Abstract
Stem cells are primitive self renewing undifferentiated cell that can be differentiated into various types of specialized cells like nerve cell, skin cells, muscle cells, intestinal tissue, and blood cells. Stem cells live in bone marrow where they divide to make new blood cells and produces peripheral stem cells in circulation. Under proper environment and in presence of signaling molecules stem cells begin to develop into specialized tissues and organs. These unique characteristics make them very promising entities for regeneration of damaged tissue. Day by day increase in incidence of heart diseases including left ventricular dysfunction, ischemic heart disease (IHD), congestive heart failure (CHF) are the major cause of morbidity and mortality. However infracted tissue cannot regenerate into healthy tissue. Heart transplantation is only the treatment for such patient. Due to limitation of availability of donor for organ transplantation, a focus is made for alternative and effective therapy to treat such condition. In this review we have discussed the new advances in stem cells such as use of cord stem cells and iPSC technology in cardiac repair. Future approach of CB cells was found to be used in tissue repair which is specifically observed for improvement of left ventricular function and myocardial infarction. Here we have also focused on how iPSC technology is used for regeneration of cardiomyocytes and intiating neovascularization in myocardial infarction and also for study of pathophysiology of various degenerative diseases and genetic disease in research field.
Keywords: Stem cells, Myocardial infarction, Cardiac regeneration, Umbilical cord blood, iPSC technology
Introduction
Nowadays stem cells are becoming very important in various research centers. Stem cells are self renewable, and can be differentiate into specialized cells like nerve cell, skin cell, intestinal cell, blood cell etc. Adult stem cells, embryonic stem cells, umbilical cord stem cells, iPS cells are the different sources of stem cells for treatment of various diseases (1–5). Worldwide increase in incidence of various heart diseases including ischemic heart disease (IHD), congestive heart failure (CHF) are the major cause of death. Adult stem cell has natural regenerative property but due to limited regenerative potential as compare to pluripotent stem cells they are found to be less useful (6). Many scientist and researchers are giving their contribution in this field. In this review cord blood (CB) and induced pluripotent stem (iPS) are majorly highlighted for repairing of damaged cardiac tissue. Much of work is done on animal models and various clinical trials are performed to prove regenerative efficiency of CB stem cells. CB stem cells are collected from newborns and are able to use as a regenerative medicine with reduced graft versus host reaction and other immune rejection. CB contains mesenchymal cells which play major role on cardiomyocyte regeneration and angiogenesis. iPS cells are derived from insertion of defined factors such as Oct3/4, Sox2, c-Myc and Klf4, in somatic cells. iPS cells play important role in restoring the function of infarcted cardiomyocytes by replacing damaged myocardium (7). Thus CB stem cells and iPS cells are the ideal candidates for their use as regenerative medicine, tissue engineering, and cell replacement therapies a good approach for the treatment of heart disease.
