Abstract
Background
In severe chronic stages of emphysema the only treatment is lung transplantation. SO, an urgent need exists for the development of effective treatments. Stem cells therapy arises as a new therapeutic approach.
Aim of the Work
To investigate whether bone marrow mononuclar cells (BMMNCs) can promote lung regeneration and decrease apoptosis in lipopolysaccharide (LPS) induced pulmonary emphysema in C57Bl/6 mice.
Material and Methods
14 weeks old female mice (C57Bl/6), weighing around 25 g were used in this study. The mice were divided into 4 groups (10 in each group): group A: mice received no treatment, group B: mice received intranasal instillation of LPS with no further treatment, group C: mice received intranasal instillation of LPS then given a dose of BMMNCs and evaluated 21 days later and group D: the mice that received intranasal instillation of LPS then given a dose of Dulbecco’s Modified Eagle’s Medium (DMEM) and evaluated 21 days later. Imaging analysis was done using imagej program. To measure apoptotic index, Anti–caspase 3 polyclonal antibody staining was done.
Results
Analysis of the mean of airspace equivalent diameters (D0) and its statistical distribution (D1) for the different groups allowed to observe that group treated with BMMNCs (group C) showed the significant improvement in D0 and D1 than the group received LPS only (group B). Analysis of apoptotic index showed significant difference between BMMNCs treated group (group C) and that received LPS only (group B).
Conclusions
BMMNCs effectively promote lung regeneration and reduction of apoptosis in pulmonary emphysema.
Keywords: Emphysema, Stem cells, Mononuclear cells, Apoptosis, Lipopolysaccharide
Introduction
Background
Emphysema (a COPD phenotype) defined as irreversible destruction of the alveoli and associated with inflammation in the airways and lung parenchyma (1, 2). In addition to the well-known impact of emphysema on the lungs, extra pulmonary systemic effects have also been described (3). Despite the status of COPD as a major global health problem, no currently available therapies can limit COPD progression. In severe chronic stages the only treatment that remains is lung transplantation, representing a procedure with high levels of morbidity and mortality. The severity of its pathology together with the lack of any effective treatment transforms emphysema into a great medical challenge. Therefore, an urgent need exists for the development of new and effective treatments for COPD (4, 5). Despite significant progress in understanding of lung stem cells and their functional capacities over the past decade, much remains unknown about the processes involved in lung repair (6).
Aims of study
This study was planned to evaluate the efficacy of BMMNCs in treatment of LPS-induced pulmonary emphysema.
Materials and Methods
Selected animals
14 weeks old female mice (C57Bl/6) (40 mice completed the study), weighing around 25 g were included in the study. The mice raised and maintained at the Mansoura Medical Research Center (MERC) and were provided with rodent diet and water. The study was conducted accordance with Institutional Review Board. Mice were divided into 4 groups (10 mice for each group). Group A: included the mice that received no treatment. Group B: included the mice that received intranasal instillation of lipopolysaccharide (LPS) for 8 weeks without further treatment. Group C: included the mice that received intranasal instillation of LPS for 8 weeks followed by one week recovery period then given a dose of bone marrow mononuclear cells (BMMCs) intravenously and evaluated 21 days later. Group D: included the mice that received intranasal instillation of LPS for 8 weeks followed by one week recovery period then given a dose of Dulbecco’s Modified Eagle’s Medium (DMEM) intravenously and evaluated 21 days later.
Emphysema induction
Lipopolysaccharide was purchased as purified lyophilized powder, prepared by phenol extraction from Escherichia coli serotype 0111:B4 from Sigma (St. Louis, MO). LPS was reconstituted with phosphate buffered saline (PBS) (1 mg/mL) and stock aliquots (1 mg/ml) were stored at −20°C. Immediately before use, 1.5 ml LPS stock was diluted in 50 ml PBS, solution was vortexed for at least 30 minutes to redissolve the adsorbed product. The mice were held in upright position and by the means of glass dropper, intranasal instillation of LPS (3 μg/dose) was done. Then the mice were held in this position for 2~5 minutes after instillation to facilitate the deposition of LPS into the mice lungs. Intranasal instillation was repeated three times per week for a period of 8 weeks and after the last dose the mice were given a recovery period of one week to eliminate the direct effect of LPS administration.
