Significance
Tobacco smoking causes emphysema, a fatal disease involving extensive structural damage of the lung. Besides directly oxidizing lung proteins, tobacco smoke activates Rtp801, a proinflammatory cellular factor that induces overproduction of NO by inducible NOS and consequent lung protein nitration and damage. Such oxidized or nitrated lung proteins become susceptible to breakdown by lung proteases causing emphysema. On interacting with the antioxidant vitamin C, tobacco smoke loses its ability to cause lung protein oxidation or damage, indicating that its oxidant(s) are primarily responsible for triggering lung injury. Vitamin C provides comprehensive protection against tobacco smoke-induced emphysema by attenuating direct damage of the lung by its oxidant(s) and their ability to activate cellular damage machineries including Rtp801 and lung proteases.
Keywords: cigarette smoke, emphysema, vitamin C, ascorbate, Rtp801
Abstract
Cigarette smoking causes emphysema, a fatal disease involving extensive structural and functional damage of the lung. Using a guinea pig model and human lung cells, we show that oxidant(s) present in tobacco smoke not only cause direct oxidative damage of lung proteins, contributing to the major share of lung injury, but also activate Rtp801, a key proinflammatory cellular factor involved in tobacco smoke-induced lung damage. Rtp801 triggers nuclear factor κB and consequent inducible NOS (iNOS)-mediated overproduction of NO, which in combination with excess superoxide produced during Rtp801 activation, contribute to increased oxido-nitrosative stress and lung protein nitration. However, lung-specific inhibition of iNOS with a iNOS-specific inhibitor, N6-(1-iminoethyl)-L-lysine, dihydrochloride (L-NIL) solely restricts lung protein nitration but fails to prevent or reverse the major tobacco smoke-induced oxidative lung injury. In comparison, the dietary antioxidant, ascorbate or vitamin C, can substantially prevent such damage by inhibiting both tobacco smoke-induced lung protein oxidation as well as activation of pulmonary Rtp801 and consequent iNOS/NO-induced nitration of lung proteins, that otherwise lead to increased proteolysis of such oxidized or nitrated proteins by endogenous lung proteases, resulting in emphysematous lung damage. Vitamin C also restricts the up-regulation of matrix-metalloproteinase-9, the major lung protease involved in the proteolysis of such modified lung proteins during tobacco smoke-induced emphysema. Overall, our findings implicate tobacco-smoke oxidant(s) as the primary etiopathogenic factor behind both the noncellular and cellular damage mechanisms governing emphysematous lung injury and demonstrate the potential of vitamin C to accomplish holistic prevention of such damage.
Cigarette smoking is recognized as the predominant cause of emphysematous lung damage associated with chronic obstructive lung disease (COPD), which presently accounts for almost one-fourth of the global mortality and is projected to become the third largest killer by the year 2020 (1). This revelation mandates serious clinical scrutiny of the mechanism underlying the pathogenesis of this disease and exploration of its possible prevention.
We had previously demonstrated that cigarette smoke (CS) can directly oxidize animal lung proteins both in vitro (2) and in vivo (3) and, thereby, render them susceptible to increased proteolytic damage consistent with that observed in CS-induced emphysema. However, we were then unable to identify the precise cellular factors involved in the damage process (3–5). Although the involvement of various endogenous cellular mediators of oxidative stress (6–8) and lung tissue damage including protease–antiprotease imbalance (9), antioxidant depletion (10, 11), protease activation (12–14), alveolar inflammation, and apoptosis (15–17) has been reported thereafter to explain the pathogenesis of such disease, none have been able to comprehensively demonstrate how the noncellular and cellular machineries involved in CS-induced lung damage are activated following inhalation of CS and the extent to which they contribute to the damage process.
Indeed, oxidative stress has been long identified as a major factor behind CS-induced emphysematous lung damage, in which the possible involvement of cellular reactive oxygen species (ROS) and reactive nitrogen species (RNS) have been typically indicated (18–20). CS-induced oxidative stress releases inflammatory cytokines like IFN-γ, TNF-α, IL-1β, and IL-8, resulting in inflammatory lung damage by neutrophils and nuclear factor κB (NF-κB) (21, 22) and the overexpression of inducible NOS (iNOS) and its product, nitric oxide (NO), contributing to extensive oxido-nitrosative lung damage (23, 24). More recent work has shown that Rtp801, which promotes oxidative stress-induced cell death through activation of NF-κB, is significantly overexpressed in human smokers (6). Oxidants apparently produced in the lung in response to smoking have been implicated with decreased expression of the anti-apoptotic protein, vascular endothelial growth factor (VEGF) (25, 26), increased apoptosis (27, 28), and lung cell autophagy (29). Curiously, a recent report has ascribed lung protein nitration by peroxynitrite, generated from iNOS-derived NO, exclusively to CS-induced lung injury, and even claimed reversal of such damage through global inhibition of iNOS (23). Although such studies have separately identified various cellular factors sustaining CS-induced emphysema, they have perhaps fallen short of identifying the decisive trigger behind such damage and the precise share of the cellular mediators of such damage compared with the direct xenobiotic-induced lung injury inflicted by CS itself.
In fact, what precisely triggers CS-induced emphysema still remains an open question. Amid this query, our present study reveals that even the major cellular pathway associated with CS-induced emphysema, involving the pulmonary Rtp801-mediated activation of NF-κB and iNOS and consequent oxido-nitrosative damage of lung proteins and cellular apoptosis (6), is in fact triggered by the CS oxidants itself. Indeed, CS extract divested of its oxidative potency or neutralized by the antioxidant, ascorbate, or vitamin C failed to elicit characteristic oxido-inflammatory damage of human lung cells, indicating that even the cellular mediators of CS-induced lung damage functionally depend on the oxidant(s) furnished by CS, thus establishing them as the primary trigger for CS-induced emphysema.
Moreover, our finding that vitamin C cannot only scavenge the harmful CS oxidant(s) that inflict direct lung injury but can also attenuate the associated cellular response(s) modestly contributing to the lung damage and suppress the up-regulation of matrix-metalloproteinase-9 (MMP-9), the major lung protease involved in the damage (30), also underscores the prospects of achieving comprehensive prevention of CS-induced emphysema using this antioxidant.
Results
Tobacco Smoke Induces Emphysematous Lung Damage.
Exposure of guinea pigs to CS for 28 d resulted in time-dependent alveolar lung damage (Fig. 1 A, v–viii) as depicted by the mean linear intercept (Lm) (Fig. 1B) and destructive index (DI) (Fig. 1C) of the lung alveolar air-space expansion, along with extensive breakdown of the major lung structural protein, elastin, and overexpression of MMP-9, the primary lung metalloproteinase involved in protein degradation during emphysema (30) (Fig. 1E and Fig. S1 A and B). The Lm and DI values increased by almost four and six times, respectively, for the CS-treated animals from the 28-d sham-control (air-exposed) values of 0.67 ± 0.03 (Lm) and 13.83 ± 2.32 (DI) to that of 2.44 ± 0.22 (Lm) and 80.76 ± 3.11 (DI). Such alveolar damage was accompanied by increased cellular apoptosis, evidenced through TUNEL assay (Fig. 1D and Fig. S1C) and concurring changes in Bax and Bcl-2 expression levels (Fig. 1E) and the corresponding Bax:Bcl-2 ratio (Fig. 1F).
Fig. 1.
Tobacco smoke induces apoptosis and emphysematous lung damage in guinea pigs. (A) Representative histology from hematoxylin and eosin-stained lung sections of air exposed (sham controls) for 0 (i), 14 (ii), 21(iii), and 28 (iv) d against that of CS exposed for 0 (v), 14 (vi), 21 (vii), and 28 (viii) d, respectively, highlighting morphological changes in lung alveolar airspace (10 fields, n = 6). Alveolar morphometric changes depicted in A were expressed in terms of Lm (B) and Di (C) as indicators of lung damage. (D) Representative lung section profiles of the above treatment groups depicting alveolar cell apoptosis (green), as analyzed by (TUNEL) assay. Nuclei were stained with DAPI (blue) (10 fields, n = 6). (E) Immunoblots depicting time-dependent changes in lung elastin and MMP-9 levels with increasing CS exposure with corresponding change in pulmonary Bax (proapoptotic marker) and Bcl-2 (anti-apoptotic marker) expression levels. (F) Consequent increase in Bax/Bcl-2 expression ratio with time-dependent increase in CS exposure against sham controls shows virtually no change in levels of Bax or Bcl-2 (n = 6). Data were statistically analyzed by paired Student’s t test. Significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions. (Scale bars: A, 50 µm; D, 100 µm.)
Fig. S1.
Histograms depicting relative levels (intensities) of the proteins elastin (A) and MMP-9 (B) in the immunoblots shown in Fig. 1E, normalized against tubulin as internal standard. C represents the relative number of apoptotic lung cells as evidenced through the TUNEL assay in Fig. 1D. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions.
Tobacco Smoke Exposure Increases Pulmonary Expression of Rtp801, NF-κB, NOS and Oxido-Nitrosative Stress in the Lung.