Sources of stem cells
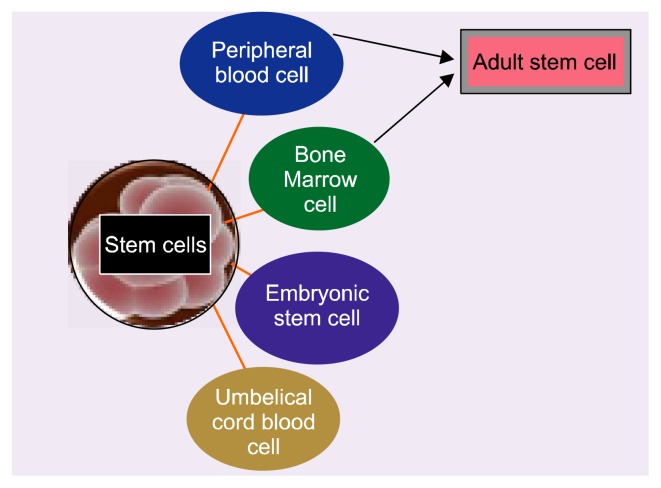
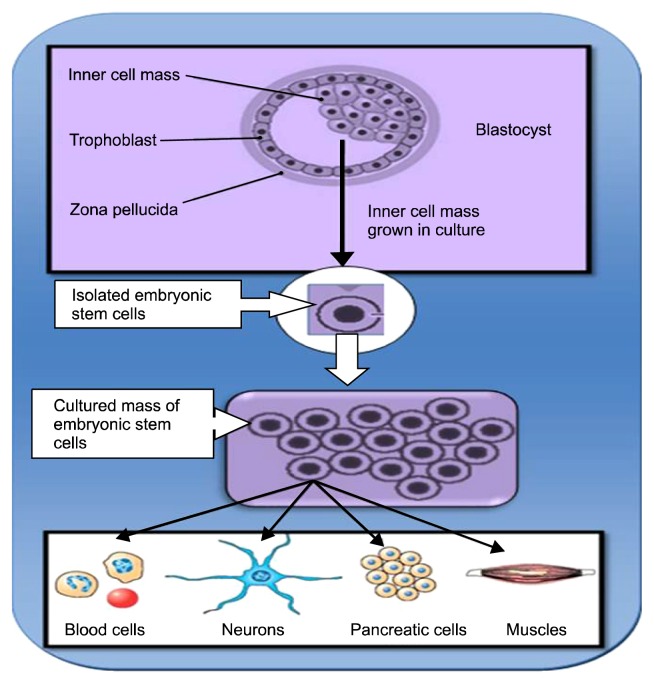
There are many different types of stem cells that originate from different places in the body and are formed at different time. Stem cell may be adult stem cells and embryonic stem cells (Fig. 1). A] Adult stem cells can be derived from bone marrow, peripheral blood and certain other organs like brain, liver, skeletal muscle, and dermal tissue. 2] Peripheral stem cells are very less in number but it can be increased by giving some white cell growth factors. Embryonic stem cells can be derived from inner cell mass of blastocyst, one of the embryonic stages and consist of 150 cells. Embryonic stem cells are pluripotent stem cells have ability to develop into three germ layers namely ectoderm, mesoderm and endoderm which give rise to different cells of the body (Fig. 2). Ectodermal layer forms skin, neural, and ocular cells while mesodermal layer forms cardiac muscle, skeletal muscle, smooth muscle and kidney cells and endodermal layer give rise to lung, pancreatic, ovarian cells, testicular cells, hepatic cells and cells of GIT tract (8).
Fig. 1.
Schematic presentation of different sources of stem cells.
Fig. 2.
Differentiation of Embryonic stem cells into various cells.
One more new source of stem cells is Umbilical cord stem cells. It is collected from umbilical cord after baby is born. These stem cells have gained their importance since last two years. It used as an alternative source of hematopoietic stem and progenitor cells capable of reconstituting the bone marrow of recipients in various hematological diseases. Nowadays cord stem cells are used for repairing of cardiac tissue damaged due to myocardial infarction and left ventricular dystrophy.
Umbilical cord stem cell
Cord stem cells are obtained from placental blood after baby’s birth. In the past the cord stem cell was usually thrown away without considering its importance. The blood in umbilical cord is collected in special collecting bags.
Almost every cell in the body display proteins named as Human leukocyte antigens (HLA) on cell surface. HLA play a major role in the immune recognition of foreign proteins by binding short peptides and presenting them to T lymphocyte. There are 2 classes of antigens. Class I antigens are expressed on the surface of almost all nucleated cells in the human body. They have been sub classified as HLA-A, HLA-B, and HLA-C. Class II antigens are expressed on the surface of immune cells and can be induced in some other cell types. These have been sub-classified as HLA-DR, HLA-DQ, and HLA-DP (9). UCB show less stringent HLA matching between patient and the donor has yielded favorable outcomes.
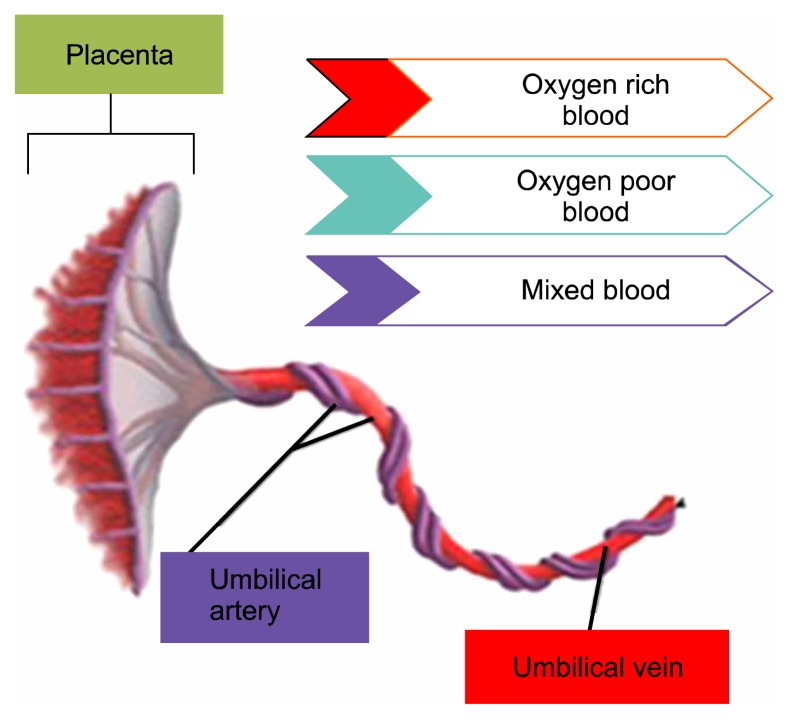
The umbilical cord consists of two arteries and one vein arranged in a helical structure (Fig. 3) (10). Two arteries of the umbilical cord spiral around the umbilical vein, making them stiffer and more difficult to rupture. Umbilical cord arteries and veins carry deoxygenated blood to the placenta and oxygenated blood from the placenta to the developing fetus, respectively (11).
