Abstract
Deciphering the geographic context of diversification and distributional dynamics in continental biotas has long been an interest of biogeographers, ecologists, and evolutionary biologists. Thirty years ago, the approach now known as comparative phylogeography was introduced in a landmark study of a continental biota. Here, I use a set of 455 studies to explore the current scope of continental comparative phylogeography, including geographic, conceptual, temporal, ecological, and genomic attributes. Geographically, studies are more frequent in the northern hemisphere, but the south is catching up. Most studies focus on a Quaternary timeframe, but the Neogene is well represented. As such, explanations for geographic structure and history include geological and climatic events in Earth history, and responses include vicariance, dispersal, and range contraction-expansion into and out of refugia. Focal taxa are biased toward terrestrial or semiterrestrial vertebrates, although plants and invertebrates are well represented in some regions. The use of various kinds of nuclear DNA markers is increasing, as are multiple locus studies, but use of organelle DNA is not decreasing. Species distribution models are not yet widely incorporated into studies. In the future, continental comparative phylogeographers will continue to contribute to erosion of the simple vicariance vs. dispersal paradigm, including exposure of the widespread nature of temporal pseudocongruence and its implications for models of diversification; provide new templates for addressing a variety of ecological and evolutionary traits; and develop closer working relationships with earth scientists and biologists in a variety of disciplines.
Keywords: biogeography, biodiversity conservation, evolutionary biology, diversification, ecology
A landmark study 30 y ago (1) used mitochondrial DNA (mtDNA) variation to reveal a suite of “cryptic,” geographically structured evolutionary lineages embedded within four codistributed species of freshwater fishes in the southeastern United States and attributed interspecific similarities in lineage distributions to shared histories of isolation and divergence, i.e., vicariance, across putative geographic barriers. Comparative phylogeography as applied to continental biotas subsequently has expanded in terms of the variety of questions addressed, theory and methods applied, number of regions on different continents explored, and variety of taxa and genomes used. However, of course, biogeographers had long before understood the importance of exploring geographic patterns across multiple codistributed taxa.
Continental Biogeography Before Comparative Phylogeography
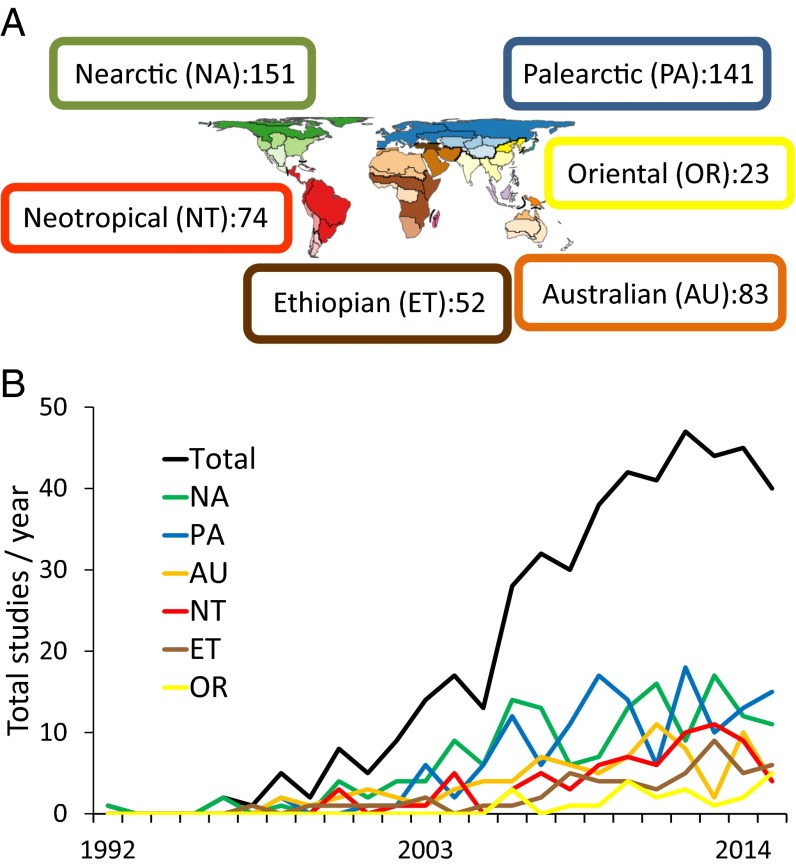
In the late 19th century, Alfred Russel Wallace mapped distributions of multiple vertebrate and invertebrate taxa to develop the system of terrestrial zoogeographic regions and subregions used more or less intact to this day (2), although modifications continue to be proposed using new datasets, concepts, and methods (3) (Fig. 1A). However, Wallace was interested in more than pattern, and in particular how earth features and history (geological and climatic) might be responsible for generating geographic patterns of distribution and diversification. In his consideration of continental biotas, he speculated on the influence of barriers: for example, the uniqueness of “zoological districts” on opposite sides of the Rocky Mountains and Andes (2) and the influence of large Neotropical rivers on the distributional limits of several species of monkeys (5). He associated distributional changes and extinction of animals in Europe and North America primarily with the warm and cold climate cycles of “Glacial Epochs,” but was largely wrong about the role of geological history, dismissing the continental drift model of Earth history (6) nearly a century before the plate tectonics framework was introduced. He did, however, predict that geologists eventually would catch up to biologists in reconstructions of earth history at focal spots such as the biogeographic transition zone between the Neotropical and Nearctic regions (5).
Fig. 1.
(A) Six terrestrial biogeographic regions of the world, basically dating to Wallace (2), but this depiction (map modified with permission from ref. 4) with boundaries modified modestly and with proposed subregions (see original paper for additional terminology). Numbers refer to distribution of comparative phylogeographic studies of continental biotas using references database described further in text. (B) Growth of continental comparative phylogeographic studies 1992–2015, total and tabulated by biogeographic region using references database described further in text (numbers for 2015 not complete because database was downloaded from Web of Science in December 2015).
In his last grand statement on biogeography in his masterful 1880 book Island Life, Wallace turned his primary focus away from continents and to islands:
In islands we have the facts of distribution often presented to us in their simplest forms, along with others which become gradually more and more complex; and we are therefore able to proceed step by step in the solution of the problems they present…it is not too much to say that when we have mastered the difficulties presented by the peculiarities of island life we shall find it comparatively easy to deal with the more complex and less clearly defined problems of continental distribution [ital. not original] (6, p. 234).
Islands, in his view, with their simplified (less diverse) biotas and discreet boundaries, could provide insights into the development of continental biotas that would be difficult to extract from the latter alone.
Nevertheless, biogeographers continued to use a “multiple codistributed taxa” approach as applied to continental biotas in the century between Wallace’s Island Life and the comparative phylogeography introduced in 1986 (1). Jordan and Grinnell used comparative evidence (7, 8) when examining the role of geographic isolation across barriers in the production of new species; indeed, Grinnell (8, p. 253) remarked that “By such a study, of comparative distribution, it seems possible that the ranges of birds and mammals may become subject to satisfactory explanation.” Wallace’s keen interest in the influence of Glacial Epochs was expanded by Adams (9) into an examination of postglacial distributional shifts in five geographically separated North American biotas out of glacial age “biotic preserves” (in modern terms, refugia). Haffer (10) proposed a Pleistocene species pump model of avian diversification in the Amazonian region resulting from repeated cycles of forest expansion and contraction. Remington (11) proposed 13 “suture zones” between major biotic assemblages in North America and suggested some level of hybridization might be occurring in secondary contact between previously isolated species and subspecies. Rosen (12) used North and Middle American biotas, in conjunction with paleogeographic reconstructions of the region, to outline newly forming theory in “vicariance biogeography.” Interactions between biotas across biogeographic regions included a classic study of biotic interchange by Simpson (13), who focused on the transition zone between Neotropical and Nearctic regions with an accounting of the historical assembly of the South American mammal fauna that included a relatively recent wave of immigration from North America. Darlington (14) took a more global view than Simpson on dispersal among biogeographic regions, developing perhaps the last preplate tectonics and distinctly nonvicariance model, wherein waves of “dominant” animals would disperse away from an Old World tropics center of origin, displacing inferior species as they invaded northern temperate and southern regions. Investigators increasingly discovered value in use of molecular markers to resolve geographic variation within species and among higher taxa (15), and perhaps the best example of examining codistributed species before the advent of mitochondrial DNA-based phylogeography was a 1984 study that used microcomplement fixation of serum albumin to place geographic isolation and divergence of several Australian frogs within a pre-Quaternary timeframe (16).
