Significance
We investigated the hypothesis that the perception of time passing can exert a stronger influence on blood glucose level compared with the passage of actual time in people with type 2 diabetes. Our findings suggest that manipulation of participants’ perception of time resulted in blood glucose levels changing in accordance with how much time participants believed had passed, instead of how much time had actually passed. These results are an important example of the influence psychological processes can directly exert on the body. Mindsets and expectations may play an increasingly important role in type 2 diabetes management.
Keywords: perceived time, blood glucose levels, diabetes, expectations, false-clock paradigm
Abstract
The current study investigates whether perceived time has an effect on blood glucose level in people with type 2 diabetes. The hypothesis is that perceived time will have a greater influence over blood glucose level than actual time. Changes in blood glucose levels were measured in 46 participants with diabetes while they completed simple tasks during a 90-min period. Participants’ perception of time was manipulated by having them refer to clocks that were either accurate or altered to run fast or slow. Blood glucose levels changed in accordance with how much time they believed had passed instead of how much time had actually passed. These results are an example of the influence psychological processes can directly exert on the body.
The relationship between expectations and physiological responses has received much attention in the study of the placebo effect (1, 2), a phenomenon producing physiological changes in the body without specific biological stimulation (3). For example, the expectation that an activity leads to a decrease in weight may result in an actual reduction of weight (4), and perceptions also change physiological responses to food consumption (5). Similarly, study participants role-playing air force pilots, a group expected to have excellent vision, had better vision than control participants (6). Despite a broad range of data describing the influence of mental states over the body, the role of psychological processes, especially when dealing with chronic health conditions, has been frequently underestimated. The connection between mind and body has received particularly limited attention in the study of metabolic disorders, such as diabetes.
Type 2 diabetes, the most common form of diabetes, is a chronic disease that affects millions (7). Its symptoms include periodic rises in blood glucose levels (BGLs) because the body produces insufficient insulin and/or resists the effects of insulin, leading to short-term severe shock and multiple long-term complications including strokes, neuropathies, kidney disease, and vision problems (8). Genetic factors appear to be a strong biological trigger (9), and obesity seems to be a powerful environmental trigger (10).
Although recognized as relevant psychosocial elements in diabetes management, few psychological factors have been studied for the effect they can exert on diabetic physiology. The majority of studies concerning psychological issues and diabetes have focused on depression, a serious comorbid condition (11), or on the negative effect of distress on disease management (12). Apart from studies on depression and distress, limited efforts have been made to investigate the effect of psychological variables on blood sugar regulation. No studies to our knowledge have investigated the potential for psychological mechanisms to directly influence BGLs.
Glucose levels in people with type 2 diabetes follow a particular time course, but how is the course determined? Current models suggest it is determined solely by physiological factors (13). There is, however, reason to believe this may not necessarily be the case in general. We often feel hungry, for example, when we see it is lunchtime, despite having felt sated moments before (14).
The purpose of the present study is to investigate the hypothesis that perceived time affects BGLs. It has been reported that the manipulation of time perception can influence the intensity of perceived pain (15), as well as emotional responses (16). If perceived time can also influence glucose levels, the research will provide further evidence of the inextricable relationship between mind and body. Positive findings would suggest that perceived time exerts a significant effect on the pace and rhythm of some natural physiological processes, including BGLs.
Materials and Methods
We used flyers and local advertisements to recruit 47 volunteers who have type 2 diabetes mellitus (≥12 mo duration; 24 women; mean age = 54.1 y, SD = 12.2 y) and who were being treated with diet and metformin, a biguanide antidiabetic medication. At least a week before they came to the laboratory, we sent participants a package of forms and instructions, including a brief survey about medical history and conditions, a daily glucose diary, a glucose fluctuation chart, and fasting instructions. The protocol was approved by the Harvard University Institutional Review Board. Participants provided informed consent.
Participants were instructed to record their BGLs before and after every meal and to complete a daily blood glucose change chart for a week before the experiment. The procedure allowed them to become more familiar with their BGL fluctuations. To minimize potential BGL variability, we asked them to fast for at least 8 h before they came to the laboratory for the studies, beginning at 9:00 AM (mean fasting hours: 11 h 47 min; SD: 1 h 34 min).
