Significance
We describe a means of obtaining spectral information using the principles of physical optics and an off-axis pupil shape without requiring spectrally distinct photoreceptor classes. The mechanism described here offers a possible solution to a long-standing puzzle in marine animals: cephalopods dramatically change color for both producing chromatically matched camouflage and signaling to conspecifics, despite having a single photoreceptor channel. The ability of these animals to achieve such excellent color matching to their surroundings, despite being “color blind” in the traditional sense, can be understood if they exploit chromatic aberration to deduce spectral information. The bizarre off-axis pupils of these animals can be understood as an adaptation that maximizes spectral information, even at the expense of image acuity.
Keywords: spectral discrimination, chromatic aberration, color vision, pupil shape, cephalopod
Abstract
We present a mechanism by which organisms with only a single photoreceptor, which have a monochromatic view of the world, can achieve color discrimination. An off-axis pupil and the principle of chromatic aberration (where different wavelengths come to focus at different distances behind a lens) can combine to provide “color-blind” animals with a way to distinguish colors. As a specific example, we constructed a computer model of the visual system of cephalopods (octopus, squid, and cuttlefish) that have a single unfiltered photoreceptor type. We compute a quantitative image quality budget for this visual system and show how chromatic blurring dominates the visual acuity in these animals in shallow water. We quantitatively show, through numerical simulations, how chromatic aberration can be exploited to obtain spectral information, especially through nonaxial pupils that are characteristic of coleoid cephalopods. We have also assessed the inherent ambiguity between range and color that is a consequence of the chromatic variation of best focus with wavelength. This proposed mechanism is consistent with the extensive suite of visual/behavioral and physiological data that has been obtained from cephalopod studies and offers a possible solution to the apparent paradox of vivid chromatic behaviors in color blind animals. Moreover, this proposed mechanism has potential applicability in organisms with limited photoreceptor complements, such as spiders and dolphins.
We show in this paper that, under certain conditions, organisms can determine the spectral composition of objects, even with a single photoreceptor type. Through computational modeling, we show a mechanism that provides spectral information using an important relationship: the position of sharpest focus depends on the spectral peak of detected photons. Mapping out contrast vs. focal setting (accommodation) amounts to obtaining a coarse spectrum of objects in the field of view, much as a digital camera attains best focus by maximizing contrast vs. focal length. We note that a similar phenomenon has been advanced as a possible explanation for color percepts in red/green color-blind primates (1); however, primates have not evolved the off-axis pupil shape found in nearly all shallow water cephalopods that enhances this effect.
The only other known mechanism of color discrimination in organisms involves determining the spectrum of electromagnetic radiation using differential comparisons between simultaneous neural signals arising from photoreceptor channels with differing spectral acceptances. Color vision using multiple classes of photoreceptors on a 2D retinal surface comes at a cost: reduced signal to noise ratio in low-light conditions and degraded angular resolution in each spectral channel. Thus, many lineages that are or were active in low-light conditions have lost spectral channels to increase sensitivity (2).
Octopus, squid, and cuttlefish (coleoid cephalopods) have long been known to be among the most colorfully active organisms, vividly changing color to signal conspecifics and camouflage. In 350 BCE, Aristotle (3) remarked that the octopus “seeks its prey by so changing its color as to render it like the color of the stones adjacent to it; it does so also when alarmed.”
Cephalopods use their control of skin coloration to become (i) inconspicuous by camouflaging against local backgrounds (Fig. 1, Fig. S1, and Movie S1) or (ii) highly conspicuous during colorful mating and threat displays (Fig. 1, Fig. S2, and Movie S2). Despite this chromatically active behavior, genetic and physiological studies (4–7) show that (with one exception) cephalopods lack multiple photoreceptor types. Cephalopods also fail certain behavioral trials (7–11) designed to test for color vision by opponent spectral channels.
Fig. 1.
Cephalopod behavior and pupil shapes. Figs. S1 and S2 and Movies S1 and S2 show additional examples. Many shallow water cephalopods produce colorful displays [(A) Australian giant cuttlefish Sepia apama] to conspecifics and accurately color-match natural environments to camouflage [(B and C) broadclub cuttlefish Sepia latimanus]. Their pupil shapes [(D) S. bandensis] maximize chromatic blur. Images courtesy of (A) Klaus Stiefel, (B) Flickr/Lakshmi Sawitri, (C) Ken Marks, and (D) Roy Caldwell.
Fig. S1.
Color matching and pupil shape in cephalopods. Fig. 1 and Movie S1 show more examples. (A–D) The coral reef broadclub cuttlefish Sepia latimanus lives in one of the most chromatically complex ocean environments and accurately color-matches natural environments to camouflage. More examples of color matching in cephalopods are found in Movie S1. (E) The pupil in shallow water squids (such as this Sepioteuthis) maximizes off-axis light when imaging in the horizontal plane. (F) Shallow water octopus species, such as this Octopus vulgaris, maximize off-axis light when imaging the bottom. Images courtesy of (A) Ken Marks, (B–D) Flickr/Lakshmi Sawitri, (E) Klaus Stiefel, and (F) Alexander Stubbs.
Fig. S2.
Examples of highly colorful signaling in cephalopods. Fig. 1 and Movie S2 show more examples. (A–C) The big-fin reef squid Sepioteuthis lessoniana vividly changes color while signaling to members of its own species. (D) The Australian giant cuttlefish Sepia apama also uses a colorful display, and the fine network of black lines allows for an easy determination of chromatically induced defocus and thus, chromatic information. During displays, the cuttlefish pupil is typically maximally contracted as a semiannulus, suppressing other sources of image degradation while maximizing chromatically induced blurring. Images courtesy of (A and C) © Gary Bell/OceanwideImages.com, (B) Ria Tan/Wildsingapore.com, and (D) Richard Ling.
We are faced with two distinct but related paradoxes: (i) how can these animals with a single photoreceptor achieve good background color matching, and (ii) why would they break camouflage to produce risky colorful mating displays (readily visible to predators with color vision) unless this chromatic information was visible to conspecifics and carried some selective advantage?
Previous attempts to reconcile these apparent paradoxes include suggestions that (i) the animals do not actually match natural background colors (12) or (ii) multiple photoreceptor types could exist (13, 14) in the animal’s skin. Neither of these explanations resolves the puzzle of “color-blind camouflage,” and researchers remain in search of a mechanism that allows for this ability (7, 14–16). We are unaware of a proposal for how natural selection would drive the evolution and maintenance of colorful intraspecific displays in these soft-bodied mollusks if this information was not available to the animals themselves.
Contradictory Evidence: Chromatic Behavior but a Single Opsin
The extent of color matching in cephalopods remains somewhat controversial in some circles, but we assert that shallow water cephalopods often match the coloration of natural backgrounds (Fig. 1, Fig. S1, and Movie S1), and we encourage readers to examine Movie S1, which shows cephalopod camouflage in their natural habitat, and reach their own conclusions. Some had claimed (12) that these organisms simply match the brightness and spatial scale of patterns in their environment, tricking the human visual system without actually requiring a color match. Numerous studies (14, 17–21) show, however, that cuttlefish and octopus actively vary their spectral reflectance in response to background color rather than simply modulating their luminance.
Kühn (21) conducted a series of behavioral experiments comparing the octopus and cuttlefish camouflage responses when placed on a series of greyscale and colored substrates. His data show statistically significant evidence that these organisms expand their long wavelength-reflecting chromatophores when on spatially variable red or yellow backgrounds but that they primarily expand black chromatophores when on corresponding greyscale backgrounds (21). Kühn (21) concluded that these organisms must have the ability to discriminate spectral content.
Contemporary laboratory and field observations (17–20) show that octopus and cuttlefish produce high-fidelity color matches to natural backgrounds (Fig. 1). The most definitive recent evidence for color matching in a laboratory setting used (14) a hyperspectral imager in conjunction with spectral angle mapping to show that cuttlefish varied their spectral reflectance (chromatic properties) to maintain excellent spectral matches to a diversity of natural backgrounds and interestingly, maintained poorer matches in brightness (luminance). These studies (14, 17–20) corroborate the earlier result by Kühn (21): cephalopods vary their spectral reflectance by active control over their chromatophores in response to natural backgrounds rather than simply varying their luminance.
Some have suggested that cephalopod skin might contribute to spectral discrimination through either undiscovered additional opsins (14) or filtering the single known opsin response. The recently published octopus genome (22) did not identify any additional opsins using both whole-genome sequencing and transcriptome sequencing of skin tissue, despite a focus on identifying G protein-coupled receptors. Across a diversity of taxa, all cephalopod studies to date have found rhodopsin transcripts in the skin identical to those in the eye (23), and the skin’s spectral response to light is nearly identical to that of the retina (24). Given multiple strong lines of evidence against additional undiscovered skin opsins and no described mechanism for spectral discrimination arising from rhodopsin alone, this competing hypothesis is not currently viable. Additionally, absent a focusing element, detectors on the skin act as wide-angle nonimaging light sensors and cannot provide useful information regarding background coloration or signals produced by conspecifics.