Mononuclear cells harvesting
Bone marrow mononuclear cells used for the study were taken from 4 donors C57Bl/6 male mice 8 weeks old. The mice were euthanized using intraperitoneal injection of sodium thiopental (120 mg/kg) 3 mg/mouse. The long bones were sterilized by immersion in 70% ethanol. The ends of the bones was cut and bone marrow was extruded by inserting a needle in one end through the bone shaft and injection of tissue culture media (Dulbecco’s modified Eagle’s medium-DMEM-, Sigma Chemical Comp. St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma). After a homogeneous cell suspension was achieved, cells were centrifuged for 10 min, resuspended in DMEM and added to Ficoll-Hypaque and again centrifuged and resuspended in phosphate buffered saline (PBS). The isolated cells were counted in a Neubauer chamber with Trypan Blue for evaluation of viability. Aliquot of mononuclear cells was used for immunophenotypic characterization of the injected cell population. Cell characterization was performed by flow cytometry using antibodies CD45 (leukocyte), CD34 (hematopoietic precursors), CD8 and CD4 (T lymphocyte), CD14 (monocytes and macrophages), CD11b, CD29 and CD45 (mesenchymal stem cells) (7).
Mononuclear cells and Dulbecco’s modified Eagle’s medium transplantation
Cell transplantation performed 9 weeks after the start of LPS administration. The mononuclear cells injected intravenously through tail vein. The treated group was given 50 μl/mouse of cell suspension at a concentration of 2× 106 cells/ml. Group D was injected with vehicle Dulbecco’s modified Eagle’s medium alone intravenously through tail vein. The sacrifice of mice for analysis performed 21 days after treatment.
Imaging Analysis
The mice were euthanized using intraperitoneal injection of sodium thiopental (dose/mice) (120 mg/kg) about 3 mg/mouse. The lungs of each mouse were expanded through intratracheal injection of 10% phosphate-buffered formalin and then dissected, fixed in 3% buffered formaldehyde and paraffin embedded. Three-μm-thick slices from both lungs were prepared and stained with hematoxylin-eosin. Airspace enlargement was quantified by measuring the air space equivalent diameter according to Parameswaran et al., 2006 (8) using computer-assisted imaging analysis (imagej 1.48q). Three digital images from different lobules of each mouse were created. The total number of images acquired and analyzed was 120. Images were visualized by a video camera (nikonACT-1 version 270) with a resolution of 640×480 pixels, magnification 10 xs adapted to a microscope (Olympus BX40, Japan). The original image was separated into tissue and airspace. To do this, the original image was thresholded to obtain a binary image, with black representing tissue and white representing airspace. To measure airspace area and diameter, the area of each airspace (Ai) was measured in binary image using (Analyze>Tools>ROI manager). The corresponding equivalent airspace diameter (di) was calculated as:
This is equal to the diameter of a circle with area Ai. To compare normal and emphysematous tissue sections using d, D0 and D1 were measured, defined as the ratio of two moments of d where <···> indicates the arithmetic mean.
For υ=0, D0=<d> is simply the arithmetic mean of the airspace diameters Apoptosis assay: Three-μm-thick slices were cut and stained with Anti–caspase 3 polyclonal antibody (Caspase 3 (CPP32) Ab-4, Rabbit Polyclonal Antibody, Thermo scientific). Images were visualized also by a video camera (nikonACT-1 version 270) with a resolution of 640×480 pixels, magnification 20× adapted to a microscope (Olympus BX40, Japan). Apoptotic index was calculated using image j as a percent ratio between the number of active caspase3–positive alveolar cell nuclei and total number of cell nuclei in 8 fields of each BMMNC treated mice, and in those received LPS only. Structures of non parynchemal cells e.g. bronchi and blood vessels were not involved (9).
Statistical Analysis
The statistical analysis of data was done by using SPSS programs statistical package for social science version 16. The description of the quantitative data concerning D0, D1 and apoptotic index of mean±SD. The analysis of the data was done to test statistical significant difference between groups. For quantitative data student t-test was used to compare between 2 groups. One way ANOVA test was used to compare the results of the four groups and LSD post-hoc test was used to compare the results between each two groups.