When volunteers arrived at the laboratory, we explained that we were interested in enhanced cognitive functioning among people who have type 2 diabetes. Volunteers were randomly assigned to one of three experimental conditions: Fast, Normal, and Slow. We asked them to leave behind all devices that indicated time (e.g., phones, tablets, watches, etc). Then we took them to a separate laboratory room where they played three simple video games for 90 min and for approximately the same amount of time for each game.
Participants in the Normal condition viewed a clock on the desk that indicated the actual time passing while they played video games; that is, their clocks correctly showed that 90 min had passed by the end of the task period. Participants in the Slow condition, however, viewed a clock rigged to run two times slower than actual time, so that it indicated only 45 min passed by the end of the task period. Participants in the Fast condition viewed a clock that ran two times faster than actual time, so it indicated that 180 min had passed by the end of the task period. Previous studies using a “false-clock” manipulation have shown this to be a reliable and effective method of distorting time perception (15).
In all cases, participants were not aware of the name or status of their treatment group, nor of the properties of the modified clocks. Participants were asked to switch the game they were playing every 15 min, which kept them aware of the passage of time. We measured BGL immediately before and after they played the games. After the intervention, we also assessed the level of their stress and hunger. Stress was assessed with a single item asking “Please use the sliding scale to indicate your current level of stress at this moment,” using a slide scale rated 1–10. We used the Satiety Labeled Intensity Magnitude, a validated scale, to measure hunger (17). Finally, to check for the success of our time manipulation, we asked participants to guess the current time at the end of their participation.
Results
The three groups did not significantly differ on demographic variables or on any other variable at the beginning of the study, including fasting hours and BGL before the intervention (P > 0.05).
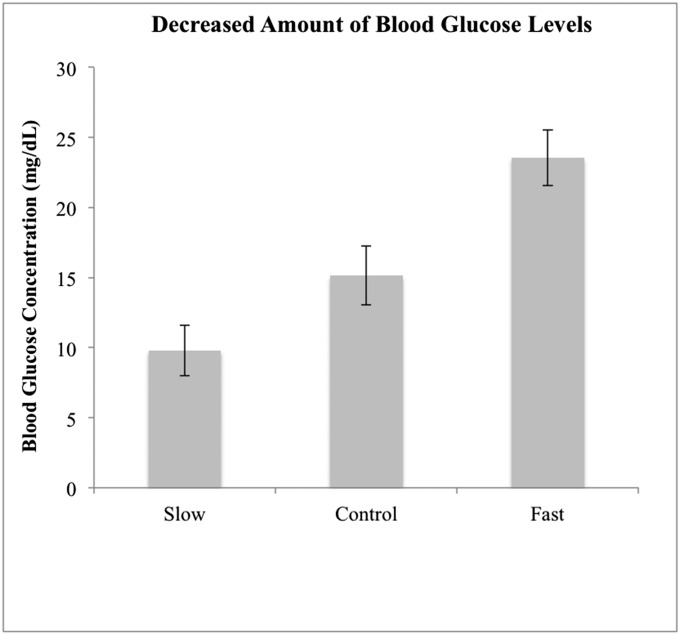
To check for the success of our time manipulation, we asked participants to guess how many minutes had passed at the end of their participation. Participants in the Slow, Normal, and Fast conditions guessed an average of 59.3 (SD = 19.3), 111.7 (SD = 21.1), and 174.6 (SD = 40.4) min had passed, respectively. Fig. 1 presents the mean change in BGL during the time manipulation period.
Fig. 1.
Decreased amount of mean blood glucose concentration during the time manipulation period. Error bars represent ± SE.
A mixed between-within subjects ANOVA was conducted to assess the effect of the time manipulation on BGLs. There was a significant effect of the time on the overall BGL [Wilks’ λ = 0.18; F(1, 43) = 193.55; P < 0.001; ηp2 = 0.82] and a significant interaction between time and groups [Wilks’ λ = 0.64; F(2, 43) = 12.07; P < 0.000; ηp2 = 0.36]. Participants who had been watching fast clocks showed a greater decrease in BGL (M = 23.5 mg/dL) than those in the Normal group (M = 15.1 mg/dL), and those with slow clocks showed a lesser decrease in BGL (M = 9.8 mg/dL) than those in the Normal group. Across all participants, the magnitude of BGL decrease was associated with the duration of perceived passed time (r = 0.53; P < 0.01).