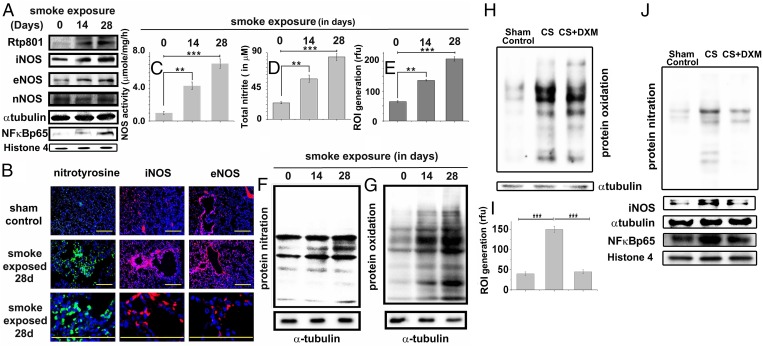
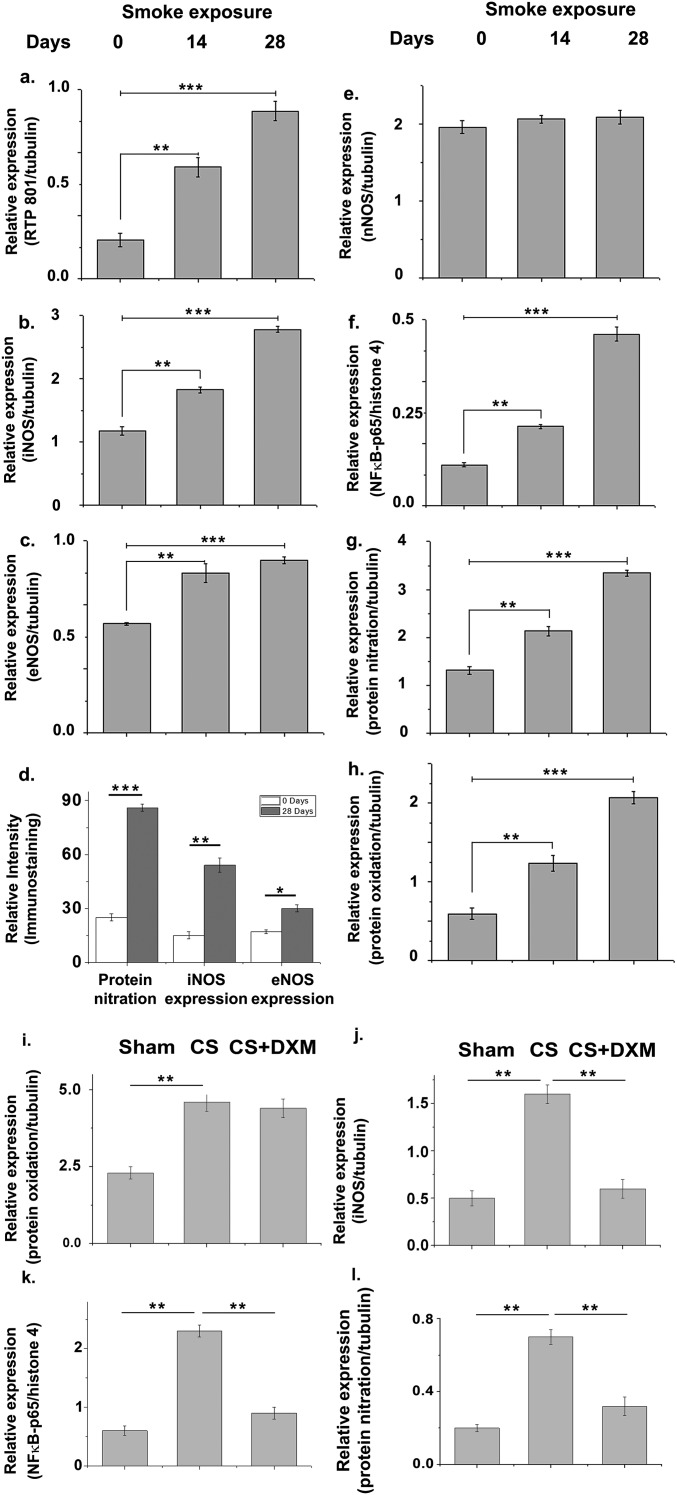
CS exposure caused time-dependent increase in the pulmonary expression of the inflammation-mediating protein, NF-κB (p65 subunit) and its activator, Rtp801 (Fig. 2A and Fig. S2 A and F). Analysis of associated changes in the pulmonary expression of the three NOS isoforms, iNOS, endothelial NOS (eNOS), and neuronal NOS (nNOS), revealed that after 28 d of CS exposure, iNOS levels increased almost 2.6-fold compared with a modest increase for eNOS (1.6-fold), and almost no change for nNOS (Fig. 2A and Fig. S2 B, C, and E). This change was corroborated by corresponding immunofluorescence depictions of iNOS and eNOS expression in the same lung tissues (Fig. 2B and Fig. S2D).
Fig. 2.
Tobacco smoke activates Rtp801 and consequent NF-κB–induced overexpression of iNOS and oxido-nitrosative stress in the lung. Immunoblots showing the levels of Rtp801, iNOS, eNOS, nNOS, and NF-κB–p65 in the whole-lung lysates of guinea pigs administered 14 and 28 d of CS exposure against air-exposed sham controls (A). (B) Representative profiles showing the relevant immunostained lung sections treated with specific antibodies followed by fluorescently tagged secondary antibodies against nitrotyrosine (green/Alexa Fluor 488), iNOS (red/Alexa Fluor 568), and eNOS (red/Alexa Fluor 568). (Scale bars: 100 µm.) (10 fields, n = 6.) Total NOS activity (C) determined in the above whole-lung lysates along with total nitrite (nitrate+nitrite) concentration in BALF (D) (measure of total NO released in the lung). Level of ROI (E) generated by freshly collected whole-lung lysates was also spectrofluorimetrically estimated by CM-H2DCFDA assay and shown in terms of relative fluorescence unit (rfu). Immunoblot shows the level of protein nitration (F) and oxidation of lung proteins (G) with increasing CS exposure (n = 6). Immunoblot showing the levels of protein oxidation (H), protein nitration, iNOS, and NF-κB–p65 expression (J) along with cellular ROI levels (I) in the lungs of animals exposed to 14 d of CS with or without DXM administration (nebulization), against air-exposed sham controls. Data were statistically analyzed by paired Student’s t test. Significant differences (**P < 0.01, ***P < 0.001) were observed in comparison with unexposed controls. Data are represented as means ± SD and represent three independent experiments done under similar conditions.
Fig. S2.
Histograms depicting relative levels (intensities) of the proteins Rtp801 (A), iNOS (B), eNOS (C), and nNOS (E) normalized against tubulin, and NF-κB–p65 (F) normalized against histone 4 as respective internal standards in the immunoblots shown in Fig. 2A along with relative levels of protein nitration (G) in Fig. 2F and protein oxidation (H) in Fig. 2G normalized against tubulin as internal standard. The relative immunofluorescence intensities for nitrotyrosine, iNOS, and eNOS expression levels as shown in Fig. 2B is depicted in D. Histograms representing relative levels (intensities) of oxidized proteins (I), iNOS (J), and nitrated protein (L) (normalized against tubulin as internal standard), and NF-κB–p65 (K) (normalized against histone 4 as internal standard) in the 14-d CS-exposed animals with and without DXM treatment against air-exposed sham controls, corresponding to the immunoblots shown in Fig. 2 H and J. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions.
Consistent with the increased expression of NOS, particularly iNOS, we observed a time-dependent increase in the total activity of NOS (Fig. 2C) and the total bronchoalveolar lavage fluid (BALF) nitrite level (measure of NO released in the lung) (Fig. 2D) in the CS-treated animals. To assess possible de novo entrapment of this NO by superoxide to produce peroxynitrite, we also measured corresponding ROI (reactive oxidative intermediate) levels in the same lung tissues using 2′7′dichlorodihydro fluorescein diacetate (H2DCFDA) (Fig. 2E). The level of increase in lung ROI levels in response to CS exposure (Fig. 2E) was found to closely match with the corresponding elevation in NO levels (Fig. 2D), raising the possibility of de novo generation of peroxynitrite (31, 32). Indeed, immunoblot analysis of the same lysates for protein nitration, a signature of peroxynitrite involvement, revealed significant increase in such modification (Figs. 2 B and F and Fig. S2 D and G). When we subjected part of the above lysates to Oxyblot analysis, for assessing the level of lung protein oxidation, we observed substantial time-dependent oxidative modification of lung proteins due to CS exposure (Fig. 2G and Fig. S2H). In an attempt to examine the relative level of lung protein oxidation caused directly by CS, in comparison with that produced by the lung oxido-inflammatory machinery during CS exposure, we administered dexamethasone, an antiinflammatory glucocorticoid into the animal’s lung through nebulization, to suppress possible contribution of oxido-inflammatory protein oxidation induced by CS exposure. We limited such exposure to only 14 d to avoid possible glucocorticoid resistance through sustained smoke exposure. Indeed, this treatment produced almost the same level of lung protein oxidation as that obtained without any antiinflammatory treatment (Fig. 2H and Fig. S2I) and little changes in levels of lung ROI (Fig. 2I) and iNOS, NF-κB, and protein nitration compared with the sham controls (Fig. 2J and Fig. S2 J–L).
Relative Role of Pulmonary iNOS-Generated NO and Tobacco Smoke Oxidants in CS-Induced Oxido-Nitrosative Lung Injury.
Because a recent report has predominantly implicated iNOS to the pathogenesis of CS-induced emphysema (23), we resolved to reexamine such findings by deploying specific pulmonary administration of N6-(1-iminoethyl)-L-lysine, dihydrochloride (L-NIL), a selective iNOS inhibitor. We primarily executed such lung-specific inhibition of iNOS considering the possible complications that may arise in interpreting results obtained through whole-body iNOS inhibition as carried out in the above study (23). Also, to examine possible involvement of CS and cell-generated oxidants besides NO, in the observed lung damage, we orally administered the CS-treated animals with ascorbate or vitamin C, an antioxidant with established capability of scavenging such oxidants de novo (2, 33).