My main objective here is to address several of the contributions of comparative phylogeography to developing a better understanding of diversification and distributional dynamics in continental biotas. To do so, I first provide an overview of the growth, both conceptually and geographically, of the scope of continental comparative phylogeography over the 30 y since its beginnings (1). I end with a few predictions regarding future directions for a comparative phylogeography of continental biotas and its potential role in the advancement of related disciplines.
The Conceptual and Geographic Scope of Continental Comparative Phylogeography
In this section, I explore the context and content of continental comparative phylogeography using a database of hundreds of titles and abstracts of publications within this arena, beginning chronologically with the paper that established firmly the conceptual framework and potential of comparative phylogeography (17).
Building the Database.
I began with three topics searches of the Web of Science online database (last search: 2 December 2015) using the following terms: “comparative phylogeograph*”; codistributed not “comparative phylogeograph*”; and codistributed not “comparative phylogeograph*.” The basic search returned nearly 900 citations, from which my first pass sought to delete those that dealt with geographies that were not concerned with continental biotas (e.g., islands, marine). Following this pass, 704 citations remained, but many included comparative phylogeograph* only in the Keywords Plus field, which adds keywords according to titles of papers cited in a paper. I therefore made a second pass through the list with the goal of removing citations that did not provide indications of being conducted within a comparative framework, which resulted in 487 citations remaining. Finally, with subsequent searches of the database to harvest information reported here, I eliminated an additional 32 citations that were not concerned with continental biotas but I had not detected as such earlier, resulting in a final database of 455 citations distributed across biogeographic regions as summarized in Fig. 1A.
One caveat and one further explanation are warranted. First, I emphasize that this collection represents a sampling and not an exhaustive list of citations that focus on comparative phylogeography of continental biotas. As an example illustrating this, two of my own studies (18, 19) would not have been included had I not inserted them manually after the initial search. Nevertheless, I would argue that this collection of 455 citations is broadly representative both conceptually and geographically. Second, many studies that are included superficially could be construed to be single-taxon phylogeographic studies. However, one of my guidelines in determining which citations to keep and which to eliminate required evidence that what appeared to be a single-taxon study was embedded within a context of knowledge about the phylogeographies of codistributed taxa from prior studies, either explicitly as a motivator for choosing a focal taxon to study or in the context of inference about the resulting geographic pattern.
Studies Are Becoming Globally Representative.
Prior reviews, most recently in 2010 (20), have pointed to a northern hemisphere bias in the distribution of phylogeographic studies. Here, continental comparative phylogeographic studies can be summarized, approximately, into northern, NA + PA + OR = 315, vs. southern, AU + ET + NT = 209 (see Fig. 1A for abbreviations), representing a more equitable 60:40 ratio. Indeed, total numbers of studies have increased over time in both hemispheres, although less rapidly in each of the southern and in the Oriental biogeographic regions (Fig. 1B).
Hotspots of Activity Are Increasing and Geographically Widespread.
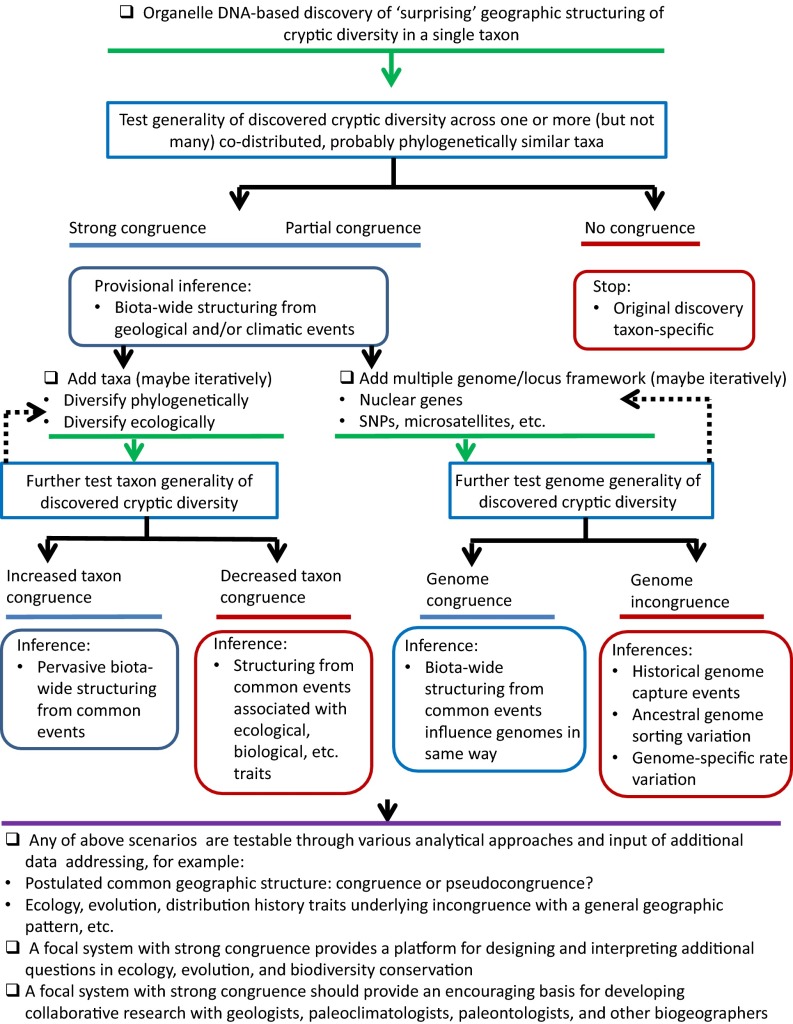
I recognize a continental comparative phylogeography hotspot as a definable geographic area and suite of taxa that either has become, or appears to be emerging, as one that attracts repeated studies, ordinarily wherein more recent studies explicitly reference known phylogeographic structure (Fig. 2). Although not all studies are dependent on such systems, they arguably offer added value for purposes of not only building a rich depiction of the pervasiveness of events in earth history on a complex history of biological diversification and distributional dynamics, but they also have potential to generate novel insights into connections between biogeographic structure and related questions in ecology, evolutionary biology, and biodiversity conservation.
Fig. 2.
Anatomy and life history of an idealized focal system in continental comparative phylogeography. A focal system is defined here as one that generates repeated studies, each time adding new taxa and/or new genomes/genetic markers.
