Significance
Siglec-8 downregulates eosinophil- and mast cell-mediated inflammatory responses upon engagement by specific self-glycans. We used solution NMR spectroscopy to determine the structure of the N-terminal lectin domain of human Siglec-8 in complex with its preferred glycan target 6′-sulfo sialyl Lewisx. Quantitative binding studies with differently sulfated glycans and structure-based mutants demonstrate that Siglec-8 simultaneously recognizes a terminal N-acetylneuraminic acid (sialic acid) and an underlying 6-O–sulfated galactose, yielding a tight and unique specificity. We offer direct structural and mechanistic insights into how the self-glycan code is deciphered by Siglec-8, emphasize the crucial role of glycan sulfation in immunological control of inflammation, and provide a rational framework for designing Siglec-8 agonists to harness its signaling pathway in allergic and inflammatory disorders.
Keywords: NMR spectroscopy, protein–carbohydrate recognition, glycan sulfation, immune regulation, glycoimmunology
Abstract
Siglec-8 is a human immune-inhibitory receptor that, when engaged by specific self-glycans, triggers eosinophil apoptosis and inhibits mast cell degranulation, providing an endogenous mechanism to down-regulate immune responses of these central inflammatory effector cells. Here we used solution NMR spectroscopy to dissect the fine specificity of Siglec-8 toward different sialylated and sulfated carbohydrate ligands and determined the structure of the Siglec-8 lectin domain in complex with its prime glycan target 6′-sulfo sialyl Lewisx. A canonical motif for sialic acid recognition, extended by a secondary motif formed by unique loop regions, recognizing 6-O–sulfated galactose dictates tight specificity distinct from other Siglec family members and any other endogenous glycan recognition receptors. Structure-guided mutagenesis revealed key contacts of both interfaces to be equally essential for binding. Our work provides critical structural and mechanistic insights into how Siglec-8 selectively recognizes its glycan target, rationalizes the functional impact of site-specific glycan sulfation in modulating this lectin–glycan interaction, and will enable the rational design of Siglec-8–targeted agonists to treat eosinophil- and mast cell-related allergic and inflammatory diseases, such as asthma.
Discrimination between self and nonself constitutes one of the most challenging tasks for the immune system. Innate immunity relies on an array of germ-line–encoded receptors that detect conserved features on invaders, providing immediate defense against infection, whereas adaptive immunity ensures to keep pace with the vast diversity of constantly evolving pathogenic threats. To prevent self-destructive immune responses, both systems are counterbalanced by inhibitory receptors that down-regulate immune activation upon sensing molecular markers of self. Glycans decorating the surfaces of all living cells are at the forefront of such recognition processes, providing with their unique and complex structures highly discriminative signatures of cellular identity. Terminal sialic acids are an immunological hallmark of vertebrate self-glycans (1–3), and recent evidence suggests glycan sulfation to be another self-marker specific to mammals (4).
Sialic acid-binding Ig-like lectins (Siglecs) represent the largest family of mammalian innate-immune cell surface receptors that recognize self-associated glycans and convert these extracellular recognition events into inhibition of immune cell function (2, 3, 5). To date, 14 human Siglecs have been identified, mostly expressed on various leukocyte populations. Each Siglec displays extracellularly a unique N-terminal lectin domain that binds distinct sialic acid-containing glycan (sialoglycan) ligands, and most members contain conserved immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (6) in their cytoplasmic tails. Ligand-induced activation results in ITIM-phosphorylation by Src family tyrosine kinases and recruitment of SH2-containing phosphatases (SHPs), which interfere with activation pathways of the underlying cell (2). Although Siglecs are generally known to control and balance immune cellular responses by acting as inhibitory receptors, recent studies intriguingly demonstrated that signaling of specific members is implicated in the regulation of the life span of immune cells, expanding their function as immune modulators to potential mediators of immune homeostasis (7).