Results
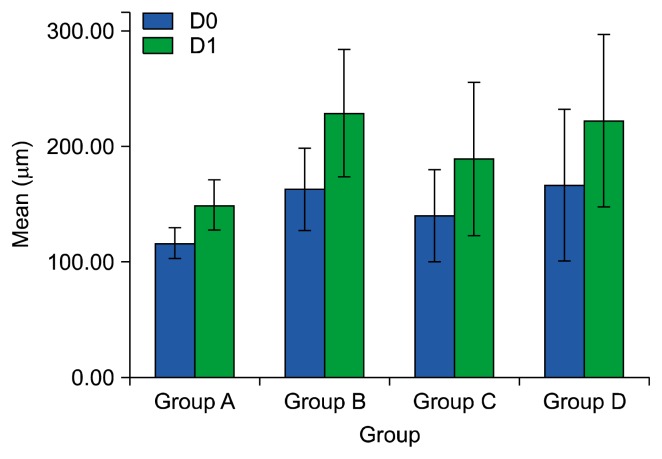
Concerning the mean of air space equivalent diameter (D0) and its statistical distribution (D1) for the different groups allowed to observe that mice group treated with BMMNCs (group C) showed the significant improvement in D0 and D1 (D0 mean: 139.70 μm±19.9 and D1 mean: 188.22 μm±33.1) than the group received LPS only (group B) (D0 mean: 162.10 μm±17.8 and D1 mean: 228.21 μm± 27.7) (p value 0.001). Also, there was insignificant differences between group (B) (D0 mean: 162.10 μm±17.8 and D1 mean: 228.21 μm±27.7) and that received DMEM medium (group D) (D0 mean: 165.58± 32.8 μm and D1 mean: 221.78 μm±37.7) (D0: p=0.59 and D1: p=0.49) (Fig. 1, 2).
Fig. 1.
Cluster bar graph represents means and standard deviations of the mean of airspace equivalent diameters (D0) and its statistical distribution (D1) in studied groups. Group A: (the mice that received no treatment), group B (the mice received intranasal instillation of LPS), group C (the mice received BMMNCs after LPS instillation) and group D (the mice received DMEM media after LPS instillation).
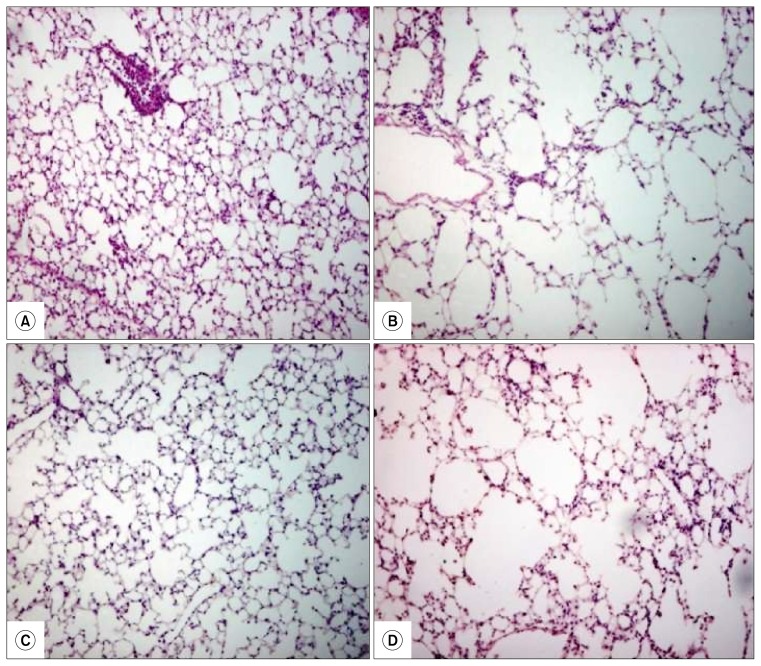
Fig. 2.
Lung parenchyma stained with hematoxylin-eosin, (A) represents group A (the mouse that received no treatment), (B) represents group B (the mouse that received LPS) with an overall increase in air space size compared to group A, (C) represents group C (the mouse received BMMNCs after LPS) with decrease in air space size compared to group B and (D) represents group D (the mouse received DMEM media after LPS instillation) with an overall increase in air space size similar to group B.
Analysis of apoptotic index showed significant difference between BMMNCs treated group (group C) (24.2%± 5.62) and that received LPS only (group B) (43.7%±8.29) (p=0.001) (Table 1, Fig. 3).
Table 1.
Comparison of apoptotic index between group (B) and group (C)
| Group (B) | Group (C) | p-value (Test of significance) | |
|---|---|---|---|
| Apoptotic index Mean (%)±SD | 43.7±8.29 | 24.2±5.62 | 0.001 |
Group B (the mice received intranasal instillation of LPS), group C (the mice received BMMNCs after LPS instillation).
