Abstract
Background and Objectives
Mesenchymal stem cells (MSCs) have been shown to ameliorate cisplatin-induced acute kidney injury (AKI). The present study compares the efficacy of different routes of MSCs administration on kidney damage and regeneration after cisplatin-induced AKI.
Methods
A single intraperitoneal injection of cisplatin (5 mg/kg) was used to induce AKI in 160 rats. MSCs (5×106) were given by either intravenous, intra-arterial or kidney sub capsular injection one day after cisplatin injection. Suitable control groups were included. Rats were sacrificed at 4, 7, 11 and 30 days after cisplatin injection. Kidney function parameters, kidney tissue oxidative stress markers, and scoring for renal tissue injury, regeneration and chronicity were all determined.
Results
MSCs by any routes were able to ameliorate kidney function deterioration and renal tissue damage induced by cisplatin. The overall results of the three routes were equal. Differences between the different routes in one parameter were transient and inconsistent with other parameters.
Conclusions
Changing the route of MSCs injection does not have a major influence on the outcome. Future evaluation should focus on differences between the routes of administration considering the long term safety.
Keywords: Acute kidney injury, Cisplatin, Mesenchymal stem cells, Routes of administration, Sub capsular
Introduction
The successful use of mesenchymal stem cells (MSCs) in treating acute kidney injury (AKI) in small animal models suggested that these cells might be a potential new therapy of AKI (1–3). In murine models, MSCs were capable of ameliorating renal damage induced by cisplatin (4, 5). It is now widely accepted that the renoprotective effect of MSCs is due to growth factors and cytokines which are secreted by these cells and were proven to have trophic, immunomodulatory, and anti-apoptotic effects (1, 6).
However, the suitable route to administer MSCs to gain their maximum benefits was not yet evaluated. In systemic administration through intravenous (i.v.) injection, most of the stem cells were found to accumulate within filtering organs such as the lung, liver, or spleen (7). Still, part of these cells could reach the kidney through homing of MSCs (8). In addition, it was suggested that homing of systemically administered MSCs in certain organs, as the spleen, could provide additional benefits as the immunomodulatory effect of MSCs could inhibit inflammatory cells in this inflammatory cell reservoir (9). Inflammatory cells influx from different reservoirs significantly contributes to kidney injury in cisplatin model of AKI (10, 11). On the other side, local injection of MSCs in the injured kidney would ensure the delivery of more MSCs to the kidney. Nevertheless, direct intra-arterial injection of MSCs was reported to have some limitations (12).
The present study was conducted to evaluate the effect of different routes of MSCs administration on the outcome of cisplatin-induced AKI. Assessment of histologic changes in the kidney has been conducted using a scoring system that considers different features of injury, inflammatory cell infiltration, regeneration, and chronic changes.
Materials and Methods
The experimental protocol was approved by the Local Ethical Committee, Faculty of Medicine; Mansoura University.
Reagents
Cisplatin was obtained from Hospira (Hospira UK Limited, Warwickshire, UK) in 1 mg/ml concentration. Dulbecco’s modified Eagle’s medium (DMEM) and 10% fetal bovine serum (FBS) were obtained from Lonza (Verviers, Belgium). Fluorescent labeled antibodies for flowcytometric analysis were obtained from eBioscience (San Diego, CA, USA).
Preparation and expansion of bone marrow mesencyhmal stem cells
Adult male Sprague-Dawley rats weighing 180 to 210 g served as bone marrow donors; MSCs were prepared as described previously (13). Bone marrow was obtained from long bones which were sterilized by immersion in 70% ethanol. The ends of the bones were cut and bone marrow was extruded by inserting a needle in one end through the bone shaft and injection of DMEM containing 10% FBS. The effluent was collected in sterile tubes and gentle pipetting resulted in generation of a single cell suspension. Bone marrow cells was counted and plated with a concentration of 10×106/ml in T-75 flasks and cultured in DMEM containing 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere that contained 5% CO2. Medium was changed after 4 days and every 3 days thereafter. Non-adherent cells were removed with medium changing. After a mean of 7 days, cells reached sub-confluence and were detached with trypsin/EDTA, reseeded at 4×103 cells/cm2, and used for experiments after the third passage. MSC features were demonstrated by typical spindle-shaped morphology.
Characterization of MSCs
Fibroblast-like colony-forming unit assay
Fibroblast colony growth was evaluated on primary cells grown on tissue culture in six-well dishes (14). Bone marrow-derived cells were plated at the density of 25×106 cells/well. After 7 days, the capability of MSCs to form fibroblast-like colonies was assessed.
In vitro differentiation assays
MSCs were grown until confluence and the growth medium was replaced with the inductive medium consisting of Iscove’s modified Dulbecco’s medium (Invitrogen), 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and β-mercaptoethanol supplemented with specific differentiation reagents as follows:
Osteogenesis assay
Cultures were fed twice a week for 3 weeks with 10 mM β-glycerolphosphate, 50 μg/ml ascorbic acid, and 10−9 M dexamethasone (15). Then cells were fixed with 10% formalin for 20 min at room temperature and the presence of calcium-rich hydroxyapatitein the extracellular matrix was assessed by staining for 20 min with Alizarin Red S (16).