Furthermore, scores from Satiety Labeled Intensity Magnitude indicated that participants with fast clocks (M = 56.87; SD = 23.78) reported being hungrier at the end of the study than those with normal (M = 37.53; SD = 10.45) or slow (M = 29.4; SD = 18.11) clocks [F(2, 43) = 9.14; P < 0.000]. Perceived stress, however, did not vary across the groups [Fast: M = 3.0 (SD = 1.55); Normal: M = 2.80 (SD = 2.07); Slow: M = 2.67 (SD = 1.63); F(2, 43) = 0.141; P = 0.87].
Discussion
We demonstrated that BGLs in people with type 2 diabetes were affected more by subjects’ false impressions of how much time had passed than by the actual passage of real time: Glucose level was lower in participants who thought more time had passed than in those who thought less time had passed. These results support the claim that psychological processes, such as the perceived passage of time, can exert considerable influence over natural, physiological processes.
We ruled out several competing explanations for how the observed results might be explained by methodological artifacts or other confounding factors. The artificial time distortion treatment itself was effective: When asked how much time they thought had actually passed, participants in each group reported estimates in line with the intended manipulation. The task itself did not have any unusual effect on BGL, as participants in the normal clock condition exhibited BGL decrease at a rate similar to what would be expected of patients with diabetes at resting, postfasting BGLs (18, 19).
We considered the possibility that participants who thought they had been playing games for 3 h might have become more stressed or agitated than those who only believed 45 or 90 min had passed. Elevated stress, in turn, could speed rates of BGL decrease, so this seemed a plausible alternative explanation (20). Participants, however, reported no difference in perceived stress levels across conditions. This gives us reasonable cause to reject elevated stress levels as an effect mechanism. Perceived stress and actual stress are not perfectly correlated (21), however, and our study data do not give any insight into how physiological reactions to stress, such as cortisol or dehydroepiandrosterone sulfate (DHEAS) hormone changes, might mediate changes in BGLs. This is a relevant hypothesis that merits further investigation. We also found that perceived time influenced participants’ perceived hunger, which is highly related to sugar consumption (22). Stress levels can also be affected by perceived hunger (23), so future studies should address potential mediating effects of hunger-related hormones (e.g., ghrelin, peptide tyrosine-tyrosine) on the relation between perceived time and BGLs.
Another potential confounding factor concerns the imbalanced number of times each group switched video games. Participants switched games every 15 min, according to the clocks they were given, so participants in the fast clock group switched more frequently than participants in other groups. One might propose that the increased switching in the fast clock group might have led to greater activity and exertion, causing blood glucose to decline more rapidly. The switching process entailed loading up a different video game on a computer, which is not a particularly demanding task. Even so, we controlled for this, to a degree, by instructing participants to alert the experimenters when it was time to change games, at which point the experimenters performed the actual switching. No actual effort was required of participants to make the change, aside from alerting the experimenter. It seems implausible that the effort required to signal the experimenter at switching times could have played a significant role in the intergroup differences observed.
These findings may be relevant for advancing the care and treatment of individuals suffering from diabetes. Official standards for care and treatment of diabetes make no explicit mention of the influence of subjective cognition on diabetic metabolism (24), but our results indicate otherwise. Our finding that the mind can actually adjust the body’s glucose levels suggests new avenues for treatment and intervention. Further studies should explore potential clinical implications and more deeply investigate the relationship between BGL and psychological variables such as cognitive styles, mindfulness, and coping strategies.
We selected participants with type 2 diabetes for a number of reasons. First, people with diabetes represent a clinical population that stands to greatly benefit from these findings. In particular, type 2 diabetes is the most common form of diabetes, and so would make for easier recruitment. Moreover, the pancreas of patients with type 2 produces insulin, but the body is not able to use it (insulin resistance); we assumed that the link between expectations and physiological responses would have been easier to detect in people with type 2 diabetes than in those with type 1 diabetes. People from this population often check their glucose level, allowing an association between time and physiological parameters, creating the expectation that a certain amount of time corresponds to a certain change in BGL. The validation of whether the same pattern of results would emerge among people with type 1 diabetes (or even those without diabetes), however, would be essential steps for future models.