Apoptotic index: a percent ration between the number of active caspase3–positive nuclei in alveolar septa and total number of cell nuclei in 8 fields of each BMMNC treated mice and in those received LPS only.
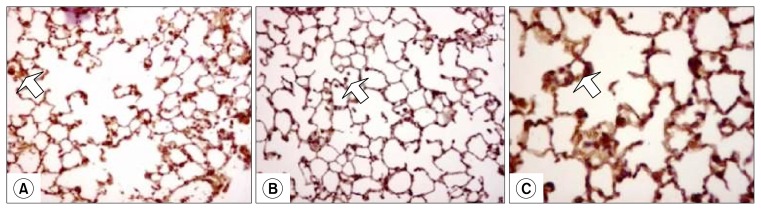
Fig. 3.
Lung sections stained with immunohistochemistry for activated caspase3; (A) showing increased number of caspase3 positive cells in the alveolar septa (arrows) of mouse received LPS only (group B); (B) BMMNCs treated mouse lung (group C), showing decreased number of caspase3 positive cells in the alveolar septa in the lung (arrow); (C) 40× magnification to show caspase-3 positive cells.
Discussion
The current study was designed to isolate and culture BMMNCs from male C57Bl/6 mice and to evaluate their benefit in reduction of apoptosis and promotion of lung regeneration in a simple model of LPS induced pulmonary emphysema.
C57Bl/6 mice were selected for this study because of the close similarities with the human genome and the relatively low cost of its breeding, housing, and maintenance (10). There are many studies such as those reported by Obot et al., 2004 and Phillips et al., 2015 (11, 12); utilized C57Bl/6 mice in pulmonary emphysema induction. However, they used male C57Bl/6 mice instead of female mice used in this study. Similar to this study, Phillips et al., 2015 used female C57Bl/6 mice but 10 weeks old mice were used instead of 14 weeks old. Pulmonary alveoli in female mice are estrogen-responsive structures and oophorectomy in adult mice results in alveolar loss, and estradiol replacement induces alveolar regeneration (13) so, we select female mice in this study. 14 weeks age used because this age is located in the adult age group of mice. This age group represents the reference for any age change, whether the change is developmental, maturational, or aging. For these reasons we use this age group in this study similar to Tibboel et al., 2013 (14).
Because of availability and relatively low cost of LPS, it was used in emphysema induction in this study instead of pancreatic elastase. The dose of LPS used in this study was 3 μg/dose, repeated three times per week for a period of 8 weeks chosen according to our pilot study. Three microgram per dose of LPS used in this study corresponds to a dose of smoking 15 cigarette delivered to the human lung (15). Mice in Brass et al., 2008 (16) were exposed to aerosolized LPS (5 μg/m3 of air) for 4 hours per day for 4 weeks by Jet Nebulizer. In our pilot study, nebulizer was experimented but emphysematous changes in mice lung were heterogeneous and less than those resulted from intranasal instillation of LPS. Therefore, intranasal instillation used in this study instead of nebulizer. Pulmonary emphysema was induced in Anneke et al., 2008 (17) by using LPS (5 μg/mouse), administered intranasally to mice, which were anesthetized with halothane twice a week for a period of 8 weeks. In this study the frequency of intranasal instillation increases in contrast to Anneke et al., 2008 to compensate the amount of LPS wasted by the movement of non anesthetized mouse.
Previous studies demonstrated that single intratracheal LPS challenge results in rapid but transient infiltration of neutrophils from the pulmonary vascular bed into the respiratory air spaces. Long-term LPS exposure also resulted in a chronic inflammatory response in the parenchymal area, which was comprised of activated macrophages–indicated by increased expression of MHC class II molecules on the cell surface and CD8T cells. The absence of neutrophils at 1 week after the last LPS exposure indicates that neutrophil infiltration is rather a transient process triggered by each LPS exposure instead of being a chronic factor (15). So, in this study BMMNCs injected after one week recovery period to eliminate direct effect of LPS (neutrophilic infiltration).
Therefore, the method of emphysema induction using multiple intranasal instillations LPS by glass dropper and without anesthesia used in this study is simple and less invasive which by extension led to reduction in the rate of animal mortality and the cost of the study as in Pera et al., 2011 (18). Inspite of similarity between this study and Pera et al., 2011, the mouse was used as an experimental animal in this study instead of guinea pig.