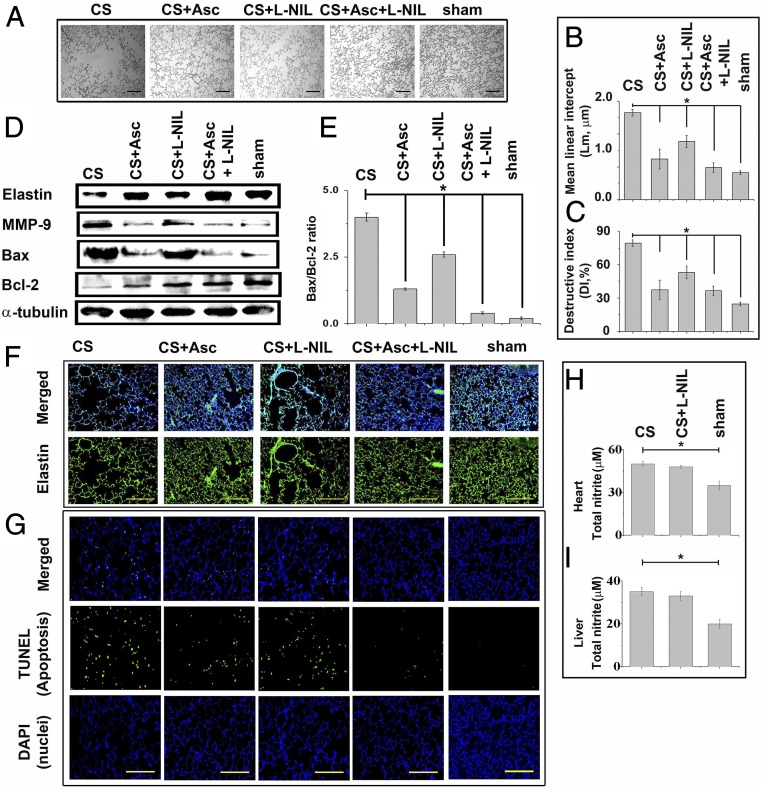
Morphometric analysis of alveolar airspace expansion (Fig. 3A) as well as Lm (Fig. 3B) and DI (Fig. 3C) of the relevant lung tissues revealed almost 46% and 78% protection in lung damage through specific inhibition of lung iNOS by L-NIL and ascorbate treatment, respectively. The protection accomplished through a combination of both L-NIL and ascorbate, however, was significantly higher (86%) than achieved individually by the two (Fig. 3 A–C). Notably, the degree of prevention of the breakdown of lung elastin (Fig. 3 D and F and Fig. S3 A and C), as well as MMP-9 overexpression (Fig. 3D and Fig. S3B) by ascorbate treatment, were consistent with corresponding levels of protection evidenced through morphometric analysis of lung damage (Fig. 3 A–C). The CS-induced lung cell apoptosis, evidenced through TUNEL assay (Fig. 3G and Fig. S3D) and evaluation of corresponding Bax:Bcl-2 ratio (Fig. 3E), also showed consistency in such protection. Lung-specific administration of L-NIL didn’t significantly affect iNOS activity in either the heart (Fig. 3H) or liver (Fig. 3I) of the treated animals, confirming specific and targeted inhibition of lung iNOS through lung-specific administration of L-NIL.
Fig. 3.
Ascorbate prevents tobacco smoke-induced elastin breakdown, MMP-9 overexpression, and consequent emphysematous lung damage. (A) Representative histology from hematoxylin-stained lung sections of CS-exposed, CS+Asc (ascorbate), CS+L-NIL, CS+Asc+L-NIL and sham control guinea pigs (10 fields, n = 6). CS-induced alveolar damage is morphometrically represented in terms of Lm (B) and Di (C). (D) Immunoblots show levels of elastin breakdown, MMP-9 overexpression, and Bax and Bcl-2 expression levels in the whole-lung lysates due to CS exposure under the indicated treatment conditions along with corresponding ratios of Bax and Bcl-2 expression levels (E). (F) Lung sections immunostained with anti-elastin primary antibody and secondary antibody conjugated with Alexa Fluor 568 (green) (10 fields, n = 6). (G) Indicated lung sections subjected to TUNEL analysis against nuclear staining with DAPI (blue) (10 fields, n = 6). Activity levels of iNOS in the heart (H) and liver (I) tissues of CS-treated animals subjected to lung-specific L-NIL treatment (n = 6). Data were statistically analyzed by paired Student’s t test. Significant differences (*P < 0.05) were found between groups as indicated. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions. (Scale bars: 100 µm.)
Fig. S3.
Histograms depicting relative levels (intensities) of the proteins elastin (A) and MMP-9 (B) in the immunoblots depicted in Fig. 3D normalized against tubulin as internal standard. C and D represent the relative immunofluorescence intensities of lung elastin and the number of TUNEL-positive cells in Fig. 3 F and G, respectively, under different treatment conditions. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions.
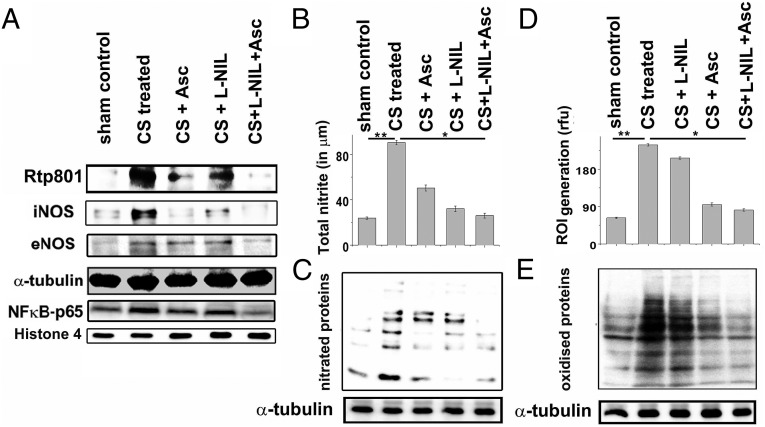
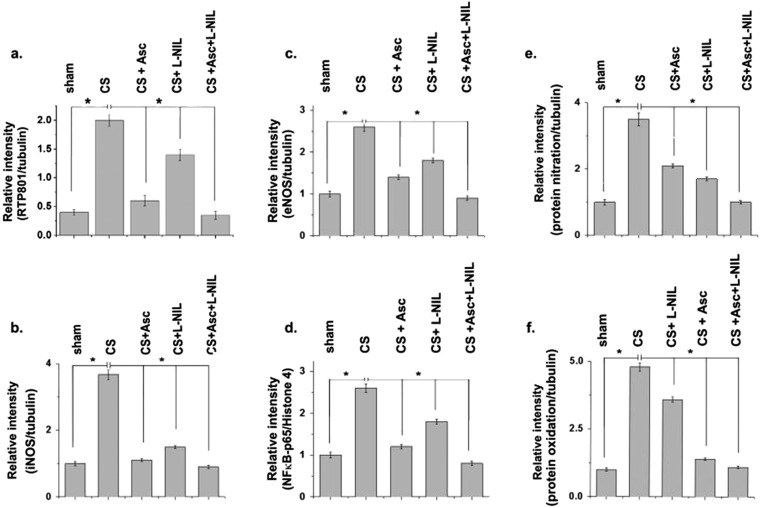
We also examined the effect of ascorbate and L-NIL on pulmonary expression of Rtp801 and NF-κB–p65, two major and associated cellular mediators of CS-induced inflammatory lung injury. Whereas ascorbate was able to substantially restore levels of both Rtp801 and NF-κB–p65 close to that of sham controls, L-NIL could not elicit significant protection (Fig. 4A and Fig. S4 A and D), indicating that inhibition of iNOS was not sufficient for prevention of the inflammatory cellular effectors of CS-induced lung damage.
Fig. 4.
Comparative prevention of tobacco smoke-induced pulmonary activation of Rtp801, NF-κB, and NOS expression as well as consequent oxido-nitrosative lung protein damage by ascorbate and L-NIL. (A) Immunoblot shows the pulmonary expression levels of the inflammatory proteins Rtp801 and NF-κB–p65 and the primary NO-producing NOS isoforms in the lung, iNOS, and eNOS in CS-exposed, CS+Asc, CS+L-NIL, and CS+Asc+L-NIL treated guinea pigs against air-exposed sham controls. (B) The total NO generated in the lung in terms of detectable total nitrite concentration in the BALF in the above samples (n = 6). Immunoblots depicting the levels of lung protein nitration (C) and the amounts of ROI generated (D) in the lung of the above-treated animals (n = 6). (E) Immunoblots indicating the levels of lung protein oxidation in the above-mentioned groups (n = 6). Data were statistically analyzed by paired Student’s t test. Significant differences (*P < 0.05, **P < 0.01) were observed between the groups as indicated. Data are represented as means ± SD and represent three independent experiments done under similar conditions.
Fig. S4.
Histograms depicting relative levels (intensities) of the proteins Rtp801 (A), iNOS (B), and eNOS (C) in the immunoblots shown in Fig. 4A, normalized against tubulin as internal standard; and NF-κB–p65 (D) normalized against histone 4 as the internal standard. E and F respectively show the relative levels of protein nitration and protein oxidation in Fig. 4 C and E normalized against corresponding tubulin expression. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions.