Chromatic Blurring and the Importance of Pupil Shape
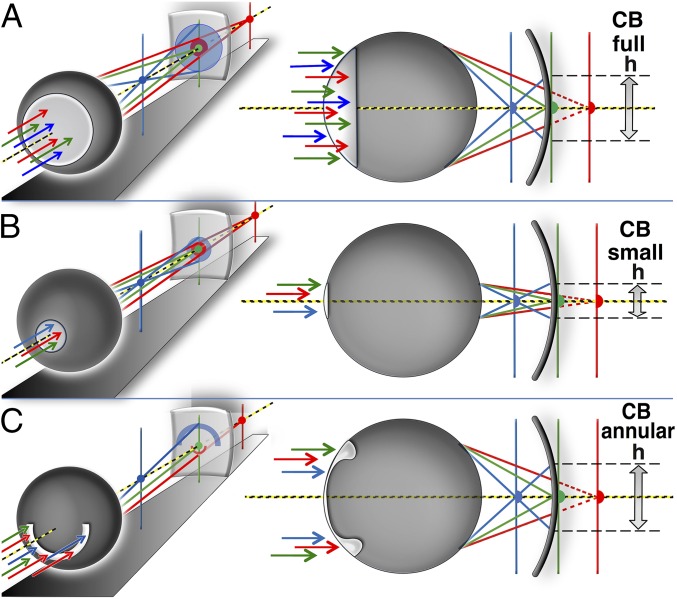
Fig. 2 shows the mechanism that we are proposing for how chromatic aberration can be exploited to achieve spectral sensitivity. As we show below, the off-axis pupils of cephalopods combine with the wavelength dependence of the lens index of refraction to generate chromatic blur; different wavelengths come into focus at different distances from the lens. The spectral content of a structured scene can be deduced by sweeping through focus (i.e., changing the lens to retina distance) and seeing how the image blurring varies. A key element in our argument is the observation that the off-axis pupils common in cephalopods actually maximize the chromatic blurring in their visual system (Table S1). These animals would have better acuity if they had evolved a small, on-axis pupil, such as the one in the eye of the reader. Instead, they seem to have sacrificed overall acuity in favor of chromatic blurring, which we suggest here as a mechanism for spectral discrimination. This mechanism is similar to that in the recent observation (25) that vertical and horizontal pupils produce astigmatic blurring.
Fig. 2.
Chromatic blur and pupil geometry. The (A) full and (C) annular aperture pupils produce more chromatic blurring (CB) than (B) the small on-axis pupil, because they transmit rays with a larger ray height h. Vertical lines show best focus positions for blue, green, and red light.
Table S1.
Cephalopod retinal image quality budget
| Term | FWHM (μm) | h Dependence | λ Dependence |
| Photoreceptor size | 5 | None | None |
| Retinal surface displacement | 1 | ∝h | None |
| Cross-talk between photoreceptors | Neglected (in the text) | ∝h | Unknown |
| Residual spherical aberration | 10 at full aperture | ∝Δh for annular pupil | None |
| Diffraction with d = 1 mm; on-axis pupil | 6 | ∝h−1 | ∝λ |
| Diffraction with d = 8 mm; on-axis pupil | 0.75 | ∝h−1 | ∝λ |
| Other achromatic aberrations | 20 at full aperture | Unknown, likely ∝h | None |
| Chromatic aberration with d = 1 mm; on-axis pupil | 6 | ∝h | None |
| Chromatic aberration with d = 8 mm; on-axis pupil | 48 | ∝h | None |
| Chromatic aberration with semiannular pupil | 61 | ∝h | None |
The columns present the various aberration phenomena, the resulting Gaussian–PSF-equivalent FWHM, and the dependencies on ray height h and wavelength-λ. Chromatic aberration is the dominant contribution to image blurring down to 1-mm pupil diameters. The quadrature sum of the various contributions for the semiannular pupil is 65 μm and dominated by chromatic blurring. These values correspond to an f/1.2 lens with a 12-mm diameter.
SI Experimental Procedures provides a detailed description of the numerical modeling that we performed to assess the quantitative variation of blurring vs. spectral structure and focal spacing. These calculations were based on the measured optical properties of cephalopods, and we show that (despite claims to the contrary in previous works by others) chromatic blurring dominates the image quality for these animals. This chromatic aberration is what affords them the opportunity to exploit this mechanism for achieving color sensitivity.
SI Experimental Procedures
Chromatic Aberration Computation.
Chromatic blurring (Fig. 4) was computed with an MATLAB code adapted from a program initially written by C.W.S. to investigate the out of focus properties of the large synoptic survey telescope. The point spread function (PSF) and encircled energy diagrams (Fig. S4) shown were computed with a related program. Both are available on request.
Fig. 4.
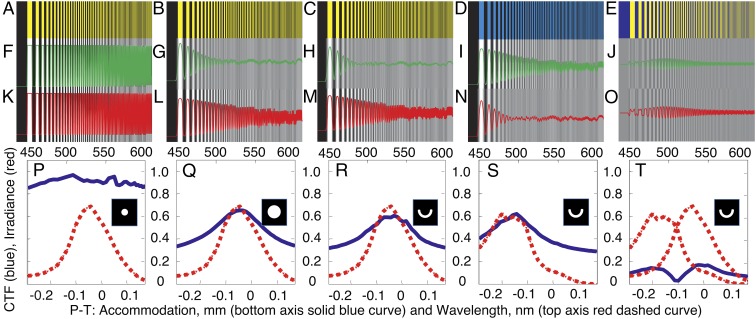
Chromatic blur simulations for semiannular pupil. Test patterns (A–C in black and yellow, D in black and blue, and E in blue and yellow) are used to simulate chromatic blur vs. accommodation. Examples are shown of detected intensity variations and contrast at best focused wavelengths of (F–J) 470 and (K–O) 550 nm. CTF is extracted from line plots of intensity (traces in green in F–J and red in K–O). (P–T) CTF (blue) vs. accommodation (lower x scale) tracks the spectrum of detected photons (red) vs. wavelength (upper x scale), with a spectral resolution that depends on pupil shape. The pupil dependence of spectral resolution (width of blue CTF traces) is shown for (P) small (d = 1 mm) and (Q) full (d = 8 mm) on-axis pupils and (R–T) the semiannular (6 mm < d < 6.66 mm) pupil. (R–T) The CTF peak tracks the spectrum for the semiannular pupil. (T) The flat CTF vs. accommodation obtained from (J and O) the line plots of intensity for (E) the blue to yellow test pattern precludes spectral discrimination for this case.
Fig. S4.
