Significance
The epigenome influences gene regulation and genome evolution. The DNA methylomes of Arabidopisis hybrids are distinct from both parents; however, how the parental methylomes interact in hybrids is poorly understood. We discovered pervasive, nonadditive DNA methylation changes (“methylation interactions”) throughout the genome in hybrids of Col and C24 Arabidopsis accessions. Methylation interactions correlated with high levels of small interfering RNAs, known components of the RNA-directed DNA methylation (RdDM) pathway. Indeed, abrogation of RdDM activity abolished methylation interactions in filial 1 (F1) hybrids. Methylation interactions have distinct polymorphism frequencies: Regions with increased methylation compared with the parents are highly conserved, whereas regions with decreased methylation are divergent. Our results show that RdDM is required for DNA methylation interactions in hybrids.
Keywords: DNA methylation, RdDM, siRNA, hybrid vigor, methylation interaction
Abstract
DNA methylation is a conserved epigenetic mark in plants and many animals. How parental alleles interact in progeny to influence the epigenome is poorly understood. We analyzed the DNA methylomes of Arabidopsis Col and C24 ecotypes, and their hybrid progeny. Hybrids displayed nonadditive DNA methylation levels, termed methylation interactions, throughout the genome. Approximately 2,500 methylation interactions occurred at regions where parental DNA methylation levels are similar, whereas almost 1,000 were at differentially methylated regions in parents. Methylation interactions were characterized by an abundance of 24-nt small interfering RNAs. Furthermore, dysfunction of the RNA-directed DNA methylation pathway abolished methylation interactions but did not affect the increased biomass observed in hybrid progeny. Methylation interactions correlated with altered genetic variation within the genome, suggesting that they may play a role in genome evolution.
DNA methylation is a conserved epigenetic mark in many eukaryotes (1–6). In plants and mammals, DNA methylation plays important roles in genome stability, genomic imprinting, paramutation, and gene regulation during development and diseases (1–6). Parental genetic alleles interact in the filial 1 (F1) progeny in a Mendelian manner. DNA methylation may affect this interaction such that methylated epialleles may show nonadditive interactions. This notion is supported by recent observations that hybridization results in nonadditive changes in the F1 plant DNA methylome (7, 8). It has been proposed that genome-wide interactions between parental epialleles in F1 hybrids are critical for hybrid vigor, the superior performance of hybrids compared with their parents (8–13). Epigenome interactions may confer nonadditive transcriptional and epigenetic activities in hybrid offspring compared with the parental lines. In contrast to additive regulation, which leads to midparent value (MPV) levels equal to the average of the two parental lines, nonadditive regulation results in a deviation from the MPV, such that the F1 hybrid resembles the high- or low-expression parent. In addition, nonadditive regulation can be transgressive, which is beyond the range of the parental levels (14).
Studies in rice and Arabidopsis have identified nonadditive changes in DNA methylation levels at loci where parental methylation levels are different [differentially methylated regions (DMRs)] (7, 8, 11, 12, 15, 16). These “methylation interactions” were attributed to two mechanisms, transchromosomal methylation (TCM) and transchromosomal demethylation (TCdM), whereby the methylation level of one parental allele is altered to resemble the methylation level of the other parental allele (7, 8, 17). At some genomic loci, the transmethylation events in F1 were shown to be inherited to the next generation, as observed for paramutation (17). Nonetheless, a thorough profile of DNA methylation interactions across the whole Arabidopsis genome has yet to be determined. In particular, it remains unclear whether DNA methylome interactions occur only at DMRs between the two parents.
Plant cytosine DNA methylation occurs in all three sequence contexts, including the symmetrical CG and CHG (H = A, C, T) contexts and the asymmetrical CHH context (2). Establishment of DNA methylation in all three cytosine contexts can be mediated through the RNA-directed DNA methylation (RdDM) pathway, in which the complementary pairing between small interfering RNAs (siRNAs) and scaffold RNAs is thought to determine the target specificity of the DNA methyltransferase domains rearranged methyltransferase 2 (DRM2). The plant-specific RNA polymerase (Pol) Pol IV is essential for producing the majority of 24-nt siRNAs in the RdDM pathway, and another plant-specific RNA polymerase, Pol V, is responsible for transcription of the long noncoding RNAs that function as scaffold RNAs while being tethered to the chromatin (4, 6, 18).
In Arabidopsis F1 hybrids, DNA methylation interactions frequently correlate with changes in siRNA levels (7, 8, 12, 15, 16). Similarly, DNA methylation interactions in rice F1 hybrids were shown to be strongly associated with regions where siRNA production is unequal in the two parents (11). An association between changes in DNA methylation levels and transgressive siRNAs has also been observed in tomato hybrids (19). These observations strongly suggest a role for RdDM in hybrid methylome interactions. However, genetic evidence directly linking RdDM with methylome interactions in hybrids has been lacking. In addition, the role of RdDM in hybrid vigor is unclear.
We analyzed Arabidopsis DNA methylomes and small RNAs (sRNAs) of Col, C24, and their F1 hybrids in both the WT background and in a mutant background that is defective in RdDM. Our analyses yielded important insights into DNA methylome interactions in hybrids, in addition to identifying patterns that are consistent with previous reports. We identified hundreds of TCM and TCdM DMR loci in the F1 progeny. Unexpectedly, however, our results revealed that hybrid methylome interactions are dominated by TCM at thousands of loci, where DNA methylation levels are similar between Col and C24 [similarly methylated regions (SMRs)]. We demonstrate that the RdDM pathway is essential for methylome interactions. Interestingly, TCdM and TCM are positively and negatively linked with genetic variations, suggesting that highly divergent siRNAs from one allele cannot induce methylation of the other allele, whereas conserved siRNAs can trigger methylation. We found that DNA methylation levels change at both parental alleles in hybrids, suggesting that communication between alleles is important for RdDM. Some of the allelic communication is independent of diffusible siRNAs, and our data suggest that RdDM may involve interallelic pairing.
Results
Pipeline to Identify DNA Methylome Interactions in Col/C24 DMRs.
To understand DNA methylation interactions in the F1 hybrids of Col and C24, we generated single-base resolution maps of the DNA methylomes from Col, C24, and their reciprocal F1 hybrids using 14-d-old plants. The overall cytosine methylation levels of Col and C24 are 6.75% and 7.05%, respectively, which are consistent with earlier studies (8, 20). We observed increased DNA methylation levels (8.91% and 9.6%) in reciprocal F1 hybrids of Col and C24 relative to their parents (SI Appendix, Fig. S1A). DNA methylation increased in all three sequence contexts (CG, CHG, and CHH) in both F1 hybrids relative to their parents (SI Appendix, Fig. S1A). The increased methylation level in F1 hybrids relative to parents is consistent with previous observations in Ler × C24 hybrids (7, 8).
To identify regions of methylation interactions in F1 hybrids, we compared the methylomes of each F1 hybrid and the methylomes of their parents. Previous studies had suggested that methylome interactions in F1 hybrids occur in DMRs between parental alleles (7, 8). We identified 10,581 DMRs between Col and C24 parents (Fig. 1A). Among these DMRs, 7,487 showed higher methylation levels in Col than C24, and are referred to as Col_DMRs hereafter. Similarly, the remaining 3094 DMRs with higher methylation levels in C24 are referred to as C24_DMRs.
Fig. 1.
Identification of methylation interactions in hybrids. (A) Pipeline used to determine methylation interactions in F1 hybrids. The Arabidopsis genome was divided into 200-bp bins, where DMRs and SMRs were identified by comparing methylation levels of Col and C24. For each 200-bp DMR or SMR, the methylation level of F1 was compared with the PAV to determine TCM and TCdM regions. The region numbers of each group are indicated at the bottom. (B) Schematic model of methylation patterns of different types of loci, including TCM DMR, TCdM DMR, NI DMR, TCM SMR, and NI SMR. (C) Compositions of NI DMRs, TCM DMRs, TCdM DMRs, NI SMRs, and TCM SMRs.