Fig. 3.
The umbilical cord arranged in a helical structure.
Collection of UCB
Cord blood is collected at the time of delivery by one of 2 techniques: either in vivo or in vitro in a specialized apparatus. The cord is wiped clean and held slightly away from the perineum to avoid contamination with maternal blood. A large bore needle is then inserted into the umbilical vein. This is connected to a closed collection bag that contains an anticoagulant (usually citrate-phosphate-dextrose) (11).
Storage
Cord blood can be stored in public or private bank depending on availability. Stem cells are preserved in nitrogen freezer till transplantation. The 20 ml blood is then immediately ready for addition of a cryopreservent that is generally a 5 ml solution of 55% w/v dimethyl sulfoxide (DMSO) to prevent damage of collected cells, 5% dextran-40 and water resulting in a final volume of 25 ml. Initially CB units are placed in a controlled rate freezer (CRF) that is programmed to take the cells down to a temperature of −80°C through a freeze curve validated by each center to prevent detrimentation of CB units. In most cases, however, CB units are stored in the vapour phase of a standard liquid-nitrogen supplied storage tank maintained at a temperature of −196°C (12).
Clinical applications of UCB
Cord blood consists of hematopoietic stem cells and mesenchymal stem cells. It has high proliferating capacity and so can be used for treatment of disease in adult human being. CD34+ cells from cord blood possess higher proliferative potential in vitro (13). Cord blood also contains potent angiogenesis stimulating cells. In addition to endothelial progenitors, mesenchymal stem cells, which are found in cord blood, are known to secrete numerous cytokines and growth factors such as VEGF and FGF-2 which stimulate angiogenic process (14). Human UCB-derived stem/progenitor cells can be used as an alternative cell source to rejuvenate the infarcted myocardium, enhance healing, and improve left ventricular (LV) function. Leor et al and co-author in their work came with new findings that intravenous delivery of human UCB-derived CD133+ progenitor cells can prevent scar thinning, attenuate systolic dilatation, and improve LV function after MI. The transfused cells were able to migrate, colonize, and survive in the infarcted myocardium. However, the therapeutic effect was independent of trans-differentiation and direct myogenic or angiogenic contribution (15).
Cord blood-derived mesenchymal stem cells have demonstrated ability to differentiate into a wide variety of tissues in vitro including neuronal, hepatic, osteoblastic, and cardiac tissue (16–21). The existence of cells with such pluripotency in cord blood was also observed by Kogler and colleagues who identified an Unrestricted Somatic Stem Cell (USSC) with capability of differentiation into functional osteoblasts, chondroblasts, adipocytes, hematopoietic and neural cells (22). UCB stem cell therapy in MI are under investigation: including improved myocardial perfusion, attenuation of cardiac remodelling, reduction of inflammatory responses by limiting expression of TNF-α, MCP-1, MIP and INF- α, and cardiac regeneration (23).
Indeed, the study laboratory has shown that hUCB cells release angiogenic factors in vitro under hypoxic conditions. This is supported by the data consistent with a previous report that showed increased expression of VEGF 164 and 188 accompanied by angiogenesis and improved remodelling after administration of hUCB mononuclear cells into the myocardium (24).
Expansion capacity of cord blood mesenchymal stem cells is 20 times whereas for adipose derived cells expansion capacity is 8 times and bone marrow derived cells expanded 5 times (25).
To improve regenerative capacity it necessary to administer of cord blood cells in combination with activators of endogenous stem cells. For example, clinically used activators such as thalidomide (26), valproic acid (27), or 5-aza-cytidine (28, 29) all have demonstrated ability to induce proliferation of CD34+ stem cells in vitro and/or in vivo. Some endogenous chemo attractants are secreted by the injured tissue. For example, following myocardial infarction, as well as stroke, there is a period of time at which concentration of local stem cell chemo attractants are so high for repair of tissue.