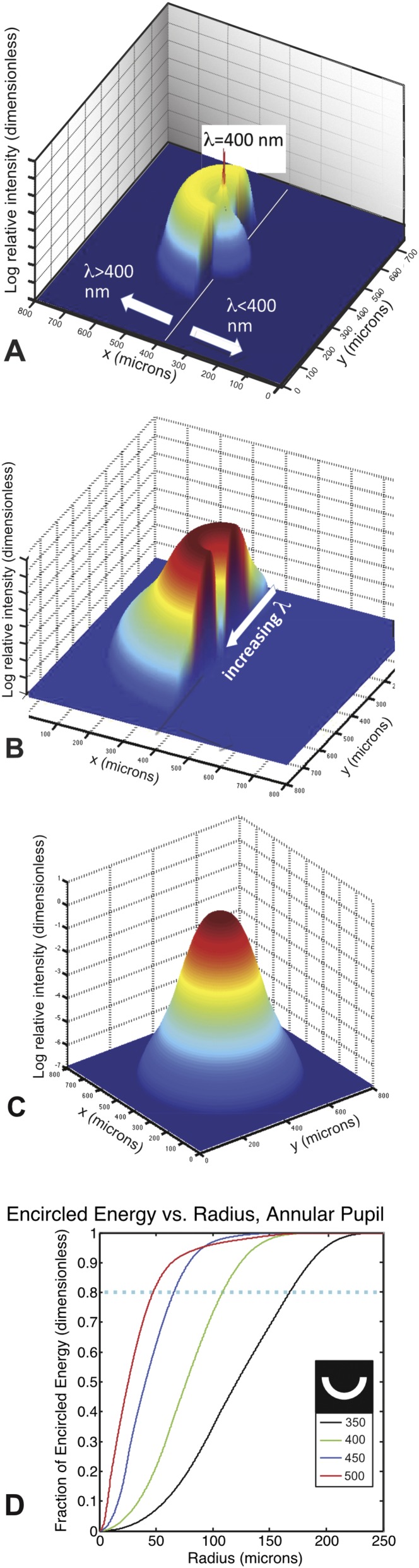
PSF and encircled energy results from chromatic aberration computations. (A) Chromatic PSF for 400-nm best focus, semiannular pupil. This figure shows the computed photon intensity on the retinal surface for a point source at infinity, with a solar spectrum filtered to a 3-m depth, convolved with the opsin sensitivity function. The spatial units are micrometers. This PSF is for the half-annular pupil geometry, so that light with λ > 400 nm has yet to come to a focus, whereas light with λ < 400 nm has already passed through its position of best focus. The spike at the center corresponds to the best focused photons with λ = 400 nm, which have the highest surface brightness. (B) Chromatic PSF for 700-nm best focus, semiannular pupil. This PSF is computed for the same half-annular pupil as in A, but the PSF has been rotated about the optical axis by 180° for clarity. All of the rays have passed through their position of best focus, and therefore, there is a monotonic relationship between wavelength and distance from the axis of symmetry as indicated. The radial plot of photon surface brightness amounts to a dispersed spectrum of the incident light, with the redder light being closest to being in focus. (C) Chromatic PSF for 700-nm best focus, full pupil. As distinct from the semiannular case, the center of the PSF is filled in with polychromatic light that passes through the center of the pupil and suffers minimal chromatic blurring, whereas the outer edges of the PSF are illuminated exclusively by the photons of the shortest wavelength. There is, therefore, a radial gradient in spectral purity and a complex relationship between position and spectral content. (D) Encircled energy vs. radius for various accommodation settings. This result is for the semiannular pupil at different accommodation settings indicated as the best focus wavelength in nanometers. The plot shows the integrated enclosed energy within the PSF for the semiannular pupil geometry. This result was computed for a white reflector illuminated by the depth-attenuated solar photon spectrum for 350 < λ < 650 nm. The red curve yields an 80% encircled energy radius of 47 μm, which corresponds to a Gaussian–PSF-equivalent FWHM of 61 μm at the accommodation setting of sharpest focus, which corresponds to a best focused wavelength of 500 nm.
The detected light intensity I(i,j) in each pixel (i,j) of the simulated retinal image is given by
where Φsolar(λ) is the solar photon irradiance spectrum; the exponential term accounts for the reduction in down-welling photon flux at a water depth D with an attenuation length z(λ); R(x,y,λ) is the spectral reflectance of the portion of the scene at a location x,y; PSF(x,y,i,j,λ) is the wavelength-dependent PSF of the visual system at pixel (i,j) for light arriving from location (x,y) in the scene; and O(λ) is the sensitivity function of the photoreceptor opsin. The limits of integration span the spatial extent of the scene within the field of view and the spectral range of interest [in this case, band-limited by the opsin response O(λ)].
We explored the performance of the cephalopod visual system for different pupil shapes, colored test patterns, and accommodation (lens to retina) distances with a numerical simulation that
-
i)
Computed the spectrum of solar illumination after taking into account the filtering properties of seawater at the chosen depth of 3 m,
-
ii)
Constructed simulated test patterns with reflectance spectra characteristic of the marine environment to provide a standardized framework for quantitative comparison of image sharpness,
-
iii)
Determined the amount of light at each of a set of discrete wavelengths that was out of focus for each choice of pupil, test pattern coloration, and accommodation value,
-
iv)
Computed the fraction of light detected because of the limited spectral response of the single opsin in the retina,
-
v)
Summed up the wavelength by wavelength blurred images to arrive at a final simulated retinal image, and
-
vi)
Computed an image sharpness metric for each situation analyzed.
We then assessed the extent to which variation in image contrast with accommodation can be used to extract information about the spectral characteristics of the scene. Of particular interest was the comparison of spectral information for different pupil shapes to explore the evolutionary advantage of the peculiar off-axis annular pupils that are common in cephalopods.
We describe each of these aspects of the image simulation and analysis in more detail below.
Illumination.
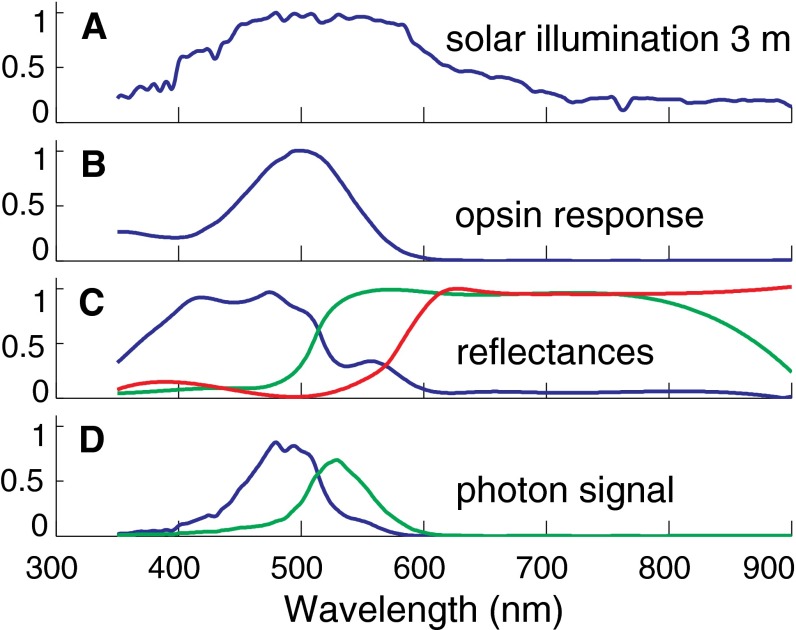
We used a ground-level spectrum of solar irradiance from https://www2.pvlighthouse.com.au/resources/optics/spectrum%20library/spectrum%20library.aspx multiplied by λ to convert to relative photon irradiance. To account for illumination attenuation at a 3-m depth D, we used the Pacific seawater optical attenuation length data from the work by Morel and Maritonrena (51). We used the attenuation lengths appropriate for 0- to 2-m depths, which correspond to 0.043-mg m−3 chlorophyll density. The resulting photon irradiance spectrum is presented in Fig. S3A.
Fig. S3.
Spectra used in simulations showing the wavelength dependence (in nanometers) of multiple quantities of interest. All plotted quantities are dimensionless and normalized. (A) Depth-attenuated solar photon spectrum. (B) The sole opsin’s relative photon sensitivity response function from the work by Chung (6). (C) The three fish reflectance spectra that we used from blue, green, and red Australian reef fish (50). (D) Detected photon flux for the two bluest reflectance spectra that were used for the test patterns shown in Fig. 4.
Reflectance Spectra and Test Image Generation.
Each pixel in the simulated test image was assigned a reflectance spectrum that was a weighted superposition of template reflectance spectra. To simulate the colors encountered in marine settings, we use measured (50) reflectance spectra of blue and yellow tropical fish.
The reflectance spectra are shown in Fig. S3C. We produced a variety of dual-color bar charts (shown in Fig. 4 A–E) with varying spatial frequency to assess the acuity of the resulting images. The spatial scale of these test images was set to 5 μm/pixel, which corresponds to the typical diameter of the photosensitive structures (rhabdomes) that pave the retinal surface of cephalopods. We produced bar chart test patterns with 112- to 2-pixel bright bar widths. At our sampling of 5 μm/pixel, this resolution corresponds to 165- and 10-μm widths, respectively, on the retina.
PSF Computation.
The PSF at each wavelength determines the sharpness of the image formed on the retina. The PSF, in turn, is determined by the combination of the pupil shape, the refractive properties and configuration of the optical components, the spectrum of light reflected by the scene, and the shape and location of the retinal surface. Rays first propagate through the pupil, which determines both the collecting area and the off-axis distances h of the rays that are imaged onto the retinal surface. The cephalopod lens is well-approximated by a sphere with a radial gradient in the index of refraction that produces a remarkably effective correction for spherical aberration (26). The wavelength dependence of the index of refraction does, however, produce chromatic aberration. Different wavelengths have different effective focal lengths. The blur induced by this depends on the angle at which the rays intersect the optical axis, which in turn, scales with the ray’s distance off axis. Pupils that transmit a large proportion of off-axis rays produce more chromatic blurring than pupils that are predominantly on axis.
When chromatically out of focus rays emanating from a point source at infinity of a given wavelength intersect the retina, they produce a PSF that is a scaled image of the pupil (Fig. 2).
Our MATLAB program uses the wavelength dependence of the focal length of the Octopus australis eye as reported by Jagger and Sands (26). Their laboratory measurements show a subpercentage perturbation in focal length caused by residual spherical aberration but a chromatic fractional shift in focal length, δf/f, of 4.1% between 450 and 700 nm (26). A second-order polynomial fit to the data in the work by Jagger and Sands (26) yielded δf/f = −5.4676 × 10−5λ2 + 0.0794λ − 27.1047, with δf/f = 0 at 550 nm.