Adipogenesis assay
Cells were incubated for 3 weeks with 5 μg/ml insulin (Sigma) and 10−9 M dexamethasone. Adipogenic differentiation was visualized in phase-contrast microscopy by the presence of highly refractive intracellular lipid vacuoles (15). Oil Red O (Sigma) staining was used to assay the accumulation of lipid droplets in these vacuoles (16).
Chondrogenesis assay
MSCs were harvested and 6×105 cells were centrifuged to form a pellet on the bottom of a 15-ml polypropylene tube (Falcon). The micro mass was cultured in 500 μl of chondrogenic medium that consisted of 50 μg/ml ascorbic acid 2-phosphate and 1 ng/ml TGF-β1 (Sigma) (15). After 3 weeks of culture, cell clumps were harvested, embedded in paraffin, cut into 3-μm sections, and stained for glycosaminoglycans using 0.1% safranin O (Sigma).
Real-time quantitative reverse transcriptase-polymerase chain reaction (RT–PCR)
Differentiation of MSCs to adipocytes was demonstrated by RT-PCR analysis of adipocytic markers expression. The selected markers included lipoprotein lipase, leptin, adiponectin and PPAR-γ. Total RNA was isolated from MSCs and real-time reverse transcriptase-PCR was performed as described previously (17). Selective primer sequences were used (Table 1). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard.
Table 1.
Primers for real-time RT-PCR conducted to detect adipocytic markers in mesenchymal stem cells
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Lipoprotein lipase | GTACAGTCTTGGAGCCCATGC | GCCAGTAATTCTATTGACCTTCTTGTT |
| Liptin | TTCACACACGCAGTCGGTATC | GTGAAGCCCGGGAATGAAG |
| Adiponectin | GGGATTACTGCAACCGAAGG | CCATCCAACCTGCACAAGTTT |
| PPAR-γ | CATACATAAAGTCCTTCCCGCTG | TTGTCTGTTGTCTTTCCTGTCAAGA |
| GAPDH | ACAAGATGGTGAAGGTCGGTG | AGAAGGCAGCCCTGGTAACC |
Flow cytometry for cell surface expression assay
Bone marrow-derived MSCs at passage 3 were released by trypsinization and analyzed by flow cytometry. The cells were centrifuged at 1,200 rpm for 5 minutes, and were then solved in phosphate buffered saline (PBS) at the concentration of (1×106/ml). Fluorescent labeled antibodies directed against CD34, CD45, CD29, CD44 and CD90 surface markers (10 μl for each sample) were added and incubated for 30 minutes at room temperature. Labeled cells were washed with PBS, fixed in flow buffer, and analyzed using a flow cytometer (Coulter).
Stem cell labeling
MSCs were marked by incorporation of 5-bromo-2 deoxyuridine (BrdU; Sigma-Aldrich, Taufkirchen, Germany). MSCs were incubated with BrdU (10 μM/L) for 48 h at 37°C to label more than 90% of the incubated cells (18).
Study design
The study was conducted on 160 adult female Sprague-Dawley rats weighing 180~210 g. Rats were divided into 4 groups. Except for group I, all animals were injected with cisplatin (single dose 5 mg/kg intraperitoneal, i.p. in 1 ml saline). One day latter, group III received MSCs while group IV was injected with fresh culture media alone.
Group I (negative control) (n=20): rats were injected with normal saline alone i.p. (1 ml).
Group II (cisplatin injected group) (n=20): animals received only cisplatin with no further interventions.
Group III (cisplatin injected and stem cells treated group) (n=60): This group was further divided into three subgroup according to the route of stem cell injection:
- Subgroup IIIa (n=20): rats were treated with 0.5 ml of culture media containing 5×106 MSCs injected i.v. in the tail vein.
- Subgroup IIIb (n=20): rats were treated with 0.5 ml of culture media containing 5×106 MSCs under the capsule of the left kidney.
- Subgroup IIIc (n=20): rats were treated with 0.5 ml of culture media containing 5×106 MSCs injected intra-arterially (i.a.) in the artery of the left kidney.
Group IV (cisplatin injected and fresh medium treated group) (n=60): This group was further divided into three subgroup according to the route of media injection:
- Subgroup IVa (n=20): rats were injected with 0.5 ml culture media i.v. in the tail vein.
- Subgroup IVb (n=20): rats were injected with 0.5 ml of culture media under the capsule of the left kidney.
- Subgroup IVc (n=20): rats were injected with 0.5 ml of culture media i.a. in the artery of the left kidney.
At days 4, 7, 11, and 30 after cisplatin injection, five rats were sacrificed at each time interval in groups I and II as well as each subgroup for groups III and IV. Blood samples were collected from the retro-orbital vein. Before sacrifice, 24 hr-urine was collected using a metabolic cage. Rats were sacrificed using an over dose of thiopental. Blood samples were collected from the heart then the left kidney was removed for preparation of renal tissue homogenate and for histopathological evaluation.