In fact, expression levels of both iNOS and eNOS decreased almost close to that of the sham controls in the CS-exposed animals treated with L-NIL and ascorbate conjointly (Fig. 4A and Fig. S4 B and C). Whereas pulmonary iNOS levels decreased by almost 97% and 82% compared with the CS-treated animals through separate treatment of ascorbate and L-NIL, respectively, corresponding eNOS levels decreased by approximately 76% and 52% (Fig. 4A and Fig. S4 B and C). Animals treated exclusively with L-NIL and ascorbate, respectively, showed almost an 86% and 59% decrease in whole-lung NO generation compared with only CS-treated counterparts (Fig. 4B). Moreover, levels of NO (Fig. 4B) and ROI generated (Fig. 4D) had strong compliance with corresponding lung protein nitration levels (Fig. 4C and Fig. S4E) with the lowest nitration witnessed in the CS-exposed animals treated with both L-NIL and ascorbate (98%), followed by L-NIL (72%) and then ascorbate (66%) (Fig. 4C and Fig. S4E). In contrast, although the highest level of protection against lung protein oxidation (Fig. 4E and Fig. S4F) was again observed in the CS-exposed animals conjointly treated with L-NIL and ascorbate (99%), ascorbate alone produced much larger protection (90%) compared with only L-NIL (31%) (Fig. 4E). Such observations indicated the possible involvement of diverse cellular RNS and ROS/oxidants, besides the oxidants present in CS, in CS-induced lung damage.
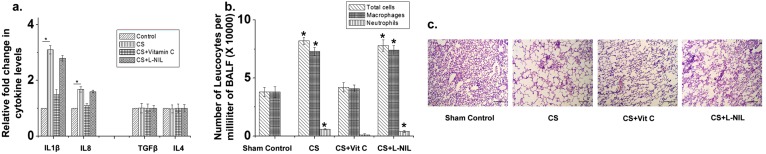
We also evaluated the relative levels of important M1 (IL-1β and IL-8) and M2 (TGF-β and IL-4) immune response signature cytokines in the BALF and total leukocyte infiltration into the BALF and lung alveoli in the 28-d CS-exposed animals with and without L-NIL and ascorbate treatment. Here, also we found that ascorbate could arrest the CS-induced inflammatory responses, whereas L-NIL was virtually ineffective (Fig. S5).
Fig. S5.
Histograms depicting relative fold changes in the expression levels of the cytokines IL-1β, IL-8, TGF-β, and IL-4 in the BALF of guinea pigs exposed to CS for 28 d with and without vitamin C (CS + Vit C) or L-NIL (CS + L-NIL) supplementation, against air-exposed sham controls (A), and the number of inflammatory cells (total leukocytes, macrophages, and neutrophils) in the BALF of the above groups of animals (B). C depicts representative histology from hematoxylin and eosin-stained lung sections of air-exposed (sham controls) guinea pigs against that of 28-d CS-exposed guinea pigs with and without vitamin C (CS + Vit C) or L-NIL (CS + L-NIL) supplementation, showing prominent cellular infiltration in the alveolar region of the CS-exposed and CS + L-NIL–treated guinea pigs. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions. (Scale bars, 50 µm.)
Tobacco Smoke Induces Protein Degradation in Normal Human Lung Epithelial Cells.
Indeed, the act of tobacco smoking by humans entails inhalation of smoke directly into the lung by the smoker where the CS first interacts with a layer of buffer called the respiratory tract lining fluid (RTLF) covering the lung epithelial cells, which is the first cellular interface the CS interacts and predisposes to damage. Thus, studying the effect of CS on the lung epithelial cells is perhaps closely replicated by diffusing CS into a buffer that mimics such RTLF and then incubating relevant amounts of the generated CS buffer with cultured lung epithelial cells. Because smoking is claimed to cause lung cancer, we used normal lung epithelial cells to avoid any bias in the etiopathological response of the cell to CS exposure. We examined possible degenerative effect of CS on such cells by incubating normal human lung epithelial cells (NL-20) with 1% CS extract (CSE) (Materials and Methods) in the presence and absence of ascorbate (200 µM) and L-NIL (1 mM) and then subjecting their lysates to 2D-DIGE (2D difference gel electrophoresis) analysis for evaluation of possible protein degradation by CS. The basis for using 200 μM ascorbate for such experiments is based on our observation that the level of the vitamin in the plasma of the guinea pig does not increase beyond such threshold levels no matter how much ascorbate they are fed.
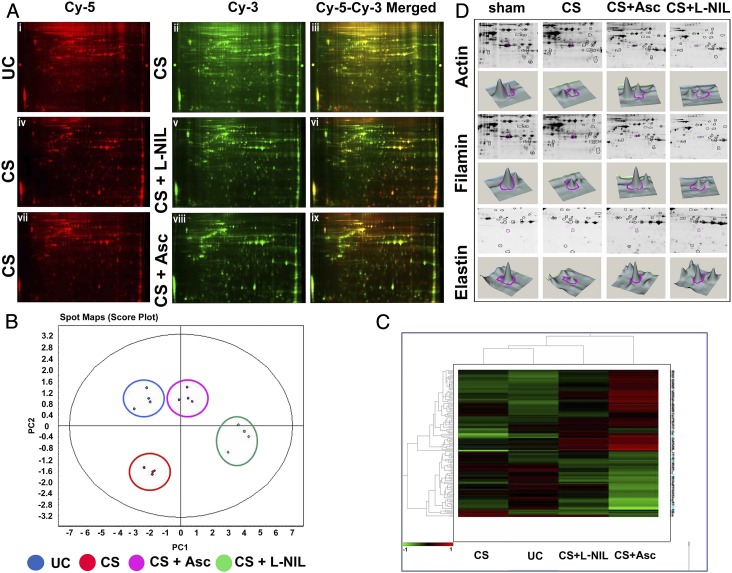
For the DIGE analysis, the CS-untreated control (UC) group of the NL-20 cells (Fig. 5 A, i) was compared against the CS-treated (CS) group (Fig. 5 A, ii) whereas the CS+L-NIL (Fig. 5 A, v) and the CS+ascorbate [CS+Asc] (Fig. 5 A, viii) groups were compared with their respective CS-treated counterparts (Fig. 5 A, iv and vii). The principal component analysis (PCA) was performed on the DIGE within the Extended Data Analysis (EDA) module of DeCyder 2D software. Fig. 5B depicts replicate spot maps of the four groups in which the CS-treated (CS, red) showed a pronounced decrease in protein expression across the four spot maps compared with the UC group (blue). The expression distribution of the spot maps in the CS+Asc group (purple) was similar to the UC group, confirming the profound protective effect of ascorbate against the decrease in protein expression in the CS group (red). The CS+L-NIL group (green), however, showed a much-decreased protection compared with ascorbate. Hierarchical clustering analysis (Fig. 5C) of the DIGE data also corroborated such results. Twenty-four proteins that underwent significant degradation (>60%) due to CS exposure were selected through the EDA analysis and corresponding t tests and ANOVA filters and, thereafter, were identified by MALDI-TOF analysis. These data confirmed that CS treatment caused extensive degradation of lung proteins, and ascorbate produced substantial protection against it compared with L-NIL. In fact, of the numerous proteins undergoing CS-induced degradation, three important lung structural proteins, elastin (2.4-fold decrease, P << 0.01), actin (2.14-fold decrease, P << 0.01), and filamin (1.77-fold decrease, P = 0.0053) were prominently identified, all of which were significantly protected from such degradation by ascorbate and not L-NIL (Fig. 5D). Accuracy provisions characterizing such DIGE analysis strongly vindicated the results obtained through the animal experiments in our present study.
Fig. 5.
Ascorbate prevents proteolysis of human lung proteins in normal human lung epithelial cells treated with CSE. (A) DIGE analysis shows lung protein degradation on CS exposure and comparative prevention achieved through ascorbate and L-NIL pretreatment. The first row depicts the comparative DIGE profiles of the CS-untreated (Cy-5 red, i) lung epithelial proteins from the NL-20 cells against their CS-treated counterparts (Cy-3, green, ii) along with their corresponding overlay (Cy-5–Cy-3 yellow, iii). The second and third rows similarly depict the comparative DIGE profiles of the respective CS-treated (Cy-5 red, iv and vii) against their CS + L-NIL (Cy-3 green, v) and CS + Asc (Cy-3 green, viii) treated counterparts along with their corresponding overlays (Cy-3–Cy-5 yellow, vi and ix). Replicate spot maps of the above four experimental groups were filtered with ANOVA < 0.001 and subjected to DeCyder EDA with PCA (B) and hierarchical clustering (C). (D) CS-induced degradation of lung proteins identified by DeCyder followed by MALDI-TOF analysis. Among many proteins, three important lung structural proteins, namely actin (2.14-fold decrease; P < 0.01), filamin (1.77-fold decrease; P = 0.0053) and elastin (2.4-fold decrease; P < 0.01), were identified to undergo extensive degradation and were protected by ascorbate but not L-NIL.
Tobacco Smoke Oxidant(s) Are the Primary Trigger Behind Emphysematous Lung Damage.