We modeled a cephalopod lens with a 10-mm diameter d and a focal length of f = 12 mm at 550 nm measured from the center of the lens. The spectral range used in the computer model was restricted to 450 nm < λ < 650 nm to avoid making an extrapolation from the measured chromatic focal changes reported by Jagger and Sands (26). We ran through this spectral range with a step size of Δλ = 5 nm. Substantial amounts of illumination (15% of the photons) can be between 350 and 450 nm, depending on the transmission through the water column. The chromatic focus perturbations are enhanced at short wavelengths; therefore, for our PSF estimates (but not for the image contrast calculations), we do extrapolate the data from Jagger and Sands (26) down to 350 nm using the expression given above in conjunction with the attenuated photon spectrum and the opsin response. We used the opsin photon sensitivity curve as a function of wavelength from Chung (6).
We performed PSF calculations for three different planar pupil masks. One corresponds to the full useful aperture of the lens with an 8-mm pupil diameter. The second mask is an axially centered pupil with a 1-mm diameter, the size at which diffraction and chromatic effects are comparable. The third mask approximates the U-shaped semiannular pupil seen in many free-swimming diurnal cephalopods under bright illumination (shallow water squid and cuttlefish), with a 6-mm i.d., a 6.66-nm o.d., and a polar angle extent of 180°.
Representative PSFs for the semiannular pupil and a point-like white reflector, R(x,y,λ) = δ(x,y), are shown in Fig. S4 A and B. The PSF that we computed when 400-nm light is brought to a sharp focus (Fig. S4A) shows the spike from the point source at 400 nm superimposed on the out of focus pupil images from the other wavelengths from either side of focus. The PSF obtained when the lens is far out of focus (Fig. S4B) is, in effect, a radially dispersed image of the point source. These intensity distributions are not well-represented by Gaussian PSFs, and therefore, we used encircled energy (52) (Fig. S4D) computations to determine a Gaussian-equivalent FWHM for these PSFs.
The PSF produced by a large circular pupil (Fig. S4C) is axisymmetric and breaks the relationship between radial position and wavelength. The light at the center of the PSF contains all wavelengths but only from the rays that pass close to the optical axis.
CTF Analysis.
We simulated this visual system by computing the retinal image that would be produced using various combinations of pupils (and their corresponding PSFs) and test pattern scenes. The focal plane image at each wavelength is a convolution of the appropriate wavelength-dependent PSF with the test pattern image produced on the retina. We computed this convolution for a discrete set of wavelengths (each 1 nm across the opsin response) and summed the resulting hyperspectral synthetic image along the spectral direction to arrive at a final full spectrum simulated image. We then computed an image quality metric (a quantitative indicator of image sharpness) by measuring the contrast in the simulated image.
Our image simulation program used an outermost loop that stepped through a sequence of lens to retina separations. For each of these accommodation values (i.e., lens to retina separation), we then iterated through a set of discrete wavelengths integrated over illumination wavelengths and the opsin response function for 450 nm < λ < 650 nm and computed the appropriate focus offset and corresponding blur for that wavelength. The sum, in the wavelength direction, of the blurred hyperspectral image stack produced a 2D simulation of the test pattern image on the retina,. We took care to introduce appropriate parity flips of the annular pupil (apparent in Fig. S4 A and B) according to the sign of the focal length offset at each wavelength. The wavelength-summed images were normalized so that the intensity value in a resolved test bar was unity.
The blurred test pattern images were each analyzed to assess the sharpness of the image using line profile plots across the images (Fig. 4). We defined a CTF metric that has the merit of being simple and that suppresses aliasing artifacts from the bar pattern and the pupil shape; we computed two times the SD of the pixel values in each image. A crisp image has a bimodal normalized intensity histogram (predominantly ones and zeros) and a high SD. A highly blurred image has an intensity histogram peaked at the mean pixel value and a low SD. By mapping out this CTF metric vs. lens to retina spacing, we can quantitatively assess the extent to which image sharpness can be used to deduce scene spectral content. These results are presented as CTF vs. accommodation plots for various pupil shapes and simulated scene spectral content in Fig. 4.
The animation in Movie S3 shows how the contrast of the simulated image depends on focal setting for the black to yellow test pattern shown in Fig. 4.
Determination of the Image Quality Budget.
To assess whether the chromatic aberration effects really do dominate the sharpness of the images, we produced an image quality budget that includes other sources of potential image blurring.
We evaluated the various terms in the image quality budget (Table S1) using geometrical or diffractive optics principles as appropriate. Each entry is provided as Gaussian-equivalent FWHM in the focal plane in units of micrometers for the f/1.2 spherical lens and a 12-mm focal length, with a radial gradient in index of refraction that compensates for spherical aberration. To convert to an equivalent angular resolution (which is independent of lens diameter for those terms that are in the geometrical optics regime), FWHM∠ = 2arctan(FWHM/(2FL)).
Adding all of the blur contribution in quadrature for the annular pupil case yields an angular resolution of FWHM∠ = 0.3°. This estimate is in broad agreement with behavioral experiments (53) that indicated a dynamic minimum separable angle (MSA) measured for 80-mm mantle-length animals in bright broadband light in the cephalopod Sepia officinalis of MSA = 0.6 ± 0.2°. A determination of cephalopod acuity on O. australis was performed by Muntz and Gwither (54) using static resolution targets. If we take the minimum detectable static contrast for cephalopods to be (7) 15%, then their results correspond to an angular FWHM of 0.2°, again in basic agreement with our image quality budget estimates, showing that there is no other term greatly compromising the image quality perceived by these organisms.
The geometric optics approximation (and the diameter independence of FWHM∠) breaks down if a physical length scale becomes important. There are two limiting cases where that occurs. (i) For sufficiently small (d < 1 mm) pupils, the wavelength of light becomes important, and diffraction dominates the image quality budget. (ii) The fixed photoreceptor size limits angular resolution for lens diameter d < 1 mm.
Despite the single photoreceptor type, chromatic aberration dominates the image quality budget, except for a small, on-axis pupil shape.
Photoreceptor size.
The typical diameter for the rhabdomes tiled across the cephalopod retina is reported (12) to be 5 μm. This length scale sets a limit on spatial sampling in the focal plane. The propagation of light rays across adjacent rhabdomes (1) (“rhabdome cross-talk”) would induce additional (and potentially chromatic) image degradation, but studies of cuttlefish retina concluded (27, 55) that their rhabdomes are clad in pigmented sheathing that may suppress this potential source of image degradation. We, therefore, elected to not include any potential image degradation from rhabdome cross-talk. If rhabdome cross-talk were a significant contributor to image blur, this effect would not favor the annular pupil shape seen in these animals.
Retinal displacement.
Cross-sectional light micrograph images of cephalopod retinal structure indicate (55) an rms axial displacement of, at most, a few micrometers over spatial scales of tens of micrometers. This displacement translates into a defocus blur of order 1 μm for the full aperture pupil. The retinal displacement along the optical axis would have to be comparable with the chromatic focus shift (300 μm) (Fig. 3) to produce image degradation comparable with the chromatic blur. The “retinal bump” in cephalopods could provide spectral information at fixed accommodation if the line of sight is varied (37) so as to shift the scene across this perturbation in effective focal length or if the object of interest’s image on the retina spans the retinal bump.
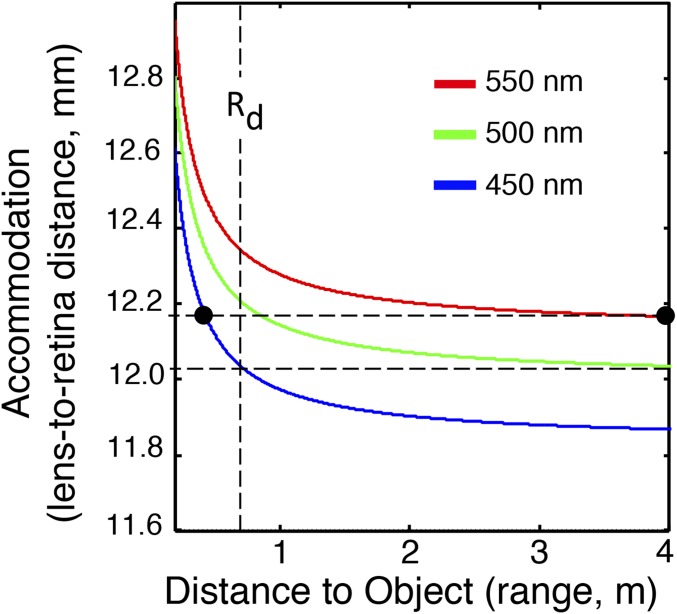
Fig. 3.