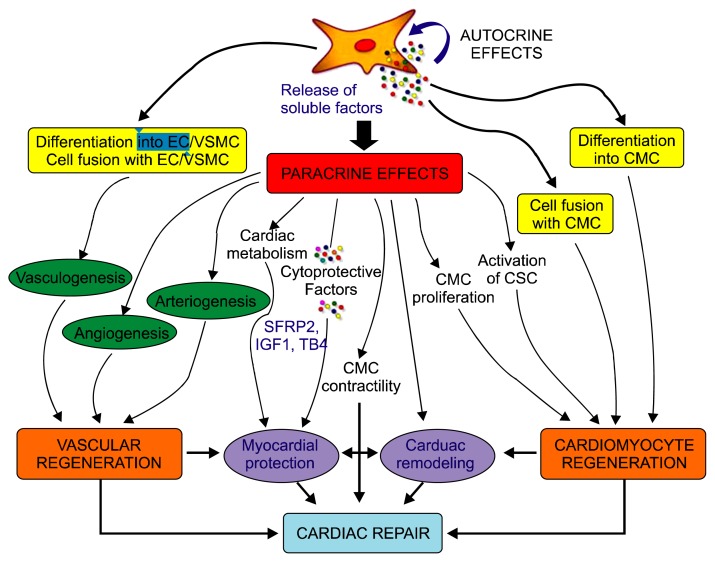
Massimiliano Gnecchi and coworkers explained three mechanism of action of MSC cell in cardiac repair. Among them are cardiomyocyte regeneration, vasculogenesis and paracrine effects. They also explained MSC cells reduces scar size, improved left ventricular function, induced reverse remodeling, Most importantly, engrafted MSC secrete a wide array of soluble factors that mediate beneficial paracrine effects and may greatly contribute to cardiac repair (Fig. 4) (30, 31). Gaballa and co-workers in myocardial infracted rats showed that CD34+ CB stem cells induced blood vessel formation, reduced infarct size and restored heart function. However these effects are thought to be due to release of angiogenic factors like VEGF, EGF and angiopoietin 1, 2 and induction of angiogenesis/vasculogenesis. It has been observed in many of research that process of neovascularization is due to pre-existing endothelial cells eitherat the site of injury or from circulatory endothelial cells (32, 33). UCB contains mesenchymal stem cells that secrete some soluble factors at damaged site creating microenvironment that regulate biological activities including angiogenesis, repair of damaged tissue eg. in myocardial infarction.
Fig. 4.
In a study using culture medium supplemented with the demethylating agent 5-azacytidine (5- AZA) at a concentration of 3 μmol/L for 24 h. Makino and collaborators reported that the morphology of almost 30% of the cells changed from fibroblast-like shape to a ball-like form and, with time, to the characteristic rod-shape of myofibers, and started expressing fetal CMC markers. Electron microscopy revealed a CMC-like structure, such as the presence of sarcomeres, centrally positioned nuclei, and atrial granules (34).
David T Harris and his colleague in their review showed that CB contains multiple populations of pluripotent stem cells and capable of giving rise to hematopoietic, epithelial, endothelial and neural tissues both in vitro and in vivo (35). Thus, CB stem cells are nowadays can be able to treat a wide variety of diseases including cardiovascular, ophthalmic, orthopedic, neurologic, and endocrine diseases.
There is ongoing research to find more diseases that can be helped by cord stem cells. Because these stem cells are primitive in nature, they have a lower rate of complications than with other stem cells transplant as that in bone marrow.
Advantages
Cord blood is easily collected following permission of the newborn’s parents. There is no pain while collecting cord blood stem cell as that of marrow stem cells. It is an safe procedure there is no harm to the baby while collecting blood.
They are more abundant than stem cells in red bone marrow.
They are less likely to cause graft-versus-host disease, so that there is no issue of match between donor and recipient cells as that required in bone marrow transplant. This provides a larger number of potential donors. Because newborns are in a relatively immunodeficient state, the risk for developing Graft versus Host Disease (GvHD) is reduced.
They are less likely to transmit infections.
They can be stored indefinitely in public or family cord-blood banks and can be made easily available whenever required.
It also reduces matching problem highly observed in Bone marrow transplantation (BMT) where transplantation is from HLA-matched unrelated donors or HLA-matched unrelated donors (36).
Disadvantages
Cord blood may act as an carrier for certain genetic disorder from parents.
Storage Cost for Cord stem cells banking is usually between $1,000~$ 2,000 and annual storage fee of approximately $100~$150.
Induced pluripotent stem cell
Induced pluripotent stem cells are reprogrammed cells and obtained by inserting the transcription factor in adult stem cells. The resulting iPS can differentiate into three germ layers in vitro. Takahashi and Yamanaka has demonstrated induction of pluripotent stem cells from mouse embryonic or adult fibroblasts by introducing four defined factors, Oct3/4, Sox2, c-Myc and Klf4, under ES cell culture conditions. Some research also showed reprogramming of cell by inserting factors NANOG, and LIN28. These reprogrammed cell exhibits same morphology, proliferative, differentiating capacity and gene markers as that of ES cells. It is observed that there is teratoma formation after subcutaneous transplant of iPS cells into nude mice (37). Each established iPS cell line can be characterized by several methods. Reverse-transcription polymerase chain reaction, immunocytochemistry and antibody staining can be used to evaluate specific genes or proteins further microarray analysis is useful for gene expression of iPS cells. Both in vivo and in vitro differentiation should be assessed to evaluate the pluripotency of each iPS cell line (38). Teratoma formation is an established assay that can be used to determine the in vivo differentiation. Embryoid body formation can be used to assess the in vitro differentiation of iPS cells (39, 40).