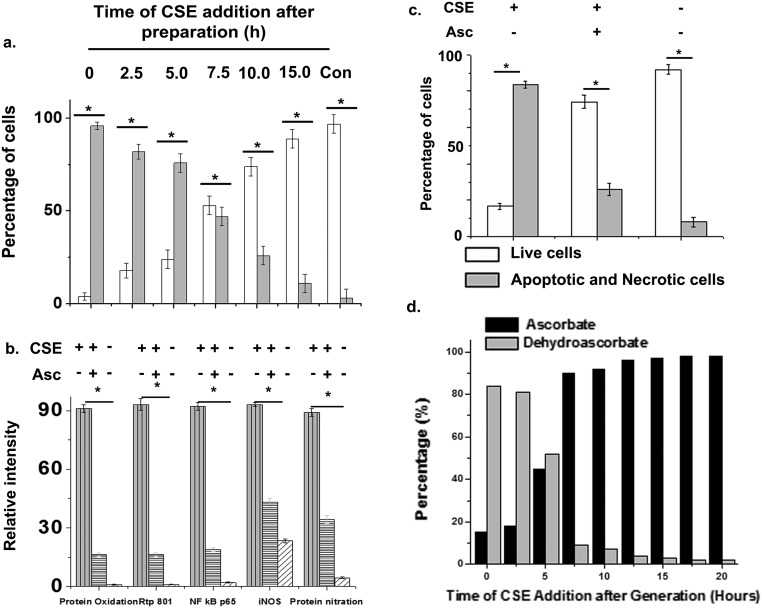
We postulated that the sustained oxidative potency of CS may be primarily involved in the pathogenesis of CS-induced emphysema. Although CS extract could directly oxidize the pure protein, BSA, in vitro, it, however, failed to cause its nitration (Fig. 6A), thus partly overruling the direct role of CS in the nitration of lung proteins, observed in vivo (Fig. 2F). To examine the possible causal association between the oxidative potency of CS and activation of cellular effectors of inflammatory lung damage, freshly prepared 1% CS extract (Materials and Methods) was added to NL-20 cells as described above in a time-dependent manner following its preparation, over a period of 15 h, and the treated cells were thereafter incubated overnight at 37 °C. This treatment produced a gradual time-dependent decrease in oxidation of lung proteins with corresponding decrease in the cellular expression levels of Rtp801, NF-κB–p65, iNOS, and protein nitration (Fig. 6B) as well as associated apoptosis/necrosis in the treated lung cells (Fig. 6C and Fig. S6A). Indeed, once the CS extract ceased to retain its oxidative potency from approximately 10 h of its preparation (also tested through its capability to oxidize the pure protein BSA), it not only failed to produce any protein oxidation, but was also unable to elicit any change in the expression levels of Rtp801, NF-κB–p65, iNOS, and protein nitration (Fig. 6B) or induce any observable apoptosis/necrosis in the treated lung cells (Fig. 6C and Fig. S6A). When the CS extract was added to the NL-20 cells in a media containing the antioxidant, ascorbate (200 μM), it also lost its capability to produce the above changes significantly (Fig. 6 D and E and Fig. S6 B and C). This observation clearly indicated a causal association between the capability of CS to oxidize lung proteins and the corresponding activation of the major lung cellular apparatus required for triggering and sustaining CS-induced lung damage.
Fig. 6.
Tobacco smoke oxidant(s) act as the key trigger for Rtp801 activation and consequent oxido-nitrosative stress and apoptosis/necrosis of lung cells exposed to CS. (A) CSE was first directly incubated with a pure protein BSA (1 mg) for 15 min and then subjected to Oxyblot analysis or immunoblot analysis for nitrotyrosine formation in which prenitrated BSA was used as the positive control (+ve con). (B) CSE (1%), either freshly prepared or kept in an airtight flask for 15 h following preparation (for time-dependent deactivation of its oxidative potency) was added at a regular intervals of 2.5 h to cultured NL-20 cells followed by overnight incubation at 37 °C. Cell lysates prepared from the treated cells were then subjected to immunoblot analysis by using anti-Rtp801, anti-NF-κB–p65, anti-iNOS and anti-nitrotyrosine antibodies or oxyblot analysis using anti-DNP antibody and relevant secondary antibodies. Such treated NL-20 cells were also separately stained with a solution of AO and EtBr for assessing their apoptosis/necrosis levels. (C) Representative sections of NL-20 cells showing overall levels of apoptosis/necrosis (10 fields). (D) In a separate experiment, NL-20 cells pretreated with 200 μM ascorbate were incubated overnight with CSE (1%) and their lysate were probed for the same markers as in B. (D) A portion of the same NL-20 cells used in the above experiment were stained with a solution of AO and EtBr to evaluate their apoptosis/necrosis levels. (E) Representative sections of NL-20 cells showing levels of apoptosis/necrosis (10 fields). Normal cells appeared green, apoptotic cells showed condensed/fragmented orange chromatin (red arrow, Inset), and necrotic cells showed complete orange nucleus (yellow arrow, Inset). Data shown are representative of three independent experiments done under similar conditions. (Scale bars: C and E, 100 μm; E, Inset, 50 µm.)
Fig. S6.
Histograms depicting relative numbers of apoptotic and necrotic human epithelial lung cells shown in Fig. 6C after time-dependent addition of freshly prepared CSE (A) and shown in Fig. 6E with and without Asc addition during CSE-treatment (C). B shows the relative levels (intensities) of protein oxidation, Rtp801, iNOS, and protein nitration normalized against tubulin along with NF-κB normalized against histone 4 as internal standards, in the immunoblots shown in Fig. 6D. D represents relative levels of conversion of ascorbate to dehydroascorbate in the media containing fixed concentration of vitamin C (200 µM) and exposed to CSE at different time points after its preparation. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions.
Discussion
Oxidative modification of lung proteins has been primarily implicated with its increased proteolysis and consequent loss of elasticity and structural integrity of the lung, culminating to fatal breathing dysfunctions during tobacco smoke- or CS-induced emphysema (5, 34–36). However, most studies have attributed such CS-induced oxidative damage mainly to the endogenous RNS/ROS generated in the lung during smoking (18–20, 34, 37, 38) without analyzing the comparative role of oxidants furnished by the CS itself in such damage process.
Although several mechanisms associating oxidative damage of the lung to the pathogenesis of CS-induced emphysema has emerged over the years, none have conjointly addressed the role of CS oxidants along with the lung-generated oxido-nitrosants in the disease process (23, 38, 39). Indeed, our present work reveals the thus far unanalyzed diverse share and comparative role of the CS oxidants and cellular oxido-nitrosants in emphysematous lung damage inflicted by CS.
Given that CS interacts with the lung epithelia almost directly during smoking (40), we examined whether the intrinsic oxidative potency of CS plays any role in the initiation or sustenance of the lung damage associated with emphysema. Our findings clearly indicate that CS is capable of directly oxidizing lung proteins even in the absence of cellular generators of ROS or RNS. Moreover, once devoid of its oxidative potency, CS was incapable of not only oxidizing proteins in the treated lung cells, but also triggering the major inflammatory pathway governing CS-induced lung damage, involving Rtp801/NF-κB/iNOS and the characteristic oxido-nitrosative protein modification and lung apoptosis/necrosis. In fact, effective neutralization of CS oxidants with the antioxidant ascorbate substantially reduced the capability of CS to both initiate and sustain such damage. This finding clearly indicates that the primary trigger behind CS-induced emphysematous lung damage involving extensive protein modification apparently resides in the oxidative potency of CS itself. Although CS could directly oxidize a pure protein like BSA, it was incapable of eliciting its nitration, thus signifying that lung-generated RNS is probably responsible for the lung protein nitration observed in vivo. Notably, CS has already been reported to up-regulate the mammalian target of rapamycin signaling inhibitor, Rtp801, which is also an activator of NF-κB (6). Such proposed role of Rtp801 as a key cellular response factor for oxidative activation by CS oxidants is also supported by lack of any change in the levels of NF-κB and consequent inflammation or lung damage in Rtp801 knockout mice exposed to CS (6). In fact, our findings support a strong causal link between the oxidative potency of CS and corresponding activation of Rtp801 in the triggering of the cellular machinery involved in emphysematous lung damage.
Indeed, we observed little difference in the level of oxidative damage of the lung proteins even when the primary CS-induced oxido-inflammatory machinery comprising of the Rtp801/NF-κB/iNOS was apparently blocked by the antiinflammatory glucocorticoid, dexamethasone, again suggesting that the major part of the CS-induced oxidative lung damage is caused directly by oxidants furnished by CS. This observation also indicated that such direct oxidative modification of lung proteins by CS oxidants is probably more determining for the pathogenesis of CS-induced emphysema than the subsequent oxido-nitrosative modification mediated by ROS/RNS generated in the lung, in response to CS exposure. The fact that CS directly bubbled into BSA solution failed to elicit any protein nitration, and lung-specific L-NIL treatment could reduce BALF nitrite levels by almost 86% and substantially arrest the lung protein nitration even after extensive CS exposure, clearly demonstrated that the NO/RNS present in CS is probably not significantly involved in the observed lung protein nitration caused by CS.
Nonetheless, our results indicate that even the observed lung protein nitration apparently caused by the cellular RNS during smoking at best partially contributes to the major lung protein modification or oxidation already inflicted by CS oxidants and has a comparatively minor involvement in accomplishing the overall CS-induced emphysematous lung injury. The relative incapability of L-NIL (iNOS inhibitor) compared with ascorbate (established scavenger of both CS oxidants and intracellular ROS/RNS) to significantly minimize CS-induced oxidative protein damage in the lung largely confirms this fact.
Interestingly, a recent report has claimed almost absolute prevention and reversal of CS-induced emphysematous lung damage through whole-body or global inhibition of iNOS in a mouse model (23). However, our study shows that lung-specific inhibition of iNOS cannot significantly prevent such lung damage in the guinea pig, whose four-lobed lung architecture and physiological responses to CS exposure are more comparable to humans than mouse, which has a two-lobed lung (41, 42). Moreover unlike mouse, guinea pigs resemble humans with respect to their response to oxidative stress because they cannot synthesize ascorbate or vitamin C de novo to dynamically attenuate such stress (43). Notably, no reversal of lung damage was observed in severely emphysematous guinea pigs even after sustained L-NIL treatment as reported by the above study (Fig. S7). In contrast, treatments that accomplished specific reduction in the expression of Rtp801 (ascorbate treatment) witnessed substantial reduction in the lung injury, whereas those that elicited exclusive inhibition of iNOS (L-NIL treatment) failed to do so, again signifying a more critical role of Rtp801 as a cellular effector of lung damage compared with iNOS. Indeed, this result is in consonance with our observation that L-NIL, while inhibiting pulmonary iNOS, was incapable of significantly preventing CS-induced activation of the inflammation triggering factors, Rtp801 or NF-κB, in the lung. More importantly, holistic iNOS inhibition is likely to compromise physiological functions based on multiple organ cross-talk involving the lung, which can severely disrupt the overall cellular and systemic responses governing the etiopathogenesis of CS-induced emphysema (44, 45), a factor that could well account for the difference in observations between the above study (23) and that of ours.