Range–color–focus relationship for a 10-mm-diameter cephalopod lens. Colored lines show the accommodation vs. range relationship at the 500-nm opsin peak sensitivity and the FWHM of the spectral resolution that we estimate for the chromatic focusing hypothesis. For objects more distant than Rd, there is an unambiguous relationship between wavelength and the accommodation setting that makes the sharpest image. For objects closer than Rd, an independent determination of range is needed to break the degeneracy between range and color.
Residual spherical aberration.
Although a spherical lens of uniform index of refraction produces pronounced spherical aberration, numerous studies have shown that the lenses of fishes and cephalopods have a radial variation in the index that largely compensates for spherical aberration. Jagger and Sands (26) show a typical FWHM from on-axis residual spherical aberration in octopus of less than 5 μm at full aperture. That measurement was for lenses a factor of two smaller than our 12-mm focal length model, and therefore, we have scaled this up to 10 μm for the entry in the image quality budget. We note also that this is for full aperture imaging and that the annular pupil greatly reduces the radial span of rays in the system. This calculation is, therefore, a conservative overestimate for the annular pupil geometry, because the residual spherical aberration scales (56) as Δh.
Chromatic aberration.
The experimental data (26) clearly indicate a wavelength-dependent focal shift in the lens of the octopus. We used our quadratic fit to the fractional chromatic focal length shift measured for octopus lenses from the work by Jagger and Sands (26) to perform a numerical computation of the 80% encircled energy radius for a point source at infinity, with best focus accommodations corresponding to wavelengths between 350 and 650 nm, for the three different pupil geometries that we studied. For this computation, we were interested in the entire wavelength range of interest, and therefore, we extended the focal length dependence on wavelength down to 350-nm wavelengths. Representative PSFs for accommodation settings that correspond to 400 and 700 nm are shown in Fig. S4 A–C. The encircled energy as a function of distance from the centroid is shown in Fig. S4D. We converted from the 80% encircled energy radius, R80, to a Gaussian-equivalent FWHM = 1.3 × R80. This calculation produced FWHM Gaussian equivalents of 6, 48, and 61 μm for the small, full, and annular pupils, respectively, at the best focus wavelength of 500 nm, the peak of the opsin curve.
Diffraction.
The diffraction limit on the focal plane has a spatial FWHM given by FWHMdiff = (f/#)(λ). For our full pupil with d = 8 mm, at the wavelength of peak opsin sensitivity, this calculation gives FWHMdiff = 1.5 × 0.5 μm = 0.75 μm. Stopping down the pupil to a smaller diameter d increases this term by a dimensionless multiplicative factor of 8 mm/d. Our smallest circular pupil diameter of d = 1 mm produces a diffraction limit of 6 μm FWHM, which is equal to the chromatic aberration term at that small aperture.
Other achromatic aberrations.
To constrain the magnitude of aberrations other than those listed above, we turned to the narrowband measurements of cephalopod PSFs performed by Gagnon et al. (31). They measured the FWHM produced in collimated 550-nm light (with a 10-nm bandwidth) at full aperture (31). Their data show a strong correlation between FWHM and f number (31). For their results on f/1.2 lenses, such as the one modeled here, when scaled to a 12-mm focal length, they observe an FWHM of 20 μm. We have, therefore, entered this value in Table S1 as other achromatic aberrations. We do not know the ray height dependence of these other aberrations, but unless the blurring induced by these other sources of wave-front error is independent of ray height h, they will remain subdominant for all pupil geometries. These other contributions add order of 10% (when taken in quadrature) to the total FWHM, and therefore, the image quality budget is dominated by the chromatic term.
Translation of the Paper by Kühn (21) from the Original German.
A 2015 translation by J. Schoeneberg of the relevant section of the 1950 paper by Kühn (21) states
Both the statistical recordings of the observed color, that are shown in figures and tables, and the immediate observations of the chromatophore changes show, that colored environments provoke different reactions than non-colored luminances. The differences in gray value on the grayscales get answered by a differential expansion of the black chromatophores. Their differential expansion also shows a different ‘luminance-value’ in the colored environment; yet added to that is a counteracted behavior of the colored chromatophores: if the color of the substrate is of short wavelength (blue, green), the colored chromatophores, the orange colored ones extremely, become small, if the color is of long wavelength (yellow, red), the colored chromatophores become extended, the more, the longer the wavelength of the light that they reflect. These reactions prove a ‘Sense of color’ of the cuttlefish, i.e. the capability to discriminate certain wavelength qualitatively from luminances of mixed light that appears colorless.
Results
Ideally, a set of monochromatic measurements of the point spread function produced by a cephalopod lens for different pupil sizes and lens to retina spacing would establish an empirical determination of the chromatic blur seen by these creatures. We are unaware of an appropriate comprehensive dataset, and therefore we have used the available laboratory measurements to produce a computer model of the chromatic properties for a representative cephalopod. Because the primary eye design features (complex pupil shape, spherical gradient index lens, and single-opsin retina) are common across coleoid cephalopods, we will use this model as representative of this class of animals.
Using measured (26) optical properties of Octopus australis, we performed a simulation by constructing a hyperspectral image cube [at 5 μm/pixel in the spatial directions, corresponding to a typical cephalopod rhabdome diameter (12, 27), and 200 planes spanning 450 nm < λ < 650 nm in the spectral direction at Δλ = 1 nm]. We modeled an f/1.2 spherical lens with a 10-mm diameter, but our computed chromatic blurring results are independent of this choice of length scale. For each lens to retina focal distance, which brings a single wavelength into crisp focus, we computed the pupil-dependent chromatic image blur at the other wavelengths. We summed up the blurred image cube along the wavelength direction [weighted by the product of the seawater-filtered solar photon illumination, the reflectance spectrum, and the opsin response curve (Fig. S3)] to arrive at a final simulated chromatically blurred image on the retina. This procedure was repeated for three different pupil shapes for a sequence of accommodation values.
Chromatic Blur Dominates Image Quality Budget.
A variety of factors determine the blurring of the image formed on the retina, including diffraction through the pupil, aberrations in the optical system, and retinal limitations (2, 25–34). These terms comprise the image quality budget and determine the sharpest image that can be formed. The eyes of O. australis are particularly well-studied (26), and we used data from this species as a proxy for other shallow water cephalopod eyes to make quantitative assessments of the image quality budget. The O. australis lenses have two properties shared by all other studied cephalopods: (i) they are remarkably well-corrected for spherical aberration, and (ii) the index of refraction varies with wavelength, inducing chromatic aberration. This chromatic aberration is uncorrected and found in all studied (26, 28, 29) cephalopod lenses. In some other animals, radial multifocal zones may produce a partial chromatic correction (30).
Wavelength dependence of the index of refraction induces (26) chromatic blurring, because different wavelengths have different focal lengths. This effect dominates the image quality budget (Table S1). The extent of chromatic blurring depends on both chromatic focal shift and the angle at which rays strike the optical axis (Fig. 2). This angle depends on the ray’s height h; the off-axis ray distribution determines the extent of chromatic blur. Although the single opsin restricts the range of wavelengths detected, our analysis shows that, when integrated over the wavelength response, chromatic blurring dominates image quality, except for small, on-axis pupils or when the lens diameter is so small that the granularity of the photoreceptors dominates. A monochromatic point source generates a scaled image of the pupil, with both size and parity determined by the amount of defocus (Fig. 2 and Fig. S4 A–C).
Range, Color, and Best Focus.
Organisms routinely determine the best focus for objects of interest in their visual field by varying focal length and comparing relative image quality. This focus aberration can be used as an accurate range-finding mechanism as shown in chameleons (35) and jumping spiders (36). If chromatic blur dominates the image quality budget, there is an interrelationship between range, color, and best focus (Fig. 3). For example, jumping spiders misjudge (36) distance depending on the illumination spectrum. Differential image blurring has been proposed (37) as a range-finding mechanism for squid, but chromatic aberration [not considered in the work by Chung and Marshall (37)] drives a strong relationship between spectrum, range, and best focus. Even in this narrowband system, chromatic aberration can compromise the determination of range based on best focus values.
Coleoid cephalopods use (38) binocular convergence and stereopsis to judge distance when striking prey items with their projectile tentacles, independent of image acuity. Thus, the combination of a determination of best focus and an independent determination of range allows for spectral discrimination.