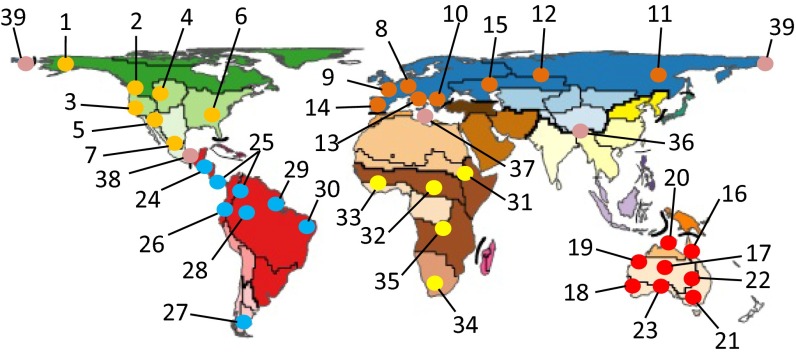
Here, I identify two advances since a 2010 review paper (20) listed continental comparative phylogeography hotspots including Europe, Pacific Northwest, California, Neotropical rain forests, Baja California, East Africa, Southeast North America, and Australian Wet Tropics. First, I recognize a global proliferation of recognizable or emerging hotspots distributed across biogeographic regions (Fig. 3), although none were detected in India or the Middle East, and the “hottest” area of activity in the Oriental region is transitional between it and the Palearctic in the vicinity of the Tibetan Plateau and Eastern Himalayas. Second, as with overall growth of studies in the southern hemisphere described above, my assessment indicates a more even distribution of established or emerging hotspots between hemispheres. Transition zones between biogeographic regions also are represented (Lower Central America between Neotropical and Nearctic; Mediterranean between Ethiopian and Palearctic; Qinghai-Tibetan Plateau between Oriental and Palearctic; and Beringia between Palearctic and Nearctic), signaling an emergent comparative phylogeographic approach to revealing the history of biotic interchange between regions that so captivated Simpson (13), but with the added realization that such transition zones often cross active tectonic boundaries between continental plates.
Fig. 3.
Continental comparative phylogeography hotspots or emerging hotspots (replicate studies using cumulatively increasing numbers of taxa) as inferred from scan of references database used here. Each set of colored dots represent a biogeographic region, except for those numbers that represent transition zones between regions: 36, OR-PA, Eastern Himalayas/Qunghai-Tibet Plateau; 37, ET-PA, Mediterranean Basin; 38, NT-NE, Middle America; 39, PA-NA, Beringea. All other localities identified as follows: 1, Alaska/East Beringea; 2, Pacific Northwest; 3, California; 4, Rocky Mountains; 5, Southwestern Aridlands; 6, Southeastern/Eastern; 7, Trans Mexican Volcanic Belt; 8, European Alps/Carpathians; 9, Western Palearctic; 10, Balkan Peninsula; 11, Eastern Asia; 12, Eurasia; 13, Italian Peninsula; 14, Iberian Peninsula; 15, Central Asia/Ponto-Caspian; 16, Australian Wet Tropics; 17, Interior Deserts; 18, Southwestern Australia; 19, Pilbara Region; 20, Monsoonal Tropics; 21, Souteastern Australia/Tallaganda; 22, Endorheic Basins; 23, Southern Australia; 24a, Lower Central American lowlands; 24b, Lower Central American highlands; 25, trans-Andean; 26, Andes; 27, Patagonia; 28, Amazon Rain Forest; 29, Caatinga/Cerrado; 30, Atlantic Rain Forest; 31, Eastern Arc (Afromontane) Mountains; 32, Central African Forest; 33, West African Forests/Savannas; 34, Cape Region; 35, Tropical African Forests/Savannas. Map modified with permission from ref. 4.
The Topics Addressed Are Diverse.
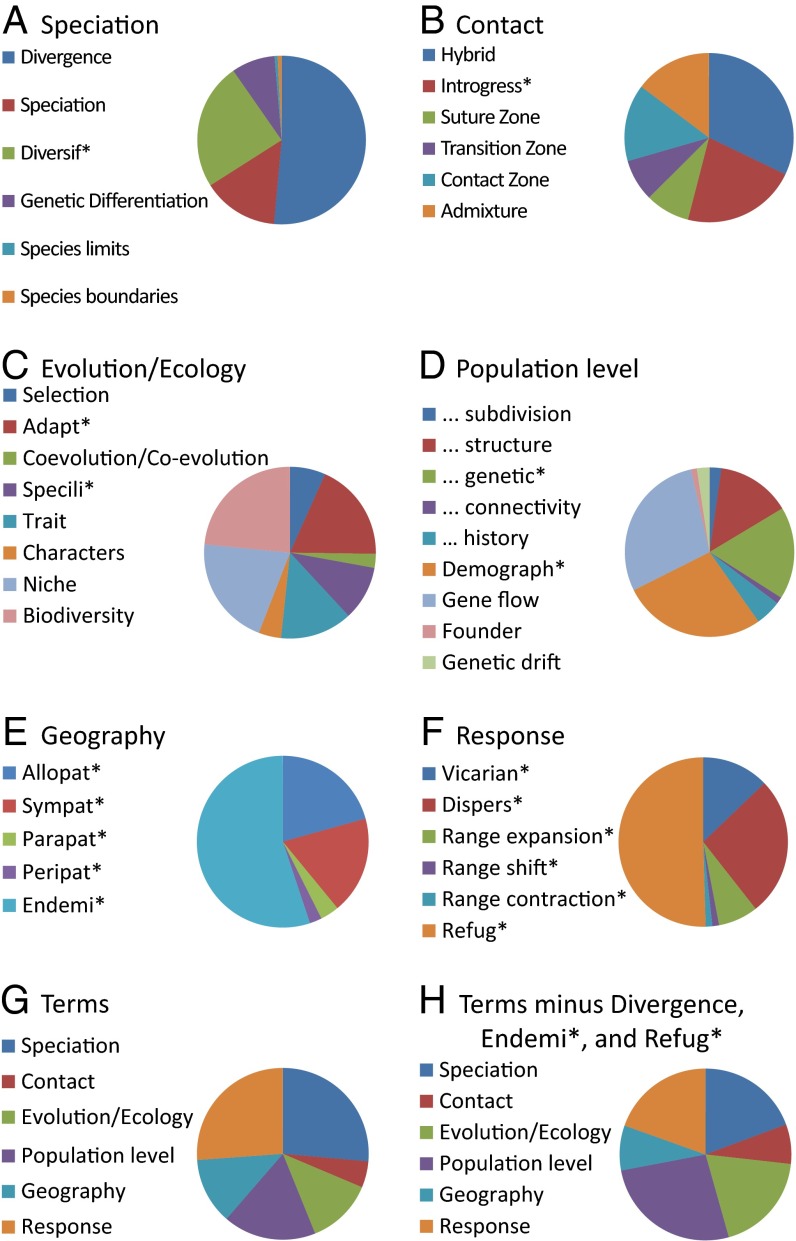
Here, I explore the frequencies of terminology generally associated with concepts in evolution, ecology, biogeography, and biodiversity conservation to assess their relative importance in continental comparative phylogeography, summarized under six categories designated a priori (Fig. 4): speciation, contact (between once isolated lineages), evolution/ecology (other than speciation), population level, geography, and response (to drivers such as geological and climatic changes). Very frequently used terms are Divergen* under speciation, Endemi* (somewhat surprisingly) under geography, and Refug* under response. However, a summary of the frequency of the six main categories (Fig. 4G) suggests that, although topics under speciation and response are most frequently mentioned, the distribution becomes more even among speciation, evolution/ecology, population level, and response if the overwhelming influences of Divergen*, Endemi*, and Refug* are removed (Fig. 4H). As with phylogeography generally, continental comparative phylogeography addresses a wide range of core concepts in modern biology in a geographic context.
Fig. 4.
Frequency of terms generated through searches of references database used here, embedded within six categories (A–F) considered here to represent a range of primary foci of continental comparative phylogeography. Those followed by an asterisk denote root terms that find any variation (e.g., allopat* will find allopatric, allopatry, allopatrically). The total frequencies of the six primary foci (G and H) differ because the latter was generated after removing three terms of overriding frequency, showing a more even distribution across the primary foci.
Focal Taxa Are by Definition Geographically Codistributed, but Might Also Have an Ecological or Biological Relationship.