Because of sequence variations between Col and C24 ecotypes, the amplification efficiency during sequencing and the ratios of mapped reads to sequenced reads could be different between Col and C24 alleles for the same genomic region (SI Appendix, Fig. S1B). To account for these differences in the predicted additive methylation level in F1 hybrids, we determined the weighted methylation levels [predicted additive value (PAV)] instead of the mean methylation level (MPV) for hybrid progeny, as previously described (21) (Materials and Methods). We compared the methylation levels of F1 hybrids with the calculated PAVs for all of the 10,581 DMRs. We found that the methylation levels in F1 hybrids are not significantly different from PAVs [false discovery rate (FDR) > 0.01] at 91.4% of DMRs (9,669), suggesting that there is no methylation interaction at these regions (Fig. 1 A and B). Hereafter, these regions are referred to as noninteraction (NI) DMRs. In contrast, the F1 DNA methylation levels at the remaining 912 DMRs were significantly different (either higher or lower) from PAVs (Fig. 1A), suggesting methylation interactions at these regions. Based on a study by Greaves et al. (7), we classified the methylation interactions as TCM, in which the methylation level in F1 is significantly higher than PAV (FDR < 0.01), and TCdM, in which the methylation level of F1 is significantly lower than PAV (FDR < 0.01) (Fig. 1 A and B). TCM and TCdM refer to allelic interactions of specific chromosomal loci. Of the 912 DMRs, there were 680 TCM DMRs and 232 TCdM DMRs (Fig. 1A). In contrast to NI DMRs, which are enriched within genic regions, TCM and TCdM DMRs tend to reside in transposable elements (TEs), intergenic regions, and 1 kb upstream and 1 kb downstream of gene regions (Fig. 1C).
Characterization and Validation of DNA Methylation Interactions in Col/C24 DMRs.
To characterize TCM and TCdM DMRs further, we calculated the DNA methylation levels of high-parent alleles, low-parent alleles, and reciprocal F1 hybrids. We also calculated the PAV and MPVs of F1 hybrids for each DMR. We found that the F1 methylation levels are significantly higher and lower than PAVs/MPVs at TCM and TCdM DMRs, respectively (Fig. 2A and SI Appendix, Fig. S6A). We also analyzed allele-specific DNA methylation of NI DMRs, which revealed parental levels of allele-specific methylation in F1 and Mendelian segregation in F2 progenies (SI Appendix, Fig. S1C and Table S1).
Fig. 2.
Characterization of methylation interactions in DMRs. (A) Methylation levels of TCM DMRs and TCdM DMRs are shown. The high parent is the parent with a higher methylation level at the DMR. The low parent is the parent with a lower methylation level at the DMR. (B) Allele-specific analysis of methylation levels of TCM DMRs and TCdM DMRs. The high allele is the allele from the high parent. The low allele is the allele from the low parent. DNA methylation levels of a TCM DMR (C) and a TCdM DMR (D) (boxed regions) in Col, C24, F1 hybrids, nrpd1nrpe1 (Col), nrpd1nrpe1 (C24), and nrpd1nrpe1 F1 hybrids. Integrative Genomics Viewer (IGV) screen shots of whole-genome bisulfite sequencing data are shown. Vertical bars on each track indicate DNA methylation levels. (Bottom) Levels of DNA methylation and siRNAs of boxed regions were quantified from the whole-genome data and are shown. F1 and F1r indicate where the maternal line is Col and C24, respectively. Chr, Chromosome.
At TCM DMRs, both high-parent and low-parent alleles of F1 have higher methylation levels relative to their parents and it seems that both alleles contribute to the increased methylation levels in F1 hybrids (Fig. 2B). For example, at the TCM DMR (a C24_DMR) near At3g20830, the Col allele is unmethylated in the Col parent but gains methylation in reciprocal F1 hybrids, and the C24 allele is highly methylated in the C24 parent and hybrids (Fig. 2C and SI Appendix, Fig. S2A). We further assayed the DNA methylation status of F2 offspring at this locus, along with another TCM-DMR locus (a Col_DMR; SI Appendix, Fig. S2D), using allele-specific methylation-sensitive restriction enzyme PCR (Chop-PCR). Interestingly, all of the samples of F2 populations are methylated at the two loci (Table 1), thus displaying a non-Mendelian inheritance pattern that is reminiscent of paramutation (22).
Table 1.
Segregation of DNA methylation in F2 for TCM DMRs
| Loci | F2 types | WT | nrpd1nrpe1 | |||
| High | Low | High | Low | P value (χ2 test) | ||
| Chr.3:7286624–7286946 | Col × C24 | 44 | 0 | 32 | 12 | 3:1, P = 0.73 |
| C24 × Col | 44 | 0 | 34 | 10 | 3:1, P = 0.73 | |
| Chr.5:9280943–9281312 | Col × C24 | 43 | 0 | 31 | 13 | 3:1, P = 0.49 |
| C24 × Col | 43 | 0 | 32 | 12 | 3:1, P = 0.73 | |
Chr., Chromosome.
We also analyzed allele-specific methylation of TCdM DMRs and found that the decreased methylation level of these regions relative to the PAV in F1 arises mainly from a reduction in methylation of high-parent alleles (Fig. 2B). For example, at the TCdM DMR near At5TE58360, the methylation of the low-parent (Col) allele remains low in F1, whereas the high-parent (C24) allele shows a decreased methylation level in F1 relative to the C24 parent (Fig. 2D). This pattern of methylation change was validated by Chop-PCR and cleaved amplified polymorphic sequence (CAPS)-PCR (SI Appendix, Fig. S2B).
Identification and Characterization of Methylation Interactions in Col/C24 SMRs.
We noticed that the methylation levels of genome-wide mCG, mCHG, and mCHH were elevated in F1 hybrids relative to parents (SI Appendix, Fig. S1A); however, we identified only 680 TCM DMRs (Fig. 1A), which show increased DNA methylation in F1. Non-DMRs (or SMRs) are more widespread than Col/C24 DMRs in the Arabidopsis genome (575,977 SMRs vs. 10,581 DMRs), so we investigated whether methylation interactions occur in SMRs. Using the same approach that identified TCM and TCdM at DMRs, we uncovered 2,457 TCM and 15 TCdM SMRs (Fig. 3A).
Fig. 3.
Characterization of methylation interactions in SMRs. (A) Pie chart of methylation interaction regions in F1. (B) Methylation levels of mCG, mCHG, mCHH, and total mC of TCM SMRs in Col, C24, and F1 hybrids. (C) Allele-specific analysis of methylation levels of TCM SMRs in F1 hybrids. (D) DNA methylation levels of a TCM SMR (boxed region) in Col, C24, F1 hybrids, nrpd1nrpe1 (Col), nrpd1nrpe1 (C24), and nrpd1nrpe1 F1 hybrids. (Bottom) DNA methylation levels of the boxed region were quantified from the whole-genome bisulfite sequencing data and are shown.
As expected, the methylation levels of Col and C24 parents are comparable at SMRs (Fig. 3B). Compared with NI SMRs, TCM SMRs have significantly higher DNA methylation levels in F1 hybrids relative to both parents (Fig. 3B). As in DMRs, we observed methylation interactions in SMRs at all three cytosine contexts (i.e., mCG, mCHG, mCHH) (Fig. 3B). Further, allele-specific analysis revealed that DNA methylation is increased in both alleles at TCM SMRs in F1 hybrids (Fig. 3C). We evaluated a specific TCM SMR on chromosome 4 and found that the parents have similar methylation levels, whereas the F1 hybrids showed increased methylation levels relative to PAV and to both parents (Fig. 3D), consistent with the statistical results (Fig. 3C).
We determined that NI SMRs are enriched within genic regions, whereas the majority of TCM SMRs are within TEs, intergenic regions, and 1 kb upstream or downstream of genes, similar to TCM and TCdM DMRs (Fig. 1C). However, TCM SMRs are mainly located in heterochromatic pericentromeric regions, whereas TCM/TCdM DMRs are distributed in euchromatin (SI Appendix, Fig. S3A). We also found that TCM SMRs are enriched for rolling circle transposons/Helitrons and depleted for LTR/Gypsy TEs (SI Appendix, Fig. S3B). Further analysis showed that TCM SMRs are enriched at the edges of long TEs (SI Appendix, Fig. S3C), which are targets of RdDM (23), suggesting a potential connection between the methylation interactions and RdDM.
Methylation Interactions Associate with siRNAs.