Biochemical measurement
Biochemical parameters were measured using an automated spectrophotometer (Slim Plus, Italy) and specific kits for creatinine (Diamond Diagnostics, Egypt), blood urea nitrogen (BUN; Stanbio Lab., Texas, USA), serum calcium (Biomerieux) and serum albumin (Egyptian American Company for Laboratory Services, Egypt). Lipid peroxidation (malondialdehyde ‘MDA’ production), reduced glutathione (GSH) contents and superoxide dismutase activity (SOD) in the kidney tissue homogenate were determined according to slandered methods (19, 20).
Renal morphology
The kidneys were perfused in a retrograde fashion through the abdominal aorta using saline 0.9% followed by 10% neutral buffered formalin for in situ fixation. Samples for tissue homogenate were obtained before the use of formalin. Renal samples were processed for light microscopic observation. Histopathological changes were analyzed in the different regions of the kidney (cortex, outer stripe of outer medulla “OSOM”, inner stripe of outer medulla “ISOM”, and inner medulla using hematoxylin and eosin (H&E). In each layer, the scoring system considered active injury changes, regenerative changes, and chronic changes. Active injury changes include necrotic tubules and interstitial infiltration by inflammatory cells. Regenerative changes include presence of mitosis, solid cellular sheets between the tubules, and regenerating tubules. Chronic changes include atrophic tubules with flat lining, casts, thick basement membrane and interstitial fibrosis (Table 2).
Table 2.
Pathological scoring of active injury, regenerative, and chronic changes in the kidney
| Finding | Score | |
|---|---|---|
| Active injury score | ||
| No of necrotic tubules/HPF | 1~3 | 1 |
| 4~5 | 2 | |
| 6~10 | 3 | |
| >10 | 4 | |
| No of rows of interstitial inflammatory cells | 1~3 | 1 |
| 4~5 | 2 | |
| >5 | 3 | |
| Regenerative changes score | ||
| No of regenerating tubules/HPF | 1~2 | 1 |
| 3~5 | 2 | |
| >5 | 3 | |
| No of mitotic figures/HPF | 1~2 | 1 |
| 3~5 | 2 | |
| >5 | 3 | |
| No of solid cellular sheets/HPF | 1~2 | 1 |
| 3~5 | 2 | |
| >5 | 3 | |
| Chronic changes score | ||
| Interstitial fibrosis by the ×100 | <25% | 1 |
| 25~50% | 2 | |
| 50~75% | 3 | |
| >75% | 4 | |
| No of atrophic tubules/HPF | 1~5 | 1 |
| 6~10 | 2 | |
| >10 | 3 | |
Statistical analysis
Statistical analysis was carried out using the SPSS software (version 16.0, SPSS, IL, USA). Data were tested for normal distributions by Kolmogorov-Smirnov test. Biochemical parameters were evaluated by analysis of variance (ANOVA) and post hoc test for multiple comparisons (Bonferroni). Values were expressed as mean±SD. Pathological scores of different groups were evaluated using Kruskall-Wallis (K-W) followed by Mann-Whitney test for significance between individual groups. Values were expressed as median (min-max). p-values<0.05 were considered statistically significant.
Results
The media treated group (group IV) had similar biochemical, oxidative stress, and histopathological parameters values as those of the cisplatin group without significant differences. Their results were excluded for simplification. These groups indicate absence of effects of the fresh culture media by different routes of administration in comparison to the groups treated by MSCs.
Characterization of rat bone marrow-MSCs
The isolated rat bone marrow-MSCs were characterized by their adherence to plastic, a predominant spindle-shaped morphology, and formation of fibroblast-like colonies (Fig. S1A). When cultivated in the suitable differentiation media, MSCs were able to differentiate into osteoblasts, adipocytes, and chondroblasts (Fig. S1B~D). Differentiation of MSCs to adipocytes was further demonstrated by RT-PCR analysis of adipocytic markers expression. Rat MSCs expressed lipoprotein lipase, leptin, adiponectin and a transcription factor known to be involved in control of adipocytic differentiation, PPAR-γ (Fig. S1E~H). By FACS analysis, MSCs were negative for the hematopoietic lineages markers CD34 and CD45; but positive for mesenchymal markers CD29, CD44, and CD90 (Fig. S1I~N).