The majority of studies in continental comparative phylogeography use taxa that are geographically codistributed without reference to additional criteria such as specified ecological relationships, e.g., contrasting habitat specializations, but several studies of plant comparative phylogeography in the European Alps provide a good example of doing so, with plants identified as specialists on either calcareous or silicicolous substrates (21). One interesting but not yet common approach is to investigate the historical assembly of taxa associated ecologically into trophic interactions and food webs (22). A reasonable subset of studies (NA = 8; PA = 16; AU = 1; NT = 4; ET = 5) goes farther and investigates phylogeographic congruence, or lack thereof, between species that have an obligatory relationship such as hosts and parasites or predators (23), or plants and pollinators (24): a particularly well-studied system involves oak gall wasps and their parasitoids in the Palearctic region (25).
Temporal Scope Spans Neogene and Quaternary Periods.
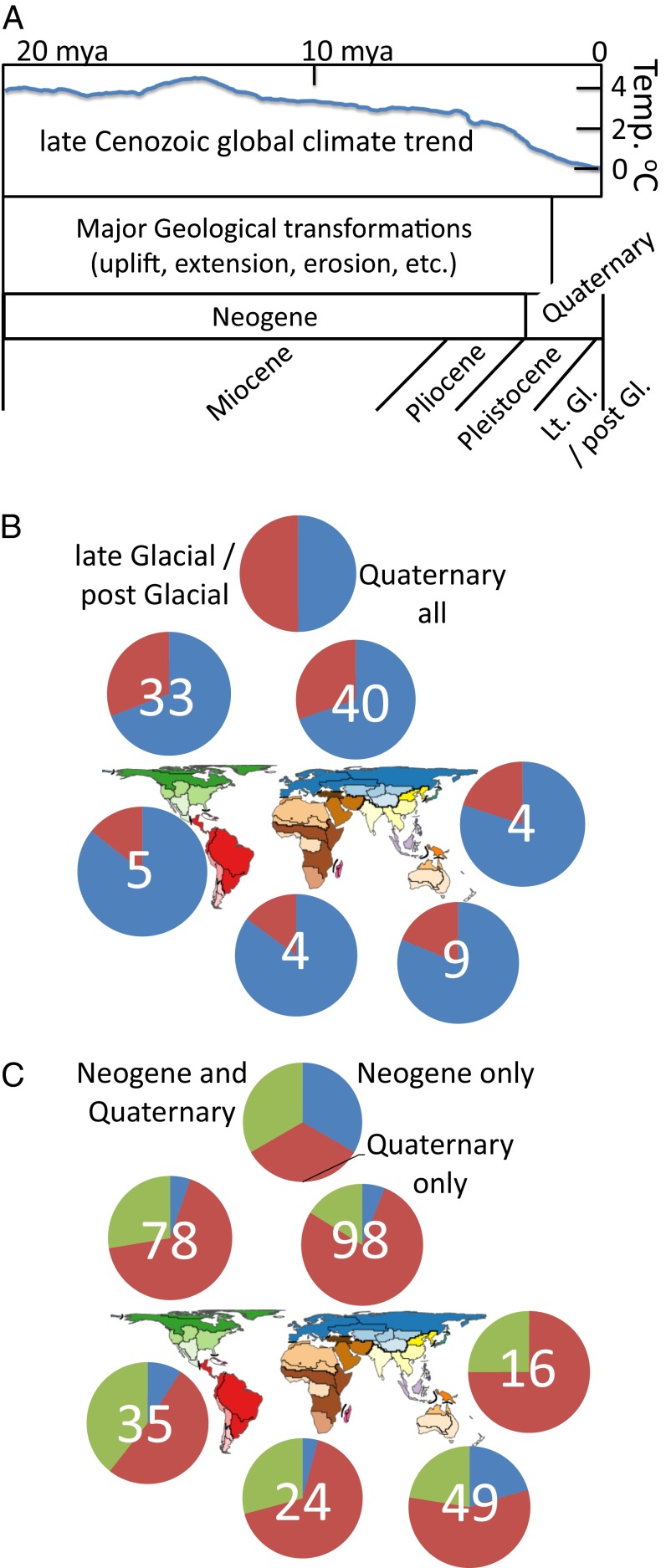
In general, the Neogene and early Quaternary periods are regarded as times of major geological activity on a number of continents, including uplift, extension, erosion, incision of large sections of continents, and episodes of marine transgressions (Fig. 5A). Major episodes of geological transformation are more localized to the Neogene in, for example, the western Nearctic and Neotropical regions, but extend into the Quaternary in the Oriental-Palearctic transition area (26). Beginning in the later Neogene and accelerating into and throughout the Quaternary, global temperatures decreased and the oscillations between glacial and interglacial climatic regimes increased in magnitude (27). As such, continental comparative phylogeography studies that reference processes the could be driving divergence and range dynamics during the Neogene and early Quaternary are likely to consider a combination of geological and climatic processes, whereas those that reference the later Quaternary, and more specifically the late glacial (e.g., last glacial maximum or LGM) into postglacial timeframes are more likely to emphasize climate changes alone, considering the geological template to be for the most part already established by this time (Fig. 5B).
Fig. 5.
The timeframes explored empirically by continental comparative phylogeography. Terms used to describe geological timeframes differ among studies (A) but are subsumed for purposes of searching the reference database as Neogene (=Miocene + Pliocene) and Quaternary (=Pleistocene + Late Glacial/post Glacial + LGM + Holocene). Late Cenozoic global climate trend traced from an online summary (www.columbia.edu/∼mhs119/Sensitivity+SL+CO2/) of a well-known figure (27). Studies using terms describing timeframes (total numbers tallied for each region overlaid on pie charts) summarized as (B) late Glacial/post Glacial vs. Quaternary all, and (C) Neogene only vs. Quaternary only vs. Neogene and Quaternary. Map modified with permission from ref. 4.
Here, I find that a number of studies reference a late glacial into postglacial timeframe specifically (Fig. 5C), but although these represent an appreciable portion of all studies referencing the Quaternary, there are many additional studies centered in a Quaternary timeframe that do not adhere to this relatively recent and temporally narrow period. I also find that out of the larger number of studies that span either Neogene, or Quaternary, or both (Fig. 5C), those limited to the Quaternary represent the highest frequency in all biogeographic regions, but each region also contains roughly a quarter (more in AU and NT, fewer in PA) studies that reference either the Neogene or both Neogene and Quaternary timeframes. These results support a search indicating that, although most studies suggest that taxa are responding to climatic change as a driver, a number of them also reference geology as a potential driver (Climat* = 638 total hits; Geol* = 103 total hits), corroborating the generality of the signature of Neogene divergence within “intraspecific and closely related species” foreseen decades earlier by, for example, Maxson and Roberts (16).
By Spanning Neogene and Quaternary Periods, an Array of Biotic Responses to Geological and Climatic Events in Earth History Are Addressed.
