Significance
This work is to our knowledge the first illustration of the ability of the high-energy resolution fluorescence-detection X-ray absorption spectroscopy (HERFD-XAS) technique to directly probe the crystal-field splitting in the 5f shell of actinides. This is a breakthrough for actinide science, allowing for the easy extraction of desired information from the spectroscopic method, which is easy to interpret and to calculate. Furthermore, the HERFD-XAS technique allows us to resolve the charge-transfer satellites in actinide spectra that were hidden before, thus enhancing the sensitivity to the covalent character of the chemical bonding. Using ThO2 as an example, we show that other common techniques underestimate the crystal-field strength and that ThO2 is not an anionic compound as previously believed.
Keywords: actinide compounds, crystal field, covalency
Abstract
Applying the high-energy resolution fluorescence-detection (HERFD) mode of X-ray absorption spectroscopy (XAS), we were able to probe, for the first time to our knowledge, the crystalline electric field (CEF) splittings of the shell directly in the HERFD-XAS spectra of actinides. Using ThO2 as an example, data measured at the Th 3d edge were interpreted within the framework of the Anderson impurity model. Because the charge-transfer satellites were also resolved in the HERFD-XAS spectra, the analysis of these satellites revealed that ThO2 is not an ionic compound as previously believed. The Th occupancy in the ground state was estimated to be twice that of the Th states. We demonstrate that HERFD-XAS allows for characterization of the CEF interaction and degree of covalency in the ground state of actinide compounds as it is extensively done for 3d transition metal systems.
For strongly electron-correlated systems, X-ray absorption spectroscopy (XAS) is viewed as a technique capable of providing information on the ground-state character despite the presence of the core hole in the final state of the spectroscopic process. In the 3d transition metal compounds, XAS at the metal 2p edge is routinely used to probe the magnitude of the crystalline electric field (CEF) splittings in the 3d shell and the degree of the 3d hybridization in the ground state (1).
However, for the shell of actinides, such a use of XAS is difficult. At the U 3d and 4d edges, where the transitions to the 5f states are probed, core-hole lifetime broadenings [full width at half maximum (FWHM)] are about 3.2 eV and 4.2 eV, respectively (2), whereas at the 5d edge, the broadening can be as large as 6.0 eV. Therefore, the conventional XAS spectra of actinide materials at the 3d and 4d edges often appear as single lines where splittings of the 5f states (e.g., due to the CEF interaction) are completely smeared out (3–8).
The CEF effects for actinides are usually characterized with the help of optical absorption (reviewed in ref. 9) and electron paramagnetic resonance (EPR) (10, 11) spectroscopies but that is often done on doped systems when the actinide ions in question are introduced into the lattice of a compound without 5f electrons. Another high-resolution technique that can provide information about the CEF splittings is inelastic neutron scattering (INS) (12, 13) but the spectra usually cover a very limited energy range. In both optical absorption and INS spectroscopies, the CEF strength and parameters are evaluated by fitting the calculated energy levels of the CEF Hamiltonian to the energies of the measured lines of the 5f–5f transitions, whereas attempts to calculate spectral intensities are rare.
On the other hand, for XAS at the threshold of actinides it is relatively straightforward to calculate both energy levels and spectral intensities due to dipole selection rules. The effects of the hybridization of the valence states of actinides with ligand states in compounds and the charge transfer between ligand and actinide sites are expected to result in the appearance of charge-transfer satellites in the XAS spectra. The interpretation of such satellites can provide information on the degree of the hybridization, covalency of the compound, and the nature of the ground state.
An advanced technique, high-energy resolution fluorescence-detection X-ray absorption spectroscopy (HERFD-XAS), has been recently used for measurements at the 3d edge of U compounds (14, 15). This has led to a tremendous improvement in the experimental resolution and revealed additional structures in the XAS spectra, not measurable before. We demonstrate, using ThO2, that the HERFD-XAS technique now allows us to directly probe the CEF splittings in the shell with the results clearly shown in the spectra. An analysis of the newly resolved charge-transfer satellites indicates significant occupancy of Th valence states, which contradicts the common view of ThO2 as an ionic system.