Fig. S7.
Representative histologies from hematoxylin and eosin-stained lung sections of air-exposed sham control and 28 d CS-exposed guinea pigs against those of guinea pigs exposed to CS for 28 d followed by vitamin C or L-NIL administration for additional 60 d, without CS exposure (A). All animals were kept under suboptimal vitamin C treatment (5 mg/kg body weight⋅animal−1⋅d−1) for the 60-d recuperation period without CS exposure to prevent the onset of scurvy. Sections highlight morphological changes in lung alveolar airspace (10 fields, n = 6) due to such treatment. Such alveolar morphometric changes were expressed in terms of Di (B) and Lm (C) as indicators of lung damage. Data were statistically analyzed by paired Student's t test. Significant differences (*P < 0.05) were observed in comparison with unexposed controls. Data are represented as means ± SD and are representative of three independent experiments done under similar conditions. (Scale bars: 50 µm.)
Emphysema is also characterized by increased proteolytic degradation of CS-modified lung proteins, primarily by the metalloproteinase, MMP-9, present in the lung (30). Here also, ascorbate was found to effectively reduce the CS-induced up-regulation of MMP-9. These data indicated that ascorbate besides preventing modification of the lung proteins by CS oxidant(s) and pulmonary oxido-nitrosant(s) could also mitigate the subsequent proteolysis of the modified proteins by endogenous lung proteases to render protection at both the major levels of lung damage underlying CS-induced emphysema.
The antioxidant property of ascorbate has been the subject of much debate largely because of its capability to act as a prooxidant in the presence of free iron (46). However, this controversy seems to be finally waning with several carefully designed studies showing that ascorbate predominantly acts as a potent antioxidant in vivo (47). Indeed, the potential of vitamin C or ascorbate to prevent CS-induced lung damage has been first reported by us through in vitro (2, 5) as well as in vivo (3) studies using guinea pig models of CS-induced emphysema. Thereafter, several other groups have vindicated our observations (26, 48, 49). However, none of these studies could successfully elucidate the oxidative targets of ascorbate. In fact, to examine whether ascorbate indeed acts as an antioxidant to neutralize CS oxidants, we modified the experiment described under Fig. 6B by adding a fixed amount of ascorbate (to a final concentration of 200 μM) into the cell culture medium before adding 1% CSE at regular intervals of 2.5 h after its preparation, for a period of 20 h (0–20 h). We found that the ability of the added CSE to convert ascorbate to dehyroascorbate (the oxidized form of ascorbate) was concordant with its residual oxidative potency (evaluated through the capability of such CSE to oxidize the treated lung cell proteins in identical ascorbate-free counterparts) (Fig. S6D and Fig. 6B). This observation clearly indicated that ascorbate was primarily acting as a reducing agent to neutralize the CS oxidants and, thereby, transforming into dehydroascorbate in the process of acting as a prophylactic against CS-induced lung damage (Fig. 6 D and E). In fact, similar redox neutralization of prominent CS electrophiles like semiquinones and aldehydes by ascorbate have also been reported (50, 51), indicating that ascorbate can effectively combat both oxidative and electrophilic toxicity of CS. To further examine whether ascorbate treatment can reverse the emphysematous lung damage inflicted by CS, we treated guinea pigs exposed to CS for 28–60 d of vitamin C treatment after CS exposure. We found there was no improvement in the lung damage compared with the CS-treated guinea pigs (Fig. S7). This finding clearly proved that vitamin C acts as a preventive and not curative against CS-induced lung damage.
However, our observation that ascorbate can suppress proinflammatory protein expressions including Rtp801, NF-κB, and iNOS, as well as the major lung protease, MMP-9, is indeed clinically encouraging. In fact, such role of ascorbate is not novel because several studies have demonstrated the ability of ascorbate to down-regulate gene expression levels including iNOS (52), NF-κB (53), and MMP-9 (54). Ascorbate probably manifests such gene regulatory role by abrogating critical oxidative triggers involved in the up-regulation of such genes (33, 47). This phenomenon certainly mandates more careful and well-designed studies because it has the potential to open up inexpensive therapeutic avenues for numerous inflammatory diseases.
Besides an enormous number of oxidants, CS contains numerous electrophilic compounds, most prominent among which are semiquinones and aldehydes, which generally form adducts with proteins (50, 51). However, it is not clear whether such protein adducts may also become susceptible to increased proteolysis like that observed for the oxidized or nitrated proteins during CS-induced emphysema. In the contrary, reports suggest that such protein-adduct formations may also render the target proteins resistant to proteolytic attack (55). Because CS is claimed to be a complex mixture of more than 4,700 components (56), of which a large share is claimed to be potential oxidants (57), it is overwhelmingly challenging to either identify or speculate the possible identity of the major oxidants involved in CS-induced lung damage. Such exercise becomes even more complex with the above numbers steadily increasing with evolving detection methods and the formidable task involved in establishing their relative bioavailability and potency at the cellular level. We have thus taken the liberty to avoid this complex exercise because we strongly feel that this job needs to be addressed with due patience and caution to be accurate and useful. Nevertheless, our present study convincingly demonstrates that although endogenously generated oxido-nitrosants in the lung partially contribute to the observed lung damage during smoking, it is the oxidants in CS that are primarily responsible for directly eliciting such damage and triggering the generation and activation of cellular mediators, including major endogenous proteases facilitating such damage. Thus, CS oxidants appear to act as the primary etiologic factor behind the initiation and sustenance of CS-induced emphysema, both at the noncellular and cellular levels.
Indeed, in unveiling the causal and damaging role of tobacco oxidants in CS-induced emphysema, our study also highlights the health risks of smoking and the undisputed benefits of quitting such practice. In fact, years of sustained social and legislative measures to restrict the practice of smoking have cut no ice with the world witnessing a phenomenal increase in the global incidence of smoking and tobacco-related diseases (1). Thus, in want of more effective and enabling measures to proscribe or eradicate this health damaging practice, the use of a preventive as inexpensive as vitamin C can be explored for salvaging the lives of millions of inveterate smokers enchained in this addictive yet suicidal exercise and reducing the global clinical burden associated with tobacco smoking.
Materials and Methods
Antibodies.
Mouse anti-nitrotyrosine, anti-MMP, and anti-DNP antibodies were obtained from Millipore, whereas all three anti-NOS antibodies were purchased from BD Biosciences. The anti-Bax, anti-Bcl-2, anti-elastin, and anti-p65 antibodies were procured from Santa Cruz Biotechnology, whereas the anti-Rtp801 antibody was procured from Abcam. Secondary horseradish peroxidize (HRP) and Alexa Fluor 488/562 conjugated anti-mouse/rabbit antibodies were obtained from GE Healthcare and Invitrogen, respectively.
Reagents and Chemicals.
All reagents including L-NIL and Asc were procured from Sigma-Aldrich. H2DCFDA and ECL Prime Western blotting reagent were purchased from Molecular Probes and GE Healthcare, respectively. All cell culture media were obtained from GIBCO.
Animals Used.
Adult male guinea pigs weighing 300–400 g were used. Animals were housed under controlled conditions having equal daylight cycle of 12 h with food and water supply ad libitum and were randomly allocated to smoke-exposed and smoke-unexposed groups comprising six guinea pigs each. Protocols for animal treatment (detailed below) were approved by the Institutional Animal Ethics Committee (Department of Biochemistry, University of Calcutta) and were in strict conformity with NIH guidelines.
Exposure of Animals to Tobacco Smoke.
CS was administered to the guinea pigs through whole-body exposure (3, 36) by using six Kentucky research grade cigarettes (3R4F) obtained from University of Kentucky [containing total particulate matter (11 mg), tar (9.4 mg), and nicotine (0.73 mg) per cigarette] per day for each treated animal at an interval of 1 h for a period of 1 mo. Animals were also exposed to CS either under administration of a selective iNOS inhibitor, L-NIL (1 mg per animal), or the antiinflammatory glucocorticoid, dexamethasone sodium phosphate (DXM) (1 mg per animal) through nebulization (Omron, NE-C25; 0.2 mL/min). The ascorbate treated groups were given monitored oral administration of the antioxidant, freshly dissolved in water (100 mg/kg body weight per animal) in conjunction with or without L-NIL nebulization. Animals were also pretreated with L-NIL, DXM, or ascorbate for 7 d before initiation of CS exposure. Age- and body weight-matched sham controls kept under identical conditions were subjected to air exposure instead of CS. To check whether vitamin C or L-NIL can reverse the CS-induced emphysematous lung damage, guinea pigs exposed to 28 d of smoke were administered 60 d of vitamin C or L-NIL without further smoke exposure, and their lung damage were evaluated and compared with those administered 28 d CS exposure and kept under no treatment for another 60 d. After treatment, the animals were euthanized by using i.p. ketamine administration and the left lungs were inflated with 10% (vol/vol) neutral-buffered formalin (NBF) (48). The fixed tissues were then paraffin embedded for morphometric analysis. The right lungs were either freshly used or snap-frozen in liquid nitrogen and stored at −85 °C for further use.
Preparation of Lung Tissues for Histological and Immunofluorescence Studies.
The inflated lung tissues were fixed in 10% (vol/vol) NBF for 48 h. After fixation, the tissue was dehydrated by transferring into 70% (vol/vol) alcohol, and after 24 h, transferred to 90% (vol/vol) alcohol for 1 h (two times) and finally to absolute alcohol for 1 h (two times). Finally, the tissue section was embedded in paraffin (Merck) and then trimmed into fine sections and mounted on grease-free glass slides. The paraffin was then heat-melted and washed with xylene. Sections were then washed sequentially with absolute alcohol, 90%, 70%, 50%, and 30% (vol/vol) alcohol and finally washed twice with either distilled water or PBS.
