Significance
Adverse life conditions are linked to increased expression of proinflammatory genes and reduced expression of antiviral genes. However, these findings have come from Western, educated, industrialized, rich, and democratic (WEIRD) societies. Therefore, we evaluated adversity-related gene regulation among former child soldiers in Nepal—a non-WEIRD population. We found that posttraumatic stress disorder (PTSD) and resilience were inversely and independently associated with gene regulation among a population exposed to war during childhood. The results suggest that gene regulation responses to adversity are not restricted to WEIRD contexts and they underscore the role of psychological resilience in determining the molecular impact of traumatic experiences. Promoting resilience, even in the absence of PTSD symptom reduction, may have benefits for physical and mental health.
Keywords: biocultural anthropology, child abuse, global mental health, low-income countries, social genomics
Abstract
Adverse social conditions in early life have been linked to increased expression of proinflammatory genes and reduced expression of antiviral genes in circulating immune cells—the conserved transcriptional response to adversity (CTRA). However, it remains unclear whether such effects are specific to the Western, educated, industrialized, rich, and democratic (WEIRD) cultural environments in which previous research has been conducted. To assess the roles of early adversity and individual psychological resilience in immune system gene regulation within a non-WEIRD population, we evaluated CTRA gene-expression profiles in 254 former child soldiers and matched noncombatant civilians 5 y after the People’s War in Nepal. CTRA gene expression was up-regulated in former child soldiers. These effects were linked to the degree of experienced trauma and associated distress—that is, posttraumatic stress disorder (PTSD) severity—more than to child soldier status per se. Self-perceived psychological resilience was associated with marked buffering of CTRA activation such that PTSD-affected former child soldiers with high levels of personal resilience showed molecular profiles comparable to those of PTSD-free civilians. These results suggest that CTRA responses to early life adversity are not restricted to WEIRD cultural contexts and they underscore the key role of resilience in determining the molecular impact of adverse environments.
Exposure to social adversity during childhood and adolescence predicts a host of physical and mental health problems across the life span (1, 2). One hypothesized mechanism of such effects involves the impact of adverse environmental conditions on development of bidirectional regulatory interactions between the nervous and immune systems (3, 4). For example, chronic exposure to adverse social environments is associated with activation of a conserved transcriptional response to adversity (CTRA) involving up-regulation of proinflammatory genes and down-regulation of type I IFN- and antibody-related genes in myeloid lineage immune cells (5–7). These molecular dynamics have been implicated in a diverse array of disease processes, including cardiovascular and neurodegenerative diseases (8, 9), metastatic cancer (10), viral infection (6, 11), and psychiatric conditions such as anxiety and depression (12). In nonhuman primates, exposure to unstable social conditions for the first 4 mo of life is sufficient to induce the CTRA profile (13), and longitudinal human studies suggest that the molecular residue of such effects can persist into middle and late adulthood (14, 15). Research has begun to clarify the basic neuroimmune signaling pathways involved in CTRA development (6, 7, 9, 16).
Mapping the sources of molecular resilience to early adversity is complicated by the fact that all previous research in this area has been conducted in Western, educated, industrialized, rich, and democratic (WEIRD) populations (17). Non-WEIRD societies differ in the degree and type of exposure to stressful and traumatic life events. For example, non-WEIRD populations are more likely to be exposed to war and political violence (18, 19). Children in non-WEIRD populations are also more likely to be exposed to war as both civilians and combatants (20). WEIRD cultures may also atypically influence the nature and sources of psychological resilience to early life adversity. Previous studies have identified some resilience factors that may protect against CTRA emergence in midadulthood, such as early-life maternal relationship quality (21) and a sense of personal purpose in life (22–24). These studies were conducted in WEIRD cultures with comparatively extreme individualistic orientations, in which resilience is seen as an attribute of the person. It is unclear whether such individualized conceptions of resilience would pertain in non-WEIRD populations that are more collectivistic in orientation (25, 26), particularly if the nature, intensity, or context of adversity also differs in these environments. In contrast to individualistic interventions, in non-WEIRD populations with high trauma exposure, social ecological resilience interventions promoting family and community recovery have been considered culturally consistent and have shown positive outcomes (27). Non-WEIRD environments may also differ from WEIRD environments in the nature of microbial exposures and resulting immune system regulatory dynamics (28, 29). Such considerations suggest that assessments of resilience developed for use in WEIRD contexts may not associate with immune cell molecular markers in other cultural and ecological contexts. Given the population bias in existing CTRA research, it remains unclear whether early life social adversity would affect CTRA gene expression similarly in a non-WEIRD low-/middle-income country (LMIC), which is more comparable with the context in which the majority of the world’s children develop (30).
Therefore, the present research examined how early life social adversity and individual psychological resilience interact with CTRA gene expression in a non-WEIRD/LMIC population exposed to adversity: specifically, former child soldiers and other war-affected youth in Nepal.
Results
To assess the roles of early adversity and self-perceived resilience in the development of CTRA gene expression profiles in a non-WEIRD/LMIC culture, we evaluated 154 former child soldiers (age 16–26 y) and 136 matched civilians (15–24 y) who grew up amid war but were not combatants (Table 1). The participants were part of a 5-y longitudinal study that began in 2007, a year after peace accords ending the decade-long People’s War in Nepal (31, 32). Two hundred fifty-eight former child soldiers were enrolled in the study before participating in nongovernmental reintegration services provided by United Nations Children’s Emergency Fund (UNICEF), and a cohort of 258 civilian children matched on demographics were enrolled in the study but did not receive intervention services. Twelve months later, 222 of the former child soldiers (86% of original sample) and 234 of the matched civilians (91%) were reinterviewed. For the current study, in 2012, 154 of the former child soldiers (60% of original participants) and 136 of the matched civilians (53%) were traceable for interviews, at which time blood spots were collected for gene expression profiling (n = 282). In 2012, posttraumatic stress disorder (PTSD) was evaluated with the Child PTSD Symptom Scale (CPSS) (33), which was culturally and clinically validated in Nepal (34). Self-perceived psychological resilience was measured using a culturally adapted abbreviated version of Wagnild and Young’s Resilience Scale (35). The prevalence of PTSD in 2012 was 16.2% among former child soldiers compared with 5.9% among matched civilians [odds ratio (OR) = 3.10, 95% confidence interval (CI) (1.35, 7.13), P = 0.008]. The mean resilience score was 12.55 (SE 0.30) among child soldiers and 13.15 (SE 0.33) among matched civilians (t = 1.35, df = 288, P = 0.18).
Table 1.
Demographics of former child soldiers and civilian youth
| Demographic | Former child soldiers (n = 154) | Civilian youth (n = 136) | Group differences |
| Age, mean (SE) | 20.43 (0.13) | 19.88 (0.15) | t = 2.75, P < 0.01 |
| Gender, n (%) | |||
| Female | 77 (50.0%) | 58 (42.6%) | χ2 = 1.57, P = 0.24 |
| Male | 77 (50.0%) | 78 (57.4%) | |
| Caste, n (%) | |||
| Upper caste | 37 (24.0%) | 35 (25.7%) | χ2 = 0.13, P = 0.94 |
| Lower caste | 38 (24.7%) | 32 (23.5%) | |
| Ethnic minority | 79 (51.3%) | 69 (50.7%) | |
| Education, n (%) | |||
| ≤5th grade completed | 37 (24.0%) | 26 (19.1%) | χ2 = 1.40, P = 0.50 |
| 5th–9th grade completed | 59 (38.3%) | 51 (37.5%) | |
| ≥10th grade completed | 58 (37.7%) | 59 (43.4%) | |
| Lifetime traumatic events, n (%) | |||
| ≤1 event | 58 (37.7%) | 93 (68.4%) | χ2 = 27.31, P < 0.01 |
| Two or more events | 96 (62.3%) | 43 (31.6%) | |
| Combat exposure, n (%) | |||
| No combat | 116 (75.3%) | 131 (96.3%) | χ2 = 25.22, P < 0.01 |
| Any combat | 38 (24.7%) | 5 (3.7%) | |
| PTSD | |||
| No (CPSS <20) | 129 (83.8%) | 128 (94.1%) | χ2 = 7.67, P < 0.01 |
| Yes (CPSS ≥20) | 25 (16.2%) | 8 (5.9%) | |
| Resilience, mean (SE) | 12.55 (0.30) | 13.15 (0.33) | t = 1.35, P = 0.18 |
Among the 282 participants from whom dried blood spot samples were collected, 254 (90.1%) participants’ samples yielded valid transcriptome profiles and had data available for all other measures analyzed. Expression of the CTRA profile was assessed using an a priori-defined contrast among 53 gene transcripts (52 of which were detectable in this sample) comprising up-regulation of 19 proinflammatory genes and down-regulation of 34 genes involved in type I IFN and antibody responses. Mixed-effect linear model analyses found CTRA gene expression to vary systematically as a function of an a priori-specified set of demographic characteristics [age, sex, ethnic minority status, low caste status, and educational attainment; F(5, 240) = 57.55, P < 0.0001] and an a priori-specified set of RNA transcripts marking the relative prevalence of major leukocyte subsets [CD4+ and CD8+ T lymphocytes, B lymphocytes, natural killer cells, and monocytes; F(8, 240) = 123.74, P < 0.0001]. Parameter estimates relating CTRA gene expression to individual demographic and leukocyte subset dimensions are reported in Table 2, model 1.
Table 2.