The spherical lens system that we modeled obeys a modified lensmaker’s equation. The image distance I is a function of the wavelength-dependent focal length f(λ) and object distance O, with
We used this expression and the measured (26) chromatic aberration for O. australis to compute the image distance I needed to achieve a focused image as a function of both object distance and wavelengths at λ = 450, 500, and 550 nm. These wavelengths correspond to the opsin peak and the FWHM of the spectral resolution that we estimated numerically using synthetic monochromatic illumination. Fig. 3 shows the relationship between object distance (range) and the lens to retina spacing (accommodation) for these chosen wavelengths. Although there is color-range ambiguity for nearby objects, range-independent spectral discrimination (defined here as discrimination between the opsin peak wavelength and the two FWHM points) can be achieved for objects at distances beyond Rd =(d/(10 mm))(0.75 m) for lens diameter d. In this geometrical optics regime, the disambiguation range Rd scales with lens diameter. Rd is about one body length in O. australis.
Beyond Rd, best focus depends only on spectrum and is independent of range. A scan through focus amounts to a spectral scan of the scene. The animal can determine the object’s color by finding the focal setting that produces the sharpest image, regardless of range. This best focus determination can be achieved by some combination of (i) displacing the lens (39) relative to the retina (accommodation), (ii) changing the distance to the object, and/or (iii) imaging the object across regions of the retinal surface with different effective focal lengths.
What occurs when objects are closer than Rd? The focal spacing creating a crisp focus of a 450-nm light source at 0.2 m also creates a sharp image of a 550-nm light source more than 4 m away (Fig. 3). Studies of range determination in cuttlefish show (38) that they use multiple methods for precisely establishing distances. Both cuttlefish and squid rely on this ability to accurately project their tentacles and capture food. This ranging ability can break the range color degeneracy, improving spectral resolution and allowing them to use image sharpness to obtain spectral information for R < Rd.
This mechanism for spectral discrimination is computationally more intensive than a differential comparison of photoreceptor outputs in opponency. We believe that this may be one factor contributing to the exceptionally large (12) optic lobes found in coleoid cephalopods.
Chromatic Blurring Evidently Favored over Visual Acuity.
Although ambient light levels influence optimal pupil area, pupil shape determines the extent of chromatic blurring (Fig. 2). Chromatic blur dominates the cephalopod image quality in low-light conditions with a fully dilated pupil (Table S1). The off-axis slit and semiannular pupils used in high-light conditions preserve this spectral discrimination mechanism across a wide dynamic range of illumination. The semiannular pupil shape (Fig. 1), common in both cuttlefish and shallow water squids, maximizes the off-axis distance of optical rays from objects in the horizontal plane around the animal. The horizontal slit pupil of shallow water octopus (Fig. S1F) species intercepts a similar ray bundle when imaging the bottom, acting as an arc-like pupil for images formed on the upper portion of the retina that has an enhanced density of photoreceptors (40).
We computed the pupil dependence of the contrast transfer function (CTF) vs. accommodation and the corresponding spectral resolution for three pupil shapes using the yellow to black test pattern (Fig. 4 A–C). The small on-axis pupil (Fig. 4P) has minimal chromatic blur and maintains a crisp CTF across the range of accommodation settings, maximizing visual acuity but with degraded spectral sensitivity. Full (Fig. 4Q) and semiannular pupils (Fig. 4R) more realistically represent cephalopod pupils under low- and high-light conditions, respectively, and have virtually identical accommodation-dependent chromatic blur and correspondingly higher spectral resolution than the small pupil.
We propose that natural selection might favor the maintenance of spectral discrimination over image acuity in these animals.
Discussion
Despite earlier behavioral results indicating color discrimination (9, 21), two lines of evidence drove (7) the prevailing view that nearly all cephalopods are color blind. First, only one photoreceptor type exists (4–6) in the retina of shallow water cephalopods. Our mechanism for spectral discrimination requires only one receptor type. Second, some behavioral experiments (7–11) designed to test for color vision in cephalopods produced negative results by using standard tests of color vision to evaluate the animal’s ability to distinguish between two or more adjacent colors of equal brightness. This adjacent color comparison is an inappropriate test for our model (Fig. 4R). Tests using rapidly vibrating (8, 9) color cues are also inappropriate. Although these dynamical experiments are effective tests for conventional color vision, they would fail to detect spectral discrimination under our model, because it is difficult to measure differential contrast on vibrating objects. These results corroborate the morphological and genetic evidence: any ability in these organisms for spectral discrimination is not enabled by spectrally diverse photoreceptor types.
Table S2 reviews cephalopod behavioral experiments that investigated color vision and their consistency with our proposed mechanism. Before the determination that cephalopods possess a single photoreceptor type, there were numerous experiments showing that they had spectral discrimination. These results were summarized and dismissed in the work by Messenger et al. (9) with the following rationale:
Table S2.
Prior behavioral cephalopod experiments testing for color vision
| Experiment | Experiment description | Consistent with model? |
| 1950: Kühn (21) | (A) Test of camouflage response on textured colored backgrounds vs. greyscale; (B) training experiment on Octopus vulgaris using stationary targets | Yes for both. Kühn (21) concludes a wavelength sensing capability; both of his experimental designs (A and B) allow for the determination of chromatically induced defocus |
| 1973: Messenger et al. (9) | (C) Training experiments on octopus under fluorescent lighting using colored rods vibrated at 3 Hz; (D) nystagmus response in octopus to alternating colored stripes | Yes, rapidly vibrating color cues would make color assessment by chromatic defocus impossible. Yes, under our model, they are unable to judge coloration absent fine-scale structure; adjacent colors are not resolvable |
| 1975: Roffe (10) | (E) Training experiment on octopus with unfocused monochromatic light projected onto a white screen without focusing cues | Yes, light projected onto uniform disk would not allow for determination of chromatic defocus |
| 1977: Messenger (8) | (F) Training experiments on octopus with rectangles of colored cues vibrated at 3 Hz | Yes, rapid vibration makes color assessment by chromatic defocus impossible |
| 1996: Marshall and Messenger (11) | (G) Camouflage assay using two adjacent colors of artificial fine gravel | Yes, under our model, adjacent colors are not resolvable |
| 2006: Mäthger et al. (7) | (H) Nystagmus tracking response in Sepia using alternating adjacent colored bars rotated around the head of the animal; (i) camouflage assay using two adjacent colors in a uniform checkerboard | Yes for both. Both lines of evidence (H and I) use adjacent colors without fine-scale structure; this degeneracy defeats a spectral discrimination model using chromatically dependent defocus as seen in Fig. 3 |
This table provides a summary of how prior laboratory cephalopod behavior and vision experiments compare with the chromatic aberration model proposed here.
…all the authors are guilty of one or more of three serious errors: failure to take into account the spectral sensitivity curve of the subject, failure to control for the difference in brightness between test objects, and, in the behavioral experiments, inadequate quantification of results, which are presented without conventional statistical analysis.
We view this critique (9) of the 1950 paper by Kühn (21) as inaccurate. The work by Kühn (21) is mischaracterized as purely a training experiment (9). A reading of the work by Kühn (21) shows that, although he did perform extensive training experiments indicating spectral discrimination in Octopus vulgaris, he also clearly showed the differential responses of cuttlefish chromatophores to differentially colored textured backgrounds. We have provided a translation of the relevant section of this paper in SI Experimental Procedures.
In our proposed mechanism, cephalopods cannot gain spectral information from a flat-field background or an edge between two abutting colors of comparable intensity (Fig. 3). This phenomenology would explain why optomotor assays and camouflage experiments using abutting colored substrates (7, 9, 11) fail to elicit a response different from a flat-field background. Similarly, experiments (10) with monochromatic light projected onto a large uniform reflector or training experiments (8, 9) with rapidly vibrating colored cues would defeat a determination of chromatic defocus.
The mechanism proposed here is readily testable by conducting behavioral experiments that assess a cepaholpod’s ability to achieve successful camouflage as a function of both the spatial and the spectral structures of the background. Although we assert that the 1950 experiment by Kühn (21) clearly shows that the cuttlefish camouflage response differs in textured backgrounds colored in shades of gray with spectrally uniform reflectance compared with colored backgrounds, we suggest repeating this classic experiment using flat-field background of uniform luminosity without any potential focusing aids for the organisms.
We predict that the animals will fail to match flat-field backgrounds with no spatial structure as previously shown in figure 3B in the work by Mäthger et al. (7) just as a photographer could not determine best focus when imaging a screen with no fine-scale spatial structure. If, for instance, their ability to spectrally match backgrounds was conferred by the skin or another potential unknown mechanism, they would successfully match on flat-field backgrounds. However, under our model, they should succeed when there is a spatial structure allowing for the calculation of chromatically induced defocus, such as in our test patterns (Fig. 4) or the more naturally textured backgrounds by Kühn (21). If, however, cephalopods truly cannot accurately match their background color but solely use luminance and achromatic contrast to determine camouflage, we would expect the response on colored substrates to be identical to that on a gray substrate of similar apparent brightness with identical spatial structure. We encourage groups with access to live cephalopods to conduct these experiments, although we caution that they should be conducted under natural illumination conditions.