Lm and DI Analyses.
Guinea pig lung sections were processed for hematoxylin and eosin staining as mentioned earlier (58). The lung alveolar destruction was measured in terms of Lm and DI as described before (59, 60).
Evaluation of Leukocyte Infiltration and Cytokines in BALF.
BALF was collected from the CS-exposed guinea pig lung by flushing with three aliquots of 4.0-mL sterile PBS (37 °C). The BALF obtained was immediately centrifuged for 10 min at 400 × g at 4 °C, and the pellet was resuspended in PBS and used for total cell counting, whereas the supernatant was kept at −80 °C. Five microliters of the cell suspension was smeared on a microscope slide, dried, and then stained with Wright–Giemsa stain for microscopic evaluation of possible leukocyte infiltration in the BALF. Cytokine analysis from the BALF was performed by using BD CBA Flex Set (BD Biosciences) according to the manufacturer’s instruction. Because signals obtained with such mouse antibody-based kits were significantly weak, we depicted the results as relative changes (Fig. S5).
Cell Culture of NL-20.
NL-20 cells were obtained from the American Type Culture Collection. The cells were cultured in Ham’s F-12 medium containing 1.5 g/L sodium bicarbonate, 2.7 g/L d-glucose, 2.0 mM l-glutamine, 0.1 mM nonessential amino acids, 0.005 mg/mL insulin, 10 ng/mL epidermal growth factor, 500 ng/mL hydrocortisone, and 4% (vol/vol) FBS. Cultures were maintained at 37 °C in 100% (vol/vol) air-atmosphere (without CO2).
Preparation of CSE.
CSE was prepared by diffusing smoke from one research grade cigarette (3R4F) into 1 mL of serum-free media supplemented with 1% FBS (59–61) (the pH adjusted to 7.4). CSE was freshly prepared for each experiment and, when required diluted, with serum-free culture media (concentration was standardized by monitoring absorbance at a wavelength of 320 nm). The control was prepared by diffusing air through 1 mL of serum-free culture media and processed identically. In another experiment, freshly prepared CSE was kept in an air tight flask for 15–20 h and added periodically to NL-20 cells or ascorbate containing medium to study possible time-dependent abrogation of its oxidative potency and mechanism of ascorbate neutralization of CS oxidants.
Measurement of Pulmonary NO Synthesis.
Pulmonary NO generation was measured in terms of total nitrite/nitrate content through Griess assay (61). One hundred microliters of Griess reagent, containing equal volumes of 0.5% sulfanilamide and 0.05% N-(1-naphthyl) ethylenediamine dihydrochloride was either added to 100 μL of BALF or whole-lung lysates following reduction of all nitrate to nitrite with 100 μL of vanadium chloride and then incubated for 30 min (25 °C). Absorbance was measured at 540 nm by using a tunable microplate reader (Versamax; Molecular Devices) and the total nitrite content was estimated against a NaNO2 standard curve, as a measure of the NO produced.
iNOS Activity Measurement.
Whole-lung lysates (50 µg) were incubated in 40 mM 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (EPPS) (pH 7.4), 3 mM DTT, 4 μM FAD, 4 μM FMN, 10 μM H4B, 10 mM Arg, 0.5 mM EDTA, 1.2 mM CaCl2, 1 μM CaM, 0.1 mg/mL BSA, 18 units/mL catalase, and 10 units/mL SOD in triplicate. NADPH (10 mM) was then used to trigger NO production at 37 °C for a period of 1 h (62, 63). The total nitrite/nitrate content was then estimated as described above, as a measure of NO produced.
Immunoblot Analysis.
Whole-lung lysates of treated guinea pigs were prepared in radioimmunoprecipitation assay buffer as described earlier (49). Nuclear extraction was performed for determination of nuclear translocation of NF-κB–p65 by standard protocols (49). Whole-lung lysates and nuclear extracts were subjected to SDS/PAGE followed by immunoblotting with relevant primary antibodies. Thereafter, the blots were washed and incubated with HRP-conjugated anti-mouse IgG or anti-rabbit IgG (GE Healthcare) for 1 h and developed with ECL reagent (GE Healthcare), and data was analyzed by using the Chemidoc XRS+ (Bio-Rad). The protein levels were normalized against tubulin except NF-κB–p65, for which histone 4 was used.
Oxyblot Assay for Detection of Lung Protein Oxidation.
Protein oxidation was assessed by using the Oxyblot Kit (Millipore). Twenty micrograms of lung lysates were derivatized with 10 µL of 1× DNPH solution in 2 M HCl and incubated for 15 min at 25 °C. The reaction was then arrested with a neutralization buffer (7.5 µL), and the samples were subjected to SDS/PAGE and further immunoblotting by using rabbit anti-DNP antibody. The membranes were then washed and incubated with anti-rabbit IgG for 1 h and further analyzed for protein oxidation after washing and treatment with ECL reagent (GE Healthcare).
TUNEL Assay for Detection of Cellular Apoptosis.
Paraffin-embedded lung tissue sections were deparaffinised as described above and treated with freshly prepared permeabilization solution (10 mM sodium citrate and 0.05% Tween 20, pH 6.0). The sections were then washed with PBS and incubated with 50 µL of TUNEL mixture (Roche) in dark at 37 °C for 1 h. After washing the TUNEL reagent with PBS, the slides were mounted with DAPI-containing mounting medium (Vectashield) for examination under a fluorescence microscope (Olympus IX 71) and data was analyzed with the Image Pro-6.3 acquisition software provided by the instrument manufacturer.
Immunofluorescence Studies.
Deparaffinized tissue sections were processed for antigen retrieval with permeabilizing solution. The slides were then blocked with 1% BSA and incubated overnight with relevant primary antibodies. After washing with PBS-T, the sections were incubated with either Alexa Fluor 488 (for nitrotyrosine and elastin) or Alexa Fluor 562 conjugated anti-mouse antibodies (for iNOS and eNOS). The slides were then examined under a fluorescence microscope (Olympus IX 71), and data was analyzed by using the Image Pro-6.3 acquisition software.
Measurement of Total ROIs.
Freshly dissected lung tissues were homogenized in Hepes buffered saline (HBS) and centrifuged at 13,000 × g for 15 min. Total ROI levels in the supernatants were measured at 37 °C by using H2DCFDA (Molecular Probes) as per manufacturer’s protocol (64, 65). Fluorescence was quantified by using 488-nm excitation and 525-nm emission wavelengths through a fluorescence spectrophotometer (Hitachi F-7000). For in situ detection of ROI, fresh lung tissue cryo-sections were incubated with 10 µmol/L H2DCFDA for 30 min, following which they were washed with HBS, mounted with Vectashield mounting medium, and finally examined under a fluorescence microscope (Olympus IX 71) for further analysis.
2D Fluorescence DIGE.
NL-20 cells were treated with either control medium (untreated control; UC) or with 1% CSE for 12 h (overnight) either alone (CS) or in the presence of 200 µM ascorbate (CS+ascorbate) or 1 mM L-NIL (CS+L-NIL). The treated NL-20 cells were lysed in DIGE lysis buffer (7 M urea, 2 M thiourea, 4% (wt/vol) CHAPS, 30 mM Tris, pH 8.5) and were labeled with CyDye DIGE fluors using an experimental design that incorporates an internal standard (comprised of equal amounts of four samples), reverse labeled individual samples (four replicates of each), and random pairings of the labeled samples across eight large-format DIGE gels. First-dimension isoelectric focusing was performed by using pH 4–7 range (24 cm) Immobiline DryStrips on an IPGPhor II (GE Healthcare) isoelectric focusing apparatus. The second dimension was performed by using Ettan DALTsix (GE Healthcare) using 12.5% (vol/vol) SDS/PAGE. The gels were then scanned on a Typhoon 9400, and the imaged data were analyzed by using DeCyder 2D differential analysis software (GE Healthcare) (66).
DeCyder Analysis.
DeCyder 2D software that allows comparison of multiple groups using statistical tools including t test and one-way ANOVA was used. The four samples (untreated control, CS exposed, CS exposed with ascorbate treatment, and CS exposed with L-NIL treatment) were compared for differences in protein expression following CS exposure and possible protective effects of ascorbate or L-NIL. Protein spots were picked based on difference seen between one or more groups with a statistical confidence of P < 0.01 and corresponding tryptic peptide digests were subjected to MALDI-TOF analysis for identification of the proteins.
EDA.
The results from DeCyder 2D analysis were imported into the EDA module imposing additional statistical tests on interpretation. Proteins present in 80% of the spot maps were analyzed as the base set, and spots with ANOVA of P < 0.001 were filtered. This ANOVA set was further analyzed by PCA and hierarchical clustering.
Ethidium Bromide and Acridine Orange Staining-Based Cell Viability Analysis.
CSE [1%], either freshly prepared or kept in an airtight flask for 15–20 h, was added to cultured NL-20 cells at an interval of 2.5 h following its preparation. The treated cells were incubated overnight either in absence or presence of ascorbate. After completion of the incubation, the cells were washed thrice with PBS and further incubated with ethidium bromide (EtBr) and acridine orange (AO) for 5 min (67). They were then washed with PBS and examined under a fluorescence microscope (Olympus IX 71) and analyzed by using the Image Pro-6.3 acquisition software. Typically, the nuclear binding dye, AO stains the nuclei green, whereas EtBr usually enters cells with damaged membrane and stains the nucleus red. Under such staining protocol, apoptotic cells display condensed and fragmented orange chromatin, whereas necrotic cells depict a structurally normal orange nucleus. Such demarcation forms the basis of highlighting and differentiation apoptotic cells from necrotic cells in this assay (68).