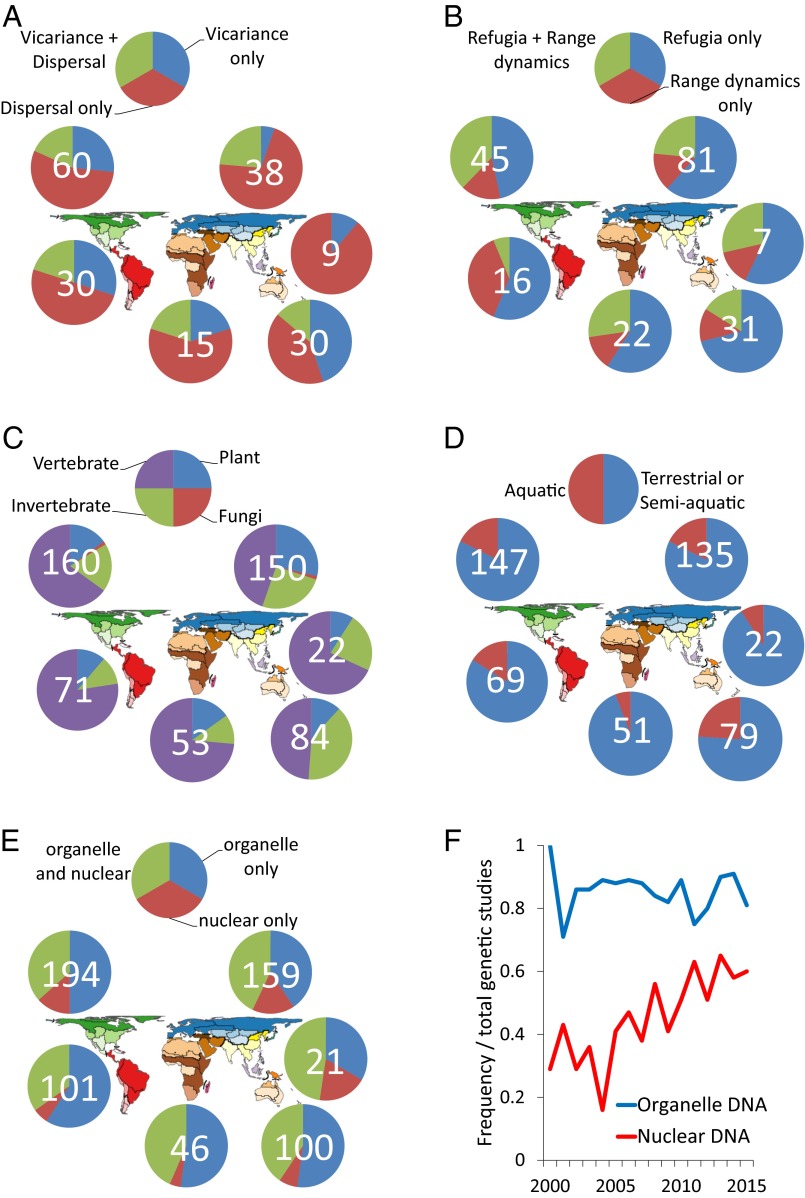
Another way to examine the role of Earth history on continental comparative phylogeographic structure is to use search terms directly referencing the full range of possible biotic responses originally envisioned within separate research paradigms, including vicariance and dispersal, e.g., Rosen (12) and Darlington (14), and refugia and range dynamics (expansion, contraction, shifting), e.g., Adams (9) and Haffer (10). First, vicariance or dispersal or both vary in frequency between regions (Fig. 6A); notably, vicariance appears most often in studies of Australian biotas and less in the Palearctic than in all other regions except the Oriental (although the sample size there is small). Within a late Quaternary timeframe, we should expect the concepts of glacial refugia and postglacial range expansion to be addressed together in a number of studies, and out of a total tabulation of papers that address one or the other or both of these concepts that connection holds true in each region, primarily in the Nearctic but not as frequently in the Neotropical region (Fig. 6B).
Fig. 6.
(A and B) Categories referenced frequently in continental comparative phylogeography of biotic responses to geological and climatic events (total numbers tallied for each region overlaid on pie charts). Studies divided to contrast terms (A) vicariance only vs. dispersal only vs. vicariance + dispersal, and (B) refugia only vs. range dynamics only vs. refugia + range dynamics. Range dynamics includes references to range expansion, range contraction, and range shift and variants of each. (C and D) Frequencies of organisms, summarized into taxonomic groups and medium inhabited, studied in continental comparative phylogeography according to search of the database used in this study. Taxa contrasted (C) as vertebrate vs. invertebrate vs. plants vs. fungi; and medium (D) contrasted as terrestrial or semiaquatic vs. aquatic organisms. (E and F) Frequencies of genomes used in continental comparative phylogeography studies. Organelle DNA (including mitochondrial DNA and chloroplast DNA, also searched as mtDNA and cpDNA) contrasted (E) with nuclear DNA (including all types of markers) as organelle only vs. nuclear only vs. organelle and nuclear. The frequency of studies (F) using markers from each genome plotted through time (numbers for 2015 not complete because database was downloaded from Web of Science in December 2015). Map modified with permission from ref. 4.
Taxa Are Biased Toward Vertebrates and Terrestrial or Semiaquatic Organisms.
Although continental comparative phylogeography began (1) with an investigation of four codistributed fresh water fish taxa, to date far more studies have emphasized terrestrial or semiterrestrial taxa, and this is true across regions (Fig. 6C). The regional distribution of taxa from five categories (vertebrates, invertebrates, plants, fungi, microbes) indicates an appreciable bias in each region toward vertebrates (Fig. 6D), although plants and invertebrates are also relatively well represented in the Palearctic and invertebrates in the Australian. Very few studies have focused on fungi, and studies of microbes appear to be nonexistent or nearly so.
The Use of Nuclear DNA and Multilocus Data Is Increasing, but Organelle DNA Is Not Going Away.
Controversy arose a few years ago with regard to the continued efficacy and importance of organelle (mitochondrial, chloroplast) genomes in phylogeography as theoretical and practical considerations argue for greater use of multilocus data from the nuclear genome (28–30). A recent argument (31) was made for the continuing importance of mtDNA in marine phylogeography even as multilocus studies grow in frequency and importance. Here, I find that a relatively small number of continental comparative phylogeographers are yet to use nuclear DNA to the exclusion of organelle DNA (Fig. 6E), particularly in the more southern Neotropical, Ethiopian, and Australian regions. However, although a higher frequency of studies use some combination of both nuclear and organelle data, a (perhaps) surprisingly high number still rely on organelle genome data alone. Indeed, the use of organelle DNA does not appear to have decreased into current times as use of nuclear DNA has clearly increased rapidly over the last decade or more (Fig. 6F).
There likely is a combination of reasons for the ongoing popularity of organelle DNA in continental comparative phylogeographic studies. First, ease and cost of data generation probably remains a central driver, particularly when a principal objective remains the goal of sampling as many geographically distributed populations and individuals as possible across multiple codistributed taxa. Second, organelle DNA, particularly mitochondrial DNA in animals, still likely delivers a very strong and heuristically valuable first approximation of geographic genetic architecture, providing a reasonable baseline for hypothesis testing when a greater diversity of genetic data from across genomes and independently sampled portions of the nuclear genome become available. Third, for purposes of contrasting and comparing signals of geographic structure across divergent taxa, perhaps the simplicity and strong geographic signal generated by organelle DNA is still something of an advantage, considering for example the number of such studies that continue to be subjected to analysis within hierarchical approximate Bayesian computation (hABC) frameworks (32, 33).
Species Distribution and Ecological Niche Models Are Promising, but Still Not Widely Used in Continental Comparative Phylogeography.
Species distribution modeling or ecological niche modeling was beginning to get introduced into phylogeography nearly a decade ago (34, 35). However, although being used commonly across phylogeography (a Web of Science search returned 419 hits for phylogeograph* and “species distribution model*” or “ecological niche model*”), that use still is somewhat limited in continental comparative phylogeography (NA = 15; PA = 8; AU = 4; NT = 7; ET = 1; OR = 2). I would expect its use in continental comparative phylogeography to grow with appreciation for developing independent, testable hypotheses for sorting alternative explanations of predicted responses (both historical and future) across codistributed taxa; potential to connect climatic and other habitat variables of specific ecological importance to target taxa; and potential to accurately model distributional responses under past and future climate models (36).