Effects of MSCs by different routes on renal dysfunction caused by cisplatin
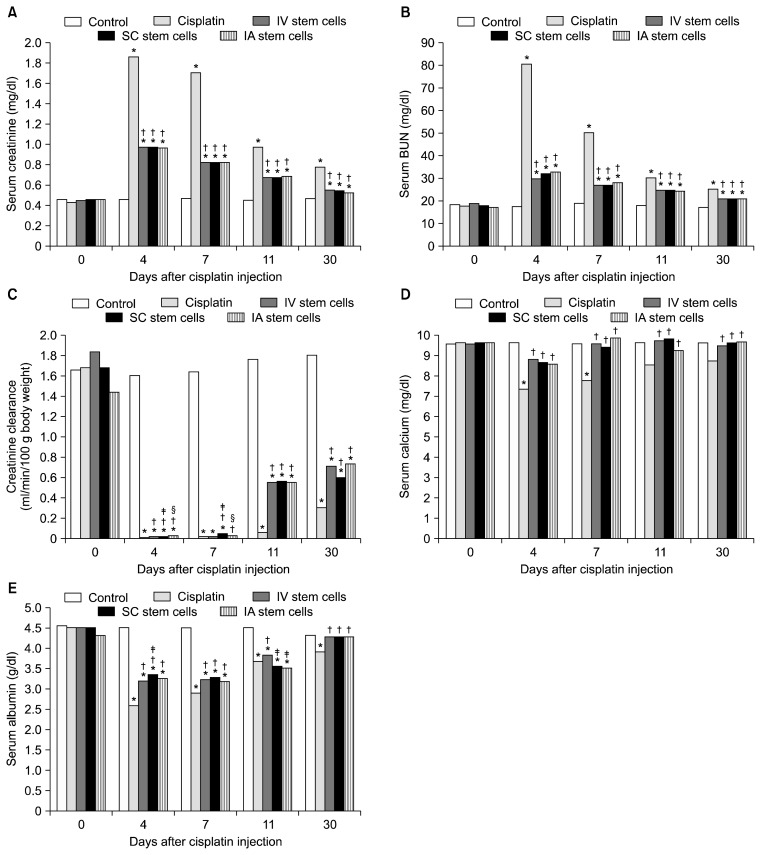
Cisplatin resulted in marked elevation of serum creatinine and BUN levels and markedly significant decrease in creatinine clearance at the 4th day. These parameters improved gradually but incompletely by the day 30. Administration of MSCs through any of the three routes resulted in gradual amelioration of such changes in these parameters. Changing the route of MSCs injection did not affect the outcome of creatinine or BUN. However, sub capsular MSCs injection was the least among all routes at day 4 but showed a moderate advantage at the 7th day (Fig. 1A~C).
Fig. 1.
Changes in serum creatinine (A), BUN (B), creatinine clearance (C), serum calcium (D), and serum albumin (E) in rats after cisplatin administration and treatment with stem cells (SC) through different routes (n=5 in each group for each time). Significant difference (p<0.05) *versus control, †versus cisplatin, ‡versus i.v. SC; §versus sub-capsular SC. Abbr.: IA: intra-arterial, IV: intravenous, SC: sub capsular.
Cisplatin resulted also into hypocalcaemia that appeared early at day 4 but improved spontaneously at day 11. MSCs by any route equally protected against the development of hypocalcaemia. Similarly, cisplatin resulted in early marked hypoalbuminaemia by day 4. MSCs by all routes also improved the hypoalbuminaemia. Of note, the effect of sub capsular stem cells on serum albumin outweighed that of i.v. stem cells at the 4th day but i.v. stem cell group took the lead over the other two groups by day 11 (Fig. 1D, E).
Effects of MSCs by different routes on the oxidative stress caused by cisplatin
Injection of cisplatin resulted in elevation of tissue levels of MDA and reduction of tissue levels of SOD and GSH. These changes were most evident by the 4th day. Treatment with MSCs through any of the three routes resulted in amelioration of such changes in these parameters. The effect i.v. stem cells on GSH outweighed that of sub capsular stem cells at the 7th day. Unexpectedly, the effect i.v. stem cells on GSH was slightly higher by the day 30. Otherwise, no statistically significant differences were evident between the i.a., i.v., and sub capsular injected MSCs (Table 3).
Table 3.
Changes in superoxide dismutase activity (SOD), malondialdehyde (MDA), and reduced glutathione (GSH) in rats kidney after MSCs injection by different routes at 4, 7, 11, and 30 days of cisplatin injection (n=5 in each group for each time interval)
| Control | Cispl. | Cispl.+i.v. SC | Cispl.+subcap.SC | Cispl.+i.a. SC | |
|---|---|---|---|---|---|
| SOD (U/g) | |||||
| 4 | 20.00±0.87 | 3.32±0.35a | 7.34±0.29a,b | 7.05±0.31a,b | 7.34±0.06a,b |
| 7 | 20.14±0.79 | 6.03±0.50a | 10.98±0.20a,b | 10.96±0.53a,b | 11.02±0.14a,b |
| 11 | 20.25±0.80 | 9.95±0.24a | 15.29±0.32a,b | 15.48±0.33a,b | 15.61±0.24a,b |
| 30 | 20.43±0.58 | 16.11±0.41a | 18.38±0.62a,b | 17.98±0.67a,b | 18.44±0.70a,b |
| MDA (nmol/g) | |||||
| 4 | 14.41±0.29 | 67.03±2.99a | 34.21±5.94a,b | 37.07±4.47a,b | 33.59±5.74a,b |
| 7 | 14.33±0.23 | 61.51±4.96a | 22.69±3.99a,b | 21.44±2.31a,b | 21.31±2.76a,b |
| 11 | 14.38±0.30 | 36.78±5.32a | 17.57±0.96b | 15.05±1.14b | 16.40±0.50b |
| 30 | 14.42±0.30 | 31.73±4.67a | 13.51±1.42b | 12.42±0.65b | 15.44±1.60b |
| GSH (mmol/g) | |||||
| 4 | 5.45±0.20 | 0.25±0.03a | 1.20±0.16a,b | 1.11±0.11a,b | 1.15±0.09a,b |
| 7 | 5.39±0.21 | 0.53±0.03a | 2.59±0.13a,b | 2.30±0.15a,b,c | 2.41±0.15a,b |
| 11 | 5.40±0.18 | 1.38±0.27a | 3.34±0.01a,b | 3.148±0.16a,b | 3.25±0.23a,b |
| 30 | 5.41±0.20 | 1.92±0.04a | 5.93±0.18a,b | 5.30±0.18b,c | 5.09±0.04a,b,c |
Significant difference (p<0.05) versus control
versus cisplatin,
versus IV SC;
Data are expressed as mean±SD; n: number of animals, Abbr.: i.a.: intra-arterial, i.v.: intravenous SC: stem cells, subcap.: subcapsular.