Materials and Methods
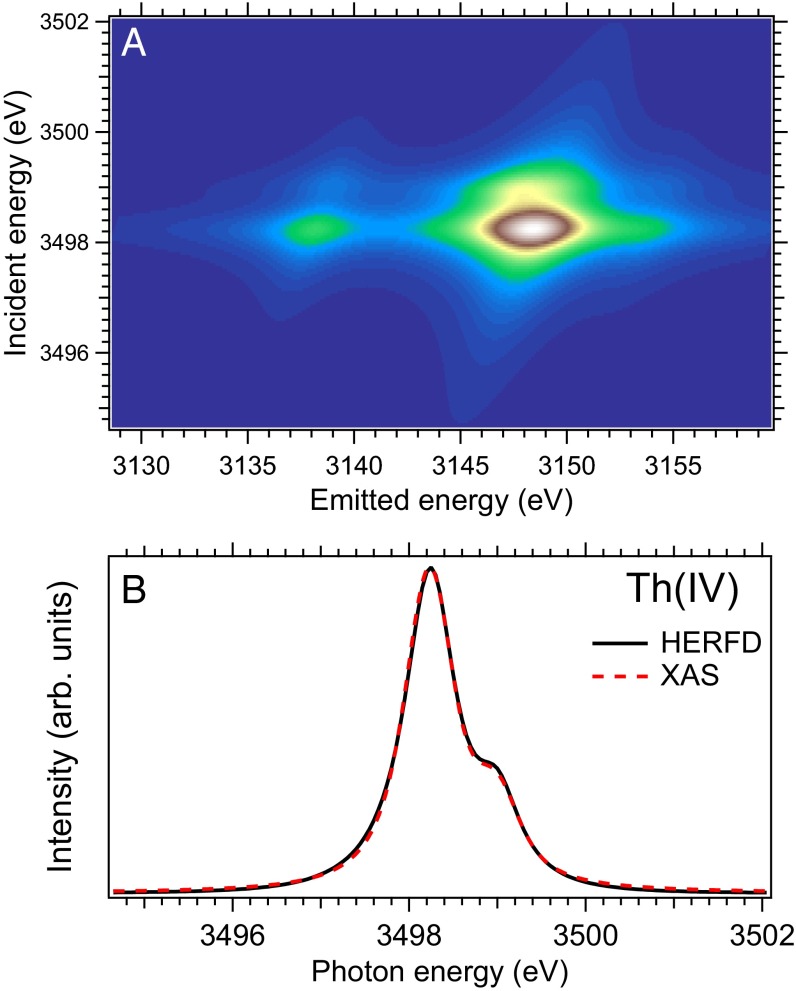
The measurements at the Th edge were performed at beamline ID26 (16) of the European Synchrotron Radiation Facility in Grenoble, France. The XAS data were measured in the HERFD mode, using an X-ray emission spectrometer (17). The HERFD spectrum of ThO2 at the Th ( transitions) edge was obtained by recording the outgoing photons with an energy of ∼3,145.7 eV as a function of the incident energy. This emission energy corresponds to the maximum of the Th ( transitions) line. The directions of the incident and emitted photons were 45° to the surface of the sample. A total energy resolution of 0.4 eV was determined by measuring the FWHM of the elastic peak. The sample of reference bulk ThO2 as characterized and used in refs. 18 and 19 was used for these experiments.
Experiments at the Ce edges ( transitions) of CeO2 were performed at beamline 7.0.1 of the Advanced Light Source, Lawrence Berkeley National Laboratory (20). The data were measured in the total electron yield (TEY) mode, using drain current from the sample. The incidence angle of the incoming photons was close to 90° with respect to the surface of the samples. The monochromator resolution was set to ∼500 meV at 890 eV. The CeO2 sample was a pressed pellet from powder acquired from Alfa-Aesar.
The calculations were performed in framework of the Anderson impurity model (21) (AIM) in a manner described in refs. 22 and 23. Besides the inclusion of the full multiplet due to actinide intraatomic interactions, the CEF acting on the shell (24) was taken into account.
To simulate a spectrum obtained in the HERFD-XAS mode, the resonant inelastic X-ray scattering (RIXS) map around the actinide line was calculated using the Kramers–Heisenberg formula
| [1] |
where and are the ground, intermediate, and final states of the spectroscopic process with energies and respectively. ω and are the energies of the incident and scattered/emitted photons with polarizations and respectively, and and are the lifetime broadenings of the intermediate and final states in terms of half width at half maximum (HWHM). Operators for optical dipole transitions (D) are expressed in terms of spherical tensor operators The HERFD-XAS spectrum is represented by a linear cut of such a RIXS map (14) along the diagonal of the plane defined by the incident energy axis and energy transfer axis or parallel to the incident energy axis at a constant emitted energy (the energy of the line maximum in this case) in the plane of the emitted vs. incident energies. The required Slater integrals and (25); spin-orbit coupling constants ζ; and matrix elements were obtained with the modified TT-MULTIPLETS package (26–28).