Generation of iPS cells
Initially for reprogramming of cells viral vectors were used which leads to insertional mutations and finally results in tumorigenesis. Therefore methods for reprogramming that do not depend upon the use of viral vectors must be established first, by making use of small molecules which are able to induce the expression of key genes. Junying Yu described the derivation of human iPS cells with the use of non-integrating episomal vectors (41). After removal of the episome, iPS cells completely free of vector and transgene sequences are derived that are similar to human embryonic stem (ES) cells in proliferative and developmental potential. Kim and his co-workers used protein delivery method and report the generation of stable iPS cells from human fibroblasts by directly delivering four reprogramming proteins (Oct4, Sox2, Klf4, and c-Myc) fused with a cell penetrating peptide (CPP). They eliminated the use of viruses, DNA transfection, and potential harmful chemical for induction of pluripotency in the cell. It has been reported that iPS cell in future could potentially provide a safe source of patient-specific cells for regenerative medicine and which can be used for clinical trials (42).
However molecular mechanisms that bring about this direct reprogramming are not known. The Takahashi and his coworkers found that Oct3/4 and Sox2 may up regulate expression of core genes associated with pluripotency and hypothesize that c-Myc and Klf4 act to modify chromatin structure to allow Oct3/4 and Sox2 access to these key target genes (43).
The ability to derive pluripotent cells from adult human tissues opens important opportunities in research and therapy. Suitable hepatocytes could be produced from iPS cells derived from individuals known to have the critical alleles for key metabolic enzymes for study of new drug during its development procedure. In addition, it will become possible to study cells in the laboratory that are equivalent to those in a patient with an inherited disease, even if the causative mutation has not been identified. These iPS cells may be used to identify the molecular mechanisms that cause a particular disease and also lead to the development of high-throughput drug screens (44).
Clinical application of iPSC
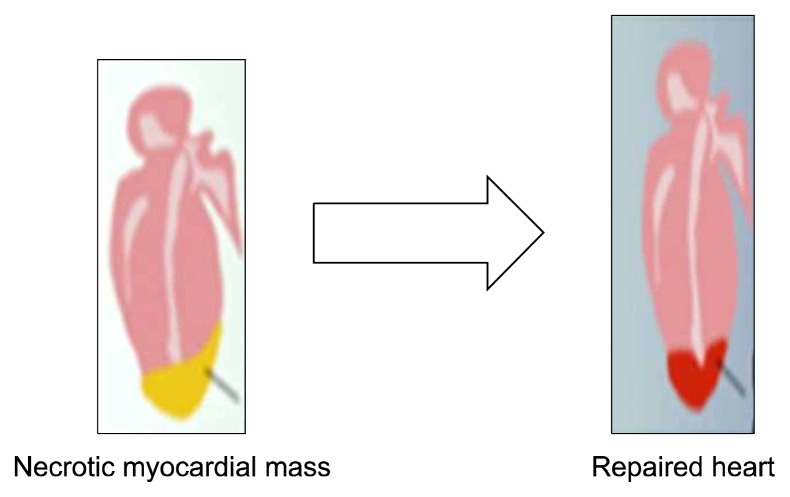
Myocardial infarction (MI) is the leading cause of death through the world. After myocardial infarction there is cardiac tissue death and restoration of cardiomyocyte is lost due to limited regenerative capacity of cell (Fig. 5) (45). ES derived Cardiomyocytes can partially remuscularize infarcted hearts and improve contractile function; however, the effect was not sustained over long follow up periods due to their limited capacity of cell division in vivo. Transplantated ES-derived cardiomyocytes disappear within several days after direct injection into hearts (46, 47). Therefore survival rate of grafted cells can be improved by administration of prosurvival factors with the grafted cells. Human iPSC cardiomyocytes when implanted in mouse model of MI shows regeneration of myocardium, smooth muscle, and endothelial tissue, restoring post ischemic contractility performance and electric stability (48).
Fig. 5.
Regeneration of myocardial tissue (45).