Difference in CTRA gene expression as a function of demographic and hematologic covariates, child soldier status, PTSD, and resilience
| Model | b (SE)* | P value |
| Model 1 (demographic and hematologic control variables only) | ||
| Age, y | −0.066 (0.026) | 0.0125 |
| Sex (female) | −1.315 (0.093) | <0.0001 |
| Ethnic minority (Janajati) | 0.114 (0.112) | 0.3100 |
| Low social caste (Dalit) | 0.122 (0.136) | 0.3706 |
| Education level (range 0–6) | 0.197 (0.032) | <0.0001 |
| CD3D | 2.605 (0.286) | <0.0001 |
| CD3E | −6.483 (0.560) | <0.0001 |
| CD4 | −4.219 (0.369) | <0.0001 |
| CD8A | 1.202 (0.220) | <0.0001 |
| CD19 | 1.833 (0.564) | 0.0013 |
| NCAM1 | −1.448 (1.067) | 0.1760 |
| FCGR3A | 1.155 (0.144) | <0.0001 |
| CD14 | −1.004 (0.123) | <0.0001 |
| Model 2 (model 1 + child soldier status) | ||
| Child soldier† | 0.198 (0.009) | 0.0281 |
| Model 3 (model 2 + PTSD) | ||
| Child soldier† | 0.154 (0.090) | 0.0901 |
| PTSD† | 0.468 (0.146) | 0.0015 |
| Model 4 (model 3 + resilience) | ||
| Child soldier† | 0.133 (0.091) | 0.1443 |
| PTSD† | 0.455 (0.146) | 0.0020 |
| Resilience (range 2–24) | −0.028 (0.012) | 0.0194 |
Partial regression coefficient from mixed-effect linear model relating average expression of 52 CTRA indicator genes to listed variables. n = 254.
Child soldier status and PTSD status included as categorical variables: former child soldier = 1, civilian youth = 0; current PTSD (CPSS total score ≥20) = 1; not current PTSD (CPSS total score <20) = 0.
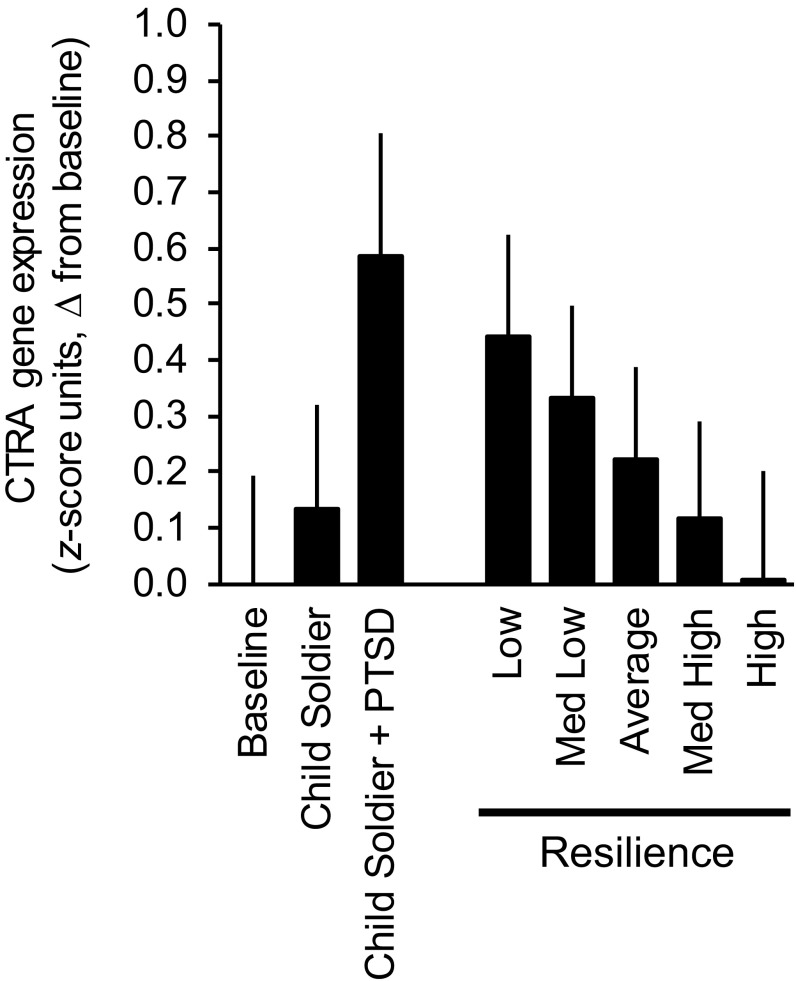
Beyond the effects of demographic characteristics and leukocyte subset distributions, CTRA gene expression was also significantly up-regulated in former child soldiers compared with civilians [F(1, 239) = 4.88, P = 0.0281] (Table 2, model 2). Subsequent analyses examining a 2D representation of war-related adversity that included current PTSD symptoms as well as child soldier status (i.e., soldier vs. civilian) found CTRA expression to vary significantly within this space [F(2, 238) = 7.64, P = 0.0006]. Dimension-specific parameters (Table 2, model 3) showed limited CTRA association with child soldier status per se [F(1, 238) = 2.90, P = 0.0901] but substantial CTRA up-regulation among those experiencing PTSD [F(1, 238) = 10.35, P = 0.0015] (Fig. 1).
Fig. 1.
CTRA gene expression as a function of early life adversity and psychological resilience. (Left three bars) Average CTRA gene expression values for civilians, former child soldiers (absent PTSD), and former soldiers suffering significant PTSD symptoms with minimal Resilience Scale scores. (Right five bars) Average CTRA gene expression values for former child soldiers with PTSD and Resilience Scale scores 2 SD below average (Low), 1 SD below average (Med Low), average (Average), 1 SD above average (Med High), and 2 SD above average (High). Estimates come from a mixed-effect linear model controlling for demographic characteristics, leukocyte subset indicators, a 2D representation of war-related adversity exposure (child soldier status and PTSD status), and continuous Resilience Scale scores. Data represent difference (+ SE) in CTRA gene expression relative to baseline.
Above and beyond the effects of demographic characteristics, leukocyte subset distributions, and the 2D representation of war-related adversity, CTRA gene expression was also down-regulated in direct proportion to Resilience Scale scores [F(1, 237) = 5.54, P = 0.0194] (Table 2, model 4 and Fig. 1). To determine how fully individual psychological resilience might buffer the effects of war-related adversity, we examined predicted CTRA gene expression levels across the observed range of individual differences in Resilience Scale scores (i.e., −2, −1, 0, +1, and +2 SD relative to the sample average). As shown in Fig. 1, buffering effects were pronounced. Among PTSD-affected former child soldiers, those with high resilience scores (+2 SD) showed CTRA gene expression levels markedly below those observed for average- and low-resilience PTSD-affected child soldiers and comparable in magnitude with levels observed in the PTSD-free civilian youth. These buffering effects did not arise from any confounding of high adversity exposure with low self-perceived resilience because Resilience Scale scores in PTSD-affected child soldiers were broadly similar to the remainder of the sample (mean 11.7 ± SD 4.1 vs. 12.9 ± SD 3.8 for the remainder; difference in means, P = 0.1675; difference in dispersion, P = 0.7737).
Discussion
These data show the emergence of persistent CTRA gene expression profiles in the aftermath of early life adversity among former child soldiers in a non-WEIRD/LMIC cultural environment. CTRA gene expression profiles were linked to the degree of trauma exposure and associated distress, as measured by PTSD symptom severity above a culturally and clinically validated cutoff point (34), more than to child soldier status per se. These relationships were independent of demographic characteristics (age, sex, ethnic status, low caste, and education) and individual differences in leukocyte subset prevalence. They also emerged in a cultural context that is likely more collectively orientated than populations previously studied in social genomics literature (36–38). Despite this collective social orientation, individual self-perceptions of personal psychological resilience were associated with markedly lower CTRA gene expression profiles, to the extent that PTSD-affected former child soldiers with high levels of self-reported resilience showed molecular profiles comparable to those of PTSD-free civilian youth. These results suggest that CTRA responses to early life social adversity may represent a relatively broad human potential that can be observed in non-WEIRD populations. Our findings echo a recent study in which a molecular association established in WEIRD populations was replicated in a non-WEIRD population: specifically, the association of stressful exposures and telomere shortening among Indian conservation refugees (39).
The present data underscore the importance of recognizing PTSD in non-WEIRD cultural groups because PTSD may herald physical and mental health problems throughout the life course. However, this contrasts with some anthropological and psychiatric perspectives arguing that PTSD is not relevant in non-WEIRD populations (40). We demonstrated an association between CTRA up-regulation and child soldier status and a stronger association between CTRA up-regulation and current PTSD. Given that both child soldiers and matched youth were exposed to war and different forms of trauma (41, 42), it is not surprising that PTSD was a stronger predictor of CTRA up-regulation than child soldier status per se. Our prior research with child soldiers in Nepal indicates that there were protective factors arising from being a child soldier, such as protection against sexual violence among girl soldiers in the People’s Army (43, 44). Child soldiers also reported solidarity and feeling supported by Maoists, which was not reported by community members (43). Therefore, despite higher trauma burden among child soldiers, the child soldier experience may confer protective factors. Similar findings of both psychological liabilities and benefits have been reported for adult soldiers in the United States (45).
A more surprising finding was the association between a resilience questionnaire designed in a WEIRD cultural context and CTRA gene expression in this non-WEIRD population. Wagnild and Young’s Resilience Scale is dominated by items related to individualistic psychological processes and does not refer to familial, community, or other collective processes (35). Items from their scale used in the Nepali version, for example, included the following: “I am able to depend on myself more than anyone else,” “I can be on my own if I have to,” and “my belief in myself gets me through hard times.” Endorsement of these beliefs was associated with down-regulated CTRA gene expression, which raises the potential that self-efficacy, whether in collectivistic or individualistic societies, could be beneficial for health.
Another important finding was that both PTSD and self-perceived psychological resilience contributed unique variance to this biological process. Critics have argued that resilience is often conflated with the absence of psychological distress after trauma (46): i.e., PTSD symptom severity is inversely proportional to resilience. Our findings support other research arguing that resilience is not merely the flipside of psychological trauma (47). For example, some child soldiers had high PTSD symptom severity, but they also reported high levels of resilience, and these soldiers displayed molecular profiles comparable with civilian children without PTSD. These results suggest that biological sequelae of trauma and adversity might be addressed by promoting resilience, even in the absence of PTSD symptom reduction. From an evolutionary perspective, these findings are important for understanding how humans have dealt with social adversity. Anthropologists suggest that much of human history was characterized by violence and small-scale warfare (48). Resilience may represent a protective psychosocial process evolved to mitigate against the long-term health effects of trauma on health (49).