Results and Discussion
The main reason for the improved resolution of the HERFD-XAS spectrum is a reduced core-hole lifetime broadening. For example, in HERFD-XAS measurements at U edges, the core hole is replaced by the core hole in the final state of the spectroscopic process (Fig. 1). This results in approximately four times better resolution because the core-hole lifetime broadening (FWHM) is estimated to be ∼0.8 eV (29) vs. ∼3.2 eV for the core hole.
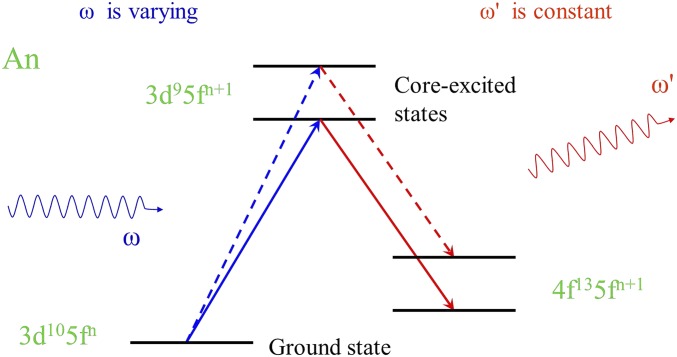
Fig. 1.
Schematic representation of HERFD process for actinides (An), using 3d-to-4f transitions.
A schematic representation of the HERFD spectroscopic process involving the electronic excitations with the consequent deexcitations at the actinide (An) atom is shown in Fig. 1, using a many-body approach. A couple of arbitrary transitions between the ground state of the system described by the electronic configuration and core-excited states of the configuration for varying incident photon energy ω are depicted. The core-excited states decay to the states of the configurations with the emittance of a photon and only photons with an energy equal to are detected.
Fig. 2A displays the RIXS map around the line of the Th(IV) ion in the cubic (eightfold ligand coordination) CEF environment calculated with Eq. 1 for the route with and set to 1.65 eV and 0.3 eV, respectively. The experimental X-ray polarization geometry was taken into account in calculations. Fig. 2B shows the calculated conventional XAS and HERFD-XAS spectra at the Th(IV) edge. The conventional XAS spectrum is represented by the transitions between the (ground state) and (excited state) configurations. The HERFD-XAS spectrum is represented by the corresponding cut in the RIXS map. The result of the cut is a one-dimensional spectrum of intensity vs. incident energy ω obtained for fixed corresponding to the main maximum of the line. In both cases, the Hartree–Fock values of the Slater integrals were scaled down to 80% (the reasoning is in ref. 30) and Wybourne’s CEF parameters (24) for the shell were set to eV and eV. Although the effects of the hybridization of Th states with ligand states (which lead to an appearance of low-intensity high-energy satellites, described below) were not taken into account, we can conclude that the conventional XAS and HERFD-XAS spectra agree with each other quite well. To simplify the computational framework, we compare the experimental HERFD-XAS data to calculations from conventional XAS.
Fig. 2.
Calculated RIXS map around the line of the Th(IV) ion in the cubic CEF environment (A) and HERFD-XAS and conventional XAS spectra at the Th(IV) edge (B).