Effects of MSCs by different routes on the histopathological changes caused by cisplatin
Active injury changes
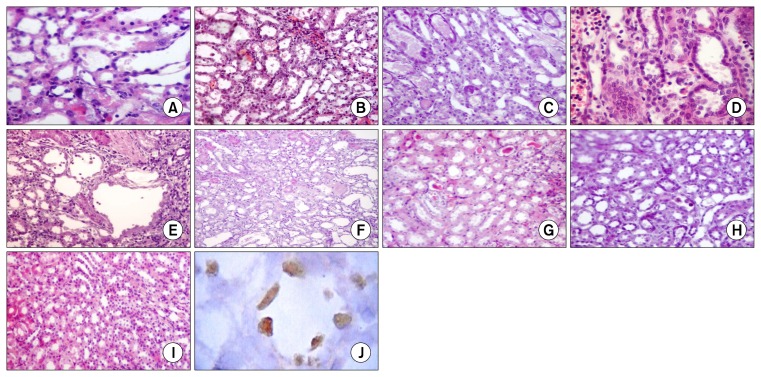
In comparison to normal (Fig. 2I), the injury induced by 5 mg/kg of cisplatin was restricted to the OSOM. Treatment with MSCs through any of the three routes resulted in attenuation of the number of necrotic tubules and inflammatory cell infiltration in comparison to the cisplatin injected group. No statistically significant differences were evident between the i.v., i.a., and the sub capsular injected MSCs at any time of the experiment (Fig. 2, Table 4).
Fig. 2.
Histological changes of the outer stripe of the outer medulla (OSOM) in the rat kidney after 4 (A~D) and 30 (E~H) days of cisplatin injection (H&E). In comparison to normal OSOM (I). Cisplatin led to tubular necrosis, degeneration, tubular casts, and cellular debris which were more evident at day 4 (A). The necrotic changes were attenuated while regenerative changes were enhanced when bone marrow mesenchymal stem cells were injected via the renal artery (B), the tail vein (C), or under the capsule of the kidney (D). Dilated tubules, some regenerating large tubules, and mild peritubular fibrosis without evidence of necrosis were all evident the cisplatin-treated group by the day 30 (E) but were much attenuated with bone marrow mesenchymal stem cells therapy in the renal artery (F), the tail vein (G), or under the capsule of the kidney (H). BrdU-labeled MSCs were traced 11 days after being injected under the capsule of the kidney (J). Cells were detected in the injured OSOM and few cells were integrated in the tubular epithelium. Magnification: ×100 (B, C, E~I); ×200 (A, D); ×400 (J).
Table 4.