Given that this study is, to our knowledge, the first study to identify both adversity and protective processes associated with CTRA profiles in a non-WEIRD population, it is important to consider limitations of the methods and analysis. First, the full Wagnild and Young Resilience Scale was not used (35). We selected items through pilot testing to determine cultural salience (50). The full 26-item version may show a different association with CTRA profiles. Second, this study examined average association of an a priori-specified set of genes and was not designed to discover novel genomic profiles or specific individual gene transcripts that show statistically reliable associations with adversity or resilience factors. Additional genomic profiles or individual transcripts may well be found to associate with early life adversity in future exploratory/discovery analyses. Third, differences between child soldiers and civilian youth may be biased by those individuals whom we located for this 5-y follow-up study. Although no differences were found in PTSD at baseline in 2007 between current participants and persons lost to follow-up in 2012 (Supporting Information), there may be other domains in which participants and persons lost to follow-up differ. Fourth, because the child soldiers in this study had received an intervention from UNICEF, the limited difference in CTRA expression relative to civilian children could be a result of reintegration services, which emphasized social connectedness and community supports (31) and yielded improvement for PTSD and depression (32).
The present study was enabled by dried blood spot (DBS) sampling under field conditions in Nepal. However, technical calibration studies suggest that DBS transcriptome profiling is less sensitive to true differences in gene expression than are analyses of immediately processed venipuncture blood samples [peripheral blood mononuclear cells (PBMCs)] (Supporting Information and ref. 51). This result implies that the Nepal DBS study may have missed some results that could have been apparent using optimal venipuncture blood samples but that the significant results that were detected are unlikely to be spurious and more likely underestimate the true magnitude of differences that would have been observed using gold standard PBMCs. An additional issue is that early exposure to parasites, which is more prevalent in non-WEIRD settings, may impact immune functioning (29) and thus the relationship of CTRA expression to social adversity. Future research should include measures of parasite burden and immune markers in relation to CTRA expression.
Regarding causal pathways, this study was not able to identify the developmental period most salient in producing current CTRA profiles (e.g., was it early childhood antecedent to becoming child soldiers, experiences during war, or postwar circumstances?). Although self-report measures were conducted longitudinally, CTRA was evaluated only at the third wave of data collection. Future studies should use longitudinal representative samples to identify associations of CTRA with adversity and resilience over the course of child development into adulthood. It is also important to note that this study is an observational analysis and cannot formally define causal relationships among child soldier status, PTSD, resilience, and gene expression.
Keeping these limitations in mind, it is useful to consider implications to improve the lives of persons exposed to early adversity in LMIC settings. The association of psychological resilience with CTRA down-regulation independent of PTSD suggests that promoting resilience, rather than just focusing on PTSD symptom reduction, may have benefits for health. This hypothesis is important because the dearth of mental health experts in LMIC settings limits the feasibility of administering intensive trauma-focused interventions (52). Resilience-promotion interventions may be more easily delivered in such contexts and may have more buy-in from beneficiaries compared with psychiatric interventions (53, 54). For example, child soldiers identified the ability to help others, to help their country, and to reduce discrimination in society as more important mental health goals than reduction of symptoms such as nightmares and flashbacks (55). This aspiration-based approach reflects previous findings in WEIRD populations linking a self-transcendent purpose in life (eudaimonic well-being) to reduced CTRA gene expression (22–24). Ultimately, these advances in understanding the role of social genomics can contribute to reducing the physical and mental health impacts of trauma and adversity worldwide.
Materials and Methods
Setting and Participants.
Nepal ranks 142 out of 174 on the human development index—near the bottom of the medium human development category (56). Nepal is characteristic of low-income countries with a high burden of infectious disease, which contributes to high rates of infant and under-5-y-old mortality (57). In addition to high exposure to infectious disease and lack of adequate health care, children in Nepal also experience adversity in the form of political violence (58). From 1996 to 2006 during the People’s War in Nepal, the Communist Party of Nepal (Maoists) People’s Liberation Army (PLA) and the Royal Nepal Army conscripted children as soldiers, sentries, spies, cooks, and porters (59, 60). Our prior research in Nepal in 2007, conducted a few months after the war ended, found that 55% of child soldiers had PTSD compared with 20% of matched civilian children, in a sample that was unique from the current cohort (41).
The current cohort began in 2007 when we used criterion sampling to select 258 child soldiers and 258 matched civilian children for this study from eight districts across Nepal (see Supporting Information for additional information on identification of study participants). In 2008, at 1-y follow-up, child soldiers (n = 222, 86% of baseline participants) and matched civilian youth (n = 234, 91% of baseline participants) were reinterviewed (32). For the current study in 2012, attempts were made to reinterview all participants from the original study. For participants over 18 y of age at the time of the current study follow-up in 2012, consent was obtained. For participants under 18 y of age, legal guardians provided consent, and the minors provided assent. The research was approved by the institutional review boards of the Nepal Health Research Council, Kathmandu, Nepal; Emory University; George Washington University; and the University of California, Los Angeles (RNA Comparison Study in Supporting Information).
Variables.
Participants completed 60- to 90-min individual interviews. Due to high illiteracy rates, research assistants read questionnaires in Nepali to the youth. The interview included culturally adapted versions of mental health assessment questionnaires and a 37-question semistructured interview, developed based on findings from qualitative research with child soldiers (43, 61).
Child PTSD Symptom Scale.
PTSD was assessed with the Child PTSD Symptom Scale (CPSS) (33), a 17-item scale corresponding to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) symptoms (scale range 0–68). The range for this sample was 0–48, mean = 5.68 (SE 0.58). The CPSS was transculturally translated and validated in Nepal and has good psychometric properties: area under the curve (AUC) = 0.77, sensitivity = 0.68, specificity = 0.73, cutoff score ≥20, Cronbach’s α = 0.94 (34). For the current analyses, participants were categorized as PTSD-positive or PTSD-negative based on the validated cutoff (34). Participants lost to follow-up did not differ from participants interviewed in 2012 with regard to baseline CPSS scores in 2007 (Supporting Information).
Wagnild and Young’s Resilience Scale.
Resilience was assessed with eight items from Wagnild and Young’s Resilience Scale (35) as follows: “I am able to depend on myself more than anyone else”; “I can be on my own if I have to”; “I usually take things in stride”; “I feel that I can handle many things at a time”; “I can usually find something to laugh about”; “My belief in myself gets me through hard times”; “I have enough energy to do what I have to do”; and “I am resilient.” These items were used based on previous piloting of the tool in Nepal to determine which items were culturally salient (50). For the eight-item Nepal version, α = 0.72, range = 2–24, mean = 12.83 (SE = 0.22). Continuous Z-scores were used for the current analyses.
CTRA Gene Expression.
Details on blood spot collection, analysis, and assay validation are provided in Supporting Information. Briefly, total RNA was extracted from DBS samples (Qiagen RNeasy) in the University of California, Los Angeles (UCLA) Social Genomics Core and converted to fluorescence-labeled cDNA (NuGEN Ovation PicoSL) and hybridized to Illumina HT-12 v4.0 BeadChips in the UCLA Neuroscience Genomics Core following the manufacturer’s standard protocol. Data are deposited in the Gene Expression Omnibus database (accession no. GSE77164). Two hundred fifty-four of 282 DBS samples (90.1%) yielded valid results, and CTRA gene expression was analyzed as previously described (6, 22), with data quantile-normalized (62), log2-transformed, and z-score–standardized within gene for mixed-effect linear model analyses (63) quantifying association between expression of 53 CTRA indicator transcripts (with inverse components weighted negatively as described below) and exposure factors including child soldier status (1/0), posttraumatic stress (1/0: CPSS score ≥20), and Resilience Scale score (continuous score). Analyses controlled for a priori-specified covariates, including systematic effects of gene, participant age (years), sex (1/0, 1 = female), ethnic minority status (1/0, 1 = Janajati), low caste (1/0, 1 = Dalit), education level (seven ordinal levels) (see Supporting Information for additional information on demographic categories), and expression of eight mRNA transcripts indicating relative prevalence of major leukocyte subsets (CD14 for monocytes; CD3D, CD3E, CD4, and CD8A for T-lymphocyte subsets; CD19 for B lymphocytes; and CD56/NCAM1 and CD16/FCGR3A for natural killer cells). The 53 CTRA indicator genes comprised two a priori-specified gene sets as follows: 19 proinflammatory genes (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, and RELB) weighted +1 as positive indicators of the CTRA profile and 34 genes involved in type I IFN responses (GBP1, IFI16, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1L, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, and OASL) and antibody synthesis (IGJ, IGLL1, and IGLL3) weighted −1 as inverse indicators (10, 22–24, 64). Data were unavailable for IFI16 due to random variations in microarray probe synthesis, leaving a final analyzed set of 52 CTRA indicator transcripts. Models were estimated by maximum likelihood using SAS PROC MIXED, with the 52 indicator genes treated as repeated measures and a heterogeneous compound symmetry covariance structure specified to accommodate correlation and heteroscedasticity across residuals (22).
Nepal Study Materials and Methods
Child Soldier and Matched Civilian Children Participants in Longitudinal Study.
Child soldier status was determined based on name lists of “children associated with armed forces and armed groups (CAAFAG)” established by human rights organizations working with UNICEF. The 2007 Paris Principles were used for the operational definition of CAAFAG: “any person below 18 years of age who is or who has been recruited or used by an armed force or armed group in any capacity, including but not limited to children, boys, and girls used as fighters, cooks, porters, messengers, spies, or for sexual purposes. It does not only refer to a child who is taking or has taken a direct part in hostilities” (65). For the purposes of this paper, we will use the more commonly used term “child soldier” instead of CAAFAG. Matched civilian children were identified through school records. Their status as non-CAAFAG was confirmed by interviews with the children and secondary review of the human rights groups’ listings.