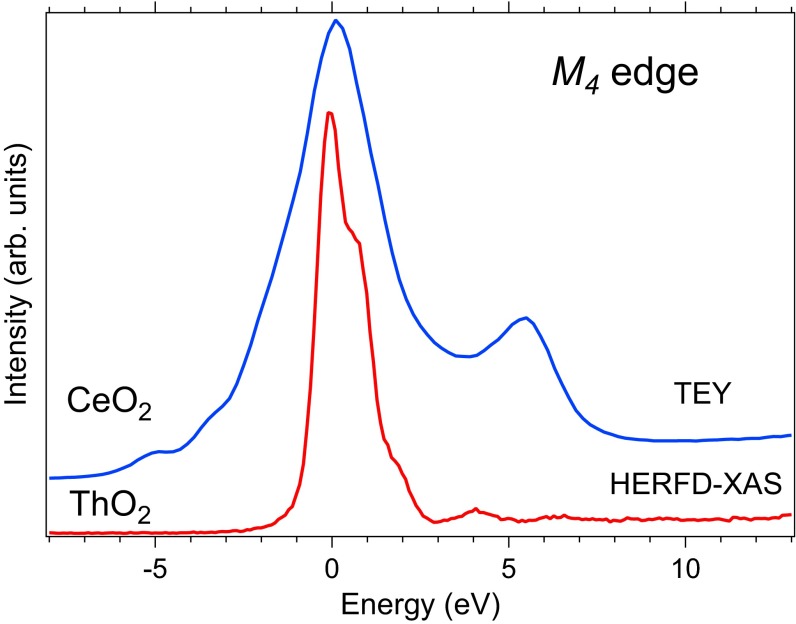
CeO2 is often used by researchers in the actinide science as a model compound and nonradioactive replacement of ThO2 (UO2) as an approximation for the physical and chemical properties and dynamic behavior of actinide materials in particular situations. However, there are significant differences between CeO2 and ThO2 from the electronic structure point of view. According to the local-density approximation with added Coulomb U interaction (LDA + U) calculations (31), the top of the valence band consists mainly of the O and Ce states whereas the bottom of the conduction band is dominated by Ce states. In ThO2, however, the unoccupied Th states lie higher in energy so that the main weight at the bottom of the conduction band is formed by the Th states. CeO2 is considered a highly covalent compound and ThO2 is not.
The spectra recorded at the edge of ThO2 and CeO2 also reveal some differences as shown in Fig. 3. Because the edge of ThO2 is represented by the transitions and the edge of CeO2 is represented by the transitions, the spectra were brought onto the same energy scale by setting the energy of the main maximum to 0 eV. The CeO2 spectrum exhibits a distinct and strong high-energy satellite at ∼5.5 eV above the main line whereas only weak high-energy structures at around 2.2 eV, 4.2 eV, and 6.4 eV above the main maximum are found in ThO2. In addition, the main line of the ThO2 spectrum has a shoulder at ∼0.8 eV. The high-energy satellite in the CeO2 spectrum has been discussed before (22, 32) and shown to arise from Ce O charge transfer as a result of strong Ce O hybridization and significant and configurational mixing in the ground state, where stands for an electronic hole in the O band. The XAS spectrum at the Ce edges of CeO2 was well reproduced by the AIM calculations for the Ce(IV) system. The CeO2 spectrum appears significantly broader than that of ThO2 because of a wider multiplet spread of the configuration caused by the stronger – interaction compared with the – interaction in ThO2 (figure 5 in ref. 32). The occupancy in the ground state was found to be about 0.5 electrons, which confirms the highly covalent character of CeO2.
Fig. 3.
XAS spectra at edge of CeO2 and ThO2.
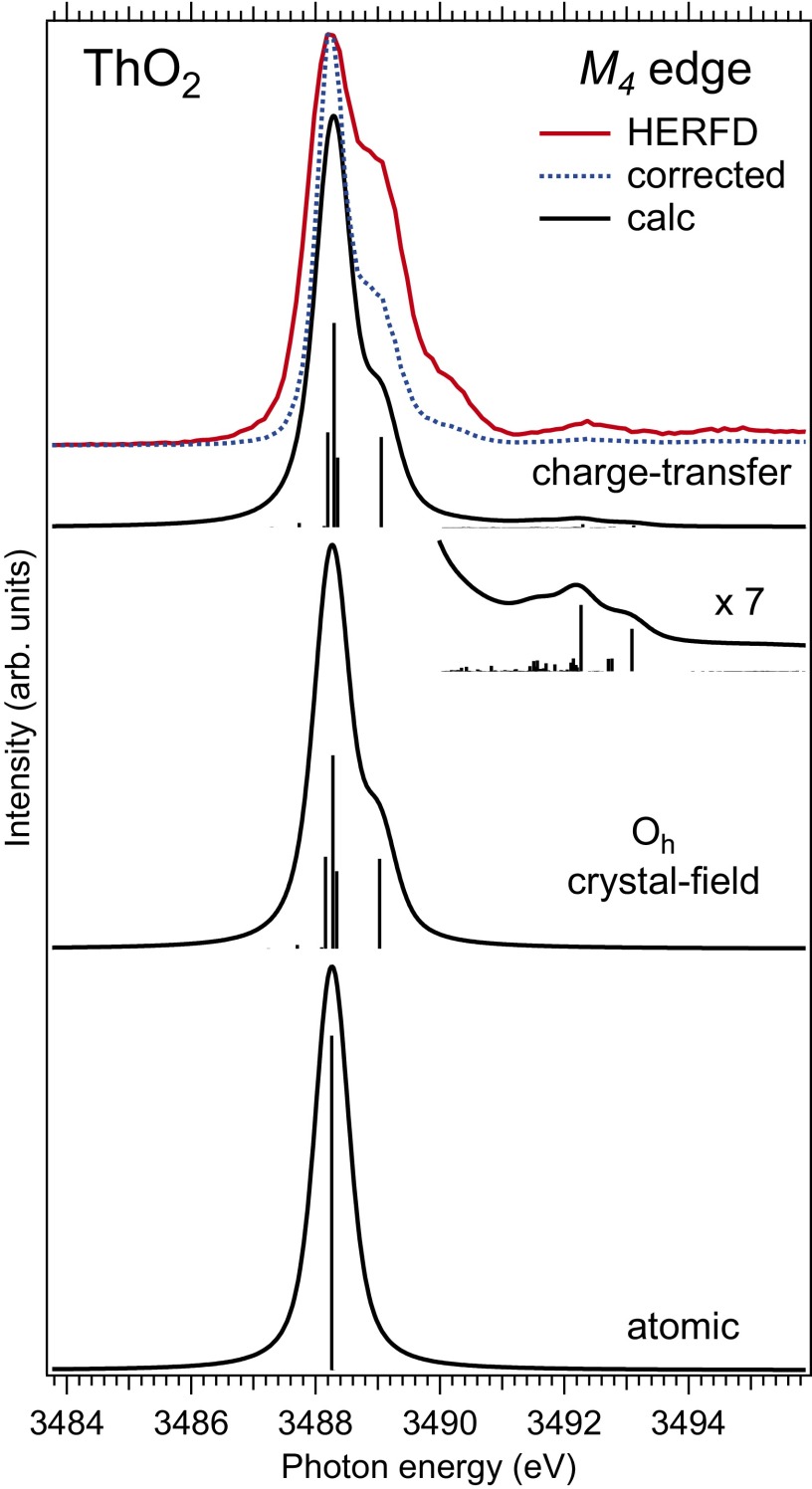