Our proposed mechanism has potential applicability in other species with a limited number of photoreceptor types and low f-number visual systems. Some dolphin species use (41) an annular pupil and a similar (42) radial gradient index of refraction lens uncorrected for chromatic aberration. They display evidence for behavioral color discrimination (43) in spectral regimes where their visual system would have difficulty (44) encoding color by opponent channels. More generally, a large number of organisms that are active both diurnally and nocturnally possesses (45) an annular pupil, and we wonder if these organisms could also benefit from color discrimination by our proposed mechanism.
Spider primary eyes also use a low f-number optical system and thus, induce high chromatic blurring. Additionally, most studied spiders image their environment with only two functional opsins (UV and green peak sensitivities) (36), although a recent study (46) showed that one genus of jumping spider may use retinal filtering to obtain some spectral discrimination. Some spiders also use an imaging system that maintains off-axis rays in high-light conditions (as in the cephalopod annular pupil) to simultaneously (47) image across multiple axially displaced focal planes. Jumping spiders can use image defocus across these focal planes to judge distance, but (as in cephalopods), this mechanism can be confounded (36) by color-range ambiguity (Fig. 3). When under natural sunlight, some jumping spiders exhibited a preference for red-colored mates (48), and crab spiders showed an ability to background match (49). However, these behaviors disappeared under fluorescent lighting (48, 49). Fluorescent lighting in these experiments created a series of line emissions that approximate δ-functions and dominated the reflected spectrum from objects in the visual field. This illumination spectrum would make spectral inferences by chromatic defocus imaging difficult.
By simultaneously comparing image quality across multiple offset focal planes, these organisms might be able to obtain more spectral information than by photoreceptors working in opponency, and indeed, tiered retinas found (2) in spiders and many deep sea fish might represent the optimal morphology for spectral discrimination using our proposed mechanism.
Conclusions
We have shown that the combination of off-axis pupil shape and chromatic aberration can be exploited to yield spectral information, albeit only in scenes that have substantial spatial/spectral contrast so that changes in chromatic blurring can be detected. A quantitative numerical model of the O. australis visual system shows the viability of this phenomenology. This spectral sensitivity mechanism offers a potential explanation for the apparent contradictions in cephalopod behavior in the wild, where these “color-blind” animals achieve remarkable color-matched camouflage and display in vivid colors. It is also consistent with the accumulated data from over 60 y of controlled laboratory vision experiments. This scenario may force us to rethink what it means to be a color-blind animal.
Experimental Procedures
We computed the relationship between image sharpness, accommodation, and spectral content. We created test patterns with different spectral characteristics and simulated the images that they would form on the single-opsin retina of O. australis for different accommodation values. The test patterns (Fig. 4 A–E) are generated with the reflectance spectra (50) of blue and yellow Australian reef fish. The side length of each pixel in the test patterns is equal to the 5-μm rhabdome diameter; our test images incorporate the sampling granularity inherent in this detector system.
We computed a CTF (Fig. 4) metric to map out image contrast as a function of accommodation, pupil shape, and the spectral content of the test image. The CTF vs. accommodation for a given test pattern tracks the underlying spectrum but with a spectral resolution that depends on intensity contrast as well as pupil shape. Fig. 4 P–T shows the spectral content of the test patterns (red lines) and the computed image sharpness vs. accommodation (blue lines). Table S1 shows quantitative values of image blurring, and SI Experimental Procedures discusses how these were computed.
Movie S3 is an animation that shows how the contrast of the image depends on focal setting (i.e., accommodation). Maximum contrast is obtained when the lens brings light at the peak of the detected photon spectrum into best focus. Two representative simulated blurred images are shown in Fig. 4 for each of five test patterns for focal settings that bring 470- and 550-nm light into focus on the retina. The amount of chromatic blurring is evident from their corresponding intensity line cuts shown as blue superimposed lines in the respective figures. We mapped image sharpness vs. accommodation setting over the full range of wavelengths. The spectral peak of the light detected from an object can be inferred from the accommodation setting where the image is best focused on the retina.
Under our model, the determination of spectral information is reliant on fine-scale intensity variations (edges, shadows, texture, etc.). This dependence imposes limitations. Cephalopods would be unable to determine the spectral content of a flat field of uniform color. They would similarly be unable to determine spectral information from abutting regions of comparable apparent intensity, differing only in spectral content (Fig. 4 E, J, O, and T). This degeneracy can account for contradictory results obtained in laboratory behavior tests for color vision (Table S2).
Natural environments rich in shadows and structure serve as focusing aids. Spectra measured (50) in marine environments often provide the spectral structure needed for this mechanism. Intraspecific displays of these organisms (Fig. 1, Fig. S2, and Movie S2) typically exhibit adjacent fine-scale black and colored regions, facilitating best focus determination. We believe that this is another adaptation that favors our model.
Supplementary Material
Acknowledgments
A.L.S. thanks University of California, Berkeley and the Museum of Vertebrate Zoology for support; Prof. J. McGuire for extensive opportunities, mentoring, and comments on this manuscript; and S. Johnsen, M. Banks, and R. Caldwell for helpful comments. C.W.S. acknowledges the support of Harvard University. We thank N.O.S.B. and the Packard Foundation for their support of science, J. Schoeneberg for translation, and C. Gregory for editorial support. The MATLAB routines used for computing the results shown here will be made available on request.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524578113/-/DCSupplemental.
References
- 1.Forte JD, Blessing EM, Buzás P, Martin PR. Contribution of chromatic aberrations to color signals in the primate visual system. J Vis. 2006;6(2):97–105. doi: 10.1167/6.2.1. [DOI] [PubMed] [Google Scholar]
- 2.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. Visual Ecology. Princeton Univ Press; Princeton: 2014. [Google Scholar]
- 3. Aristotle (350 BCE) A History of Animals, trans Thompson DW (1910) (Clarendon, Oxford)
- 4.Brown PK, Brown PS. Visual pigments of the octopus and cuttlefish. Nature. 1958;182(4645):1288–1290. doi: 10.1038/1821288a0. [DOI] [PubMed] [Google Scholar]
- 5.Bellingham J, Morris AG, Hunt DM. The rhodopsin gene of the cuttlefish Sepia officinalis: Sequence and spectral tuning. J Exp Biol. 1998;201(Pt 15):2299–2306. doi: 10.1242/jeb.201.15.2299. [DOI] [PubMed] [Google Scholar]
- 6.Chung WS. 2014. Comparisons of visual capabilities in modern Cephalopods from shallow water to deep sea. PhD dissertation (Queensland Brain Institute, University of Queensland, St. Lucia, QLD, Australia)
- 7.Mäthger LM, Barbosa A, Miner S, Hanlon RT. Color blindness and contrast perception in cuttlefish (Sepia officinalis) determined by a visual sensorimotor assay. Vision Res. 2006;46(11):1746–1753. doi: 10.1016/j.visres.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Messenger JB. Evidence that Octopus is colour blind. J Exp Biol. 1977;70(1):49–55. [Google Scholar]
- 9.Messenger JB, Wilson AP, Hedge A. Some evidence for colour-blindness in Octopus. J Exp Biol. 1973;59(1):77–94. doi: 10.1242/jeb.59.1.77. [DOI] [PubMed] [Google Scholar]
- 10.Roffe T. Spectral perception in Octopus: A behavioral study. Vision Res. 1975;15(3):353–356. doi: 10.1016/0042-6989(75)90082-6. [DOI] [PubMed] [Google Scholar]
- 11.Marshall NJ, Messenger JB. Colour-blind camouflage. Nature. 1996;382(6590):408–409. [Google Scholar]
- 12.Hanlon RT, Messenger JB. Cephalopod Behavior. Cambridge Univ Press; Cambridge, United Kingdom: 1998. [Google Scholar]