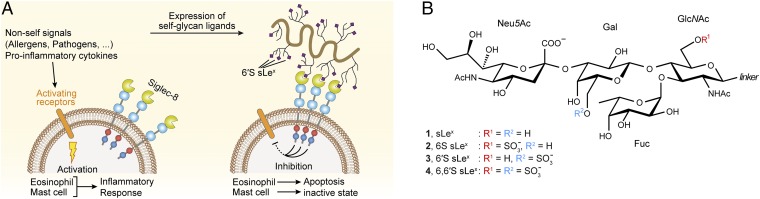
Most prominently, Siglec-8, originally identified from a cDNA library derived from a patient suffering from hypereosinophilic syndrome (8, 9), has emerged as a critical negative regulator of inflammatory response during allergic airway inflammation. It is highly and exclusively expressed on human eosinophils and mast cells and weakly on basophils and is conserved only among primates, but it lacks clear orthologs in any other mammalian species. Cross-linking of Siglec-8 on eosinophils with antibodies or a synthetic glycan ligand-coated polymer in vitro induces their rapid apoptosis (10, 11), whereas on mast cells, Siglec-8 ligation results in the inhibition of IgE/FcεRI-mediated inflammatory mediator release, without affecting their survival (12). Preactivation of eosinophils with survival-promoting, proinflammatory cytokines, a key process in allergic inflammation, not only fails to counteract Siglec-8–triggered cell death but instead potently enhances their sensitivity to undergoing apoptosis in response to Siglec-8 ligation (13–15), suggesting a particular role for Siglec-8–mediated immune suppression under inflammatory conditions. Supporting this notion, an ex vivo study showed that eosinophils isolated from the brochoalveolar fluid of allergen-challenged patients have increased susceptibility to Siglec-8–mediated apoptosis (16). Immunohistochemical analysis consistently revealed that endogenous Siglec-8 ligands are markedly up-regulated in inflamed compared with normal human airway tissues (17). Collectively, these findings led to the proposal that Siglec-8 may provide a safeguard mechanism for the immune system to selectively deplete eosinophils from inflamed tissues and simultaneously diminish mast cell inflammatory responses and may thus constitute a basic immunoregulatory pathway to trigger the active resolution of inflammation and return to homeostasis (7, 18) (Fig. 1A). There is increasing awareness that dysregulated Siglec-8 function might be critically involved in the pathobiology of allergic and chronic inflammatory disorders, including asthma, where accumulation and delayed apoptosis of activated eosinophils and mast cells in the airways are among the leading causes of persistent inflammation and tissue damage (19, 20). Indeed, Siglec-8 gene polymorphisms were identified to correlate with increased asthma risk (21), albeit the detailed molecular pathways linking Siglec-8 to disease have yet to be clarified. On account of its potent eosinophil proapoptotic and mast cell-inhibitory activities, combined with its selective expression on these key inflammatory effector cells, Siglec-8 is considered a promising target for novel antiinflammatory, proresolving treatment strategies for asthma and other disease conditions in which inappropriate and/or prolonged inflammatory responses of these cell types contributes to pathology (20, 22–25).
Fig. 1.
Proposed mechanism of Siglec-8 function in modulating eosiniphil- and mast cell-mediated immune responses and potential glycan ligands for Siglec-8. (A) Ongoing inflammation results in up-regulated expression of specific endogenous Siglec-8 sialoglycan ligands (presumably a secreted, high-molecular weight mucin, carrying multiple 6′S sLex glycan epitopes) in airway tissues. Ligand binding to the N-terminal lectin domain of Siglec-8 initiates intracellular signaling cascades, which, through phosphorylation of cytoplasmic ITIM/ITIM-like motifs and recruitment of downstream effector proteins, ultimately lead to apoptosis of eosinophils and inhibition of mast cell degranulation. (B) Chemical structures of glycan ligands used in this study: sLex (sialyl Lewisx: Neu5Acα2–3Galβ1–4[Fucα1–3]GlcNAc); 6S sLex (6-sulfo sLex); 6′S sLex (6′-sulfo sLex); and 6,6′S sLex (6,6′-disulfo sLex). The chemical linker for oligosaccharide 1 was βOCH3 and for 2–4 βO(CH2)3NH3+.
Understanding the structural basis of how Siglec-8 interacts with its carbohydrate ligand is an essential prerequisite for deciphering its molecular mechanism of action, under both physiological and pathological conditions, and will facilitate the rational design of highly specific agents to exploit its antiinflammatory signaling pathway for therapeutic purposes. Although the exact biochemical identity of its natural tissue ligands still remains unknown, glycan microarray analyses have revealed tight specificity for a unique sulfated and sialylated tetrasaccharide glycan epitope, termed 6′-sulfo sialyl Lewisx (6′S sLex) or Neu5Acα2–3[6S]Galβ1–4[Fucα1–3]GlcNAc (26, 27), whereas no binding was detected to the closely related 6S sLex (Neu5Acα2–3Galβ1–4[Fucα1–3][6S]GlcNAc) or the nonsulfated sLex, which are known ligands for E-, P-, and L-selectins (28).
Here we present the solution structures of the human Siglec-8 lectin domain in its ligand-free form and in complex with its preferred glycan epitope 6′S sLex. Our structural analysis, combined with quantitative binding studies and site-directed mutagenesis data, provides detailed insight into how Siglec-8 selectively recognizes its glycan target and demonstrates the critical influence that site-specific glycan sulfation can exert in the modulation of lectin–glycan recognition.