To investigate a possible role for RdDM and 24-nt siRNAs in hybrid methylome interactions, we sequenced siRNA libraries of Col, C24, and their reciprocal F1 hybrids (SI Appendix, Table S5). Consistent with previous studies (7, 8), we found that 21-nt siRNAs and 24-nt siRNAs were the most abundant siRNAs in all sequenced samples (SI Appendix, Fig. S4A).
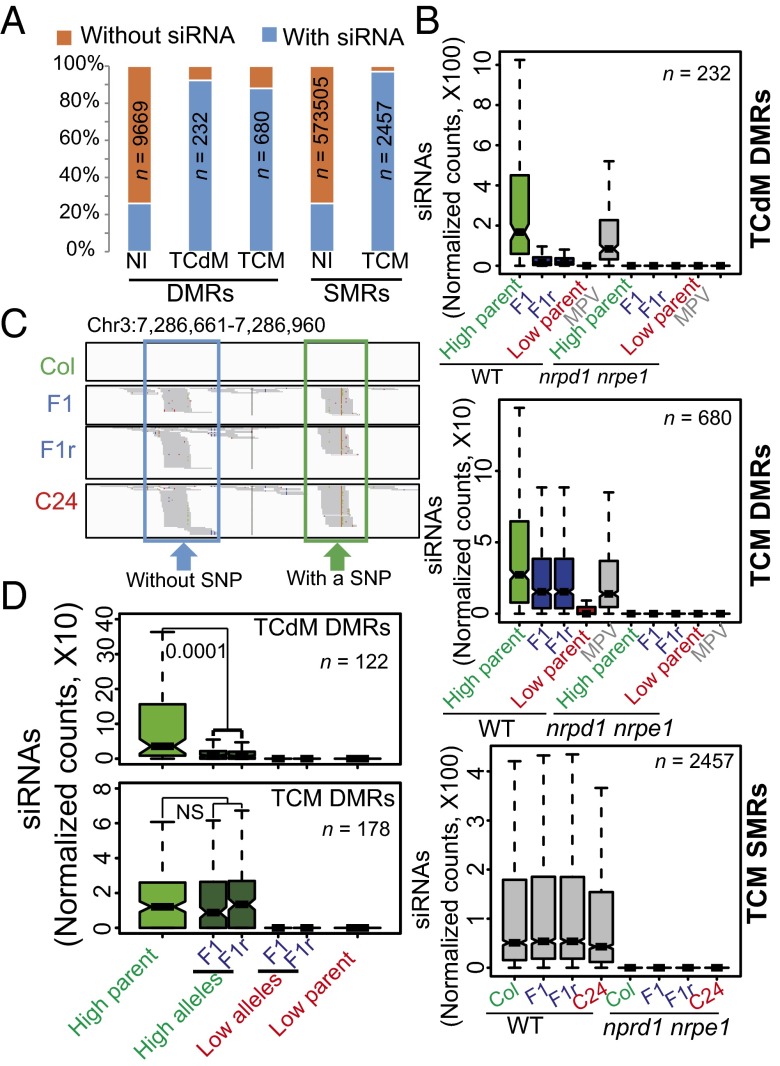
We observed 24-nt siRNAs at ∼88% (600) of TCM DMRs and 96% (223) of TCdM DMRs in F1 hybrids. In contrast, significantly fewer NI DMRs have 24-nt siRNAs in F1 hybrids (about 26%; Fig. 4A and SI Appendix, Fig. S4B). These results suggested that 24-nt siRNAs are associated with DNA methylation interactions in DMRs, which is consistent with previous findings in Ler × C24 hybrids (7, 8). We compared siRNA levels in high-parent alleles, low-parent alleles, and F1 hybrids. The MPV of siRNAs for each DMR was plotted and served as a control. The level of siRNAs in the DNA methylation high-parent allele is significantly greater than the level of siRNAs in the DNA methylation low-parent allele at both TCM and TCdM DMRs (Fig. 4B). In F1 hybrids, most of the TCM and TCdM DMRs associated with siRNAs also have siRNAs in their high-parent allele. In contrast, only about 25% of the TCM and TCdM DMRs have siRNAs in their low-parent allele. The siRNA levels are much lower at TCdM DMRs relative to the MPV in F1 hybrids (Fig. 4B), which may contribute to their decreased methylation levels (Fig. 2A and SI Appendix, Fig. S6A). TCM DMRs have similar siRNA levels relative to the MPV in F1 hybrids (Fig. 4B), suggesting that the increase in F1 DNA methylation level at TCM DMRs (Fig. 2A and SI Appendix, Fig. S6A) is not caused by an increase in siRNA levels.
Fig. 4.
Analysis of 24-nt siRNAs associated with methylation interaction regions. (A) Percentages of regions with or without siRNAs in NI DMRs, TCM DMRs, TCdM DMRs, NI SMRs, and TCM SMRs. The siRNA enrichment in the high parent was used for each DMR, and the siRNA enrichment in the Col parent was used for each SMR. (B) Abundances of 24-nt siRNAs at TCM DMRs, TCdM DMRs, and TCM SMRs in the parents and F1 hybrids in WT and nrpd1nrpe1. (C) Screen shot from siRNA sequencing that shows siRNA abundance of a TCM DMR region in Fig. 2C. The SNP in the green boxed region is indicated by a red line. (D) Allele-specific analysis of siRNAs in TCdM and TCM DMRs. siRNA abundance was calculated as reads per million (RPM). P > 0.05. NS, not significant.
Although the overall siRNA level in F1 hybrids is not significantly different from the MPV at TCM DMRs, we investigated whether siRNAs from one allele could induce siRNA biogenesis from the other allele in F1. Allele-specific analysis of siRNAs is challenging, given the low frequency of Col/C24 SNPs (around 1/200 bp, on average) relative to the short length of siRNAs (24 nt). Thus, only about 16% of TCM DMRs displayed Col/C24 SNPs within siRNA sequencing reads. At one TCM DMR that we examined, the siRNAs in F1 hybrids were generated exclusively from the C24 allele. However, the methylation level of the Col allele at this TCM DMR is increased in F1 hybrids relative to the Col parent, even though Col-specific siRNAs are not detected (Figs. 2C and 4C). We also examined other TCM DMRs, and found that when siRNAs are detected in only one parent (always the high-parent allele), all siRNAs detected in F1 hybrids are also from this parental allele (Fig. 4D). These results suggest that at F1 TCM DMRs, siRNAs generated from one allele are sufficient to trigger TCM without induction of siRNA biogenesis from the other allele. Similarly, in the TCdM DMRs, if there are siRNAs expressed in only one of the parents (always in the high-parent allele), all siRNAs detected in F1 hybrids are from this parental allele; however, the F1 siRNA levels are reduced relative to the siRNA levels in the parent (Fig. 4D).
To investigate whether siRNAs may also play a role in the TCM at SMRs, we compared siRNA levels at TCM and NI SMRs. We found that about 26% of NI SMRs contain siRNAs in F1 hybrids, compared with 97% of TCM SMRs (Fig. 4A). The levels of siRNAs at the NI SMRs (n = 157,724) are comparable between parents and F1 hybrids, and much lower than at TCM SMRs (SI Appendix, Fig. S4C). Intriguingly, although the methylation levels at TCM SMRs are higher in F1 hybrids than in their parents (SI Appendix, Fig. S3D), the siRNA levels at these regions are similar in F1 and parents (Fig. 4B and SI Appendix, Fig. S4D).
Together, our results suggest that siRNAs are associated with methylation interactions at both DMRs and SMRs, and that siRNAs from one allele may induce DNA methylation at the other allele in F1 hybrids.
Pol IV and Pol V Are Required for Methylation Interactions.
These and previous findings (7, 8) support a role for RdDM in DNA methylation interactions in hybrids. To determine if the RdDM pathway is required for DNA methylation interactions in F1 hybrids, we analyzed parent and hybrid mutant plants deficient in RdDM activity. NRPD1 and NRPE1 are the unique, largest subunits of Pol IV and Pol V, respectively. We generated single-base resolution maps of the DNA methylomes of nrpd1nrpe1 (Col), nrpd1nrpe1 (C24) (24), and their reciprocal F1 hybrids (SI Appendix, Tables S2–S4). We identified targets of NRPD1 and NRPE1 with reduced siRNA abundance in nrpd1nrpe1 (Col or C24; Materials and Methods), and found that 78% of the regions with methylation interactions overlapped with NRPD1 and NRPE1 targets.