The perception of time’s passing, even when incorrect, seems to generate measurable physiological change. We considered a number of other explanations for this result, including failure of the experimental manipulation, task-based artifacts, and elevated stress levels, but found no support in our data for these alternatives. It is possible that some mediating factor is responsible for the observed effect of perceived time on metabolism, but that such a factor is not obvious to us and was not observable in our data. We encourage future efforts to investigate mediating effects more thoroughly.
Something so abstract as the internal representation of time, how we feel the passing of minutes, might at first seem an unlikely candidate as the driver behind basic biochemical processes. Prior research, however, has reached similar conclusions about the effect of cognition over a range of physiological states, including vision and weight loss (4). What, then, sets limits on the degree to which subjective impressions can affect the workings of the human body? At the very least, our findings suggest such constraints are far less restrictive than convention dictates.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.W.S. is a guest editor invited by the Editorial Board.
References
- 1.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colloca L, Miller FG. Role of expectations in health. Curr Opin Psychiatry. 2011;24(2):149–155. doi: 10.1097/YCO.0b013e328343803b. [DOI] [PubMed] [Google Scholar]
- 3.de la Fuente-Fernández R, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 4.Crum AJ, Langer EJ. Mind-set matters: Exercise and the placebo effect. Psychol Sci. 2007;18(2):165–171. doi: 10.1111/j.1467-9280.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 5.Crum AJ, Corbin WR, Brownell KD, Salovey P. Mind over milkshakes: Mindsets, not just nutrients, determine ghrelin response. Health Psychol. 2011;30(4):424–429, discussion 430–431. doi: 10.1037/a0023467. [DOI] [PubMed] [Google Scholar]
- 6.Langer E, Djikic M, Pirson M, Madenci A, Donohue R. Believing is seeing: Using mindlessness (mindfully) to improve visual acuity. Psychol Sci. 2010;21(5):661–666. doi: 10.1177/0956797610366543. [DOI] [PubMed] [Google Scholar]
- 7.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 9.O’Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: The end of the beginning? Science. 2005;307(5708):370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 10.Mokdad AH, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 11.van Dooren FE, et al. Depression and risk of mortality in people with diabetes mellitus: A systematic review and meta-analysis. PLoS One. 2013;8(3):e57058. doi: 10.1371/journal.pone.0057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egede LE, Dismuke CE. Serious psychological distress and diabetes: A review of the literature. Curr Psychiatry Rep. 2012;14(1):15–22. doi: 10.1007/s11920-011-0240-0. [DOI] [PubMed] [Google Scholar]
- 13.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 14.Melanson KJ, Westerterp-Plantenga MS, Saris WH, Smith FJ, Campfield LA. Blood glucose patterns and appetite in time-blinded humans: Carbohydrate versus fat. Am J Physiol. 1999;277(2 Pt 2):R337–R345. doi: 10.1152/ajpregu.1999.277.2.R337. [DOI] [PubMed] [Google Scholar]
- 15.Pomares FB, Creac’h C, Faillenot I, Convers P, Peyron R. How a clock can change your pain? The illusion of duration and pain perception. Pain. 2011;152(1):230–234. doi: 10.1016/j.pain.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Lake JI. Recent advances in understanding emotion-driven temporal distortions. Curr Opin Behav Sci. 2016;8:214–219. doi: 10.1016/j.cobeha.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardello AV, Schutz HG, Lesher LL, Merrill E. Development and testing of a labeled magnitude scale of perceived satiety. Appetite. 2005;44(1):1–13. doi: 10.1016/j.appet.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303(5661):1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 19.Suckale J, Solimena M. Pancreas islets in metabolic signaling—Focus on the beta-cell. Front Biosci. 2008;13:7156–7171. doi: 10.2741/3218. [DOI] [PubMed] [Google Scholar]
- 20.Surwit RS, et al. Stress management improves long-term glycemic control in type 2 diabetes. Diabetes Care. 2002;25(1):30–34. doi: 10.2337/diacare.25.1.30. [DOI] [PubMed] [Google Scholar]
- 21.van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Loeber S, Grosshans M, Herpertz S, Kiefer F, Herpertz SC. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite. 2013;71:32–39. doi: 10.1016/j.appet.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Crowther JH, Sanftner J, Bonifazi DZ, Shepherd KL. The role of daily hassles in binge eating. Int J Eat Disord. 2001;29(4):449–454. doi: 10.1002/eat.1041. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]