Measurement of Dehydroascorbic Acid.
Dehydroascorbate (DHA) content of the cell culture media was measured by a standardized HPLC-based technique (69). The ascorbate (reduced form) content of the media was initially determined. The corresponding dehydroascorbate in the media was subsequently reduced to ascorbate by 10 mM DTT, followed by HPLC determination of the total ascorbate content (reduced + oxidized ascorbate), from which the DHA (oxidized ascorbate) quantity was calculated by subtracting the initially determined ascorbate (reduced).
Statistical Analysis.
All values are expressed as mean ± SEM. Data were analyzed by the one-way ANOVA and the two-tailed Student’s t test. A P value of ≤0.05 was considered significant for most experiments.
Acknowledgments
We thank Jing Huang and Phil Beckett (Proteomics Applications Laboratory, GE Healthcare) for excellent technical support in the 2D-DIGE experiments and Dr. Mamta Chawla Sarkar (Indian Council of Medical Research-National Institute of Cholera and Enteric Diseases, Kolkata) for her critical suggestions. This work was supported by research grants from the Council of Scientific and Industrial Research, Government of India (to K.P.), as well as a Biocon Foundation fellowship (to I.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600056113/-/DCSupplemental.
References
- 1.Mannino DM, Buist AS. Global burden of COPD: Risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 2.Panda K, Chattopadhyay R, Ghosh MK, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke induced oxidative damage of proteins and increased proteolysis. Free Radic Biol Med. 1999;27(9-10):1064–1079. doi: 10.1016/s0891-5849(99)00154-9. [DOI] [PubMed] [Google Scholar]
- 3.Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med. 2000;29(2):115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SD. Proteolysis in the lung. Eur Respir J Suppl. 2003;44:30s–32s. doi: 10.1183/09031936.03.00000903a. [DOI] [PubMed] [Google Scholar]
- 5.Panda K, Chattopadhyay R, Chattopadhyay D, Chatterjee IB. Cigarette smoke-induced protein oxidation and proteolysis is exclusively caused by its tar phase: Prevention by vitamin C. Toxicol Lett. 2001;123(1):21–32. doi: 10.1016/s0378-4274(01)00376-9. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida T, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16(7):767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 8.Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53(7):601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kukkonen MK, et al. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med. 2013;13:36. doi: 10.1186/1471-2466-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen CA. Proteinases and oxidants as targets in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(4):373–385, discussion 394–395. doi: 10.1513/pats.200504-029SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122(6) Suppl:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 12.Churg A, Wright JL. Proteases and emphysema. Curr Opin Pulm Med. 2005;11(2):153–159. doi: 10.1097/01.mcp.0000149592.51761.e3. [DOI] [PubMed] [Google Scholar]
- 13.Segura-Valdez L, et al. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117(3):684–694. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 14.Finlay GA, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax. 1997;52(6):502–506. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 16.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006;7:53. doi: 10.1186/1465-9921-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokohori N, Aoshiba K, Nagai A. Respiratory Failure Research Group in Japan Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125(2):626–632. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 19.Wijnhoven HJ, et al. Oxidative and nitrosative stress in the diaphragm of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(2):173–179. doi: 10.2147/copd.2006.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am J Respir Crit Care Med. 2000;162(2 Pt 1):701–706. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 22.Marwick JA, et al. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31(6):633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 23.Seimetz M, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147(2):293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Hesslinger C, et al. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37(Pt 4):886–891. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara Y, et al. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163(3 Pt 1):737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 26.Koike K, et al. Vitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol. 2014;50(2):347–357. doi: 10.1165/rcmb.2013-0121OC. [DOI] [PubMed] [Google Scholar]
- 27.Carnevali S, et al. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L955–L963. doi: 10.1152/ajplung.00466.2001. [DOI] [PubMed] [Google Scholar]
- 28.Tuder RM, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol. 2003;29(1):88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 29.Ryter SW, Chen ZH, Kim HP, Choi AM. Autophagy in chronic obstructive pulmonary disease: Homeostatic or pathogenic mechanism? Autophagy. 2009;5(2):235–237. doi: 10.4161/auto.5.2.7495. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson JJ, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183(7):876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101(12):4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sittipunt C, et al. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(2):503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- 33.Padayatty SJ, et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 34.Wright JL, Churg A. Current concepts in mechanisms of emphysema. Toxicol Pathol. 2007;35(1):111–115. doi: 10.1080/01926230601059951. [DOI] [PubMed] [Google Scholar]
- 35.Groneberg DA, Chung KF. Models of chronic obstructive pulmonary disease. Respir Res. 2004;5:18. doi: 10.1186/1465-9921-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright JL, Churg A. Smoke-induced emphysema in guinea pigs is associated with morphometric evidence of collagen breakdown and repair. Am J Physiol. 1995;268(1 Pt 1):L17–L20. doi: 10.1152/ajplung.1995.268.1.L17. [DOI] [PubMed] [Google Scholar]
- 37.Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal. 2013;18(15):1956–1971. doi: 10.1089/ars.2012.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254(2):72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanzetti M, et al. Oxidative stress and nitrosative stress are involved in different stages of proteolytic pulmonary emphysema. Free Radic Biol Med. 2012;53(11):1993–2001. doi: 10.1016/j.freeradbiomed.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Thorley AJ, Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(4):409–428. [PMC free article] [PubMed] [Google Scholar]
- 41.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: Insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L612–L631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 42.Wright JL, Churg A. A model of tobacco smoke-induced airflow obstruction in the guinea pig. Chest. 2002;121(5) Suppl:188S–191S. doi: 10.1378/chest.121.5_suppl.188s. [DOI] [PubMed] [Google Scholar]
- 43.Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem. 1992;267(30):21967–21972. [PubMed] [Google Scholar]
- 44.Ponnuswamy P, et al. Oxidative stress and compartment of gene expression determine proatherosclerotic effects of inducible nitric oxide synthase. Am J Pathol. 2009;174(6):2400–2410. doi: 10.2353/ajpath.2009.080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedbala W, et al. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci USA. 2007;104(39):15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podmore ID, et al. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392(6676):559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 47.Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13(9):1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 48.Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- 49.Yang SR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh A, et al. Causation of cigarette smoke-induced emphysema by p-benzoquinone and its prevention by vitamin C. Am J Respir Cell Mol Biol. 2015;52(3):315–322. doi: 10.1165/rcmb.2013-0545OC. [DOI] [PubMed] [Google Scholar]
- 51.Arai H, Uchida K, Nakamura K. Effect of ascorbate on acrolein modification of very low density lipoprotein and uptake of oxidized apolipoprotein e by hepatocytes. Biosci Biotechnol Biochem. 2005;69(9):1760–1762. doi: 10.1271/bbb.69.1760. [DOI] [PubMed] [Google Scholar]
- 52.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R50–R56. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 53.Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Golde DW. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002;41(43):12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 54.Kaul D, Baba MI. Genomic effect of vitamin ‘C’ and statins within human mononuclear cells involved in atherogenic process. Eur J Clin Nutr. 2005;59(8):978–981. doi: 10.1038/sj.ejcn.1602203. [DOI] [PubMed] [Google Scholar]
- 55.Burcham PC, Kuhan YT. Diminished susceptibility to proteolysis after protein modification by the lipid peroxidation product malondialdehyde: Inhibitory role for crosslinked and noncrosslinked adducted proteins. Arch Biochem Biophys. 1997;340(2):331–337. doi: 10.1006/abbi.1997.9903. [DOI] [PubMed] [Google Scholar]
- 56.Perfetti TA, Rodgman A. The complexity of tobacco and tobacco smoke. Contrib Tob Res. 2011;24(5):215–232. [Google Scholar]
- 57.Repine JE, Bast A, Lankhorst I. Oxidative Stress Study Group Oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156(2 Pt 1):341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 58.Yao H, et al. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172(5):1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax. 1962;17(4):320–328. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saetta M, et al. Destructive index: A measurement of lung parenchymal destruction in smokers. Am Rev Respir Dis. 1985;131(5):764–769. doi: 10.1164/arrd.1985.131.5.764. [DOI] [PubMed] [Google Scholar]
- 61.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 62.Stuehr DJ, Cho HJ, Kwon NS, Weise MF, Nathan CF. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: An FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci USA. 1991;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor β. J Exp Med. 1993;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duarte FV, Teodoro JS, Rolo AP, Palmeira CM. Exposure to dibenzofuran triggers autophagy in lung cells. Toxicol Lett. 2012;209(1):35–42. doi: 10.1016/j.toxlet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 65.Ma J, et al. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res. 2013;97(1):77–87. doi: 10.1093/cvr/cvs309. [DOI] [PubMed] [Google Scholar]
- 66.Rozanas CR, Loyland SM. Capabilities using 2-D DIGE in proteomics research: The new gold standard for 2-D gel electrophoresis. Methods Mol Biol. 2008;441:1–18. doi: 10.1007/978-1-60327-047-2_1. [DOI] [PubMed] [Google Scholar]
- 67.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 68.Renvoizé C, Biola A, Pallardy M, Bréard J. Apoptosis: Identification of dying cells. Cell Biol Toxicol. 1998;14(2):111–120. doi: 10.1023/a:1007429904664. [DOI] [PubMed] [Google Scholar]
- 69.Ngkeekwong FC, Ng LL. Two distinct uptake mechanisms for ascorbate and dehydroascorbate in human lymphoblasts and their interaction with glucose. Biochem J. 1997;324(Pt 1):225–230. doi: 10.1042/bj3240225. [DOI] [PMC free article] [PubMed] [Google Scholar]