To investigate the influence of NRPD1 and NRPE1 mutations on methylation interactions in F1 hybrids, we examined the methylation status of TCdM DMRs, TCM DMRs, and TCM SMRs in nrpd1nrpe1 F1 hybrids. At TCdM DMRs, WT plants showed reduced methylation levels in F1 hybrids relative to PAV and MPV (Fig. 2A and SI Appendix, Fig. S6A), whereas the methylation levels at these regions in nrpd1nrpe1 F1 hybrids were not significantly different from PAV or MPV (Fig. 5A and SI Appendix, Fig. S6B). At TCM DMRs and TCM SMRs, WT F1 hybrids have significantly increased methylation levels relative to PAV and MPV (Figs. 2A and 3 B and C and SI Appendix, Fig. S6A); however, this increase disappeared in nrpd1nrpe1 F1 hybrids (Fig. 5A and SI Appendix, Figs. S5A and S6B). We compared log2fold change (F1/PAV) of mCG, mCHG, and mCHH levels for WT and nrpd1nrpe1 F1 hybrids. The observed deviation of methylation levels in F1 from PAV and MPV in WT plants was diminished in nrpd1nrpe1 at all three sequence contexts, as indicated by the peak at “0,” which represents no methylation interaction (Fig. 5B and SI Appendix, Fig. S6C). This pattern was consistently observed for TCM DMRs, TCdM DMRs, and TCM SMRs (Fig. 5B), suggesting that all methylation interactions were dependent on Pol IV and Pol V. This conclusion is also supported by the examination of individual loci (Figs. 2 C and D and 3D).
Fig. 5.
Characterization of TCM DMRs, TCdM DMRs, and TCM SMRs in Arabidopsis mutants with dysfunctional Pol IV and Pol V. (A) Methylation levels of TCdM DMRs, TCM DMRs, and TCM SMRs in nrpd1nrpe1 (Col), nrpd1nrpe1 (C24), and nrpd1nrpe1 F1 hybrids. Compared with Figs. 2A and 3B, the change of methylation level in F1 hybrids relative to the PAVs diminishes in the absence of Pol IV and Pol V. (B) For TCdM DMRs, TCM DMRs, and TCM SMRs, the distribution of log2FC(F1/PAV) for mCG, mCHG, and mCHH is shown for WT and nrpd1nrpe1. A peak at “0” corresponds to no methylation interaction. (C) Levels of siRNAs and mCHH at TCdM DMRs, TCM DMRs, and TCM SMRs in Col, nrpd1, nrpe1, and nrpd1nrpe1. Only regions with siRNAs in Col are used. *P ≤ 0.01 indicates statistical significance (two-tailed t test). (D) Abundance of siRNAs for each indicated group of regions. (Left) Targets of Pol IV and Pol V have higher siRNA accumulation than regions that are not targets of Pol IV and Pol V. (Right) Among targets of Pol IV and Pol V, regions with methylation interactions in F1 have higher siRNA levels than other target regions of Pol IV and Pol V. P values < 0.001 indicate statistical significance (two-tailed t test). (E) Distribution of methylation interaction regions in four groups of Pol IV/Pol V targets that are ranked by siRNA levels. Regions with higher siRNA levels are enriched for methylation interactions. The pie chart indicates the proportions of different siRNA quartiles in methylation interaction regions that contain siRNAs.
Although the methylation levels of TCM DMRs, TCdM DMRs, and TCM SMRs in nrpd1nrpe1 F1 hybrids were not significantly different from PAV, it was possible that there were too few methylation interactions in nrpd1nrpe1 F1 to achieve statistical significance by the box plot analysis. To test this possibility, we used our original approach to identify methylation interactions using the methylomes of nrpd1nrpe1 (Col), nrpd1nrpe1 (C24), and nrpd1nrpe1 F1 hybrids. Only 0.9% of the TCM DMRs, 5% of the TCdM DMRs, and 2% of the TCM SMRs remained to be defined as regions with methylation interactions in nrpd1nrpe1 F1 hybrids. The nrpd1nrpe1 (Col) and nrpd1nrpe1 (C24) are strong RdDM mutants; therefore, the difference in methylation between nrpd1nrpe1 (Col) and nrpd1nrpe1 (C24) might be less than the difference in methylation between Col and C24, which may decrease statistical power to identify TCM and TCdM in the mutant hybrids compared with the WT hybrids. To exclude this possibility, we examined the methylation difference between nrpd1nrpe1 (Col) and nrpd1nrpe1 (C24) by identifying DMRs. However, we did not observe a decreased difference in methylation between mutant parents relative to WT parents. We identified 11,113 DMRs between nrpd1nrpe1 (Col) and nrpd1nrpe1 (C24), which is comparable with the number (10,581) between WT parents. These findings indicate that nearly all methylation interactions depend on Pol IV and Pol V, and thus on the canonical RdDM pathway.
The DNA methylation patterns in WT hybrids at TCM-DMR loci showed paramutation-like inheritance (Fig. 2C and Table 1). To examine whether this inheritance pattern is affected in nprd1nrpe1 mutants, we examined the allele-specific DNA methylation patterns of their F2 offspring for the two TCM-DMR loci in the nrpd1nrpe1 double mutant. Mutations in NRPD1 and NRPE1 resulted in a 3:1 ratio of methylated samples to unmethylated samples in the F2 population, indicating that the paramutation-like inheritance of methylation patterns in the TCM DMR loci is also dependent on Pol IV and Pol V (Fig. 2C and Table 1).
Previous studies have shown that some 24-nt siRNAs were reduced in Pol IV and Pol V mutants (Pol IV/Pol V siRNA), whereas other 24-nt siRNAs were reduced only in the Pol IV mutant (Pol IV-only siRNA) (25). We characterized TCM DMR, TCdM DMR, and TCM SMR-associated siRNAs, and found that TCdM DMR- and TCM SMR-associated siRNAs depend on both Pol IV and Pol V (Fig. 5C). In contrast, TCM DMR-associated siRNAs seemed to depend on Pol IV but not Pol V (Fig. 5C). A similar pattern was observed by using siRNA data from Law et al. (25) (SI Appendix, Fig. S5B). TCdM DMRs, which are associated with Pol IV/Pol V siRNAs, have dramatically reduced mCHH methylation levels in Pol IV and Pol V mutants, whereas TCM DMRs, which are associated with Pol IV-only siRNAs, have slightly reduced mCHH methylation levels in Pol IV and Pol V mutants (Fig. 5C). Intriguingly, the absence of Pol IV/Pol V siRNAs in TCM SMRs caused only a slight change of mCHH methylation levels in Pol IV and Pol V mutants (Fig. 5C). These results indicate that the features of siRNAs and mCHH methylation in a region (DMR or SMR) help to characterize TCM and TCdM in F1 hybrids.
Next, we investigated whether all of the targets of NRPD1 and NRPE1 show methylation interactions in the F1 hybrids. We found that of the 70,157 regions targeted by RdDM (200-bp bins showing reduction in siRNAs in nrpd1nrpe1; Materials and Methods), only 2,288 regions correspond to TCM DMRs, TCM SMRs, or TCdM DMRs. We found that the siRNA abundance was significantly higher at RdDM target regions that show methylation interactions (Fig. 5D). We ranked all Pol IV/Pol V target regions by their siRNA abundance from high to low into four groups, and found that the majority (70%) of the methylation interaction regions were among the higher ranked groups (first- and second-ranked groups) (Fig. 5E). Therefore, methylation interactions in hybrids are characterized by high levels of siRNAs.
Genetic Variations Between Col and C24 Associate with Methylation Interactions.