Effect of MSCs by different routes on acute injury, regenerative and chronicity scores in the outer stripe of the outer medulla in rat kidney after 4, 7, 11, and 30 days of cisplatin injection (n=5 in each group for each time interval)
| Control | Cispl. | Cispl.+i.v. SC | Cispl.+subcap. SC | Cispl.+i.a. SC | |
|---|---|---|---|---|---|
| Necrotic tubule | |||||
| 4 | 0.0 (0.0~0.0) | 4.0 (4.0~4.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b | 2.0 (2.0~3.0)a,b |
| 7 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b | 2.0 (2.0~2.0)a,b |
| 11 | 0.0 (0.0~0.0) | 3.0 (3.0~4.0)a | 2.0 (2.0~3.0)a,b | 2.0 (2.0~3.0)a,b | 2.0 (2.0~2.0)a,b |
| 30 | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b |
| Inflammatory cells | |||||
| 4 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b |
| 7 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 1.0 (1.0~2.0)a,b | 1.0 (1.0~2.0)a,b | 1.0 (1.0~2.0)a,b |
| 11 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b |
| 30 | 0.0 (0.0~0.0) | 2.0 (2.0~3.0)a | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b | 0.0 (0.0~0.0)b |
| Regenerative tubules | |||||
| 4 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 1.0 (1.0.1.0)a,b | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b |
| 7 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b |
| 11 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a,b | 3.0 (2.0~3.0)a,b | 3.0 (2.0~3.0)a,b |
| 30 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 3.0 (3.0~3.0)a,b | 3.0 (3.0~3.0)a,b | 3.0 (3.0~3.0)a,b |
| Mitosis | |||||
| 4 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) |
| 7 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 1.0 (1.0~2.0)a,b | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b |
| 11 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b |
| 30 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b |
| Solid sheets | |||||
| 4 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b | 2.0 (2.0~2.0)a,b |
| 7 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b |
| 11 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~3.0)a,b |
| 30 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 3.0 (3.0~3.0)a,b | 2.0 (2.0~3.0)a,b,c | 3.0 (2.0~3.0)a,b |
| Interstitial fibrosis | |||||
| 4 | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) | 0.0 (0.0~0.0) |
| 7 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a |
| 11 | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b |
| 30 | 0.0 (0.0~0.0) | 2.0 (2.0~2.0)a | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b | 1.0 (1.0~1.0)a,b |
| Atrophic tubules | |||||
| 4 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 1.0 (1.0~1.0)a,b,c,d |
| 7 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b | 2.0 (2.0~2.0)a,b |
| 11 | 0.0 (0.0~0.0) | 3.0 (3.0~3.0)a | 2.0 (1.0~2.0)a,b | 2.0 (1.0~2.0)a,b | 2.0 (1.0~2.0)a,b |
| 30 | 0.0 (0.0~0.0) | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a | 1.0 (1.0~1.0)a |
Significant difference (p<0.05) versus control
versus cisplatin
versus IV SC
versus subcapsular SC;
data are expressed as median (min-max). Abbr.: i.a.: intra-arterial, i.v.: intravenous, SC: stem cells, subcap.: subcapsular.
Regenerative changes
The appearance of regenerative changes after cisplatin was delayed to day 11 (mitotic figures) and day 30 (regenerating tubules and solid sheets). Treatment with MSCs through any of the three routes resulted in appearance of features of regeneration as early as the 4th day (regenerating tubules and solid sheets) and the 7th day (mitotic figures). Only the i.v. stem cells was superior to sub capsular stem cells in enhancing the appearance of solid sheets by the 30th day. Otherwise, no statistically significant differences were evident between the three routes (Table 4).
Chronic changes
In comparison to the saline-injected group, fibrosis was evident in all groups starting from the 7th day. At 11 and 30 days after cisplatin injection, scores of fibrosis were significantly lower in the treated groups in comparison to the cisplatin injected group. All routes were equally effective. Regarding the atrophic tubules, the numbers of atrophic tubules were significantly lower in the treated groups in comparison to the cisplatin injected group. i.a. stem cells were superior to the other treated groups at day 4 only (Fig. 2, Table 4).
Immunohistochemical detection of BrdU-labeled MSCs
Kidneys of rats which were treated with sub capsular MSCs were subjected to immunohistochemical staining to detect BrdU-labeled MSCs. In the 11th day, BrdU-positive cells were detected in the left kidney. Cells were distributed under the capsule, in the interstitium, and even were integrated in the tubules (Fig. 2J).
Discussion
The use of stem cells has emerged in the past few years as an effective strategy to reduce AKI in small animal models (2). Recovery of the kidneys from AKI after treatment with MSCs was attributed to the ability of MSCs to secret growth factors and cytokines that enhance recovery and reduce tissue damage (1, 6).
As in many kidney diseases, the pathogenesis of cisplatin-induced AKI is complex and involves both local events in the kidney as well as recruitment of the circulating inflammatory cells (21). The complexity of cisplatin-induced AKI may explain why a single pharmacological agent cannot provide complete protection against cisplatin nephrotoxicity (21).
Although the success of MSCs in treating cisplatin-induced AKI was evident in several studies (1, 4, 5), none of these studies addressed the effect of different routes of MSCs administration on renal recovery. Comparing the route of MSCs administration might give indirect evidence about the main mechanism of action of MSCs in this model; is it by local effect on the renal cells, by reducing infiltration of the inflammatory cells, or through both ways?
After systemic administration of MSCs, only a small fraction reach the kidney (7). Most of the i.v. injected MSCs into mice or rats were found to be rapidly trapped as microemboli in the lung (22, 23) and could be potentially lethal (24). However, Lee et al. (25) found that i.v. infusion of human MSCs was associated with improvement of myocardial infarction in mice because the cells which were trapped as emboli in lung expressed the anti-inflammatory factor TNF-α-induced protein-6 (TSG-6). In a parallel way, systemically administered MSCs additionally localize in the spleen (26). It was suggested that these MSCs could reduce inflammatory cells influx from bone marrow (9). Inflammation is major contributor in cisplatin-induced AKI (10, 11).