In the baseline study in 2007–2008, eight districts were participating in UNICEF-sponsored reintegration programs for child soldiers (31). The Nepali nongovernmental organization (NGO) Transcultural Psychosocial Organization (TPO) Nepal implemented the formative research with child soldiers and intervention from 2006 to 2009, along with the currently described follow-up study in 2012. The 258 participants in the quantitative study were former child soldiers who returned home after serving with the Maoist People’s Army (32). Selection criteria in 2007 for the target population included at least 1 mo of participation in an armed group, being younger than 18 y of age during initial enrollment in the study, and having a consenting caregiver. The first 30 former child soldiers enrolled for NGO services in each district were invited to participate in the study.
By the aforementioned selection criteria, 258 former child soldiers, including 159 male participants and 99 female participants, were selected for this study. The average age of participants at study entry in 2007 was 15.59 y (SD = 1.35 y, range = 11–17 y). All child soldiers were given the opportunity to participate in reintegration programs through UNICEF partners. The main packages were education, vocational training, apprenticeship, and income-generating activities (31, 32, 66).
Participants lost to follow-up in 2012 did not differ from participants interviewed with regard to baseline CPSS scores in 2007 although there was a trend for child soldiers who were lost to follow-up to have lower baseline PTSD symptom severity compared to child soldiers who participated in 2012 [child soldiers participating in 2012 (n = 154) baseline 2007 CPSS score = 18.00, 95% CI 16.42, 19.58; child soldiers lost to follow-up in 2012 (n = 104) baseline 2007 CPSS score = 15.13, 95% CI 13.20, 17.05, P > 0.05; civilian children participating in 2012 (n = 136) baseline 2007 CPSS score = 9.42, 95% CI 8.31, 10.54; civilian children lost to follow-up in 2012 (n = 122) baseline 2007 CPSS score = 8.30, 95% CI 7.11, 9.50, P > 0.05].
Research Team and Training.
Research was conducted by collaborative teams, including the Nepali research team of the TPO Nepal, and foreign researchers, including two psychologists and a psychiatrist–anthropologist, all of whom had spent more than a decade working in Nepal (67, 68). The research team obtained verbal consent or assent from all participants. Participants were compensated with small donations of school supplies or household goods. Participants with suicidal ideation or other acute mental health needs were referred to TPO Nepal counselors.
Demographics in Analyses.
Age, gender (male vs. female), caste/ethnicity, and education level were included in the current analyses because all of these factors have demonstrated associations with mental health outcomes in prior studies with other child soldiers and war-affected child populations in Nepal (41). Caste/ethnicity was coded into three groups: high Hindu castes (Brahman and Chhetri), low Hindu castes (Dalit, previously referred to as “untouchable” groups), and ethnic minorities (Janajati, referring to Hindu, Buddhist, and other groups categorized outside of the Hindu high/low dichotomy). Caste/ethnicity was coded according to national census categories, and the variable was included in the study because of prior associations with mental health outcomes (69–72). Education was categorized into seven ordinal levels: 0 = no schooling, illiterate; 1 = no schooling, literate; 2 = primary schooling; 3 = lower secondary schooling; 4 = upper secondary schooling; 5 = completed secondary leaving certificate; 6 = any higher education.
Blood Spot Collection.
Blood was collected from all consenting participants. Minilancets were used to make microincisions on participants’ fingers to collect five blood drops on newborn screening cards. Collection was performed twice during the interview process, 30 min apart (mean = 27 min, SD = 9). Dried blood spot (DBS) cards were stored in sealed containers with desiccant and then transferred within 3 d to a central facility in Kathmandu for refrigerated storage at 3 °C. Accumulated batches of DBS samples were periodically shipped at ambient temperature from Nepal to Emory University in the United States in accordance with the Centers for Disease Control and Prevention (CDC) regulations. Days in transit averaged 2.37 (SD = 0.95, range = 1–4). Duplicate biological samples were stored in Nepal according to Nepal Health Research Council regulations. Upon arrival at the Emory laboratory, samples were stored at −30 °C. The total number of days between field DBS collection and freezing at Emory averaged 13.6 (SD = 7.3, range = 4–38). Once all samples were accumulated, each was assessed for potential validity issues (e.g., overlapping blood spots, irregular shapes suggesting contact between the finger and the collection card, and evidence of fly bites), and uncompromised samples were then shipped overnight on dry ice to the UCLA Social Genomics Core Laboratory for RNA extraction. At UCLA, DBS samples were stored at −80 °C, and RNA was extracted within 8 wk of arrival (mean = 7 wk, SD = 1, range = 5–8). Total time between blood spot collection and RNA extraction thus ranged from 6 to 16.8 mo, with samples maintained at ≤4 °C for the majority of that period and samples exposed to ambient temperature for ≤1 wk. Previous studies indicate that DBS storage at ambient temperature for periods of 3–10 y has minimal effect on the calibration of microarray transcript abundance estimates (73, 74). Nevertheless, the present study sought to minimize the duration of ambient temperature storage and secured all DBS samples below 4 °C for the remaining duration to RNA extraction.
RNA Profiling.
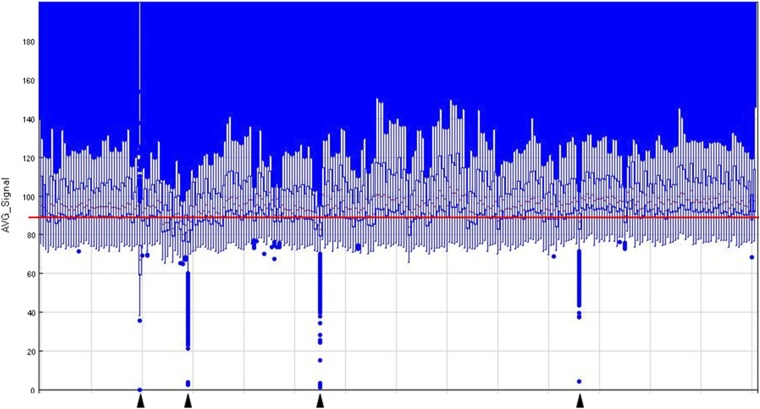
Total RNA was extracted from DBS samples by cutting all available blood spots for each participant (mean 3.4 spots, SD = 0.6, range 1–4), using a separate razor blade for each sample to prevent contamination, depositing all filter paper spots into 370 μL of QIAgen RLT buffer in an RNase-free sterile 1.5-mL microcentrifuge tube, incubating the tube for 30 min at 37 °C with agitation (1,000 rpm), and transferring tube contents (including filter paper) into a QIAshredder column for 60-s microcentrifugation at maximum speed, after which the 360 μL of remaining RLT was processed through the QIAcube nucleic acid extraction system using RNeasy Micro Kit reagents, the manufacturer’s standard operating protocol (including DNase treatment), and a 20-μL elution volume. Extracted RNA was stored at −80 °C before simultaneous transcriptome profiling. RNA was subject to cDNA amplification (NuGEN Ovation PicoSL WTA V2 System; NuGEN), fragmentation, fluorescent labeling (NuGEN Encore BiotinIL Module for Illumina Whole Genome Expression BeadChips), and hybridization to Illumina HT-12 v4.0 BeadChips (Illumina Inc.) in the UCLA Neuroscience Genomics Core following the manufacturer’s standard protocol for cDNA analysis. Data are deposited in the Gene Expression Omnibus database (accession no. GSE77164). Two hundred fifty-four of 282 assayed samples (90%) yielded valid results as determined by an a priori-defined endpoint quality criterion for raw hybridization intensity distributions (Fig. S1). As detailed below in Blood Spot Calibration Study, DBS samples yield insufficient RNA mass for preassay direct assessment of RNA integrity: e.g., by capillary electrophoresis methods such as the Agilent TapeStation. As such, all available DBS samples were submitted to assay, and sample validity was assessed using an assay endpoint criterion based on the distribution of hybridization fluorescence intensity values. The sample validity criteria were median AVG_Signal intensity values exceeding an a priori-specified threshold of 90. This endpoint criterion provides an integrated assessment of all stages of collection, storage, processing, and assay for a given sample, including input sample RNA mass and integrity, as well as downstream technical influences such as reagent failure (e.g., poor amplification) and sample loss/degradation during assay procedures. This endpoint criterion readily detects input sample deficiencies, such as insufficient RNA mass or integrity, because such samples fail to generate sufficient full-length cDNA and thus yield markedly reduced fluorescence intensity signals (as shown in Fig. S1).
Fig. S1.
Endpoint sample quality analysis of fluorescence intensity distributions in the Nepal study. Boxplots indicate the range of raw transcript abundance estimates for the 282 available DBS RNA samples in the Nepal study. Boxes span the 25th–75th percentile; midbars indicate the 50th percentile; blue dots indicate extreme values under normal distribution. Sample median expression values fell below the a priori-specified quality threshold of 90 AVG_Signal units (solid red line) for 28 of the 282 total samples (10%), leaving a total sample of n = 254 valid samples for analysis (90%). Lower margin triangles indicate highly aberrant distributions (failed RNA amplifications). RNA integrity analysis before cDNA amplification was infeasible due to DBS RNA masses falling below the limit required for valid assay (e.g., by capillary electrophoresis).