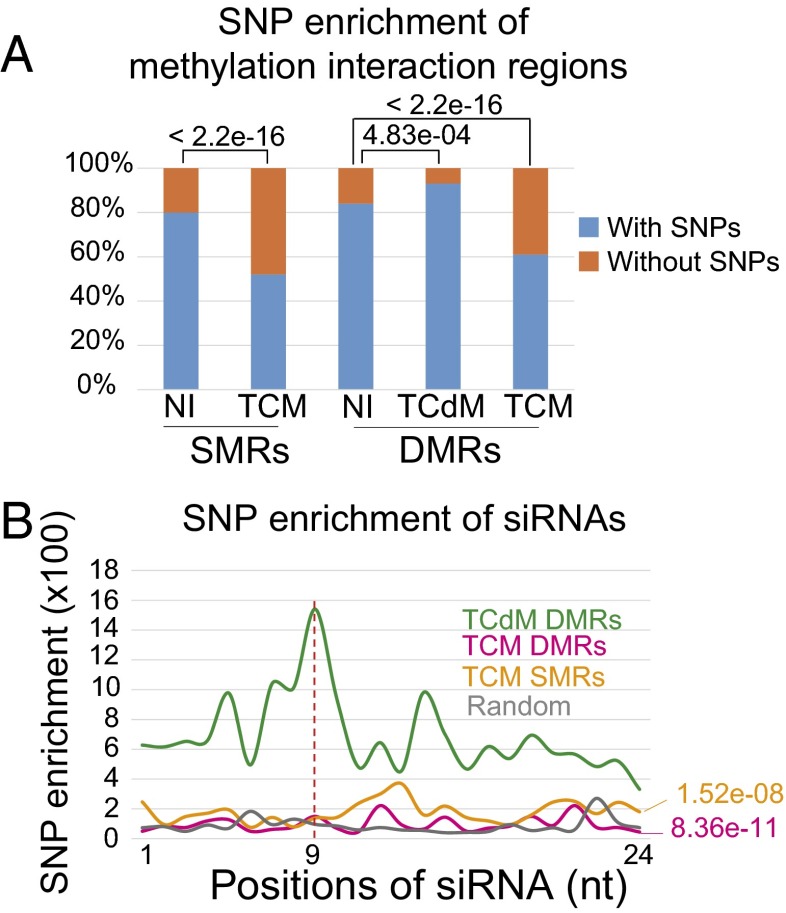
Genetic variation has been suggested to be associated with epigenetic variation, such as differential DNA methylation (26, 27). We asked whether genetic variation is also associated with methylation interactions in F1 hybrids. Col/C24 SNPs were used to estimate the genetic variation for a region. The number of SNPs within a region and its flanking 1-kb region was calculated as described previously (28). We found that about 87% of NI DMRs and 80% of NI SMRs contained SNPs (Fig. 6A). Interestingly, this percentage was 95% for TCdM DMRs, which is significantly different from the NI DMRs (Fig. 6A), but decreased to 62% in TCM DMRs and to 53% in TCM SMRs (Fig. 6A). These results indicate that TCdM DMRs tend to occur in regions with more genetic variations between the two parents, whereas TCM DMRs and SMRs are enriched in regions with fewer genetic variations.
Fig. 6.
Methylation interaction is associated with genetic variation. (A) Percentages of regions with or without SNPs in NI SMRs, NI DMRs, TCdM DMRs, TCM DMRs, and TCM SMRs. The P values were calculated by the Fisher exact test and are shown in the corresponding pairwise comparisons. (B) Distribution of SNPs along siRNAs that are associated with TCdM DMRs, TCM-DMRs, and TCM SMRs. The dashed red line indicates a peak of SNP at the ninth nucleotide position of TCdM-associated siRNAs. For enrichment analysis, number of siRNA reads with an SNP at each position was normalized by the total number of siRNA reads (SNP enrichment = 1 million × SNP reads/total siRNA reads). The random line was obtained from values of siRNAs randomly selected from the whole genome. The P values corresponding to differences between TCdM DMRs and corresponding regions were calculated using a z-test, and are shown beside the corresponding line with color highlights.
We suspected that genetic variations between the two parents may affect the targeting of siRNAs from one parental allele to the other parental allele in F1 hybrids. Less genetic variation would be predicted to facilitate mutual targeting of siRNAs from one allele to the other allele to cause TCM at DMRs and SMRs; on the other hand, more genetic variation would be predicted to impede the targeting of siRNAs from one allele to the other allele, thus triggering TCdM at DMRs. To test this hypothesis, we identified SNPs within siRNA sequences and aligned siRNAs from TCM DMRs, TCM SMRs, and TCdM DMRs to perform SNP enrichment analysis. We found that TCM DMRs and TCM SMRs have comparable SNP enrichment in their associated siRNAs (Fig. 6B). In contrast, TCdM DMRs have a much higher SNP enrichment in siRNAs compared with TCM DMR and SMR regions, with a peak at the ninth nucleotide of siRNAs (Fig. 6B). Therefore, TCdM DMRs and TCM DMR/SMRs are positively and negatively associated with genetic variations between the parents, respectively, and TCdM DMRs correlate with an exceedingly high polymorphism in siRNAs.
RdDM Is Not Required for Establishment of Hybrid Vigor in Early Seedling Growth.
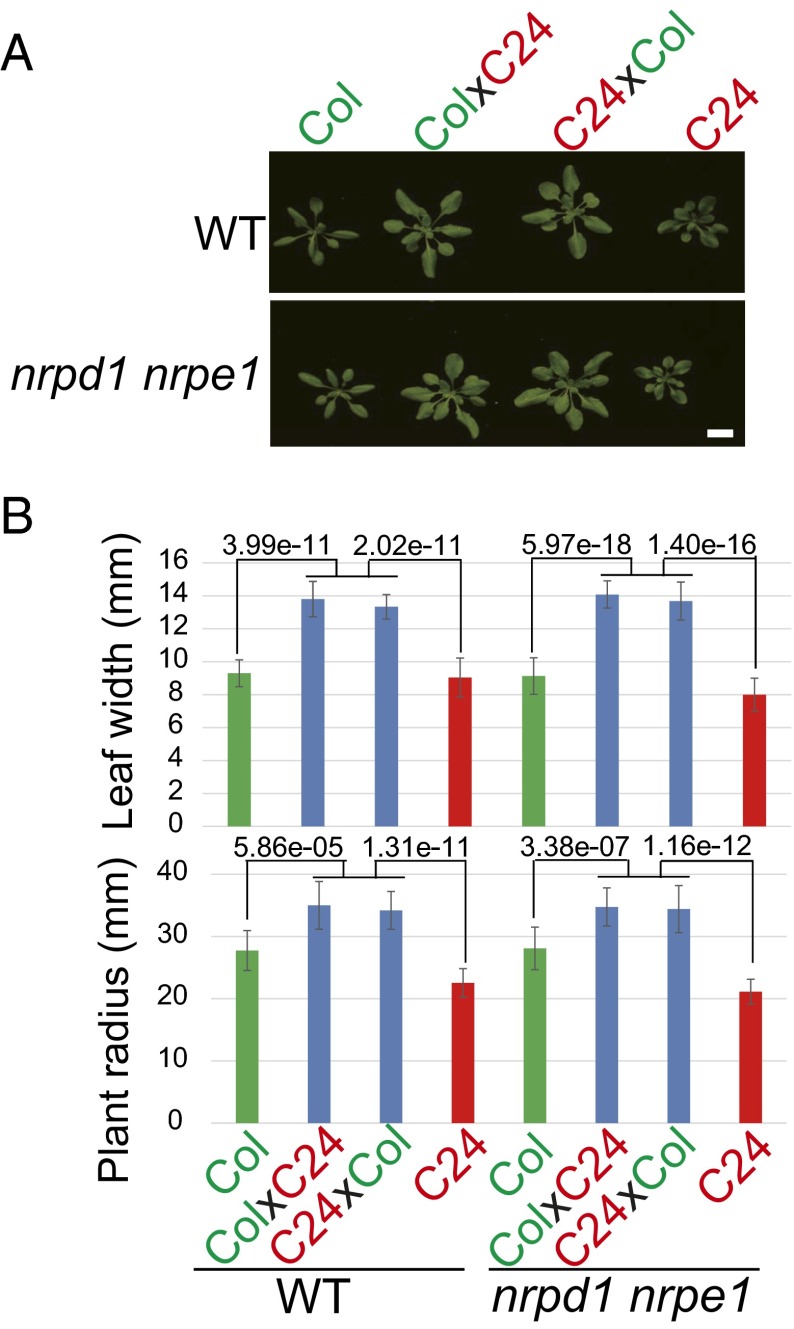
Previous studies suggested that the RdDM pathway may be involved in the regulation of hybrid vigor of Arabidopsis in early developmental stages (8, 12). Our current findings show that RdDM is required for DNA methylation interactions in the hybrids. To examine the effects of RdDM pathway on hybrid vigor genetically, we compared F1 hybrids derived from the nrpd1nrpe1 double mutant with F1 hybrids from WT plants, focusing on several traits related to early seedling growth vigor, such as leaf and rosette sizes. Consistent with previous reports (29, 30), F1 seedlings from the WT crosses showed obvious heterosis compared with both Col and C24 parents in plant and leaf sizes (P < 0.0001, t test; Fig. 7). In the nrpd1nrpe1 double-mutant background, the mutant F1 seedlings were all also bigger than their parents (Col and C24) (P < 0.0001, t test; Fig. 7), with a similar growth pattern to the growth pattern of WT F1 hybrids (P > 0.05, t test). Therefore, the RdDM pathway and RdDM-mediated methylation interactions are not required for heterosis in Arabidopsis F1 hybrids in early seedling growth.