Direct infusion of MSCs to the injured kidney would ensure delivery of more cells to the kidney. It was suggested that most of the regenerating cells come from the proliferating endogenous stem cells of the kidney (27) and the role of MSCs is to enhance the ability of these cells to proliferate (28). If local injection of MSCs was not the superior route, it will, at least, reduce the number of MSCs needed to be injected. However, the effect of local MSCs injection on inflammatory cells in comparison to the systemically injected MSCs was not evaluated. Of note, the i.a. injection of MSCs was reported to increase the probability of microvascular occlusions, which is termed “passive entrapment” (12). Intra-arterial injection of MSCs in a rat model of acute renal failure was capable of ameliorating the disease but after 2 months, the MSCs treated kidneys showed more collagen and α-smooth muscle actin. In addition, about 20% of the glomeruli contained MSC-derived adipocytes with pronounced surrounding fibrosis (29). Although these effects were attributed to the model itself, the influence of injecting large number of MSCs in the renal artery could not be excluded.
Our results did not reveal any differences between the different routes of MSCs administration regarding their improvement in serum creatinine and BUN. However and for many reasons, serum creatinine and BUN are considered a suboptimal marker of AKI (30, 31). Creatinine clearance represents a more reliable assessment of the kidney function than serum creatinine (32) and it is affected early in cisplatin-induced AKI (33). However, even such sensitive marker did not reveal a striking difference between the three routes. Similarly, none of the three routes was superior in ameliorating the changes in serum calcium and albumin or oxidative stress markers in the renal tissue. Most of the differences were temporary and limited to one time interval. In addition, such differences were not consistent with any other parameter. Of note, we should not ignore that certain changes in cisplatin-induced AKI are independent. For example, it was found that exposure of rat kidney cortical slices to cisplatin significantly increased the concentration of cytosolic Ca2+; this increase in cytosolic Ca2+ is related to decrease in Ca2+ uptake, depletion of SH-groups, but independent of lipid peroxidation (34).
In the present study, we were able to demonstrate the ability of MSCs reduce oxidative stress and enhance the antioxidant reduced GSH and SOD as early as the 4th day. Previous studies demonstrated similar results in ischemia/reperfusion injury in the kidney (35, 36). We believe that the detected early antioxidant activity of MSCs might be an important factor to set up an environment favoring proliferation of dedifferentiated epithelial cells surviving the injury or resident stem cells activity. In our laboratory, we were able to demonstrate that oxidative stress markers might be a more sensitive marker of kidney damage than serum creatinine or BUN (data are submitted for publication). Still, such sensitive markers did not differentiate between the three routes of MSCs administration.
The pathological changes were evaluated using a score that entails not only evaluating the degree of tubular damage in the OSOM but also evaluate regenerative and chronic features as well as inflammatory cell infiltration. As previously discussed, an advantage of systemic administration of MSCs is that homing of these cells in the spleen would effectively reduce inflammatory cells efflux to the injured tissue (9). However, we did not find any differences between the three routes regarding the number of infiltrating inflammatory cells. Similarly, direct injection of MSCs in the kidney did not have any additional advantage regarding both tubular injury score and regeneration score. Of note, the study was extended to 30 days in order to reveal any differences in the three routes in the terms of fibrosis. It was found that bone marrow-derived cells can give rise to myofibroblasts and may contribute to fibrosis in an experimental model of renal ischemia/reperfusion injury (37). Renal fibrosis could be evoked by a single dose of cisplatin (38). In the present study, all the three routes reduced cisplatin-induced fibrotic changes in the kidney and none of the three methods appeared to have an advantage or to be more hazardous in this context. A direct and simple explanation of our results is that MSCs act mainly through secreting growth factors and that homing is unnecessary (1, 6, 39).
However, three points should be considered in the present study before final judgment about the best route for administering MSCs. First, the model; cisplatin-induced AKI involve both local injury in the kidney as well as activation and recruitment of inflammatory cells (21). It might be more valuable to use a simple model with less complex pathology in order to find out the differences between the routes of administration. Second, we had to fix the number of the used cells to exclude such factor from giving an advantage of one way over the others. Generally, local injection requires lower number of cells. It is important to notice that the large number of MSCs which were injected locally did not provide any additional benefits. As mentioned earlier, most of the regenerating cells in the kidney originate from the cells of the kidney (27) and the role of MSCs is to enhance the ability of these cells to proliferate (28). Thus, the extent of kidney regeneration after AKI might be limited by the regenerative potential of its endogenous cells regardless the number of MSCs injected. Finally, comparing the different routes of administration at the molecular level; namely proliferation, apoptosis, and inflammatory cells markers; might reveal significant differences between different routes of administration.
Only one study used renal progenitor-like cells to treat cisplatin-induced AKI and compared two different routes of administration (40). It was found that injecting renal progenitor-like cells under the capsule of the kidney was effective and resulted in amelioration of renal damage induced by cisplatin in comparison to the intra-arterial route which was found to be ineffective. The authors did not give an explanation to these results but it should be noted that even the improvement provided by the used cells, renal progenitor-like cells, was mild.
Generally, an overlook on the results of the present study will reveal that each route produced favorable results of a certain parameter at a certain time interval. This might indicate that the “responsiveness interval” of the kidney to stem cell therapy might be more than expect. A second observation is that MSCs were able to affect early events as serum creatinine, BUN, oxidative stress parameters, and injury score, as well as, late events as formation of solid sheets. From these two points, this study presents some arguments favoring a booster dose of MSCs instead of the single dose.