CTRA gene expression was analyzed as previously described (6, 22), with data quantile-normalized (to remove batch effects and differential loading) (62), log2-transformed, and z-score–standardized within gene for mixed-effect linear model analyses (63) quantifying association between expression of 53 CTRA indicator transcripts (with inverse components weighted negatively as described below) and exposure factors including child soldier status (1/0), posttraumatic stress (1/0, CPSS score ≥20), and Resilience Scale score (continuous score). Analyses controlled for a priori-specified covariates, including systematic effects of gene, participant age (years), sex (1/0, 1 = female), ethnic minority status (1/0, 1 = Janajati), low caste (1/0, 1 = Dalit), education level (seven ordinal levels), and expression of eight mRNA transcripts indicting relative prevalence of major leukocyte subsets (CD14 for monocytes, CD3D, CD3E, CD4, and CD8A for T-lymphocyte subsets, CD19 for B lymphocytes, and CD56/NCAM1 and CD16/FCGR3A for natural killer cells). The 53 CTRA indicator genes comprised two a priori-specified gene sets as follows: 19 proinflammatory genes (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, and RELB) weighted +1 as positive indicators of the CTRA profile and 34 genes involved in type I IFN responses (GBP1, IFI16, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1L, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, and OASL) and antibody synthesis (IGJ, IGLL1, and IGLL3) weighted −1 as inverse indicators (10, 22–24, 64). Data were unavailable for IFI16 due to random variations in microarray probe synthesis, leaving a final analyzed set of 52 CTRA indicator transcripts. Models were estimated by maximum likelihood using SAS PROC MIXED, with the 52 indicator genes treated as repeated measures and a heterogeneous compound symmetry covariance structure specified to accommodate correlation and heteroscedasticity across residuals (22).
Blood Spot Calibration Study
To assess the reliability of DBS-based assessment of CTRA gene expression, we compared results from DBSs, peripheral blood mononuclear cells (PBMCs) (the gold standard reference point), and salivary RNA (negative reference point given its known partial degradation) (75) collected in parallel from 59 community dwelling adults from the Los Angeles metropolitan area in an “RNA Comparison Study.” The sample was diverse in age (mean = 45 y, SD = 22, range = 21–98), sex (81% female), race/ethnicity (12% African American, 23% Asian, 9% Hispanic, 56% Caucasian), and body mass index (BMI) (mean = 25.7, SD = 4.9, range 17–39). Written informed consent was provided by all participants, and all procedures were approved by the UCLA Medical Institutional Review Board.
Blood and saliva samples were collected from participants after at least 5 min of seated rest. DBS samples were collected using sterile minilancets to make microincisions on participants’ fingers to collect five blood drops onto filter paper cards (Whatman no. 903; GE Healthcare). Cards were dried for 3 h at room temperature, sealed in Ziploc bags with a dessicant packet, and stored at room temperature for ∼10 wk (to approximate the maximum duration of ambient temperature storage involved in field collection and shipment). Approximately 10 wk after collection, total RNA was extracted from DBS samples by cutting two to five blood spots from the five-spot filter paper for each sample (using a separate razor blade for each sample to prevent contamination, as described above), depositing all filter paper spots from each sample into 370 μL of QIAgen RLT buffer in an RNase-free sterile 1.5-mL microcentrifuge tube, incubating the tube for 30 min at 37 °C with agitation (1,000 rpm), and transferring tube contents (including filter paper) into a QIAshredder column for 60-s microcentrifugation at maximum speed, after which the 360 μL of remaining RLT was processed through the QIAcube nucleic acid extraction system using QIAgen RNeasy Micro Kit reagents, the manufacturer’s standard operating protocol (including DNase treatment), and a 20-μL elution volume. PBMC samples were derived from 8 mL of blood drawn by antecubital venipuncture into two Becton-Dickinson CPT cell preparation tubes, which were immediately centrifuged to separate PBMCs following the manufacturer’s standard protocol. Cell pellets were resuspended in QIAgen RLT buffer and frozen at –80 °C for subsequent RNA extraction on a QIAcube instrument within 2 wk of collection. Two milliliters of saliva was produced into Oragene RNA Collection Kits and refrigerated at 4 °C before RNA extraction on a QIAcube instrument within 2 wk of collection.
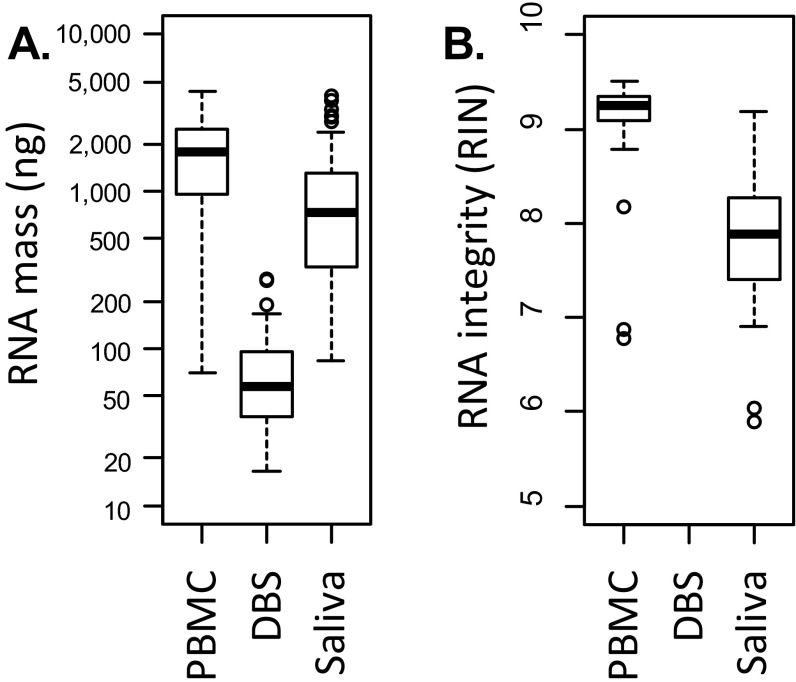
RNA mass was assessed in all samples using a Nanodrop ND1000 spectrophotometer (Fig. S2A). RNA integrity was assessed in PBMC and saliva samples using capillary electrophoresis (Agilent TapeStation) to yield a standard RNA Integrity Number (RIN) (Fig. S2B) ranging from 10 (completely intact) to 0 (totally degraded). No attempt was made to assess RNA integrity in DBS samples because total RNA quantity was expected to fall below the lower limit of detection for a reliable electrophoretic assay. As expected based on previous research (74, 76, 77), DBS samples yielded a total RNA mass less than 1/10th that of PBMCs (Fig. S2A). Saliva yielded total RNA mass on the same order of magnitude as PBMCs but with notably higher levels of RNA degradation (i.e., lower RIN scores) (Fig. S2B), as previously observed (75).
Fig. S2.
RNA mass and integrity in the DBS calibration study. Total RNA mass (A) and RNA integrity (B) were assessed in PBMC, DBS, and saliva samples collected in parallel from n = 58 community dwelling adults. Compared with the PBMC gold standard, DBSs yielded significantly lower quantities of RNA (mean = 1,792 ng for PBMCs vs. 75 ng for DBSs; difference P < 0.0001 by Wilcoxon test). Saliva yielded total RNA mass on the same order of magnitude as PBMCs (mean = 1,029 ng), but with greater levels of degradation (mean RIN score = 9.1 for PBMCs vs. 7.9 for saliva, difference P < 0.0001 by Wilcoxon test). RIN scores range from 0 (fully degraded) to 10 (fully intact) and are unavailable for DBSs (B) because sample RNA mass fell below the limit required for valid assay.
All available DBS RNA and 40 ng of RNA from saliva and PBMC samples were submitted to cDNA amplification using the NuGEN Ovation PicoSL WTA V2 System, and 100 ng of the resulting cDNA was fragmented and fluorescently labeled using the NuGEN Encore BiotinIL Module for Illumina Whole Genome Expression BeadChips. The resulting fluorescent target sample was assayed using Illumina Human HT-12 v4.0 BeadChips following the manufacturer’s standard protocol for cDNA hybridization, with scanning on an Illumina iScan instrument in the UCLA Neuroscience Genomics Core. DBS samples yielded insufficient RNA mass for direct assessment of RNA integrity before assay, so all available DBS samples were submitted to assay and sample validity was instead assessed using an endpoint distribution criterion as described above for the Nepal Study (median AVG_Signal >80, reflecting adjustment for differences in iScan calibration at the time of assay). Based on this validity criterion, one DBS sample was removed from subsequent quantitative calibration analyses along with its corresponding PBMC and saliva samples (leaving a total of n = 58 cases in final analyses). Raw and normalized data are deposited in the Gene Expression Omnibus database (accession no. GSE792699). Raw gene expression data were quantile-normalized and log2-transformed for analysis.
General Correspondence Between DBS and PBMC.
Initial analyses calibrated DBS-derived gene expression values against results from the PBMC gold standard by computing the Pearson correlation across 33,046 validly assayed gene transcripts. (To avoid bias arising from systematic algorithms, we did not attempt to impute values for observations deemed undetectable by Illumina Genome Studio data processing software.) Across all 1,916,668 observations derived from each RNA source (G = 33,046 transcripts assessed in n = 58 individuals), results showed a strong correspondence of DBS-derived estimates of transcript abundance with PBMC gold standard values (r = 0.80, P < 0.0001). In contrast, saliva-derived RNA showed notably poorer correspondence to PBMCs (r = 0.47, P < 0.0001). The latter result was expected due to the effects of partial RNA degradation on assay signal-to-noise ratios (75), and results from salivary RNA sampling thus served as a negative reference point for evaluating the quality of DBS RNA quantification (given its unknown/unassayable level of RNA integrity). Similar results emerged in analyses that averaged transcript-specific expression values over the n = 58 individuals to quantify aggregate transcript detection performance independent of individual biological variation. Results again showed strong correspondence between DBSs and PBMCs (r = 0.89, P < 0.001). By contrast, salivary RNA showed quantitatively weaker correspondence with PBMCs (r = 0.69, P < 0.0001).
Correspondence in CTRA Expression.