Fig. 7.
Seedling growth heterosis in WT and nrpd1nrpe1 plants. (A) Representative plants of Col, C24, and their F1 hybrids in WT and nrpd1nrpe1. Twenty-day-old seedlings are shown. (Scale bar, 2 cm.) (B) Heterosis was quantitatively measured as leaf width (Upper) and rosette radius (Lower) in WT and nrpd1nrpe1. Error bars are SD (n = 12–20). P < 0.001 indicates statistical significance (two-tailed t test).
Discussion
Recent studies have emphasized DNA methylome interactions in an effort to understand the molecular basis of hybrid vigor (8, 12). DNA methylation interactions in F1 hybrids have been identified at some DMRs between the two parents, where TCM or TCdM alters DNA methylation on one allele to resemble the other allele. Consistent with these reports, our analyses also identified methylation interactions at hundreds of DMRs. Unexpectedly, our analyses revealed that DNA methylation interactions also exist in thousands of SMR loci, demonstrating a dominant role of transgressive DNA methylation in hybrid methylome interactions.
In our analysis, methylation interaction regions were identified as chromosome fragments of at least 200 bp, so as to focus on clustered TCM or TCdM cytosines within DMRs or SMRs. This analysis pipeline is consistent with the notion that heritable changes in the methylation status of cytosine clusters are functionally more relevant than individual cytosines (31, 32). In addition to identifying SMRs as major loci with methylation interactions, our genetic analyses using the nrpd1nrpe1 double mutant demonstrated a critical role for RdDM in hybrid methylome interactions. RdDM is required for almost all DNA methylation interactions, as shown by the loss of TCM and TCdM patterns at DMRs and SMRs in the nrpd1nrpe1 mutant F1 hybrids (Fig. 5 A and B). On the other hand, only a subset of RdDM target loci constitutes methylation interaction regions. TCM/TCdM RdDM loci have higher levels of siRNAs than RdDM loci without methylation interactions (Fig. 5D). Indeed, the relative abundance of siRNAs at RdDM loci indicates the probability of methylation interactions in Arabidopsis hybrids.
Our analyses revealed that TCdM DMRs are characterized by high genetic variation between the two parents (Fig. 6A) such that the siRNAs from the high-parent allele are too divergent and likely cannot target the low-parent allele to induce methylation. Intriguingly, methylation of the high-parent allele is not maintained (Fig. 2B), thus causing an overall decrease in methylation in the hybrids. This observation suggests that RdDM of one allele may require the participation of the other allele. It seems that at RdDM-dependent DMRs, a productive interaction between the alleles is required to maintain the DNA methylation of the high allele, but this productive interaction does not happen at the TCdM loci due to the divergence of the two alleles. Our analyses also revealed that overall siRNA levels at TCM DMRs in hybrids are similar to parental siRNA MPVs (Fig. 4B), whereas methylation levels at these regions are higher than parental methylation MPVs. This increase in DNA methylation without increase in siRNAs may be due to transallelic action of siRNAs (i.e., siRNAs from one parent causing DNA methylation not only at the parent allele but also at the other allele, resulting in increased total DNA methylation but not siRNA levels). In addition to causing the methylation increase of the low-parent allele, our analysis showed that there is an increase in the methylation level of the high-parent allele at TCM DMRs in the hybrids (Fig. 2B). The newly gained methylation in the low-parent allele may be important for the methylation increase in the high-parent allele.
We do not know how the low-parent allele may participate in the RdDM of the high-parent allele. However, because the role of the low-parent allele is not to provide the mobile siRNAs, we propose that the two alleles must interact through physical association. Our results thus suggest that RdDM in plants may involve interallelic pairing. Numerous other epigenetic phenomena, such as olfactory receptor choice, X-chromosome inactivation, and gene regulation, also appear to involve interallelic pairing (33, 34). X-chromosome inactivation and RdDM have been shown to require structural maintenance of chromosomes (SMC) proteins, which are involved in higher order chromosome structure and dynamics, such as sister chromatid pairing and chromosome condensation (35, 36). SMC proteins may promote interallelic pairing and feedback regulation in these RNA-mediated epigenetic processes. Alternatively, the activity of the two alleles may be controlled by epistatic interactions or developmental regulation.
Our analyses of allele-specific DNA methylation revealed that DMRs without methylation interactions showed parental levels of allele-specific methylation in F1 and Mendelian segregation in F2 progenies (SI Appendix, Fig. S1C and Table S1), indicating that these DMRs are inherited stably, and thus can be used as epigenetic markers in genetic analysis. For instance, ddm1-induced DMR markers have been applied in mapping complex traits, including root length and flowering time, that are associated with heritable epimutations in Arabidopsis (31). In contrast, the DNA methylation patterns of TCM DMRs exhibited a paramutation-like behavior in F1 and F2 (Fig. 2C, Table 1, and SI Appendix, Fig. S2D). Remarkably, RdDM dysfunction changed the pattern of inheritance from paramutation-like to Mendelian inheritance. A previous study (37) did not find significant evidence for paramutation in soybean F2s, possibly because the methylome sequencing had low coverage.
Notwithstanding the critical role of RdDM in hybrid DNA methylome interactions, our analyses provided genetic evidence that RdDM is not required for hybrid vigor in Arabidopsis Col × C24 hybrids, at least at the stage of early seedling growth (Fig. 7). This result is not inconsistent with previous findings. For instance, although F1 hybrids that are defective in RdDM lose the parent-of-origin effects on circadian rhythm, they still display hybrid vigor compared with their parents (38). Greaves et al. (7) also described methylation interactions in hybrids. Changes in DNA methylation were found to correlate with changes in gene expression, but a role for the methylation interactions in hybrid vigor was not tested. It has been shown that the RNA methyltransferase HEN1 (8), which is crucial for the biogenesis of sRNAs, including both siRNAs and microRNAs (miRNAs), is required for hybrid vigor. However, it is unclear whether this requirement for HEN1 arises from its role in the RdDM pathway or miRNA pathway. The F1 hybrids generated by reciprocal crosses of maize B73 and Mo17 show clear hybrid vigor and an alteration of 24-nt siRNA profiles compared with their parents (39). Consistent with our findings in Arabidopsis, hybrid vigor is fully maintained even upon disruption of MOP1, the maize ortholog of RDR2, in both B73 and Mo17, despite a global reduction in 24-nt siRNAs. Thus, RdDM also does not appear to be involved in hybrid vigor regulation in maize. These conclusions are consistent with the observation that despite important roles of RdDM in establishing genomic DNA methylation patterns and in silencing TEs and transgenes, it is not critical for plant growth and development in Arabidopsis (6). However, it remains possible that certain RdDM-independent methylation interactions or other types of epigenetic interactions may be important for hybrid vigor. Recently, using a similar pipeline as ours, Rigal et al. (40) also identified more than 10,000 regions of nonadditively inherited DNA methylation in F1 epihybrids by crossing a met-induced hypomethylated genome with a normally methylated WT individual MET1+. Furthermore, although met1 and MET1+ show drastically different DNA methylation patterns and DNA methylation interaction after hybridization, their F1 epihybrids are not superior to the parents in biomass. Thus, hybrid vigor is not simply induced by DNA methylation interactions.