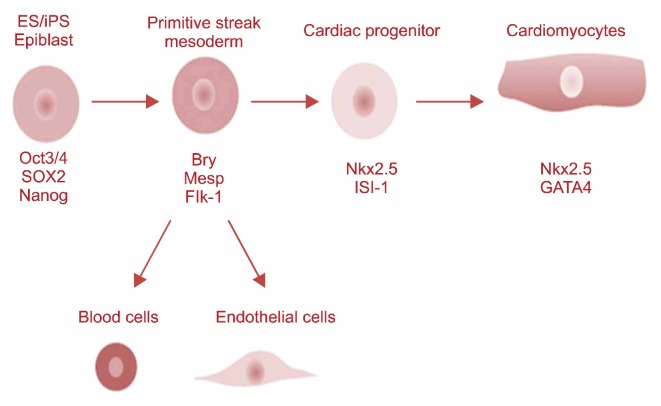
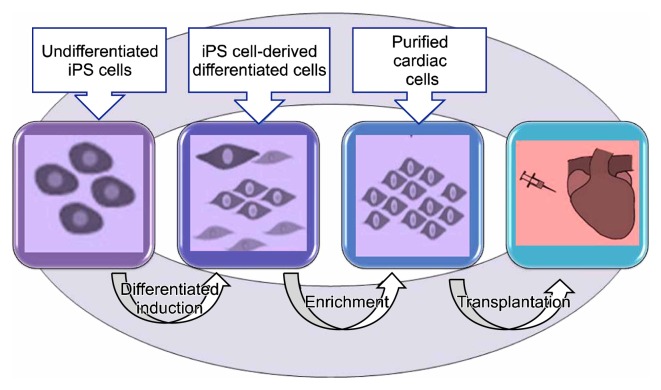
Recently iPSC technology is use to repair and replace damaged myocardium. Under suitable conditions, iPSCs could long-term propagate in undifferentiated state or differentiate into many other cell types, lincluding functional cardiomyocytes. Fig. 6, 7 shows stem cell based Cardiomyocyte differentiation from iPS cells and cardiac regeneration (49, 50). Different inducers including 5-Azacytidine, ascorbic acid and cyclosporine-A are used increase cardiac differentiation. Liu and colleagues discussed iPSC-based cardiac tissue regeneration and engineering including development of iPSC derivation, in vitro strategies for cardiac generation from iPSCs, cardiac application of iPSCs, challenges confronted at present as well as perspective in the future (51).
Fig. 6.
Fig. 7.
Jackson and their colleagues in 2001 at USA transplanted highly enriched hematopoietic stem cells, the so-called side population (SP) cells, into lethally irradiated mice subsequently rendered ischemic by coronary artery occlusion for 60 minutes followed by reperfusion. In the study they tested a novel “side population” (SP) of CD34− stem cells which is chracterised by RT-PCR and selected on the basis of Hoechst dye staining, for their capacity to regenerate cardiac myofibers and blood vessels in ischemically injured cardiac tissue. They isolated SP cells from Rosa26 transgenic mice and observed that isolated SP cells did express the Tie-2 gene, which encodes a receptor for angiopoietins 1 and 2. Paracrine signaling pathway between Ang-1 and its receptor Tie-2 is responsible for recruitment of smooth muscle cells and pericytes to stabilize newly forming endothelial tubes. In this study purified SP cells also expressed the early hematopoietic/endothelial cell transcription factor Tal-1/SCL, as well as three isoforms of VEGF-A and angiopoietin- 1 (Ang-1). Purified SP cells marked with the lacZ gene regenerated the hematopoietic system (52).
A deeper analysis of the proposed paracrine effects revealed that the transplanted iPS cells basically attenuated inflammation, increased angiogenesis, reduced apoptosis of the surrounding cells and promoted recovery of the injured tissue through wound healing (53–61). So iPSCs technology is an emerging trend for cardiac repair/regeneration. Therefore a focus is made on the current status of iPSC’s based cardiac tissue regeneration and engineering.
It has been shown that iPSCs are similar to hESCs in terms of their morphology, proliferation, feeder dependence, surface markers, gene expression, epigenetic status, formation of EBs in vitro, promoter activities, telomerase activities, and in vivo teratoma formation (62). iPSCs differ from ESCs at the molecular level when comparing gene expression signatures (63, 64).
iPS cells apart from its use in cardiac repair can also be used to study pathophysiology of various diseases include adenosine deaminase deficiency-related severe combined immunodeficiency (ADA-SCID), Shwachman-Bodi-an-Diamond syndrome (SBDS), Gaucher disease (GD) type III, Duchenne (DMD) and Becker muscular dystrophy (BMD), Parkinson disease (PD), Huntington disease (HD), juvenile-onset, type 1 diabetes mellitus (JDM), Down syndrome (DS)/trisomy 21, and the carrier state of Lesch-Nyhan syndrome (65). These specific stem cells can be used for new drug development and research purpose.
Some drawback of iPSC technology: 1] Reprogramming efficiency is still low and 2] tumorigenicity is also an obstacle preventing the further application of iPSCs. Studies in mice and rats consistently indicated that intramyocardial transplantation of iPSCs is accompanied with a high tumorigenic risk due to contamination of the graft with remaining undifferentiated stemcells. To circumvent the risk of teratoma formation, it is proposed that the number of undifferentiated cells within the graft should be reduced and highly purified in order to contain only the cells destined to replace the diseased tissue. Cell surface markers could be used in flow-sorting protocols that are successfully tested and believed to eliminate the number of undifferentiated cells, leading to high yield of differentiated cells. Some studies showed that abnormal overexpression of some undesired genes. Other challenges include poor retention and survival of transplanted cells in target regions, long-term efficacy, arrhythogenic risk, and so on (66, 67), Development of cell reprogramming or iPSC technology may open up a new perspective to the quickly progressing field of cell-based therapy.
