Thus, the present case underscores the importance of revisiting old puzzling cases in the light of recent acquisitions
Second, the p.E361X mutation may provide insight into the structure-function relationship of ATGL. Because the complete structure of ATGL has not been solved, analysis of pathogenic mutations is an especially useful investigatory tool. Human ATGL is a triacylglycerol lipase of 504 amino acids. It localizes to the surface of lipid droplets,9 where it catalyzes the breakdown of triacylglycerol to diacylglycerol and free fatty acid.8 The N-terminal region of ATGL is relatively well understood from mutational analyses and from comparison with homologues with known structures. The lipolytic patatin domain occupies much of the N-terminal portion of the protein (residues 10–178); more generally, the entire N-terminal portion (residues 1–251) is typically annotated as belonging to a family of α/β hydrolase folds.5,10
However, the structure and function of the C-terminal (residues 252–504) portion is less clear. Published information on its structure suggests that it contains a hydrophobic region (variously associated with residues 310–365,11 315–360,12 or 309–3915) that may serve in binding to lipid droplets. Although, paradoxically, ablation of the C-terminus greatly increases in vitro lipolytic activity of murine and human ATGL, C-terminal mutations also abolish lipid droplet–associated lipolysis in cells, suggesting that the C-terminal region serves to regulate the rate of lipolysis in addition to facilitating localization to lipid droplets.11,12 Duncan et al11 found that a murine ATGL-GFP fusion protein truncated at W367 (human W365) showed poor lipolysis in living cells. The observations in the murine model correspond closely with the phenotype observed in the present case with the p.E361X mutation. That the present mutation has a significant deleterious effect on function in vivo, despite sparing most of the conserved hydrophobic lipolytic domain, indicates that the deleted C-terminal residues are critical for normal ATGL activity.
Acknowledgments
Funding/Support: Dr Hirano is supported by National Institutes of Health grants R01 HD056103 and R01HD057543 and by a Muscular Dystrophy Association grant. Drs DiMauro and Hirano are supported by National Institutes of Health grants P01HD032062 and RC1NS070232 and by the Marriott Mitochondrial Disorder Clinical Research Fund.
Footnotes
Author Contributions: Study concept and design: Papadimitriou, Hays, and Hirano. Acquisition of data: Ash, Papadimitriou, Hays, and Hirano. Analysis and interpretation of data: Ash, DiMauro, and Hirano. Drafting of the manuscript: Ash and Hirano. Critical revision of the manuscript for important intellectual content: Ash, Papadimitriou, Hays, DiMauro, and Hirano. Administrative, technical, and material support: Ash, Papadimitriou, Hays, DiMauro, and Hirano. Study supervision: Hirano.
Financial Disclosure: None reported.
REFERENCES
- 1.Jordans GH. The familial occurrence of fat containing vacuoles in the leukocytes diagnosed in two brothers suffering from dystrophia musculorum progressiva (ERB.) Acta Med Scand. 1953;145(6):419–423. doi: 10.1111/j.0954-6820.1953.tb07038.x. [DOI] [PubMed] [Google Scholar]
- 2.Dorfman ML, Hershko C, Eisenberg S, Sagher F. Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol. 1974;110(2):261–266. [PubMed] [Google Scholar]
- 3.Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975;1(5957):553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefèvre C, Jobard F, Caux F, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69(5):1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer J, Lefèvre C, Morava E, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39(1):28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 6.Reilich P, Horvath R, Krause S, et al. The phenotypic spectrum of neutral lipid storage myopathy due to mutations in the PNPLA2 gene. J Neurol. 2011;258(11):1987–1997. doi: 10.1007/s00415-011-6055-4. [DOI] [PubMed] [Google Scholar]
- 7.Akman HO, Davidzon G, Tanji K, et al. Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul Disord. 2010;20(6):397–402. doi: 10.1016/j.nmd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 9.Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122(pt 11):1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grӧnke S, Mildner A, Fellert S, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1(5):323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Duncan RE, Wang Y, Ahmadian M, Lu J, Sarkadi-Nagy E, Sul HS. Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells. J Lipid Res. 2010;51(2):309–317. doi: 10.1194/jlr.M000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweiger M, Schoiswohl G, Lass A, et al. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283(25):17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]