For ThO2, if the values for the AIM parameters previously used (33) to calculate the Th X-ray photoemission (XPS) spectrum are adopted for calculations of the XAS spectrum at the Th(IV) edge, it is difficult to reproduce the high-energy (charge-transfer) satellites. This is because the emphasis in the XPS calculations was on the Th O hybridization and charge transfer so that the ground state of the system was described as a mixture of the and configurations (see also ref. 34). However, the Th O charge transfer might be more energetically favorable because the Th states are dominant at the bottom of the conduction band of ThO2. The charge-transfer energy is expected to be lower so that it is easier for an electron to hop from the O 2p band into unoccupied Th states and the ground state of the system can be expressed as a mixture of the and configurations.
Fig. 4 shows that the latter approach allows one to obtain the high-energy structures in the calculated XAS spectrum at the Th edge. In particular, the structure at around 3,492.5 eV appears in the calculated spectrum upon inclusion of the Th O charge transfer via Th 6d − Th 5f − O 2p hybridization. The multiplet poles of the calculated transitions can also be found at energies around 3,490.5 and 3,493.7 eV, although their intensities are lower in comparison with the corresponding structures in the measured spectrum. Note that the dashed curve in Fig. 4 is a HERFD-XAS spectrum corrected for self-absorption effects by using the algorithm implemented in the “XANES dactyloscope” program (https://www.cells.es/en/beamlines/bl22-claess/software). We point out that self-absorption correction algorithms used by the XAS community do introduce some uncertainty for the absorption edges with sharp and strong lines and low postedge background contribution, such as edges of transition elements and edges of rare earths and actinides.
Fig. 4.
Experimental and calculated XAS spectra at the Th edge of ThO2. The spectra are calculated using atomic and CEF multiplet theory for the Th(IV) ion and the Anderson impurity model, respectively. The dashed curve represents the HERFD-XAS spectrum corrected for self-absorption effects (https://www.cells.es/en/beamlines/bl22-claess/software) and normalized to the maximum of the as-measured spectrum.