Although DNA methylation interactions are not critical for hybrid vigor, they are clearly important for the establishment of DNA methylation patterns in the hybrid. DNA methylation patterns regulate gene expression and influence genetic variations during evolution (41, 42). Thus, due to their important role in the formation of the DNA methylome in hybrids, DNA methylation interactions may represent an important factor in genome evolution and hybridization-facilitated speciation.
Materials and Methods
Plant Materials and Growth.
Arabidopsis seeds were stratified for 7 d at 4 °C and grown on 1/2 Murashige and Skoog (MS) nutrient medium containing 0.6% agar under long-day conditions (16-h light/8-h dark cycle at 22 °C). For next-generation sequencing, 2-wk-old seedlings were flash-frozen in liquid nitrogen, ground to powder, and stored at −80 °C. To analyze the effects of the RdDM pathway on hybrid vigor, 7-d-old seedlings grown on half-MS medium were moved to soil to grow for 2 wk for further quantification; the largest leaf of each plant was selected for the quantification of leaf width and plant radius.
Genotyping of Mutants and Hybrids.
Mutants and their F1 hybrids were genotyped according to nrpd1 and nrpe1 mutant information (SI Appendix, Table S2), and then at least one natural variant site was selected between Col and C24 to confirm further that the F1 progenies were really from hybridization of Col and C24. Primers and enzymes used in genotyping are listed in SI Appendix, Table S3.
Next-Generation Sequencing and Data Analyses.
Genomic DNA and RNA were extracted from 2-wk-old seedlings, and shipped to the Beijing Genomics Institute (Shenzhen, China) for whole-genome bisulfite sequencing and sRNA sequencing. The parent that was used in methylome sequencing came from the same batch of seeds as the parent used in the crosses.
For bisulfite sequencing data processing, adapter and low-quality reads (q < 20) were trimmed first. Then, the remaining clean reads were mapped to the Col-0 TAIR10 Arabidopsis thaliana genome by BSMAP aligner, allowing up to four mismatches (43). Unique mapped reads were extracted to determine the cytosine methylation level as previously described (20), and only those cytosines with more than four reads in a library were used for our study.
Analysis of sRNA data was carried out according to an earlier study (44). In brief, clean reads were aligned to The Arabidopsis Information Resource 10 (TAIR10) genome assembly using the Burrows–Wheeler alignment tool (BWA) (45) with fault arguments, and reads not mapped to tRNA and rRNA were retained. Then, we removed the sRNA reads aligned to Arabidopsis miRNA from miRBase (46), and the remaining reads were used for subsequent analysis. Counts of 24-nt siRNAs for each locus were normalized by the total number of mapping reads as the library size. About 10 million reads were generated from each library and successfully mapped to the Arabidopsis TAIR10 genome for Col, C24, and their reciprocal F1 hybrids (Col × C24 and C24 × Col) under WT and mutant background (SI Appendix, Table S4).
To discern reads from parents in F1 hybrids for allele-specific DNA methylation and siRNA profile analyses, the SNPs between Col and C24 accessions were obtained from the 1001 Genomes Project of Arabidopsis (1001genomes.org/data/MPI/MPISchneeberger2011/releases/current//C24/Marker/C24.SNPs.TAIR9.txt) (47).
Characterizing DMRs Showing Methylation Interaction in F1 Hybrids.
Identification of DMRs between Col and C24 was conducted according to our previous methods (48), with modifications. In brief, only cytosines with at least fourfold coverage in a library were considered, and then a 200-bp tiling window was used to search the whole genome. DNA methylation levels between two parents were compared pairwise through Fisher’s exact test, and then estimated FDRs were generated to adjust P values using the Benjamini–Hochberg method. Resultant windows with an adjusted P value less than 0.01 and a >1.5-fold change of the methylation level were retained for ensuing study. The DNA methylation levels of each region were average methylation levels over a standard size region, the number of methylated cytosines divided by the number of total cytosines (mC/total C). Moreover, the P value of each cytosine in the selected regions was calculated by Fisher’s exact test, and it was considered as a differentially methylated cytosine (DMC) if its P value was not greater than 0.05. Finally, regions were defined as DMRs only if they contained at least seven DMCs.
PAV/Weighted Methylation Levels.
To discover DMRs showing methylation interaction, the weighted methylation level of F1 hybrid for each DMR was calculated as the PAV according to a previous study (21), with modifications. In short, the combined parental methylated reads were divided by the combined parental total reads inside parental DMR using the following formula:
where i is the position of cytosine, n is the total number of cytosine positions in the DMR, MC is the maternal methylated reads, PC is the paternal methylated reads, MT is the maternal total reads, PT is the paternal total reads, and total reads contain the methylated and unmethylated reads.
Subsequently, Fisher’s exact test was executed on F1 offspring and their corresponding PAV to compare the DNA methylation level between parents and their progeny. The FDR was also controlled through adjustment of P values using a Benjamini–Hochberg method. Regions with P < 0.01 were retained and classified as DMRs with methylation interaction only if their methylation levels in progeny were lower or higher than their parents. The reciprocal F1 hybrids were each compared with PAV for every region, and the regions with FDR < 0.01 in both comparisons were considered as methylation interaction regions.
MPV/Mean Methylation Levels.
To determine the MPV of F1 hybrids for corresponding regions, the mean methylation levels were calculated as previously described (7, 8). In brief, the mean value of parental methylation for each region was calculated.
Characterizing SMRs Showing Methylation Interaction in F1 Hybrids.
Regions were searched on the genome other than DMRs by using a 200-bp sliding window. The DNA methylation level of F1 and its corresponding PAV were compared pairwise through Fisher’s exact test, and the P values were adjusted through the Benjamini–Hochberg method. Windows with an adjusted P value less than 0.01 were retained for further analysis. Moreover, only regions whose methylation levels in F1 are higher or lower than both parents were considered as candidates with methylation interaction.
Identification of Targets of Pol IV and Pol V.
Characterization of targets by Pol IV and Pol V were carried out as previously described (25). Briefly, to identify the sRNA clusters, we divided the genome into 200-bp bins, using bedtools to obtain the overlapping mapped 24-nt siRNA reads, and abundances of siRNA in Col and nrpd1nrpe1 double mutant were calculated. To define the targets of Pol IV and Pol V, we compared the differential expression levels of siRNA cluster between Col and nrpd1 nrpe1 double mutant using Fisher’s exact test. Then P values were adjusted to estimate the FDR using the Benjamini–Hochberg method. The regions with FDR < 0.05 were characterized as a differentially expressed siRNA cluster between Col and nrpd1nrpe1 double mutant, and were considered as targets of Pol IV and Pol V.
PCR Assay and Genotyping.
For Chop-PCR, genomic DNA (1 μg) digested with methylation-sensitive enzyme MspI overnight was used to amplify the indicated regions. Next, CAPS-PCR was performed to determine the methylation status of different alleles (SI Appendix, Fig. S1E). Genotyping was also performed with CAPS-PCF. Primers used in Chop-PCR and CAPS-PCR are listed in SI Appendix, Table S5.
Statistical, Graphics, and Visual Analyses.
The R statistical computing package was applied to perform statistical analyses and generate associated graphics (49). The genome-wide DNA methylation profile was visualized in Integrative Genomics Viewer (50) or Integrated Genome Browser (51).
Supplementary Material
Acknowledgments
We thank Dr. Angela Andersen of Life Science Editors for editorial assistance. This work was supported by the Chinese Academy of Sciences and by US NIH Grants R01GM070795 and R01GM059138 (to J.-K.Z.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data generated for this work have been deposited in the NCBI Gene Expression Omnibus (GEO) www.ncbi.nlm.nih.gov/geo (accession no. GSE72993).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607851113/-/DCSupplemental.