We utilized the air space equivalent diameter method for assessment of lung damage and repair as an alternative to the mean linear intercepts similar to what made by Arrate et al., 2012 (19). The mean linear intercept value affected by different inflation levels and airspace shapes which makes the airspace equivalent diameters more accurate in lung emphysema quantification (20).
BMMNCs are all cells present in bone marrow whose nuclei are unilobulated. Among adult BMMNCs are hematopoietic progenitor cells at different stages of maturation as well as lymphoid cells (lymphocytes, plasma cells), monocytes, macrophages and mesenchymal stromal cells (21). The treated group of mice was given 50 μl/mouse of cell suspension at a concentration of 2×106 cells/ml the concentration as reported and used by Cruz et al., 2012 (7). But Cruz et al., 2012 reported the effectiveness of early administration of BMMNCs in elastase induced pulmonary emphysema to prevent lung and heart damage not reversing lung damage which is the aim of this study.
In the present work, an increase in D0 and D1 in group B (received LPS only) and group D (received culture media) in comparison to group A (normal group). Also, there was significant improvement in D0 and D1 in group C in comparison to group B. No significant difference was detected between group D and group B. This finding was confirmed by statistical results and coincided with those of previous workers as Nathalia et al., 2012 (22) but mean linear intercepts was used instead of airspace equivalent diameters.
Other studies as Zarogoulidis et al., 2014 (23) studied the effect of mesenchymal stem cells (MSCs) in treatment of pulmonary emphysema instead of BMMNCs that used in this study. Unlike MSCs, BMMNCs can be easily obtained and do not require in vitro expansion before their use, so no way to study the effect of its paracrine effect.
Analysis of apoptotic index in this study showed significant difference between BMMNCs treated group (group C) and that received LPS only (group B) which indicates that BMMNCs led to a significant reduction in the number of lung apoptotic cells. These results were in agreement with Yuhgetsu et al., 2006 (24) but (TUNEL-positive) cells and matrix metalloproteinase (MMP)-2 expression used as indicators of apoptosis.
Assessment of stem cells homing to emphysematous lung not done in the present study so, we were not able to determine whether BMMNCs had a direct beneficial effect on the lung regeneration and apoptosis or an indirect benefit mediated by its paracrine effect. Rojas et al., 2005 reported that these effects have been attributed to immunomodulation either from cytokine release or activation of the endogenous immune system (25).
In conclusion, we present a simple model of lipopolysaccharide induced pulmonary emphysema and simple method of quantification of lung damage. Our results showed that BMMNCs therapy effective in treatment of pulmonary emphysema.
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Snider GL. Distinguishing among asthma, chronic bronchitis, and emphysema. Chest. 1985;87(1 Suppl):35S–39S. doi: 10.1378/chest.87.1.35S. [DOI] [PubMed] [Google Scholar]
- 2.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol. 2012;48:86–98. doi: 10.1016/j.arbres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZH, Kim HP, Ryter SW, Choi AM. Identifying targets for COPD treatment through gene expression analyses. Int J Chron Obstruct Pulmon Dis. 2008;3:359–370. doi: 10.2147/copd.s1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal D, Fortunato G, Klein W, Cortizo L, Vasconcelos J, Ribeiro-Dos-Santos R, Soares M, Macambira S. Alterations in pulmonary structure by elastase administration in a model of emphysema in mice is associated with functional disturbances. Rev Port Pneumol. 2012;18:128–136. doi: 10.1016/j.rppneu.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Hackett TL, Knight DA, Sin DD. Potential role of stem cells in management of COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:81–88. doi: 10.2147/copd.s7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz FF, Antunes MA, Abreu SC, Fujisaki LC, Silva JD, Xisto DG, Maron-Gutierrez T, Ornellas DS, Sá VK, Rocha NN, Capelozzi VL, Morales MM, Rocco PR. Protective effects of bone marrow mononuclear cell therapy on lung and heart in an elastase-induced emphysema model. Respir Physiol Neurobiol. 2012;182:26–36. doi: 10.1016/j.resp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol (1985) 2006;100:186–193. doi: 10.1152/japplphysiol.00424.2005. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Ma L, Wu J, Wang K, Chen X. Apoptosis of alveolar wall cells in chronic obstructive pulmonary disease patients with pulmonary emphysema is involved in emphysematous changes. J Huazhong Univ Sci Technolog Med Sci. 2009;29:466–469. doi: 10.1007/s11596-009-0415-7. [DOI] [PubMed] [Google Scholar]