In our AIM calculations for the Th(IV) system, the ground (final) state for the XAS process was described as a combination of the and ( and ) configurations. The model parameters had the following values: eV, eV, eV, eV, and eV, where () and () are the () Coulomb interaction and core-hole potential acting on the () electron, respectively, and and are hybridization terms in the ground and final states. was set smaller to take into account the configuration dependence (35) of V. Whereas in the LDA + U calculations (31, 36) for ThO2 somewhat larger values of were applied (5 eV and 6 eV, respectively), eV was deduced from the fit of the Th XPS data (33). In addition, according to estimations in ref. 37, can be as large as
In the limit of the difference between the configuration averaged energies for the ground state was eV and eV and for the final state it was eV and eV, where and are one-electron energies of Th and O levels. The Slater integrals were scaled down to 80% of their Hartree–Fock values. The CEF parameters for the Th shell were set to eV and eV and for the Th shell to eV (38). was set to 0.3 eV and an additional Gaussian broadening was applied to match the experimental resolution.
These calculations gave the contributions of the and in the ground state of ThO2 to be 20% and 11%, respectively; i.e., the occupancy of the Th states (0.20 electrons) is larger than that of the Th states (0.11 electrons). This result does not support the ionic character of ThO2 and is also in agreement with the LDA + U (36) and hybrid-density functional (39) calculations that show the occupied Th 6d density of states (DOS) in the valence band of ThO2 to be about two times higher than the occupied Th 5f DOS.
The improved resolution of the HERFD-XAS technique allows us to resolve the shoulder at ∼0.8 eV above the main maximum of the Th edge of ThO2 (conventional XAS spectra in refs. 4 and 40). The origin of this shoulder becomes clear from a comparison of the experimental data with atomic and CEF multiplet theory for the Th(IV) ion in Fig. 4. The atomic multiplet calculations for the transitions between the and configuration in the 1 ground state produce a single multiplet pole whereas putting the Th(IV) ion in the cubic (Oh) CEF environment reveals CEF split states of the configuration, thus producing the shoulder at ∼0.8 eV above the main peak in the calculated spectrum for the CEF parameter values of eV and eV. The improvement in resolution offered by HERFD-XAS now allows probing the CEF interactions for the shell and extracting the information about the CEF effects directly from the XAS data in the manner commonly used for the edges of transition metal systems.
Specifically for ThO2, it is difficult to accurately determine the CEF strength with other techniques. For commonly used methods, such as optical and EPR spectroscopies, the ThO2 sample is usually doped with a small concentration of another actinide ion into the ThO2 lattice to observe the excitation pattern in the spectra. However, this may cause a local lattice distortion around the doped actinide ion, thus generating larger uncertainties in quantities derived from the fit of the spectra. For example, our determined values for CEF parameters and are somewhat larger than those obtained for Am-doped (41) and Cm-doped (42) ThO2.
Improved energy resolution demonstrates the shape sensitivity of the actinide edge to the strength of the crystal-field interaction and to the degree of covalency in the chemical bonding. Therefore, as in case of the edges of transition metal systems, detailed analysis of the edges spectral shapes of actinide compounds with the partially filled shell enables characterization of the ground state and low-lying excited states that define physical properties of these compounds (for example, analysis of the results of the temperature-dependent measurements or measurements in varying X-ray polarization geometries).
Acknowledgments