We also assessed DBS correspondence with PBMCs over the specific subset of 53 CTRA indicator genes used in previous studies (10, 22–24, 64) (49 of which were detectable in all samples assayed here). As shown in Table S1, Cronbach’s α coefficients indicated reasonable internal consistency for a composite CTRA summary score derived from either DBS or PBMC RNA (α values ∼0.7 for DBS and ∼0.8 for PBMC). CTRA summary scores derived from DBSs also showed good correlation with PBMC-derived summary scores (r = 0.62, P < 0.0001). In contrast, CTRA summary scores derived from salivary RNA showed comparatively poor reliability (α <0.3) and weaker correspondence to PBMC summary scores (r = 0.42, P < 0.0001). The poorer performance of salivary RNA was again expected given the measurement theory implications of decreased signal-to-noise ratios (78) stemming from partial degradation of salivary RNA (75).
Table S1.
CTRA composite score reliability
| Expression data | PBMC | DBS | Saliva |
| Raw (log2) | 0.874 | 0.739 | −0.038 |
| Standardized | 0.873 | 0.717 | 0.293 |
Entries are Cronbach’s α coefficients computed on raw or standardized RNA expression data for 49 CTRA indicator transcripts (with signs inverted for inverse components of the CTRA) assayed in parallel RNA samples from PBMCs, DBSs, and saliva. n = 58 individuals.
Detecting Substantive Differences in CTRA Expression.
To assess the efficiency with which DBS RNA can be used to detect substantive differences in CTRA gene expression, we conducted parallel analyses of demographic and hematological influences on average CTRA expression using PBMCs, DBSs, and salivary RNA. Systematic variations in average expression of the 49 detectable CTRA indicator genes were analyzed using mixed-effect linear models (63) treating genes as a repeated measure and assessing fixed effect influences of age (years), sex (female = 0/male = 1), BMI (kg/m2), a four-category race/ethnicity classification (1/0 indicators for African-American, Asian-American, and Hispanic, with Caucasian serving as the reference category), and seven mRNA transcripts indicating the relative proportions of major leukocyte subsets within the blood cell RNA pool (i.e., assessing the relative prevalence of monocytes, CD4+ and CD8+ T lymphocytes, B lymphocytes, and natural killer cells based on the relative abundance of mRNA transcripts for their canonical marker molecules CD14, CD3D, CD4, CD8A, CD19, FCGR3A/CD16, and NCAM1/CD56). Missing data on race/ethnicity (n = 1) and BMI (n = 4) reduced the final number of analyzed cases to n = 53. Transcript abundance values were standardized to mean = 0 and SD = 1 within gene to facilitate maximum likelihood estimation, and statistical models were estimated using SAS PROC MIXED with a heterogeneous compound symmetry covariance structure specified to accommodate correlation and heteroscedasticity across residuals from the 49 analyzed gene transcripts (22).
As shown in Table S2, analysis of gold standard PBMC RNA identified statistically significant (P < 0.05) differences in CTRA expression as a function of both race/ethnicity and leukocyte subset distribution. Within the omnibus effect of race/ethnicity, PBMC RNA analyses indicated statistically significant differences for each of the three parameters assessing divergence of non-Caucasian groups from the Caucasian reference category.
Table S2.
Estimated CTRA association with participant characteristics
| Demographic/characteristic | PBMC | DBS | Saliva |
| Age, y | −0.003 (0.005), P = 0.5747 | 0.002 (0.002), P = 0.3337 | −0.002 (0.001), P = 0.0613 |
| Sex (male) | 0.345 (0.275), P = 0.2175 | 0.117 (0.091), P = 0.2088 | 0.025 (0.058), P = 0.6614 |
| Race/ethnicity | F(3, 39) = 30.92, P = 0.0001 | F(3, 39) = 5.81, P = 0.0022 | F(3, 39) = 3.24, P = 0.0323 |
| African American | −1.266 (0.288), P = 0.0001 | −0.354 (0.108), P = 0.0021 | −0.046 (0.063), P = 0.4681 |
| Asian | −2.033 (0.265), P = 0.0001 | −0.257 (0.087), P = 0.0051 | −0.168 (0.055), P = 0.0044 |
| Hispanic | 1.127 (0.391), P = 0.0063 | 0.002 (0.133), P = 0.9893 | −0.012 (0.083), P = 0.8863 |
| Body mass Index | 0.027 (0.023), P = 0.2459 | 0.001 (0.007), P = 0.9204 | −0.004 (0.004), P = 0.3407 |
| Leukocyte subsets | F(7, 39) = 14.59, P = 0.0001 | F(7, 39) = 2.28, P = 0.0478 | F(7, 39) = 1.32, P = 0.2673 |
Entries represent parameter estimates (SE) and P values (or omnibus F ratios and P values for multiparameter effects) from mixed-effect linear models relating average expression of 49 CTRA indicator genes to listed demographic and biological characteristics. Effects for specific race/ethnic categories represent differences relative to the Caucasian reference group; leukocyte subsets are estimated by abundance of mRNA for CD3D, CD4, CD8A, CD19, CD16/FCGR3A, CD56/NCAM1, and CD14. Data come from n = 53 individuals with complete data on all listed variables.
Parallel analyses of DBS RNA also detected significant CTRA differences as a function of race/ethnicity and leukocyte subset distributions. Within the omnibus race/ethnicity effect, two of the three group difference parameters identified as significant by PBMC RNA analyses also reached statistical significance in DBS RNA (i.e., one false-negative finding among the total five significant PBMC results in Table S2). None of the parameters estimated to be nonsignificant in the PBMC analyses were indicated to be significant in DBSs (i.e., no false-positive findings).
By comparison, parallel analyses of salivary RNA failed to detect any differential CTRA expression as a function of leukocyte subset distributions and detected fewer effects of race/ethnicity than did DBSs (i.e., only one of the three group difference parameters reached significance, yielding three false-negative findings among the total of five significant PBMC findings). Again, none of the variables estimated to be nonsignificant in PBMC analyses were declared significant by salivary RNA analyses (i.e., no false-positive effects). This overall reduction in sensitivity to detect PBMC-observed effects is consistent with measurement theory analyses showing that the reduced reliability/increased signal-to-noise ratio for salivary RNA would increase the risk of false-negative/type II errors (failure to detect results that are actually present) but would not induce false-positive/type I errors (spurious detection of results that are not actually present) (78).
As an alternative test of effect detection sensitivity, we compared the three RNA sampling modalities in terms of their ability to detect differential CTRA gene expression in each of the four race/ethnicity groups relative to the sample-wide average. As shown in Table S3, analyses of PBMC RNA indicated statistically significant differences from the sample-wide average for each of the four groups analyzed. DBS RNA yielded significant differences for three of the four groups analyzed (i.e., closely approaching PBMC findings). Parallel analyses of salivary RNA detected a significant deviation from the sample-wide mean for only one of the four groups. This pattern of results verifies that (i) analysis of DBS RNA yields results that closely approximate the PBMC RNA gold standard and (ii) any quantitative increase in signal-to-noise ratio that stems from partially degraded RNA would result in a conservative failure to detect significant effects (but not an increased risk of false-positive significant findings), such as seen in salivary RNA samples.
Table S3.
Estimated CTRA deviations from sample mean
| Demographic | PBMC | DBS | Saliva |
| African American | −0.716 (0.289), P = 0.0175 | −0.243 (0.095), P = 0.0144 | −0.005 (0.057), P = 0.9327 |
| Asian | −1.483 (0.248), P = 0.0001 | −0.146 (0.071), P = 0.0474 | −0.126 (0.045), P = 0.0083 |
| Hispanic | 1.677 (0.383), P = 0.0001 | 0.113 (0.122), P = 0.3617 | 0.029 (0.077), P = 0.7044 |
| Caucasian | 0.550 (0.180), P = 0.0040 | 0.111 (0.044), P = 0.0168 | 0.041 (0.027), P = 0.1409 |
Entries represent mixed-effect linear model parameter estimates (SE) and P values for deviation of each racial/ethnic group from sample-wide average expression of 49 CTRA indicator genes. Data come from analysis of n = 53 individuals with complete data on all listed variables in Table S2.
Conclusion.
The results of this calibration analysis show that RNA derived from stored DBS samples provides a reliable basis for assessing leukocyte CTRA gene expression profiles. This finding is consistent with data from another recent study that also compared DBS with venipuncture blood samples and found a strong correlation (r = 0.85) for genome-wide transcript abundance estimates, as well as good correspondence of downstream bioinformatics results (51). These studies confirm earlier indications that microarray-based transcriptome profiling of DBS RNA yields general transcript abundance estimates that correlated reasonably well with gold standard venipuncture blood samples (77, 79). The present analyses also verified the utility of DBSs for assessing the specific subset of CTRA indicator genes. This high correspondence at the level of individual transcript quantification allowed for efficient detection of substantive demographic and hematological influences on the overall CTRA profile that were verified by gold standard PBMC assays, but were less consistently detectable in parallel analyses of salivary RNA. The relative insensitivity of CTRA assessment from salivary RNA likely stems from its partial degradation (75). The consequent decline in signal-to-noise ratios for transcript abundance estimates would be expected to reduce statistical sensitivity to detect differences that are truly present (78) (e.g., the race/ethnicity and leukocyte subset-related differences observed in PBMCs, which were reasonably well approximated in DBS RNA but were less consistently observed in salivary RNA). However, the relatively strong correspondence between DBS- and PBMC-derived estimates of individual transcript abundance, the relatively high internal consistency of CTRA composite scores derived from DBS RNA, and the relatively efficient detection of demographic and hematological influences on CTRA gene expression in DBSs all imply that DBS RNA samples retain sufficient integrity to permit reliable assays using high-efficiency whole transcriptome amplification systems such as the NuGEN PicoSL system. If DBS samples had been seriously degraded, they would have yielded weaker correlation with PBMC results, poorer internal consistency of CTRA composite scores, and failure to detect significant CTRA differences as a function of race/ethnicity and leukocyte subset prevalence (i.e., as seen in salivary RNA samples).