Conclusion
CB stem cells contain mesenchymal Stem cells for restoration of infarcted cardiac cells. Recently there has been a substantial increase in the clinical use and research investigation of umbilical cord in hematopoietic transplantation and in regenerative medicine. Stem cells made available from cord blood are highly potent as compare to adult stem cells. Induced pluripotent stem cells are produced by inserting special defined factors in somatic cells. iPS cells are able to regenerate infracted heart and shows sustained effect for long time as compare to ES derived cardiomyocytes. iPSC technology can open a new hope in regenerative medicine. Apart from various drawback of iPSC technology step should put forth to develop technique for optimization of reprogramming efficiency of induced pluripotent stemcells. iPS cells also play important role in study of pathophysiology of various diseases in research field and drug testing. The major advantage of using tissue-specific stem cells for therapy is that there is less possibility of immune rejection. However, there are several problems that need to be addressed, the commonest being the difficulties arising from their isolation and in vitro expansion and limited amounts of cells. Both CB stem cells and iPSC technology is emerging trend in repairing of damaged tissue and it can act as better alternative in regenerative medicine.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009;4:e4226. doi: 10.1371/journal.pone.0004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Mazzone T. Human cord blood stem cells and the journey to a cure for type 1 diabetes. Autoimmun Rev. 2010;10:103–107. doi: 10.1016/j.autrev.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 4.McKenna D, Sheth J. Umbilical cord blood: Current status & promise for the future. Indian J Med Res. 2011;134:261–269. [PMC free article] [PubMed] [Google Scholar]
- 5.Stem Cell Information [World Wide Web site] Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 2009. What are the potential uses of human stem cells and the obstacles that must be overcome before these potential uses will be realized? [Google Scholar]
- 6.Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 8.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moise KJ., Jr Umbilical cord stem cells. Obstet Gynecol. 2005;106:1393–1407. doi: 10.1097/01.AOG.0000188388.84901.e4. [DOI] [PubMed] [Google Scholar]
- 10.Tortora GJ, Derrickson BH. Development and Inheritance. In: Roesch B, editor. Principles of Anatomy and Physiology. 12th ed. Chichester: John Wiley & Sons; 2009. pp. 1144–1145. [Google Scholar]
- 11.Benirschke K, Kaufmann P. Anatomy and pathology of the umbilical cord and major fetal vessels. In: Benirschke K, Kaufmann P, editors. Pathology of the human placenta. 4th ed. New York: Springer-Verlag; 1995. pp. 319–377. [DOI] [Google Scholar]
- 12.Hamblin T. Stem cell banking- The growth of public and private cord blood banks. Stem cells, regenerative medicine, and society. World stem cell report. 2009:168–171. [Google Scholar]
- 13.Theunissen K, Verfaillie CM. A multifactorial analysis of umbilical cord blood, adult bone marrow and mobilized peripheral blood progenitors using the improved ML-IC assay. Exp Hematol. 2005;33:165–172. doi: 10.1016/j.exphem.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–839. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- 15.Leor J, Guetta E, Feinberg MS, Galski H, Bar I, Holbova R, Miller L, Zarin P, Castel D, Barbash IM, Nagler A. Human umbilical cord blood-derived CD133+ cells enhance function and repair of the infarcted myocardium. Stem Cells. 2006;24:772–780. doi: 10.1634/stemcells.2005-0212. [DOI] [PubMed] [Google Scholar]
- 16.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JA, Gang EJ, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Rapid neural differentiation of human cord blood-derived mesenchymal stem cells. Neuroreport. 2004;15:1731–1734. doi: 10.1097/01.wnr.0000134846.79002.5c. [DOI] [PubMed] [Google Scholar]
- 18.Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461–7465. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153–1161. doi: 10.1016/j.bbrc.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 20.Hutson EL, Boyer S, Genever PG. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005;11:1407–1420. doi: 10.1089/ten.2005.11.1407. [DOI] [PubMed] [Google Scholar]
- 21.Kadivar M, Khatami S, Mortazavi Y, Shokrgozar MA, Taghikhani M, Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 22.Kögler G, Sensken S, Airey JA, Trapp T, Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida-Porada G, Müller HW, Zanjani E, Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu KH, Zhou B, Yu CT, Cui B, Lu SH, Han ZC, Liu YL. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83:1491–1498. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 24.Hu CH, Wu GF, Wang XQ, Yang YH, Du ZM, He XH, Xiang P. Transplanted human umbilical cord blood mono-nuclear cells improve left ventricular function through angiogenesis in myocardial infarction. Chin Med J (Engl) 2006;119:1499–1506. [PubMed] [Google Scholar]