Results
Fine Specificity Toward Differently Sulfated sLex Glycan Epitopes Assessed by Solution NMR.
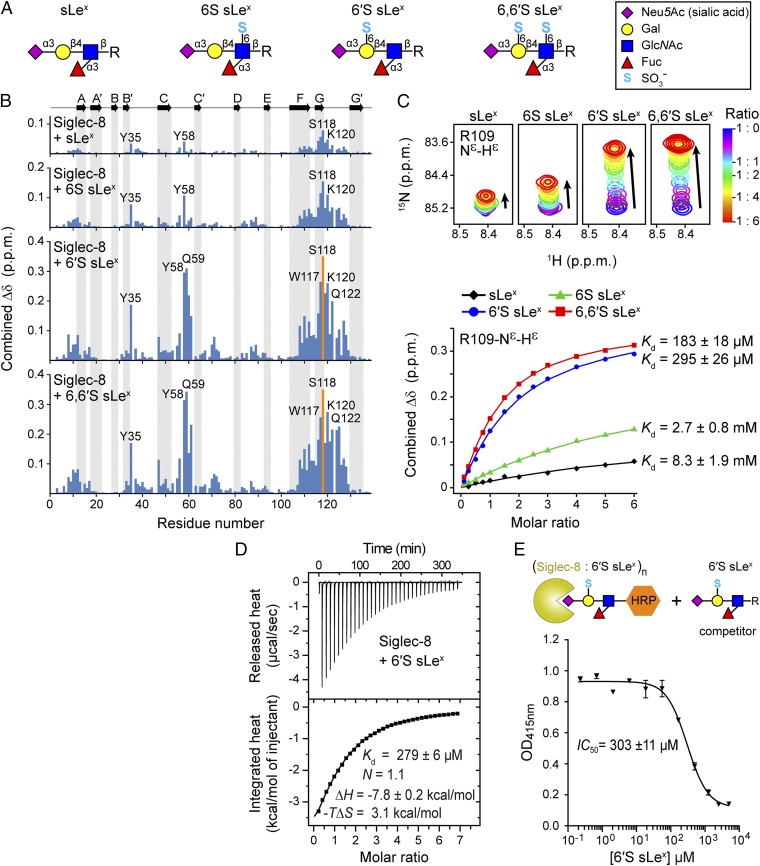
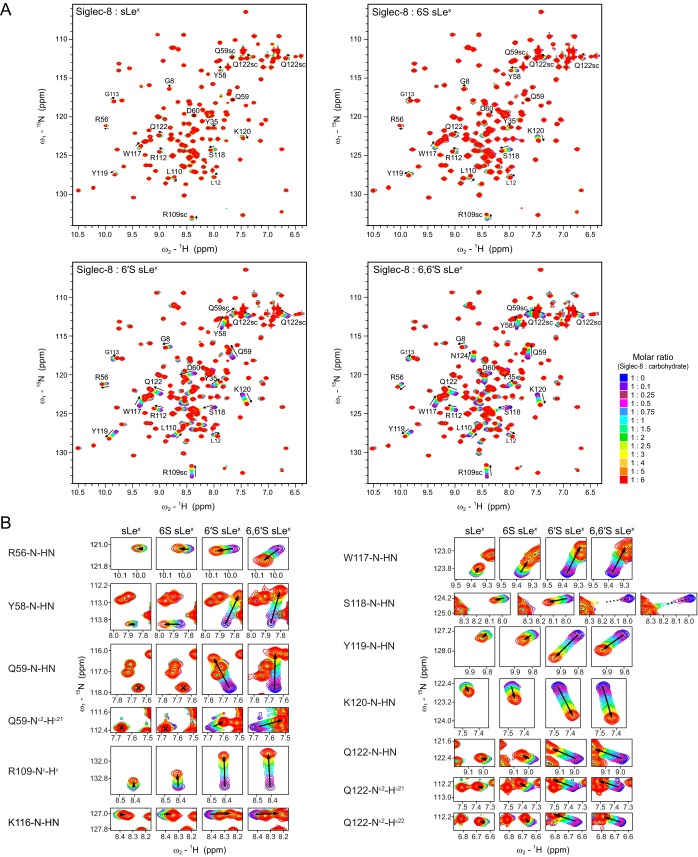
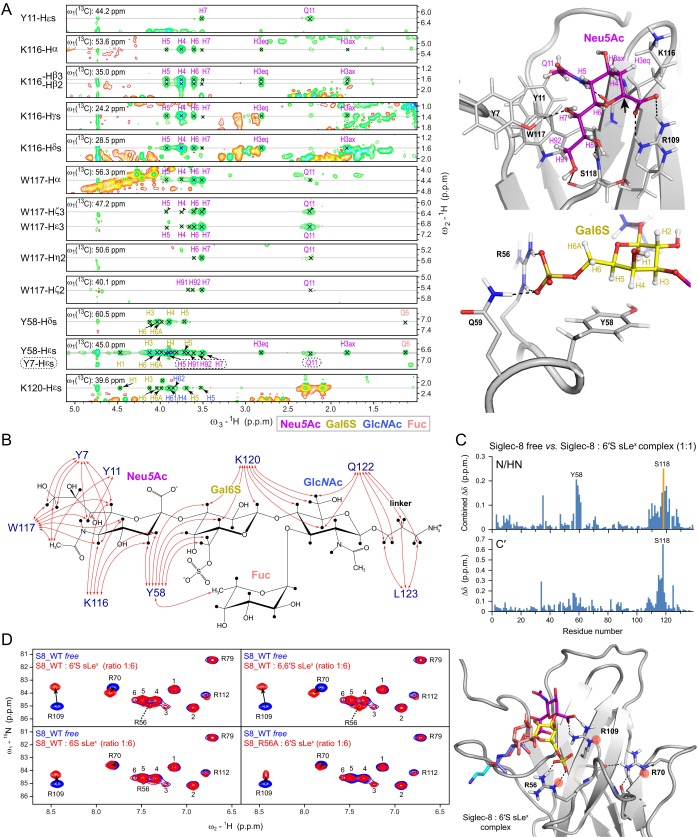
Previous glycan microarray analyses concordantly identified 6′S sLex as the best candidate ligand for Siglec-8 (26, 27), whereas a subsequent study indicated strongest binding to the disulfated 6,6′S sLex (29), which contains an additional sulfate group at the C6 position of the GlcNAc moiety. To investigate the importance of individual sulfate modifications on sLex for Siglec-8 glycan recognition, we chemically synthesized a series of monosulfated (6S sLex and 6′S sLex) and disulfated (6,6′S sLex) sialyl Lewisx variants (Figs. 1B and 2A and SI Appendix) to compare their binding with nonsulfated sLex toward a soluble recombinant 15N-labeled form of the isolated human Siglec-8 lectin domain (30). Notably, because the lectin domain of Siglec-8 (unlike those of several other Siglec family members) does not contain any potential N-linked glycosylation site in its native sequence, the recombinant Siglec-8 closely resembles the natural protein domain. We used 2D NMR spectroscopy titration experiments to monitor changes in Siglec-8 induced upon addition of increasing amounts of ligands. In the 2D 1H,15N-HSQC spectrum each NH group is represented by a cross-peak whose spectral position directly reflects its individual local chemical environment. Ligand-binding leads either to gradual changes in cross-peak positions (fast exchange) or to the appearance of new cross-peaks (slow exchange), as ligand concentration is increased. This experimental strategy