The emergence of reliable findings from DBS RNA samples after ≤1 wk of storage at room temperature suggests that field collection and delayed laboratory processing can provide valid results when care is taken to protect DBS samples from climatic and physicochemical influences (e.g., using sealed-bag storage with desiccant). Modestly increased signal-to-noise ratios were observed for DBS RNA measures, but these effects were quantitatively small and did not markedly detract from quantitative reliability or substantive analytic yield (e.g., for the CTRA gene set). Moreover, to the extent that RNA degradation did occur, the present empirical findings for salivary RNA verify theoretical analyses from measurement theory (78) in showing that such effects would induce conservative false-negative errors (i.e., type II errors, or failure to detect true effects that might otherwise have been observed using another sampling method) but would not induce false-positive errors (i.e., type I errors involving spurious detection of a result that would not be found using a more reliable assay). These results imply that significant differences in CTRA gene expression detected using DBS samples in the Nepal study are not likely to reflect spurious false-positive findings, and more likely underestimate the true magnitude of differences that would have been observed using maximally reliable PBMC analyses.
The technical conclusions of this study are limited in scope. DBS samples might show greater RNA degradation over longer storage durations and/or less well-protected storage conditions. It is unclear whether similar results would be observed using alternative RNA amplification and transcriptome assay strategies or other specific gene sets besides the CTRA. Nevertheless, when conducted using careful collection, storage, and assay conditions, DBS RNA sampling can provide valid assessment of at least some aspects of leukocyte genomic function in field study settings where venipuncture blood sampling and immediate laboratory processing (e.g., PBMC isolation) are infeasible.
Internally Consistent CTRA Indicator Set
In the course of DBS calibration study analyses of CTRA gene set reliability, Cronbach’s α internal consistency analyses of gold-standard PBMC data identified 11 transcripts in the theoretically derived CTRA indicator set that showed empirically poor correlation with overall CTRA composite scores (i.e., negative item-total correlations for both raw and standardized composites, including proinflammatory transcripts IL6, JUNB, JUND, PTGS1, REL, RELA, RELB, and TNF and antiviral transcripts IFIT1L, IFITM5, and IFNB1). When these transcripts were removed to yield a statistically purified CTRA indicator gene set, results showed enhanced reliability of PBMC-, DBS-, and saliva-based CTRA assessment (Table S4). Like the original theoretically derived CTRA indicator set, one-number summary scores based on the purified CTRA gene set showed good correlation between DBSs and PBMCs (r = 0.61, P < 0.0001), and mixed-effect linear model analyses again identified differential CTRA expression as a function of both race/ethnicity (omnibus P < 0.0001 for PBMCs and P = 0.0010 for DBSs) and leukocyte subset indicators (omnibus P < 0.0001 for PBMCs and P = 0.0202 for DBSs). Purified CTRA analysis of Nepal study DBS samples also yielded results similar to those for the original theoretically derived CTRA composite (Table S5). As such, conclusions from the Nepal study are robust to minor variations in CTRA gene set definition.
Table S4.
Purified CTRA composite score reliability
| Expression data | PBMC | DBS | Saliva |
| Raw (log2) | 0.912 | 0.789 | 0.010 |
| Standardized | 0.920 | 0.800 | 0.477 |
Entries are Cronbach’s α coefficients computed on raw or standardized RNA expression data for 38 purified CTRA indicator transcripts (with signs inverted for inverse components of the CTRA) assayed in parallel RNA samples from peripheral blood mononuclear cells (PBMCs), dried blood spots (DBSs), and saliva. n = 58 individuals. The purified composites involved deletion of 11 transcripts that showed negative item-total correlations with the overall CTRA composite score average, leaving a total of 38 empirically purified indicator transcripts. Compare results with Table S1 for the original theoretically derived (unpurified) composites.
Table S5.
Purified CTRA gene set expression as a function of child soldier status, PTSD, and resilience
| Model | b (SE)* | P value |
| Model 1 (demographic and hematologic control variables only) | ||
| Age, y | −0.054 (0.024) | 0.0270 |
| Sex (female) | −1.225 (0.088) | <0.0001 |
| Ethnic minority | 0.088 (0.105) | 0.4037 |
| Low social caste | 0.187 (0.127) | 0.1438 |
| Education level (range 0–6) | 0.201 (0.030) | <0.0001 |
| CD3D | 2.249 (0.268) | <0.0001 |
| CD3E | −5.087 (0.526) | <0.0001 |
| CD4 | −3.748 (0.346) | <0.0001 |
| CD8A | 1.167 (0.206) | <0.0001 |
| CD19 | 1.740 (0.529) | 0.0012 |
| NCAM1 | −1.609 (1.001) | 0.1093 |
| FCGR3A | 0.870 (0.135) | <0.0001 |
| CD14 | −0.953 (0.115) | <0.0001 |
| Model 2 (model 1 + child soldier status) | ||
| Child soldier | 0.178 (0.084) | 0.0349 |
| Model 3 (model 2 + PTSD) | ||
| Child soldier | 0.139 (0.085) | 0.1039 |
| PTSD | 0.423 (0.137) | 0.0022 |
| Model 4 (model 3 + resilience) | ||
| Child soldier | 0.120 (0.085) | 0.1601 |
| PTSD | 0.411 (0.137) | 0.0029 |
| Resilience (range 2–24) | −0.025 (0.011) | 0.0296 |
Partial regression coefficient from mixed-effect linear model relating average expression of 41 empirically purified CTRA indicator genes to listed variables. n = 254.
Acknowledgments
We thank Suraj Koirala, Mark Jordans, and TPO Nepal for assistance in study implementation; and the University of California, Los Angeles Neuroscience Genomics Core Laboratory and Paula Kincheloe (Emory Laboratory for Comparative Human Biology) for assistance in study procedures. This research was supported by the HopeLab Foundation and US National Institutes of Health Grants P30 AG017265 and F31 MH075584.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE77164).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601301113/-/DCSupplemental.
References
- 1.Felitti VJ, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Van Niel C, Pachter LM, Wade R, Jr, Felitti VJ, Stein MT. Adverse events in children: Predictors of adult physical and mental conditions. J Dev Behav Pediatr. 2014;35(8):549–551. doi: 10.1097/DBP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 3.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry. June 4, 2015 doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole SW. Human social genomics. PLoS Genet. 2014;10(8):e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole SW, et al. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci USA. 2015;112(49):15142–15147. doi: 10.1073/pnas.1514249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell ND, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic; Burlington, MA: 2010. [Google Scholar]
- 9.Heidt T, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capitanio JP, Cole SW. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philos Trans R Soc Lond B Biol Sci. 2015;370(1669):20140104. doi: 10.1098/rstb.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SW, et al. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci USA. 2012;109(50):20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Dev Psychol. 2015;51(11):1630–1644. doi: 10.1037/dev0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine ME, Cole SW, Weir DR, Crimmins EM. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med. 2015;130:16–22. doi: 10.1016/j.socscimed.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci USA. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behav Brain Sci. 2010;33(2-3):61–83, discussion 83–135. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- 18.Steel Z, et al. Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: A systematic review and meta-analysis. JAMA. 2009;302(5):537–549. doi: 10.1001/jama.2009.1132. [DOI] [PubMed] [Google Scholar]
- 19.Bornemisza O, Ranson MK, Poletti TM, Sondorp E. Promoting health equity in conflict-affected fragile states. Soc Sci Med. 2010;70(1):80–88. doi: 10.1016/j.socscimed.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Betancourt TS, et al. Psychosocial adjustment and mental health in former child soldiers: Systematic review of the literature and recommendations for future research. J Child Psychol Psychiatry. 2013;54(1):17–36. doi: 10.1111/j.1469-7610.2012.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredrickson BL, et al. Psychological well-being and the human conserved transcriptional response to adversity. PLoS One. 2015;10(3):e0121839. doi: 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fredrickson BL, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110(33):13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SW, et al. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology. 2015;62:11–17. doi: 10.1016/j.psyneuen.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewer MB, Chen Y-R. Where (who) are collectives in collectivism? Toward conceptual clarification of individualism and collectivism. Psychol Rev. 2007;114(1):133–151. doi: 10.1037/0033-295X.114.1.133. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SE, Welch WT, Kim HS, Sherman DK. Cultural differences in the impact of social support on psychological and biological stress responses. Psychol Sci. 2007;18(9):831–837. doi: 10.1111/j.1467-9280.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- 27.Tol WA, Jordans MJ, Kohrt BA, Betancourt TS, Komproe IH. 2013. Promoting mental health and psychosocial well-being in children affected by political violence: Part II—Expanding the evidence base. Handbook of Resilience in Children of War eds Fernando C, Ferrari M (Springer, New York), pp 29–38.