Into the Future
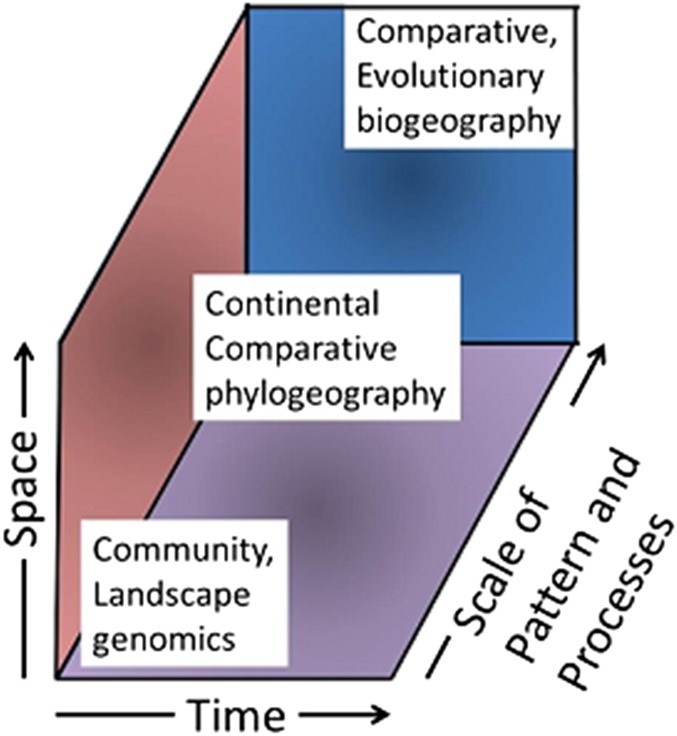
Comparative phylogeography has enjoyed a large measure of the success that eluded Wallace in 1880 when trying to understand biogeographic pattern and processes in continental biotas. Uncovering a previously unknown and unpredicted level of cryptic diversity in animals and plants and applying sophisticated analytical approaches to mapping phylogenetic and population affinities onto geography have made inroads into the questions posed by the 20th century biogeographers, ecologists, and evolutionary biologists. One ongoing challenge and promise is to continue developing connections to related disciplines that usually lie at different spatial, temporal, and pattern/process scales (Fig. 7). In doing so, continental comparative phylogeography will continue to be transformed by ongoing incorporation of, for example, species distribution modeling, the genomics revolution, geospatial statistical platforms, and an explicit hypothesis testing context (37). Here are a few predictions of some future directions and developments.
Fig. 7.
Positioning of continental comparative phylogeography within a depiction of related disciplines across spatial, temporal, and scale of pattern/process hierarchies.
Continental Comparative Phylogeography Will Continue Contributing to Erosion of the Simple Vicariance vs. Dispersal Paradigm.
Wallace was not wrong about the complexity of continental biotas, and comparative phylogeography continues to reveal that this is true in a temporal and spatial context. Pseudocongruence is a recognized pattern in biogeography (38), and in a temporal context, describes the phenomenon of a suite of taxa that superficially appear to have responded in concert to a putative barrier (e.g., a single, simultaneous vicariant event) or via mass dispersal to the opening of a dispersal route between previously isolated regions (also known by the useful but underused term “geodispersal”), but in reality were either isolated or dispersed in geographically similar fashions but at different times. One of the more detailed examples of pseudocongruence to date (33) studied 27 lineages of Neotropical birds that are codistributed with isolated populations on either side of the Andean mountains. mtDNA variation was analyzed in all individuals, and a subset was further analyzed for multilocus variation across more than 100 loci. In both datasets, hABC analyses demonstrated strong signals of pseudocongruence across the Andes, the Isthmus of Panama, and three large Neotropical rivers (Amazon, Madeira, and Negro). Trans-Andean divergence was estimated to have happened between 9 and 29 times during the Pleistocene and was attributed to dispersal rather than vicariance from earlier Andean uplift. Ecology played a role as well, in which forest canopy species, considered more capable of long-distance dispersal than understory birds, showed generally more recent times of separation of lineages across barriers.
Studies arguing for or providing evidence of pseudocongruence are becoming widespread across biogeographic regions, but several that can be highlighted in addition to the above include the following: North American southwestern aridlands (19, 39); southeastern North America (40); lower Central America (41); Beringian crossing between the Palearctic and Nearctic (42); and Australian wet tropics (43).
Implications of this growing number of examples of pseudocongruence are several, should the historically default model of simple vicariance eventually be realized to be a relatively rare event in the history of continental biotas. First, given enough data (large number of taxa and enough genetic data), it should be possible in many cases to resolve ecological trait differences that are predictive of the isolation and dispersal histories of suites of taxa within rigorous geological and climatic contexts (33). Second, investigators might turn attention to the notion that much of diversification history on continents might be explained through a model of temporally expanded “species pumps” across geographically stable barriers. In this case, a compelling question will be to address the biotic and abiotic features that combine to keep earlier isolated taxon pairs still isolated as more recent rounds of barrier formation and erosion close and open opportunities for cross-barrier dispersal, as might happen, for example, through repeated cycles of Quaternary glacial/interglacial climate oscillations (44).
More Ecological, Evolutionary, Life History, and Physiological Traits Will Be Incorporated into Continental Comparative Phylogeography.
Although some of these properties have focused the attention of continental comparative phylogeographers, perhaps from the beginning of the approach, recent studies are taking advantage of, for example, distribution modeling and massively high throughput sequencing to refine the level of resolution. A recent study (45) took advantage of ecological differences between two codistributed species of kangaroo rat (genus Dipodomys) in western North American deserts to ask whether degree of substrate specialization led to a predictable difference in demographic parameters along a postglacial range expansion front. A habitat specialist, Dipodomys deserti, was predicted to lose more genetic diversity than the generalist, Dipodomys merriami, from south [where species distribution models (SDMs) supported locations of late glacial refugia in both species] to north. These predictions were supported by both mtDNA and double-digest restriction site associated DNA (ddRADseq) SNPs datasets. Other small desert vertebrates and invertebrates have also expanded, as modeled under SDMs and in some cases shown with genetic data (46, 47) along this same expansion front, providing the intriguing potential to begin to sort among other ecological (e.g., substrate specialists) and physiological (e.g., endotherms vs. ectotherms; homeothermic vs. heterothermic endotherms) traits that are predictive with regard to distributional and demographic properties.
Note that the example above also illustrates the earlier point regarding the value of designing studies within well-studied comparative phylogeography hotspots (Fig. 2). Reinforcing this observation within the same system, one recent study (48) used the backbone general phylogeographic pattern to gain understanding into the historical development of a complex life history structure between two species of ants in the genus Pogonomyrmex. Another (49) took advantage of combining mtDNA and ddRADseq SNPs to reveal both a deep phylogeographic break (mtDNA) and recent gene flow (SNPs) across a transition zone that is coincident with at least 14 additional cryptic taxon pairs of mammals, birds, and herps (19, 44), suggesting the prospect of studying ongoing speciation processes across a transition zone (that almost certainly contains a rich history of temporal pseudocongruence) at a community scale using similar genomic tools, dramatically building on Remington’s (11) concept of suture zones.
Continental Comparative Phylogeographers Will Work More Closely with Geologists, Paleoclimatologists, and Paleontologists.
Revolutions in geology have historically signaled revolutions in biogeography: most notably, the vicariance biogeography paradigm that followed closely on the widespread acceptance of continental drift under the plate tectonics framework (50). However, Earth scientists and biogeographers often have worked independently rather than in a concerted fashion to address the inextricable links between Earth and biotic histories. Continental comparative phylogeographers have the potential to integrate more explicitly with Earth scientists to productive ends.
A good example comes from the robust phylogeographic signal of cryptic divergence between lineages distributed in the southern vs. northern parts of the Baja California Peninsula (19). A series of mid-peninsular seaways have been postulated as isolating barriers to account for these phylogeographic splits, even though geological evidence supporting existence of seaways had been largely absent. In this case, the comparative phylogeographic signal motivated geologists to investigate the plausibility of mid-peninsular seaways, concluding that regional geology is consistent with Miocene but not Plio-Pleistocene seaways (51).