- 25.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 26.De Felice L, Tatarelli C, Mascolo MG, Gregorj C, Agostini F, Fiorini R, Gelmetti V, Pascale S, Padula F, Petrucci MT, Arcese W, Nervi C. Histone deacetylase inhibitor valproic acid enhances the cytokine-induced expansion of human hematopoietic stem cells. Cancer Res. 2005;65:1505–1513. doi: 10.1158/0008-5472.CAN-04-3063. [DOI] [PubMed] [Google Scholar]
- 27.Araki H, Mahmud N, Milhem M, Nunez R, Xu M, Beam CA, Hoffman R. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin-modifying agents. Exp Hematol. 2006;34:140–149. doi: 10.1016/j.exphem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki M, Harashima A, Okochi A, Yamamoto M, Naka-mura S, Motoda R, Yamasaki F, Orita K. 5-Azacytidine supports the long-term repopulating activity of cord blood CD34(+) cells. Am J Hematol. 2004;77:313–315. doi: 10.1002/ajh.20178. [DOI] [PubMed] [Google Scholar]
- 29.Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Homing genes, cell therapy and stroke. Front Biosci. 2006;11:899–907. doi: 10.2741/1846. [DOI] [PubMed] [Google Scholar]
- 30.Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012;57:48–55. doi: 10.1016/j.vph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Gnecchi M, Zhang Z, Ni A, Dzau YJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furfaro EM, Gaballa MA. Do adult stem cells ameliorate the damaged myocardium? Human cord blood as a potential source of stem cells. Curr Vasc Pharmacol. 2007;5:27–44. doi: 10.2174/157016107779317170. [DOI] [PubMed] [Google Scholar]
- 33.Sunkomat JNE, Goldman S, Harris DT. Cord blood-derived MNCs delivered intracoronary contribute differently to vascularization compared to CD34 + cells in the rat model of acute ischemia. Stem Cells. 2007 (In Press) [Google Scholar]
- 34.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris DT, Badowski M, Ahmad N, Gaballa MA. The potential of cord blood stem cells for use in regenerative medicine. Expert Opin Biol Ther. 2007;7:1311–1322. doi: 10.1517/14712598.7.9.1311. [DOI] [PubMed] [Google Scholar]
- 36.Abotalib Z. Importance of cord blood stem cells in regenerative medicine. Saudi Journal of Biological Sciences. :1–33. Accepted Date: 25 December 2013. [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Ohnuki M, Takahashi K, Yamanaka S. Generation and characterization of human induced pluripotent stem cells. Curr Protoc Stem Cell Biol. 2009;Chapter 4(Unit 4A.2) doi: 10.1002/9780470151808.sc04a02s9. [DOI] [PubMed] [Google Scholar]
- 39.Daley GQ, Lensch MW, Jaenisch R, Meissner A, Plath K, Yamanaka S. Broader implications of defining standards for the pluripotency of iPSCs. Cell Stem Cell. 2009;4:200–201. doi: 10.1016/j.stem.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Ellis J, Bruneau BG, Keller G, Lemischka IR, Nagy A, Rossant J, Srivastava D, Zandstra PW, Stanford WL. Alternative induced pluripotent stem cell characterization criteria for in vitro applications. Cell Stem Cell. 2009;4:198–199. doi: 10.1016/j.stem.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Wilmut I. The first direct reprogramming of adult human fibroblasts. Cell Stem Cell. 2007;1:593–594. doi: 10.1016/j.stem.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Pawani H, Bhartiya D. Pluripotent stem cells for cardiac regeneration: overview of recent advances & emerging trends. Indian J Med Res. 2013;137:270–282. [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 47.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 48.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 49.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Z, Zhou J, Wang H, Zhao M, Wang C. Current status of induced pluripotent stem cells in cardiac tissue regeneration and engineering. Regen Med Res. 2013;1:6. doi: 10.1186/2050-490X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, et al. Embryonic endothelial progenitor cells expressing a broad range of pro-angiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–1578. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 54.Kupatt C, Hinkel R, Lamparter M, von Brühl ML, Pohl T, Horstkotte J, Beck H, Müller S, Delker S, Gildehaus FJ, Büning H, Hatzopoulos AK, Boekstegers P. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation. 2005;112(9 Suppl):I117–I122. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 55.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 56.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 57.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Müller S, Willhauck M, Spitzweg C, Gildehaus FJ, Münzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci U S A. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik’s cube of cell therapy for heart disease. Dis Model Mech. 2009;2:344–358. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: insights from experimental models. Thromb Haemost. 2010;104:30–38. doi: 10.1160/TH10-03-0189. [DOI] [PubMed] [Google Scholar]
- 63.Qian L, Srivastava D. Monkeying around with cardiac progenitors: hope for the future. J Clin Invest. 2010;120:1034–1036. doi: 10.1172/JCI42643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 65.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed RP, Ashraf M, Buccini S, Shujia J, Haider HKh. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen Med. 2011;6:171–178. doi: 10.2217/rme.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011;6:e19012. doi: 10.1371/journal.pone.0019012. [DOI] [PMC free article] [PubMed] [Google Scholar]