- 28.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat Commun. 2012;3:621. doi: 10.1038/ncomms1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthman CM. Inside-out and outside-in? Global development theory, policy, and youth. Ethos. 2011;39(4):432–451. [Google Scholar]
- 31.Kohrt BA, Jordans MJD, Koirala S, Worthman CM. Designing mental health interventions informed by child development and human biology theory: A social ecology intervention for child soldiers in Nepal. Am J Hum Biol. 2015;27(1):27–40. doi: 10.1002/ajhb.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohrt BA, Burkey M, Stuart EA, Koirala S. Alternative approaches for studying humanitarian interventions: Propensity score methods to evaluate reintegration packages impact on depression, PTSD, and function impairment among child soldiers in Nepal. Glob Ment Health. 2015;2:e16. doi: 10.1017/gmh.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foa EB, Johnson KM, Feeny NC, Treadwell KR. The child PTSD Symptom Scale: A preliminary examination of its psychometric properties. J Clin Child Psychol. 2001;30(3):376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- 34.Kohrt BA, et al. Validation of cross-cultural child mental health and psychosocial research instruments: Adapting the Depression Self-Rating Scale and Child PTSD Symptom Scale in Nepal. BMC Psychiatry. 2011;11(1):127. doi: 10.1186/1471-244X-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. J Nurs Meas. 1993;1(2):165–178. [PubMed] [Google Scholar]
- 36.Tol WA, Jordans MJD, Regmi S, Sharma B. Cultural challenges to psychosocial counselling in Nepal. Transcult Psychiatry. 2005;42(2):317–333. doi: 10.1177/1363461505052670. [DOI] [PubMed] [Google Scholar]
- 37.Kohrt BA, Maharjan SM. When a child is no longer a child: Nepali ethnopsychology of child development and violence. Stud Nepali Hist Soc. 2009;14(1):107–142. [PMC free article] [PubMed] [Google Scholar]
- 38.Kohrt BA, Maharjan SM, Timsina D, Griffith JL. Applying Nepali ethnopsychology to psychotherapy for the treatment of mental illness and prevention of suicide among Bhutanese refugees. Ann Anthropol Pract. 2012;36(1):88–112. [Google Scholar]
- 39.Zahran S, et al. Stress and telomere shortening among central Indian conservation refugees. Proc Natl Acad Sci USA. 2015;112(9):E928–E936. doi: 10.1073/pnas.1411902112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breslau J. Cultures of trauma: Anthropological views of posttraumatic stress disorder in international health. Cult Med Phsychiatry. 2004;28(2):113–126; discussion 211–120. doi: 10.1023/b:medi.0000034421.07612.c8. [DOI] [PubMed] [Google Scholar]
- 41.Kohrt BA, et al. Comparison of mental health between former child soldiers and children never conscripted by armed groups in Nepal. JAMA. 2008;300(6):691–702. doi: 10.1001/jama.300.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohrt BA, et al. Social ecology of child soldiers: Child, family, and community determinants of mental health, psychosocial well-being, and reintegration in Nepal. Transcult Psychiatry. 2010;47(5):727–753. doi: 10.1177/1363461510381290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohrt BA, Tol WA, Pettigrew J, Karki R. Children and revolution: The mental health and psychosocial wellbeing of child soldiers in Nepal’s Maoist army. In: Singer M, Hodge GD, editors. The War Machine and Global Health. Rowan & Littlefield; Lanham, MD: 2010. pp. 89–116. [Google Scholar]
- 44.Kohrt BA. The role of traditional rituals for reintegration and psychosocial wellbeing of child soldiers in Nepal. In: Hinton AL, Hinton DE, editors. Genocide and Mass Violence: Memory, Symptom, and Recovery. Cambridge Univ Press; Boston: 2015. pp. 369–387. [Google Scholar]
- 45.Fontana A, Rosenheck R. Psychological benefits and liabilities of traumatic exposure in the war zone. J Trauma Stress. 1998;11(3):485–503. doi: 10.1023/A:1024452612412. [DOI] [PubMed] [Google Scholar]
- 46.Theron LC, Liebenberg L, Ungar M, editors. Youth Resilience and Culture: Commonalities and Complexities. Springer; New York: 2014. [Google Scholar]
- 47.Punamaki R-L, Qouta S, El-Sarraj E. Resiliency factors predicting psychological adjustment after political violence among Palestinian children. Int J Behav Dev. 2001;25(3):256–267. [Google Scholar]
- 48.Allen MW, Jones TL. Violence and Warfare Among Hunter-Gatherers. Left Coast Press; Walnut Creek, CA: 2014. [Google Scholar]
- 49.Konner M. Trauma, adaptation, and resilience: A cross-cultural and evolutionary perspective. In: Lemelson R, Kirmayer LJ, Barad M, editors. Understanding Trauma: Integrating Biological, Clinical, and Cultural Perspectives. Cambridge Univ Press; Cambridge, UK: 2007. pp. 300–338. [Google Scholar]
- 50.Patel A. Mental health in Jumla, Nepal: A qualitative study examining the effects of war on mental health. Master’s thesis. Emory University; Atlanta: 2012. [Google Scholar]
- 51.McDade TW, et al. Genome-wide profiling of RNA from dried blood spots: Convergence with bioinformatic results derived from whole venous blood and peripheral blood mononuclear cells. Biodemogr Soc Biol. 2016;62(2):182–197. doi: 10.1080/19485565.2016.1185600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tol WA, et al. Mental health and psychosocial support in humanitarian settings: Linking practice and research. Lancet. 2011;378(9802):1581–1591. doi: 10.1016/S0140-6736(11)61094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tol WA, Jordans MJD, Kohrt BA, Betancourt TS, Komproe IH. 2013. Promoting mental health and psychosocial well-being in children affected by political violence: Part I—Current evidence for an ecological resilience approach. Handbook of Resilience in Children of War eds Fernando C, Ferrari M (Springer, New York), pp 11–27.
- 54.Kohrt B. Social ecology interventions for post-traumatic stress disorder: What can we learn from child soldiers? Br J Psychiatry. 2013;203(3):165–167. doi: 10.1192/bjp.bp.112.124958. [DOI] [PubMed] [Google Scholar]
- 55.Karki R, Kohrt BA, Jordans MJD. Child led indicators: Pilot testing a child participation tool for psychosocial support programmes for former child soldiers in Nepal. Intervention. 2009;7(2):92–109. [Google Scholar]
- 56.United Nations Development Programme . Human Development Report 2007/2008. Pallgrave Macmillian; New York: 2007. [Google Scholar]
- 57.United Nations 2008 District Health Profiles of Nepal: Nepal Public Health Association Compilation. Available at un.org.np/data-coll/. Accessed June 27, 2016.
- 58.Tol WA, et al. Political violence and mental health: A multi-disciplinary review of the literature on Nepal. Soc Sci Med. 2010;70(1):35–44. doi: 10.1016/j.socscimed.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Human Rights Watch . Children in the Ranks: The Maoists’ Use of Child Soldiers in Nepal. Human Rights Watch; Kathmandu, Nepal: 2007. [Google Scholar]
- 60.United Nations . Report of the Secretary-General on Children and Armed Conflict in Nepal. United Nations Security Council; New York: 2006. [Google Scholar]
- 61.Morley CA, Kohrt BA. Impact of peer support on PTSD, hope, and functional impairment: A mixed-methods study of child soldiers in Nepal. J Aggress Maltreat Trauma. 2013;22(7):714–734. [Google Scholar]
- 62.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 63.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. John Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 64.Vedhara K, et al. Personality and gene expression: Do individual differences exist in the leukocyte transcriptome? Psychoneuroendocrinology. 2015;52:72–82. doi: 10.1016/j.psyneuen.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. UNICEF (2007) Paris Principles: Principles and Guidelines on Children Associated with Children Associated with Armed Forces and Armed Groups (UNICEF, New York). Available at www.unicef.org/emerg/files/ParisPrinciples310107English.pdf. Accessed June 1, 2008.
- 66.Adhikari RP, et al. Protective and risk factors of psychosocial wellbeing related to the reintegration of former child soldiers in Nepal. Intervention (Amstelveen) 2014;12(3):367–378. [Google Scholar]
- 67.Upadhaya N, et al. The role of mental health and psychosocial support nongovernmental organizations: Reflections from post-conflict Nepal. Intervention. 2014;12(Suppl 1):113–128. [Google Scholar]
- 68.Kohrt BA, Jordans MJD, Morley CA. Four principles of mental health research and psychosocial intervention for child soldiers: Lessons learned in Nepal. Int Psychiatry. 2010;7(3):58–60. [PMC free article] [PubMed] [Google Scholar]
- 69.Kohrt BA, et al. Culture in psychiatric epidemiology: Using ethnography and multiple mediator models to assess the relationship of caste with depression and anxiety in Nepal. Ann Hum Biol. 2009;36(3):261–280. doi: 10.1080/03014460902839194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohrt BA. Vulnerable social groups in post-conflict settings: A mixed-methods policy analysis and epidemiology study of caste and psychological morbidity in Nepal. Intervention. 2009;7(3):239–264. [Google Scholar]
- 71.Luitel NP, et al. Conflict and mental health: A cross-sectional epidemiological study in Nepal. Soc Psychiatry Psychiatr Epidemiol. 2013;48(2):183–193. doi: 10.1007/s00127-012-0539-0. [DOI] [PubMed] [Google Scholar]
- 72.Kohrt BA, Worthman CM. Gender and anxiety in Nepal: The role of social support, stressful life events, and structural violence. CNS Neurosci Ther. 2009;15(3):237–248. doi: 10.1111/j.1755-5949.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei C, et al. Comparison of frozen and unfrozen blood spots for gene expression studies. J Pediatr. 2014;164(1):189–191.e1. doi: 10.1016/j.jpeds.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haak PT, et al. Archived unfrozen neonatal blood spots are amenable to quantitative gene expression analysis. Neonatology. 2009;95(3):210–216. doi: 10.1159/000155652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergen AW, et al. Chronic psychosocial stressors and salivary biomarkers in emerging adults. Psychoneuroendocrinology. 2012;37(8):1158–1170. doi: 10.1016/j.psyneuen.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khoo SK, et al. Acquiring genome-wide gene expression profiles in Guthrie card blood spots using microarrays. Pathol Int. 2011;61(1):1–6. doi: 10.1111/j.1440-1827.2010.02611.x. [DOI] [PubMed] [Google Scholar]
- 77.Slaughter J, et al. High correlations in gene expression between paired umbilical cord blood and neonatal blood of healthy newborns on Guthrie cards. J Matern Fetal Neonatal Med. 2013;26(18):1765–1767. doi: 10.3109/14767058.2013.804050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanyongo GY, Brook GP, Kyei-Blankson L, Gocmen G. Reliability and statistical power: How measurement fallibility affects power and required sample sizes for several parametric and nonparametric statistics. J Mod Appl Stat Methods. 2007;6(1):9. [Google Scholar]
- 79.Maeno Y, et al. Utility of the dried blood on filter paper as a source of cytokine mRNA for the analysis of immunoreactions in Plasmodium yoelii infection. Acta Trop. 2003;87(2):295–300. doi: 10.1016/s0001-706x(03)00096-2. [DOI] [PubMed] [Google Scholar]