- 13.Mäthger LM, Roberts SB, Hanlon RT. Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biol Lett. 2010;6(5):600–603. doi: 10.1098/rsbl.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiao CC, Wickiser JK, Allen JJ, Genter B, Hanlon RT. Hyperspectral imaging of cuttlefish camouflage indicates good color match in the eyes of fish predators. Proc Natl Acad Sci USA. 2011;108(22):9148–9153. doi: 10.1073/pnas.1019090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanlon R. Cephalopod dynamic camouflage. Curr Biol. 2007;17(11):R400–R404. doi: 10.1016/j.cub.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Chiao CC, Chubb C, Hanlon RT. A review of visual perception mechanisms that regulate rapid adaptive camouflage in cuttlefish. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015;201(9):933–945. doi: 10.1007/s00359-015-0988-5. [DOI] [PubMed] [Google Scholar]
- 17.Buresch KC, et al. Cuttlefish adjust body pattern intensity with respect to substrate intensity to aid camouflage, but do not camouflage in extremely low light. J Exp Mar Biol Ecol. 2015;462:121–126. [Google Scholar]
- 18.Akkaynak D, Allen JJ, Mäthger LM, Chiao CC, Hanlon RT. Quantification of cuttlefish (Sepia officinalis) camouflage: A study of color and luminance using in situ spectrometry. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199(3):211–225. doi: 10.1007/s00359-012-0785-3. [DOI] [PubMed] [Google Scholar]
- 19.Mäthger LM, Chiao CC, Barbosa A, Hanlon RT. Color matching on natural substrates in cuttlefish, Sepia officinalis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194(6):577–585. doi: 10.1007/s00359-008-0332-4. [DOI] [PubMed] [Google Scholar]
- 20.Hanlon RT, Chiao CC, Mäthger LM, Marshall NJ. A fish-eye view of cuttlefish camouflage using in situ spectrometry. Biol J Linn Soc Lond. 2013;109(3):535–551. [Google Scholar]
- 21.Kuhn A. [Color change and color sense in cephalopods] Z Vgl Physiol. 1950;32(6):573–598. [PubMed] [Google Scholar]
- 22.Albertin CB, et al. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature. 2015;524(7564):220–224. doi: 10.1038/nature14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingston ACN, Wardill TJ, Hanlon RT, Cronin TW. An unexpected diversity of photoreceptor classes in the Longfin squid, Doryteuthis pealeii. PLoS One. 2015;10(9):e0135381. doi: 10.1371/journal.pone.0135381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez MD, Oakley TH. Eye-independent, light-activated chromatophore expansion (LACE) and expression of phototransduction genes in the skin of Octopus bimaculoides. J Exp Biol. 2015;218(Pt 10):1513–1520. doi: 10.1242/jeb.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks MS, Sprague WW, Schmoll J, Parnell JAQ, Love GD. Why do animal eyes have pupils of different shapes? Sci Adv. 2015;1(7):e1500391. doi: 10.1126/sciadv.1500391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagger WS, Sands PJ. A wide-angle gradient index optical model of the crystalline lens and eye of the octopus. Vision Res. 1999;39(17):2841–2852. doi: 10.1016/s0042-6989(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Tasaki K, Sugawara Y, Tonosaki A. Fine structure of the Octopus retina. J Cell Biol. 1965;25(2):345–359. doi: 10.1083/jcb.25.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidermanns C. Messende Untersuchungen über das Formensehen der Cephalopoden und ihre optische Orientierung im Raume. Zool Jahrb Abt Anat Ontogenie Tiere. 1928;45:609–650. [Google Scholar]
- 29.Sivak JG. Shape and focal properties of the cephalopod ocular lens. Can J Zool. 1991;69(10):2501–2506. [Google Scholar]
- 30.Kröger RHH, Campbell MCW, Fernald RD, Wagner HJ. Multifocal lenses compensate for chromatic defocus in vertebrate eyes. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184(4):361–369. doi: 10.1007/s003590050335. [DOI] [PubMed] [Google Scholar]
- 31.Gagnon YL, Sutton TT, Johnsen S. Visual acuity in pelagic fishes and mollusks. Vision Res. 2013;92:1–9. doi: 10.1016/j.visres.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen S. The Optics of Life: A Biologist’s Guide to Light in Nature. Princeton Univ Press; Princeton: 2012. [Google Scholar]
- 33.Land MF, Nilsson DE. Animal Eyes. Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 34.Jagger WS, Sands PJ. A wide-angle gradient index optical model of the crystalline lens and eye of the rainbow trout. Vision Res. 1996;36(17):2623–2639. doi: 10.1016/0042-6989(95)00328-2. [DOI] [PubMed] [Google Scholar]
- 35.Harkness L. Chameleons use accommodation cues to judge distance. Nature. 1977;267(5609):346–349. doi: 10.1038/267346a0. [DOI] [PubMed] [Google Scholar]
- 36.Nagata T, et al. Depth perception from image defocus in a jumping spider. Science. 2012;335(6067):469–471. doi: 10.1126/science.1211667. [DOI] [PubMed] [Google Scholar]
- 37.Chung WS, Marshall J. Range-finding in squid using retinal deformation and image blur. Curr Biol. 2014;24(2):R64–R65. doi: 10.1016/j.cub.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 38.Cheung V, Mullins O, Nguyen P, Huberman A. 2014. Extreme binocular plasticity and dynamic strategy implementation supports vision-dependent prey capture in cuttlefish. Proceedings of the SACNAS National Conference, ed G Miranda-Carboni (Society for the Advancement of Hispanics/Chicanos and Native Americans in Science, Santa Cruz, CA), p 1159.
- 39.Schaeffel F, Murphy CJ, Howland HC. Accommodation in the cuttlefish (Sepia officinalis) J Exp Biol. 1999;202(Pt 22):3127–3134. doi: 10.1242/jeb.202.22.3127. [DOI] [PubMed] [Google Scholar]
- 40.Talbot CM, Marshall JN. The retinal topography of three species of coleoid cephalopod: Significance for perception of polarized light. Philos Trans R Soc Lond B Biol Sci. 2011;366(1565):724–733. doi: 10.1098/rstb.2010.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman LM, Peacock MF, Yunker MP, Madsen CJ. Bottle-nosed dolphin: Double-slit pupil yields equivalent aerial and underwater diurnal acuity. Science. 1975;189(4203):650–652. doi: 10.1126/science.1162351. [DOI] [PubMed] [Google Scholar]
- 42.Kröger RHH, Kirschfeld K. Optics of the harbor porpoise eye in water. J Opt Soc Am A. 1993;10(7):1481–1489. doi: 10.1364/josaa.10.001481. [DOI] [PubMed] [Google Scholar]
- 43.Griebel U, Schmid A. Spectral sensitivity and color vision in the bottlenose dolphin (Tursiops truncatus) Mar Freshwat Behav Physiol. 2002;35(3):129–137. [Google Scholar]
- 44.Fasick JI, Cronin TW, Hunt DM, Robinson PR. The visual pigments of the bottlenose dolphin (Tursiops truncatus) Vis Neurosci. 1998;15(4):643–651. doi: 10.1017/s0952523898154056. [DOI] [PubMed] [Google Scholar]
- 45.Murphy CJ, Howland HC. The functional significance of crescent-shaped pupils and multiple pupillary apertures. J Exp Zool. 1990;256(S5):22–28. [Google Scholar]
- 46.Zurek DB, et al. Spectral filtering enables trichromatic vision in colorful jumping spiders. Curr Biol. 2015;25(10):R403–R404. doi: 10.1016/j.cub.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 47.Blest AD, Hardie RC, McIntyre P, Williams DS. The spectral sensitivities of identified receptors and the function of retinal tiering in the principal eyes of a jumping spider. J Comp Physiol. 1981;145(2):227–239. [Google Scholar]
- 48.Taylor LA, McGraw KJ. Male ornamental coloration improves courtship success in a jumping spider, but only in the sun. Behav Ecol. 2013;24(4):955–967. [Google Scholar]
- 49.Llandres AL, Figon F, Christidès JP, Mandon N, Casas J. Environmental and hormonal factors controlling reversible colour change in crab spiders. J Exp Biol. 2013;216(Pt 20):3886–3895. doi: 10.1242/jeb.086470. [DOI] [PubMed] [Google Scholar]
- 50.Marshall NJ, Jennings KA, McFarland WN, Loew ER, Losey GS. Visual biology of Hawaiian reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia. 2003;2003(3):467–480. [Google Scholar]
- 51.Morel A, Maritonrena S. Bio-optical properties of oceanic waters: A reappraisal. J Geophys Res. 2001;106(C4):7163–7180. [Google Scholar]
- 52.Schroeder DJ. Astronomical Optics. Academic; San Diego: 1999. [Google Scholar]
- 53.Groeger G, Cotton PA, Williamson R. Ontogenetic changes in the visual acuity of Sepia officinalis measured using the optomotor response. Can J Zool. 2005;83(2):274–279. [Google Scholar]
- 54.Muntz WRA, Gwyther J. Visual acuity in Octopus pallidus and Octopus australis. J Exp Biol. 1988;134(1):119–129. [Google Scholar]
- 55.Hao ZL, Zhang XM, Kudo H, Kaeriyama M. Development of the retina in the cuttlefish Sepia esculenta. J Shellfish Res. 2010;29(2):463–470. [Google Scholar]
- 56.Mahajan VN. Zernike annular polynomials for imaging systems with annular pupils. J Opt Soc Am. 1981;71(1):75–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.