When a rich fossil record is available, it should be possible to use comparative phylogeographic and fossil information to test diversification hypotheses. For example, the Neogene fossil record for rodent taxa in western North America has been used to establish a relationship between topographic complexity and diversity (52): phylogeographic evidence can provide an independent approach to testing a variety of hypotheses available to account for this pattern given the high standing diversity of rodent lineages in extant biotas covering this region.
More Patterns and Inferred Processes Derived Through Continental Comparative Phylogeographic Analyses Will Be Incorporated into Biodiversity Conservation Plans.
How do we try to maximize retention of biodiversity and the ecological and evolutionary processes that underlie the origination and sustainability of that diversity in a rapidly changing world? Comparative phylogeography has been envisioned has having an important role in biodiversity conservation nearly since its beginnings (53) and continues to contribute valuable information for conservation purposes. The breadth of potential applications is enormous, for example, deriving quantitative measures of phylogenetic diversity and phylogenetic endemism in biodiversity hotspots on either end of an entire continent (54). However, value on much smaller spatial scales is also possible, illustrated by the use of phylogeographic data from 12 species of mammals, herps, and invertebrates to derive estimates of biodiversity hotspots (genetic diversity and genetic divergence) within the Mojave and Sonoran deserts in southwestern North America (55). The maps derived from this exercise should become important tools for resource managers and planners making daily decisions in the face of immediate threats to biodiversity from solar farm development, expanding urban developments, and off-road vehicle recreationists.
Continental Comparative Phylogeography Will Have an Important Role in Development of a Broadly Construed, Highly Integrative Evolutionary Biogeography.
Comparative phylogeography has been used to illustrate a multifaceted, stepwise research protocol (19), and it will continue to become more firmly embedded within a highly integrative approach to reconstructing the diversification and distributional dynamics of continental biotas that draws from the broad spectrum of analytical resources available in biogeography, systematics, population genetics, and ecology to create new bridges between, e.g., ecology and evolutionary biology (56). An “evolutionary biogeography” has been imagined (57) that, rather than continuing a long history of divisiveness between practitioners of different approaches to biogeography, placed phylogeography into a multifaceted, stepwise protocol designed to create an emergent “geobiotic scenario.” This approach identifies natural biogeographic units and analyzes the biotic relationships among them; they are next analyzed for the full suite of taxon histories, including a teasing apart of the natural pseudocongruence that is a likely result of a long history of episodes of dispersal and vicariance. A fully realized program of this nature will require innovation in how comparative phylogeography bridges and links traditionally independent research programs in a phylogenetics-based historical biogeography with a population genetics-based landscape and community genetics (Fig. 7).
A full suite of additional exciting prospects for the advancement of comparative phylogeography can be found in other contributions to this volume. Perhaps if Alfred Russel Wallace had access to the concepts and methods enjoyed by modern comparative phylogeographers, he would not have turned his focus quite as energetically toward islands to gain greater understanding of the geographic structure and history of continental biotas! Another review a decade from now will undoubtedly report on a vastly transformed continental comparative phylogeography.
Acknowledgments
I thank Profs. J. C. Avise, B. W. Bowen, and F. J. Ayala for organizing and inviting me to participate in the extraordinary colloquium that generated the basic ideas incorporated into this paper. I thank Prof. H. Kreft who provided encouragement for me to use the figure of biogeographic regions that he and Prof. W. Jetz published 6 y ago.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution X: Comparative Phylogeography,” held January 8–9, 2016, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/ILE_X_Comparative_Phylogeography.
This article is a PNAS Direct Submission.
References
- 1.Bermingham E, Avise JC. Molecular zoogeography of freshwater fishes in the southeastern United States. Genetics. 1986;113(4):939–965. doi: 10.1093/genetics/113.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace AR. The Geographical Distribution of Animals. Macmillan; London: 1876. [Google Scholar]
- 3.Morrone JJ. Biogeographical regionalisation of the world: A reappraisal. Aust Syst Bot. 2015;28(2-3):81–90. [Google Scholar]
- 4.Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions. J Biogeogr. 2010;37(11):2029–2053. [Google Scholar]
- 5.Wallace AR. On the monkeys of the Amazon. Proc Zool Soc Lond. 1852;20(2):107–110. [Google Scholar]
- 6.Wallace AR. Island Life, or the Phenomena and Causes of Insular Faunas and Floras. Macmillan; London: 1880. [Google Scholar]
- 7.Jordan DS. The origin of species through isolation. Science. 1905;22(566):545–562. doi: 10.1126/science.22.566.545. [DOI] [PubMed] [Google Scholar]
- 8.Grinnell J. Barriers to distribution as regards birds and mammals. Am Nat. 1914;48(568):248–254. [Google Scholar]
- 9.Adams CC. The postglacial dispersal of the North American biota. Biol Bull. 1905;9(1):53–71. [Google Scholar]
- 10.Haffer J. Speciation in amazonian forest birds. Science. 1969;165(3889):131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- 11.Remington CL. Suture-zones of hybrid interaction between recently joined biotas. In: Dobzhansky T, Hecht MK, Steere WC, editors. Evolutionary Biology. Plenum Press; New York: 1968. pp. 321–428. [Google Scholar]
- 12.Rosen DE. Vicariant patterns and historical explanation in biogeography. Syst Zool. 1978;27(2):159–188. [Google Scholar]
- 13.Simpson GG. History of the fauna of Latin America. Am Sci. 1950;38(3):361–389. [Google Scholar]
- 14.Darlington PJ., Jr Area, climate, and evolution. Evolution. 1959;13(4):488–510. [Google Scholar]
- 15.Avise J. Phylogeography: The History and Formation of Species. Harvard Univ Press; Cambridge, MA: 2000. [Google Scholar]
- 16.Maxson LR, Roberts JD. Albumin and Australian frogs: Molecular data a challenge to speciation model. Science. 1984;225(4665):957–958. doi: 10.1126/science.225.4665.957. [DOI] [PubMed] [Google Scholar]
- 17.Avise JC. Molecular population structure and the biogeographic history of a regional fauna: A case history with lessons for conservation biology. Oikos. 1992;63(1):62–76. [Google Scholar]
- 18.Riddle BR, Hafner DJ, Alexander LF, Jaeger JR. Cryptic vicariance in the historical assembly of a Baja California peninsular desert biota. Proc Natl Acad Sci USA. 2000;97(26):14438–14443. doi: 10.1073/pnas.250413397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle BR, Hafner DJ. A step-wise approach to integrating phylogeographic and phylogenetic biogeographic perspectives on the history of a core North American warm deserts biota. J Arid Environ. 2006;65(3):435–461. [Google Scholar]
- 20.Hickerson MJ, Meyer CP. Testing comparative phylogeographic models of marine vicariance and dispersal using a hierarchical Bayesian approach. BMC Evol Biol. 2008;8:322. doi: 10.1186/1471-2148-8-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tribsch A, Schonswetter P. Patterns of endemism and comparative phylogeography confirm palaeoenvironmental evidence for Pleistocene refugia in the Eastern Alps. Taxon. 2003;52(3):477–497. [Google Scholar]