We thank Dr. D. Hudry for providing the ThO2 sample. This research was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences Heavy Element Chemistry program (D.K.S.) and the Advanced Light Source is supported by the Director, Office of Science, Basic Energy Sciences; both supported by the US Department of Energy at Lawrence Berkeley National Laboratory under Contract DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.de Groot FMF. X-ray absorption and dichroism of transition metals and their compounds. J Electron Spectrosc Relat Phenom. 1994;67(4):529–622. [Google Scholar]
- 2.Raboud P-A, Dousse J-Cl, Hoszowska J, Savoy I. L1 to N5 atomic level widths of thorium and uranium as inferred from measurements of L and M x-ray spectra. Phys Rev A. 2000;61(1):012507. [Google Scholar]
- 3.Petiau J, et al. Delocalized versus localized unoccupied 5f states and the uranium site structure in uranium oxides and glasses probed by x-ray-absorption near-edge structure. Phys Rev B Condens Matter. 1986;34(10):7350–7361. doi: 10.1103/physrevb.34.7350. [DOI] [PubMed] [Google Scholar]
- 4.Kalkowski G, Kaindl G, Brewer WD, Krone W. Near-edge x-ray-absorption fine structure in uranium compounds. Phys Rev B Condens Matter. 1987;35(6):2667–2677. doi: 10.1103/physrevb.35.2667. [DOI] [PubMed] [Google Scholar]
- 5.Butorin SM. Resonant inelastic X-ray scattering as a probe of optical scale excitations in strongly electron-correlated systems: Quasi-localized view. J Electron Spectrosc Relat Phenom. 2000;110–111:213–233. [Google Scholar]
- 6.Nilsson HJ, Tyliszczak T, Wilson RE, Werme L, Shuh DK. Soft X-ray scanning transmission X-ray microscopy (STXM) of actinide particles. Anal Bioanal Chem. 2005;383(1):41–47. doi: 10.1007/s00216-005-3355-5. [DOI] [PubMed] [Google Scholar]
- 7.Moore KT, van der Laan G, Haire RG, Wall MA, Schwartz AJ. Oxidation and aging in U and Pu probed by spin-orbit sum rule analysis: Indications for covalent metal-oxide bonds. Phys Rev B. 2006;73(3):033109. [Google Scholar]
- 8.Tobin JG, Yu S-W. Orbital specificity in the unoccupied states of UO2 from resonant inverse photoelectron spectroscopy. Phys Rev Lett. 2011;107(16):167406. doi: 10.1103/PhysRevLett.107.167406. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Beitz JV. Optical spectra and electronic structure. In: Morss LR, Edelstein N, Fuger J, editors. Chemistry of the Actinide and Transactinide Elements. Springer; Dordrecht, The Netherlands: 2010. pp. 2013–2111. [Google Scholar]
- 10.Amoretti G, et al. Actinide and rare earth ions in single crystals of ThO2: Preparation, EPR studies and related problems. J Less Common Met. 1986;122:35–45. [Google Scholar]
- 11.Seaman LA, et al. Probing the 5f orbital contribution to the bonding in a U(V) ketimide complex. J Am Chem Soc. 2012;134(10):4931–4940. doi: 10.1021/ja211875s. [DOI] [PubMed] [Google Scholar]
- 12.Amoretti G, et al. 5f-electron states in uranium dioxide investigated using high-resolution neutron spectroscopy. Phys Rev B Condens Matter. 1989;40(3):1856–1870. doi: 10.1103/physrevb.40.1856. [DOI] [PubMed] [Google Scholar]
- 13.Nakotte H, et al. Crystal fields in UO2 - Revisited. J Phys Conf Ser. 2010;251(1):012002. [Google Scholar]
- 14.Kvashnina KO, Butorin SM, Martin P, Glatzel P. Chemical state of complex uranium oxides. Phys Rev Lett. 2013;111(25):253002. doi: 10.1103/PhysRevLett.111.253002. [DOI] [PubMed] [Google Scholar]
- 15.Kvashnina KO, Kvashnin YO, Butorin SM. Role of resonant inelastic X-ray scattering in high-resolution core-level spectroscopy of actinide materials. J Electron Spectrosc Relat Phenom. 2014;194:27–36. [Google Scholar]
- 16.Gauthier C, Solé VA, Signorato R, Goulon J, Moguiline E. The ESRF beamline ID26: X-ray absorption on ultra dilute sample. J Synchrotron Radiat. 1999;6(Pt 3):164–166. doi: 10.1107/S0909049598016835. [DOI] [PubMed] [Google Scholar]
- 17.Glatzel P, Bergmann U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes: Electronic and structural information. Coord Chem Rev. 2005;249(1–2):65–95. [Google Scholar]
- 18.Hudry D, et al. Thorium/uranium mixed oxide nanocrystals: Synthesis, structural characterization and magnetic properties. Nano Res. 2014;7(1):119–131. [Google Scholar]
- 19.Hudry D, et al. Non-aqueous synthesis of isotropic and anisotropic actinide oxide nanocrystals. Chemistry. 2012;18(27):8283–8287. doi: 10.1002/chem.201200513. [DOI] [PubMed] [Google Scholar]