Finally, the MSCs which were injected under the capsule of the kidney were labeled using BrdU. We were able to detect BrdU-labeled MSCs in the kidney at the 11th day. Few cells have been found to be integrated in the tubular epithelium. Whether this structural integration was associated with the development of differentiated functioning tubular epithelium or not was not evaluated in the present study. Previous studies demonstrated that the contribution of BMSCs to the regenerating renal cells was found to be low (27).
In summary, our data demonstrated the ability of MSCs to ameliorate cisplatin-induced renal damage in SD rats. In the term of efficacy, direct injection of MSCs does not appear to be superior to their systemic injection. Future evaluation should focus on differences between the route of administration considering the long term safety and the progress of fibrosis. At least in this model, oxidative stress markers could be a useful tool in evaluating the effect of MSCs therapy. Booster dose of MSCs might be required to improve the outcome in AKI.
Supplementary Information
Acknowledgments
This work was conducted at Mansoura Medical Experimental Research Center (MERC), Faculty of Medicine, Mansoura University.
This work was supported by science and technology development fund (STDF), grant number 1053, Minister of scientific research, Egypt.
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
Supplementary data including one figure can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/IJST/IJST-09-s002.pdf.
References
- 1.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 2.Sahu KM, Mukhiya GK, Begum F, Ahmed T, Ashrafee F, Mutawaqqel Alallah M, Hoque SM, Zayed S. Repair and recovery of acute kidney injury. Clin Query Nephrol. 2012;1:95–98. doi: 10.1016/S2211-9477(11)70013-9. [DOI] [Google Scholar]
- 3.Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant. 2014;29:1487–1493. doi: 10.1093/ndt/gft538. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Park DJ, Yun JC, Jung MH, Yeo HD, Kim HJ, Kim DW, Yang JI, Lee GW, Jeong SH, Roh GS, Chang SH. Human adipose tissue-derived mesenchymal stem cells protect kidneys from cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol. 2012;302:F1141–F1150. doi: 10.1152/ajprenal.00060.2011. [DOI] [PubMed] [Google Scholar]
- 5.Peng X, Xu H, Zhou Y, Wang B, Yan Y, Zhang X, Wang M, Gao S, Zhu W, Xu W, Qian H. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med (Maywood) 2013;238:960–970. doi: 10.1177/1477153513497176. [DOI] [PubMed] [Google Scholar]
- 6.Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 9.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 10.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 11.Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and frac-talkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther. 2008;324:111–117. doi: 10.1124/jpet.107.130161. [DOI] [PubMed] [Google Scholar]
- 12.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intra-arterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. doi: 10.1002/(SICI)1097-4644(19990315)72:4<570::AID-JCB12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 16.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 17.van Roeyen CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 2006;69:1393–1402. doi: 10.1038/sj.ki.5000332. [DOI] [PubMed] [Google Scholar]
- 18.Seghatoleslam M, Jalali M, Alamdari DH, Nikravesh MR, Hoseini M, Fazel A, Koliakos G. Optimal incubating time of in vitro bromodeoxyuridine labeling of human umbilical cord blood- mononuclear cells and their functional assessment in ICH rats. J Cell Animal Biology. 2012;6:144–153. [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 23.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 27.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 29.Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754–1764. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 30.Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002;137:744–752. doi: 10.7326/0003-4819-137-9-200211050-00010. [DOI] [PubMed] [Google Scholar]
- 31.Gill N, Nally JV, Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847–2863. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 32.Nankivell B. Creatinine clearance and the assessment of renal function. Aust Prescr. 2001;24:15–17. doi: 10.18773/austprescr.2001.009. [DOI] [Google Scholar]
- 33.Jones TW, Chopra S, Kaufman JS, Flamenbaum W, Trump BF. Cis-diamminedichloroplatinum (II)-induced acute renal failure in the rat. Correlation of structural and functional alterations. Lab Invest. 1985;52:363–374. [PubMed] [Google Scholar]
- 34.Zhang JG, Lindup WE. Cisplatin-induced nephrotoxicity in vitro: increases in cytosolic calcium concentration and the inhibition of cytosolic and mitochondrial protein kinase C. Toxicol Lett. 1996;89:11–17. doi: 10.1016/S0378-4274(96)03776-9. [DOI] [PubMed] [Google Scholar]
- 35.Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–732. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 36.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 38.Yamate J, Tatsumi M, Nakatsuji S, Kuwamura M, Kotani T, Sakuma S. Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J Comp Pathol. 1995;112:27–39. doi: 10.1016/S0021-9975(05)80087-8. [DOI] [PubMed] [Google Scholar]
- 39.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40–53. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinomura M, Kitamura S, Tanabe K, Ichinose K, Hirokoshi K, Takazawa Y, Kitayama H, Nasu T, Sugiyama H, Yamasaki Y, Sugaya T, Maeshima Y, Makino H. Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant. 2008;17:143–158. doi: 10.3727/000000008783907008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.