- 22.Garrick RC, Rowell DM, Sunnucks P. Phylogeography of saproxylic and forest floor invertebrates from Tallaganda, South-eastern Australia. Insects. 2012;3(1):270–294. doi: 10.3390/insects3010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavares C, et al. Phylogeography of the ladybird Iberorhyzobius rondensis, a potential biological control agent of the invasive alien pine bast scale Matsucoccus feytaudi. BioControl. 2015;60(1):59–69. [Google Scholar]
- 24.Espíndola A, Alvarez N. Comparative phylogeography in a specific and obligate pollination antagonism. PLoS One. 2011;6(12):e28662. doi: 10.1371/journal.pone.0028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohse K, Barton NH, Melika G, Stone GN. A likelihood-based comparison of population histories in a parasitoid guild. Mol Ecol. 2012;21(18):4605–4617. doi: 10.1111/j.1365-294X.2012.05700.x. [DOI] [PubMed] [Google Scholar]
- 26.Yue H, et al. A mitogenome of the Chevrier’s field mouse (Apodemus chevrieri) and genetic variations inferred from the cytochrome b gene. DNA Cell Biol. 2012;31(4):460–469. doi: 10.1089/dna.2011.1301. [DOI] [PubMed] [Google Scholar]
- 27.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292(5517):686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 28.Zink RM, Barrowclough GF. Mitochondrial DNA under siege in avian phylogeography. Mol Ecol. 2008;17(9):2107–2121. doi: 10.1111/j.1365-294X.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 29.Barrowclough GF, Zink RM. Funds enough, and time: mtDNA, nuDNA and the discovery of divergence. Mol Ecol. 2009;18(14):2934–2936. [Google Scholar]
- 30.Edwards S, Bensch S. Looking forwards or looking backwards in avian phylogeography? A comment on Zink and Barrowclough 2008. Mol Ecol. 2009;18(14):2930–2933, discussion 2934–2936. doi: 10.1111/j.1365-294X.2009.04270.x. [DOI] [PubMed] [Google Scholar]
- 31.Bowen BW, et al. Phylogeography unplugged: Comparative surveys in the genomic era. Bull Mar Sci. 2014;90(1):13–46. [Google Scholar]
- 32.Dolman G, Joseph L. A species assemblage approach to comparative phylogeography of birds in southern Australia. Ecol Evol. 2012;2(2):354–369. doi: 10.1002/ece3.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith BT, et al. The drivers of tropical speciation. Nature. 2014;515(7527):406–409. doi: 10.1038/nature13687. [DOI] [PubMed] [Google Scholar]
- 34.Richards CL, Carstens BC, Knowles LL. Distribution modelling and statistical phylogeography: An integrative framework for generating and testing alternative biogeographical hypotheses. J Biogeogr. 2007;34(11):1833–1845. [Google Scholar]
- 35.Waltari E, et al. Locating pleistocene refugia: Comparing phylogeographic and ecological niche model predictions. PLoS One. 2007;2(6):e563. doi: 10.1371/journal.pone.0000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reilly SB, Corl A, Wake DB. An integrative approach to phylogeography: investigating the effects of ancient seaways, climate, and historical geology on multi-locus phylogeographic boundaries of the Arboreal Salamander (Aneides lugubris) BMC Evol Biol. 2015;15:241. doi: 10.1186/s12862-015-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson MN. Natural experiments and meta-analyses in comparative phylogeography. J Biogeogr. 2014;41(1):52–65. [Google Scholar]
- 38.Donoghue MJ, Moore BR. Toward an integrative historical biogeography. Integr Comp Biol. 2003;43(2):261–270. doi: 10.1093/icb/43.2.261. [DOI] [PubMed] [Google Scholar]
- 39.Leaché AD, Crews SC, Hickerson MJ. Two waves of diversification in mammals and reptiles of Baja California revealed by hierarchical Bayesian analysis. Biol Lett. 2007;3(6):646–650. doi: 10.1098/rsbl.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soltis DE, Morris AB, McLachlan JS, Manos PS, Soltis PS. Comparative phylogeography of unglaciated eastern North America. Mol Ecol. 2006;15(14):4261–4293. doi: 10.1111/j.1365-294X.2006.03061.x. [DOI] [PubMed] [Google Scholar]
- 41.Bagley JC, Johnson JB. Testing for shared biogeographic history in the lower Central American freshwater fish assemblage using comparative phylogeography: Concerted, independent, or multiple evolutionary responses? Ecol Evol. 2014;4(9):1686–1705. doi: 10.1002/ece3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hope AG, Takebayashi N, Galbreath KE, Talbot SL, Cook JA. Temporal, spatial and ecological dynamics of speciation among amphi-Beringian small mammals. J Biogeogr. 2013;40(3):415–429. [Google Scholar]
- 43.Moreau CS, Hugall AF, McDonald KR, Jamieson BGM, Moritz C. An ancient divide in a contiguous rainforest: Endemic earthworms in the Australian Wet Tropics. PLoS One. 2015;10(9):e0136943. doi: 10.1371/journal.pone.0136943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pyron RA, Burbrink FT. Hard and soft allopatry: Physically and ecologically mediated modes of geographic speciation. J Biogeogr. 2007;37(10):2005–2015. [Google Scholar]
- 45.Jezkova T, et al. Genetic consequences of postglacial range expansion in two codistributed rodents (genus Dipodomys) depend on ecology and genetic locus. Mol Ecol. 2015;24(1):83–97. doi: 10.1111/mec.13012. [DOI] [PubMed] [Google Scholar]
- 46.Jezkova T, et al. Range and niche shifts in response to past climate change in the desert horned lizard Phrynosoma platyrhinos. Ecography. 2016;39(5):437–448. doi: 10.1111/ecog.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham MR, Jaeger JR, Prendini L, Riddle BR. Phylogeography of Beck’s Desert Scorpion, Paruroctonus becki, reveals Pliocene diversification in the Eastern California Shear Zone and postglacial expansion in the Great Basin Desert. Mol Phylogenet Evol. 2013;69(3):502–513. doi: 10.1016/j.ympev.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Mott BM, Gadau J, Anderson KE. Phylogeography of Pogonomyrmex barbatus and P. rugosus harvester ants with genetic and environmental caste determination. Ecol Evol. 2015;5(14):2798–2826. doi: 10.1002/ece3.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schield DR, et al. Incipient speciation with biased gene flow between two lineages of the Western Diamondback Rattlesnake (Crotalus atrox) Mol Phylogenet Evol. 2015;83:213–223. doi: 10.1016/j.ympev.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Lomolino MV, Riddle BR, Whittaker RJ, Brown JH. Biogeography. 4th Ed Sinauer Associates; Sunderland, MA: 2010. [Google Scholar]
- 51.Dolby GA, Bennett SEK, Lira-Noriega A, Wilder BT, Muguia-Vega A. Assessing the geological and climatic forcing of biodiversity and evolution surrounding the Gulf of California. J Southwest. 2015;57(2-3):391–456. [Google Scholar]
- 52.Badgley C, Smiley TM, Finarelli JA. Great Basin mammal diversity in relation to landscape history. J Mammal. 2014;95(6):1090–1106. [Google Scholar]
- 53.Moritz C, Faith DP. Comparative phylogeography and the identification of genetically divergent areas for conservation. Mol Ecol. 1998;7(4):419–429. [Google Scholar]
- 54.Laity T, et al. Phylodiversity to inform conservation policy: An Australian example. Sci Total Environ. 2015;534:131–143. doi: 10.1016/j.scitotenv.2015.04.113. [DOI] [PubMed] [Google Scholar]
- 55.Wood DA, et al. Comparative phylogeography reveals deep lineages and regional evolutionary hotspots in the Mojave and Sonoran Deserts. Divers Distrib. 2013;19(7):722–737. [Google Scholar]
- 56.Marske KA, Rahbek C, Nogues-Bravo D. Phylogeography: Spanning the ecology—Evolution continuum. Ecography. 2013;36:1169–1181. [Google Scholar]
- 57.Morrone JJ. Evolutionary Biogeography: An Integrative Approach With Case Studies. Columbia Univ Press; New York: 2009. [Google Scholar]