- 20.Warwick T, Heimann P, Mossessian D, McKinney W, Padmore H. Performance of a high resolution, high flux density SGM undulator beamline at the ALS. Rev Sci Instrum. 1995;66(2):2037–2040. [Google Scholar]
- 21.Anderson PW. Localized magnetic states in metals. Phys Rev B. 1961;124(1):41–53. [Google Scholar]
- 22.Butorin SM, et al. Resonant X-ray fluorescence spectroscopy of correlated systems: A probe of charge-transfer excitations. Phys Rev Lett. 1996;77(3):574–577. doi: 10.1103/PhysRevLett.77.574. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa M, Ogasawara H, Kotani A. Theory of polarization dependence in resonant X-ray emission spectra of a uranium compound. Surf Rev Lett. 2002;9(2):1149–1153. [Google Scholar]
- 24.Wybourne BG. Spectroscopic Properties of Rare Earths. Wiley; New York: 1963. [Google Scholar]
- 25.Slater JC. The theory of complex spectra. Phys Rev. 1929;34(10):1293–1322. [Google Scholar]
- 26.Cowan RD. Theory of Atomic Structure and Spectra. Univ of California Press; Berkeley: 1981. [Google Scholar]
- 27.Butler PH. Point Group Symmetry Applications: Methods and Tables. Plenum; New York: 1981. [Google Scholar]
- 28.Thole BT, van der Laan G, Butler PH. Spin-mixed ground state of Fe phthalocyanine and the temperature-dependent branching ratio in X-ray absorption spectroscopy. Chem Phys Lett. 1988;149(3):295–299. [Google Scholar]
- 29.Fuggle JC, Alvarado SF. Core-level lifetimes as determined by x-ray photoelectron spectroscopy measurements. Phys Rev A. 1980;22(4):1615–1624. [Google Scholar]
- 30.Sugar J. Potential-barrier effects in photoabsorption. II. Interpretation of photoabsorption resonances in lanthanide metals at the 4d-electron threshold. Phys Rev B. 1972;5(5):1785–1792. [Google Scholar]
- 31.Sevik C, Çaǧin T. Mechanical and electronic properties of CeO2, ThO2, and (Ce,Th)O2 alloys. Phys Rev B. 2009;80(1):014108. [Google Scholar]
- 32.Ogasawara H, Kotani A, Okada K, Thole BT. Theory of x-ray absorption spectra in PrO2 and some other rare-earth compounds. Phys Rev B. 1991;43(1):854–859. doi: 10.1103/physrevb.43.854. [DOI] [PubMed] [Google Scholar]
- 33.Kotani A, Ogasawara H. Theory of core-level spectroscopy in actinide systems. Physica B. 1993;186–188:16–20. [Google Scholar]
- 34.Gunnarsson O, Sarma DD, Hillebrecht FU, Schönhammer K. Electronic structure of the light actinide oxides from electron spectroscopy. J Appl Phys. 1988;63(8):3676–3679. [Google Scholar]
- 35.Gunnarsson O, Jepsen O. Configuration dependence of hopping matrix elements in the Anderson model. Phys Rev B Condens Matter. 1988;38(5):3568–3571. doi: 10.1103/physrevb.38.3568. [DOI] [PubMed] [Google Scholar]
- 36.Szpunar B, Szpunar JA. Application of density functional theory in assessing properties of thoria and recycled fuels. J Nucl Mater. 2013;439(1-3):243–250. [Google Scholar]
- 37.Kotani A, Jo T, Parlebas JC. Many-body effects in core-level spectroscopy of rare-earth compounds. Adv Phys. 1988;37(1):37–85. [Google Scholar]
- 38.Kvashnina KO, et al. Sensitivity to actinide doping of uranium compounds by resonant inelastic X-ray scattering at uranium L3 edge. Anal Chem. 2015;87(17):8772–8780. doi: 10.1021/acs.analchem.5b01699. [DOI] [PubMed] [Google Scholar]
- 39.Wen X-D, et al. Effect of spin-orbit coupling on the actinide dioxides AnO2 (An=Th, Pa, U, Np, Pu, and Am): A screened hybrid density functional study. J Chem Phys. 2012;137(15):154707. doi: 10.1063/1.4757615. [DOI] [PubMed] [Google Scholar]
- 40.Kelly TD, et al. The unoccupied electronic structure characterization of hydrothermally grown ThO2 single crystals. Phys Stat Sol RRL. 2014;8(3):283–286. [Google Scholar]
- 41.Hubert S, Thouvenot P, Edelstein N. Spectroscopic studies and crystal-field analyses of Am3+ and Eu3+ in the cubic-symmetry site of ThO2. Phys Rev B. 1993;48(9):5751–5760. doi: 10.1103/physrevb.48.5751. [DOI] [PubMed] [Google Scholar]
- 42.Thouvenot P, Hubert S, Edelstein N. Spectroscopic study and crystal-field analysis of Cm3+ in the cubic-symmetry site of ThO2. Phys Rev B. 1994;50(14):9715–9720. doi: 10.1103/physrevb.50.9715. [DOI] [PubMed] [Google Scholar]