References
- 1.Mathieu O, Reinders J, Caikovski M, Smathajitt C, Paszkowski J. Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell. 2007;130(5):851–862. doi: 10.1016/j.cell.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He XJ, Chen T, Zhu JK. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011;21(3):442–465. doi: 10.1038/cr.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikaard CS, Haag JR, Pontes OM, Blevins T, Cocklin R. A transcription fork model for Pol IV and Pol V-dependent RNA-directed DNA methylation. Cold Spring Harb Symp Quant Biol. 2012;77:205–212. doi: 10.1101/sqb.2013.77.014803. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Zhu JK. Active DNA demethylation in plants and animals. Cold Spring Harb Symp Quant Biol. 2012;77:161–173. doi: 10.1101/sqb.2012.77.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzke MA, Mosher RA. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat Rev Genet. 2014;15(6):394–408. doi: 10.1038/nrg3683. [DOI] [PubMed] [Google Scholar]
- 7.Greaves IK, et al. Trans chromosomal methylation in Arabidopsis hybrids. Proc Natl Acad Sci USA. 2012;109(9):3570–3575. doi: 10.1073/pnas.1201043109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, et al. Genome-wide analysis of DNA methylation and gene expression changes in two Arabidopsis ecotypes and their reciprocal hybrids. Plant Cell. 2012;24(3):875–892. doi: 10.1105/tpc.111.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birchler JA, Yao H, Chudalayandi S, Vaiman D, Veitia RA. Heterosis. Plant Cell. 2010;22(7):2105–2112. doi: 10.1105/tpc.110.076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZJ. Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet. 2013;14(7):471–482. doi: 10.1038/nrg3503. [DOI] [PubMed] [Google Scholar]
- 11.Chodavarapu RK, et al. Transcriptome and methylome interactions in rice hybrids. Proc Natl Acad Sci USA. 2012;109(30):12040–12045. doi: 10.1073/pnas.1209297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groszmann M, et al. Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc Natl Acad Sci USA. 2011;108(6):2617–2622. doi: 10.1073/pnas.1019217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnable PS, Springer NM. Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol. 2013;64:71–88. doi: 10.1146/annurev-arplant-042110-103827. [DOI] [PubMed] [Google Scholar]
- 14.Ng DW, Lu J, Chen ZJ. Big roles for small RNAs in polyploidy, hybrid vigor, and hybrid incompatibility. Curr Opin Plant Biol. 2012;15(2):154–161. doi: 10.1016/j.pbi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Greaves I, Groszmann M, Dennis ES, Peacock WJ. Trans-chromosomal methylation. Epigenetics. 2012;7(8):800–805. doi: 10.4161/epi.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Varala K, Moose SP, Hudson ME. The inheritance pattern of 24 nt siRNA clusters in arabidopsis hybrids is influenced by proximity to transposable elements. PLoS One. 2012;7(10):e47043. doi: 10.1371/journal.pone.0047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaves IK, Groszmann M, Wang A, Peacock WJ, Dennis ES. Inheritance of Trans Chromosomal Methylation patterns from Arabidopsis F1 hybrids. Proc Natl Acad Sci USA. 2014;111(5):2017–2022. doi: 10.1073/pnas.1323656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Zhu JK. RNA-directed DNA methylation. Curr Opin Plant Biol. 2011;14(2):142–147. doi: 10.1016/j.pbi.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivaprasad PV, Dunn RM, Santos BA, Bassett A, Baulcombe DC. Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. EMBO J. 2012;31(2):257–266. doi: 10.1038/emboj.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lister R, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133(3):523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz MD, Schmitz RJ, Ecker JR. ‘Leveling’ the playing field for analyses of single-base resolution DNA methylomes. Trends Genet. 2012;28(12):583–585. doi: 10.1016/j.tig.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollick JB, Chandler VL. Epigenetic allelic states of a maize transcriptional regulatory locus exhibit overdominant gene action. Genetics. 1998;150(2):891–897. doi: 10.1093/genetics/150.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zemach A, et al. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153(1):193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He XJ, et al. NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes Dev. 2009;23(3):318–330. doi: 10.1101/gad.1765209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law JA, et al. Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature. 2013;498(7454):385–389. doi: 10.1038/nature12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pecinka A, Abdelsamad A, Vu GT. Hidden genetic nature of epigenetic natural variation in plants. Trends Plant Sci. 2013;18(11):625–632. doi: 10.1016/j.tplants.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz RJ, et al. Patterns of population epigenomic diversity. Nature. 2013;495(7440):193–198. doi: 10.1038/nature11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichten SR, et al. Epigenetic and genetic influences on DNA methylation variation in maize populations. Plant Cell. 2013;25(8):2783–2797. doi: 10.1105/tpc.113.114793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto R, Taylor JM, Shirasawa S, Peacock WJ, Dennis ES. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA. 2012;109(18):7109–7114. doi: 10.1073/pnas.1204464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer RC, Törjék O, Becher M, Altmann T. Heterosis of biomass production in Arabidopsis. Establishment during early development. Plant Physiol. 2004;134(4):1813–1823. doi: 10.1104/pp.103.033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortijo S, et al. Mapping the epigenetic basis of complex traits. Science. 2014;343(6175):1145–1148. doi: 10.1126/science.1248127. [DOI] [PubMed] [Google Scholar]
- 32.Weigel D, Colot V. Epialleles in plant evolution. Genome Biol. 2012;13(10):249. doi: 10.1186/gb-2012-13-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomvardas S, et al. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126(2):403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 34.Xu N, Tsai CL, Lee JT. Transient homologous chromosome pairing marks the onset of X inactivation. Science. 2006;311(5764):1149–1152. doi: 10.1126/science.1122984. [DOI] [PubMed] [Google Scholar]
- 35.Blewitt ME, et al. SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat Genet. 2008;40(5):663–669. doi: 10.1038/ng.142. [DOI] [PubMed] [Google Scholar]
- 36.Kanno T, et al. A structural-maintenance-of-chromosomes hinge domain-containing protein is required for RNA-directed DNA methylation. Nat Genet. 2008;40(5):670–675. doi: 10.1038/ng.119. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz RJ, et al. Epigenome-wide inheritance of cytosine methylation variants in a recombinant inbred population. Genome Res. 2013;23(10):1663–1674. doi: 10.1101/gr.152538.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng DW, et al. A Role for CHH Methylation in the Parent-of-Origin Effect on Altered Circadian Rhythms and Biomass Heterosis in Arabidopsis Intraspecific Hybrids. Plant Cell. 2014;26(6):2430–2440. doi: 10.1105/tpc.113.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barber WT, et al. Repeat associated small RNAs vary among parents and following hybridization in maize. Proc Natl Acad Sci USA. 2012;109(26):10444–10449. doi: 10.1073/pnas.1202073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigal M, et al. Epigenome confrontation triggers immediate reprogramming of DNA methylation and transposon silencing in Arabidopsis thaliana F1 epihybrids. Proc Natl Acad Sci USA. 2016;113(14):E2083–E2092. doi: 10.1073/pnas.1600672113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendizabal I, Keller TE, Zeng J, Yi SV. Epigenetics and evolution. Integr Comp Biol. 2014;54(1):31–42. doi: 10.1093/icb/icu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlichting CD, Wund MA. Phenotypic plasticity and epigenetic marking: An assessment of evidence for genetic accommodation. Evolution. 2014;68(3):656–672. doi: 10.1111/evo.12348. [DOI] [PubMed] [Google Scholar]
- 43.Xi Y, Li W. BSMAP: Whole genome bisulfite sequence MAPping program. BMC Bioinformatics. 2009;10:232. doi: 10.1186/1471-2105-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gent JI, et al. Accessible DNA and relative depletion of H3K9me2 at maize loci undergoing RNA-directed DNA methylation. Plant Cell. 2014;26(12):4903–4917. doi: 10.1105/tpc.114.130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozomara A, Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneeberger K, et al. Reference-guided assembly of four diverse Arabidopsis thaliana genomes. Proc Natl Acad Sci USA. 2011;108(25):10249–10254. doi: 10.1073/pnas.1107739108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, et al. DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV. Proc Natl Acad Sci USA. 2013;110(20):8290–8295. doi: 10.1073/pnas.1300585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R Development Core Team R . A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- 50.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicol JW, Helt GA, Blanchard SG, Jr, Raja A, Loraine AE. The Integrated Genome Browser: Free software for distribution and exploration of genome-scale datasets. Bioinformatics. 2009;25(20):2730–2731. doi: 10.1093/bioinformatics/btp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.