- 10.Dawkins PA, Stockley RA. Animal models of chronic obstructive pulmonary disease. Thorax. 2001;56:972–977. doi: 10.1136/thorax.56.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obot C, Lee K, Fuciarelli A, Renne R, McKinney W. Characterization of mainstream cigarette smoke-induced bio-marker responses in ICR and C57Bl/6 mice. Inhal Toxicol. 2004;16:701–719. doi: 10.1080/08958370490476604. [DOI] [PubMed] [Google Scholar]
- 12.Phillips B, Veljkovic E, Peck MJ, Buettner A, Elamin A, Guedj E, Vuillaume G, Ivanov NV, Martin F, Boué S, Schlage WK, Schneider T, Titz B, Talikka M, Vanscheeuwijck P, Hoeng J, Peitsch MC. A 7-month cigarette smoke inhalation study in C57BL/6 mice demonstrates reduced lung inflammation and emphysema following smoking cessation or aerosol exposure from a prototypic modified risk tobacco product. Food Chem Toxicol. 2015;80:328–345. doi: 10.1016/j.fct.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Massaro D, Massaro GD. Developmental alveologenesis: longer, differential regulation and perhaps more danger. Am J Physiol Lung Cell Mol Physiol. 2007;293:L568–L569. doi: 10.1152/ajplung.00258.2007. [DOI] [PubMed] [Google Scholar]
- 14.Tibboel J, Reiss I, de Jongste JC, Post M. Ceramides: a potential therapeutic target in pulmonary emphysema. Respir Res. 2013;14:96. doi: 10.1186/1465-9921-14-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26:152–159. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- 16.Brass DM, Hollingsworth JW, Cinque M, Li Z, Potts E, Toloza E, Foster WM, Schwartz DA. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol. 2008;39:584–590. doi: 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Houwelingen AH, Weathington NM, Verweij V, Blalock JE, Nijkamp FP, Folkerts G. Induction of lung emphysema is prevented by L-arginine-threonine-arginine. FASEB J. 2008;22:3403–3408. doi: 10.1096/fj.07-096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pera T, Zuidhof A, Valadas J, Smit M, Schoemaker RG, Gosens R, Maarsingh H, Zaagsma J, Meurs H. Tiotropium inhibits pulmonary inflammation and remodelling in a guinea pig model of COPD. Eur Respir J. 2011;38:789–796. doi: 10.1183/09031936.00146610. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Barrutia A, Ceresa M, Artaechevarria X, Montuenga LM, Ortiz-de-Solorzano C. Quantification of lung damage in an elastase-induced mouse model of emphysema. Int J Biomed Imaging. 2012;2012:734734. doi: 10.1155/2012/734734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol (1985) 2007;102:459–467. doi: 10.1152/japplphysiol.00808.2006. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 2013;45:434–439. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 22.Longhini-Dos-Santos N, Barbosa-de-Oliveira VA, Kozma RH, Faria CA, Stessuk T, Frei F, Ribeiro-Paes JT. Cell therapy with bone marrow mononuclear cells in elastase-induced pulmonary emphysema. Stem Cell Rev. 2013;9:210–218. doi: 10.1007/s12015-012-9419-y. [DOI] [PubMed] [Google Scholar]
- 23.Zarogoulidis P, Hohenforst-Schmidt W, Huang H, Sahpatzidou D, Freitag L, Sakkas L, Rapti A, Kioumis I, Pitsiou G, Kouzi-Koliakos K, Papamichail A, Papaiwannou A, Tsiouda T, Tsakiridis K, Porpodis K, Lampaki S, Organtzis J, Gschwendtner A, Zarogoulidis K. A gene therapy induced emphysema model and the protective role of stem cells. Diagn Pathol. 2014;9:195. doi: 10.1186/s13000-014-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuhgetsu H, Ohno Y, Funaguchi N, Asai T, Sawada M, Takemura G, Minatoguchi S, Fujiwara H, Fujiwara T. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp Lung Res. 2006;32:413–426. doi: 10.1080/01902140601047633. [DOI] [PubMed] [Google Scholar]
- 25.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]