allowed not only detection and mapping of carbohydrate binding directly on the protein at residue-specific precision but also quantification of monovalent interactions under nearly physiological solution conditions, which is feasible even for weak-affinity interactions (Kd in the μM to mM range). Binding of each of the four tetrasaccharides induced substantial chemical shift changes in a number of Siglec-8 amide resonances and was characterized by fast exchange kinetics on the NMR time scale (Fig. S1). Perturbed residues clustered to the same discrete sequence regions of Siglec-8, indicating that each of the four sLex variants occupies the same binding surface, whereas the magnitudes of the chemical shift changes differed markedly between the four titrations (Fig. 2B and Fig. S1). Examination of the overlaid 2D 1H,15N-HSQC titration spectra revealed that most of the perturbed cross-peaks followed similar trajectories, consistent with a similar ligand orientation. However, dependent on the presence or absence of the sulfate at the C6 position of the Gal, the cross-peaks of Tyr58 and Gln59 moved into different directions (Fig. S1B), suggesting their proximity to the sulfate recognition site.
Fig. 2.
Binding of Siglec-8 to nonsulfated and differently sulfated sLex variants. (A) Schematic representations of the glycan structures shown in Fig. 1B. Monosaccharide symbols are indicated on the right. (B) Combined 1H–15N chemical shift changes (Δδ) of the 15N-labeled Siglec-8 lectin domain observed upon NMR titration with the different sLex variants (to sixfold molar excess), plotted versus residue number. Secondary-structure elements derived from subsequent structural analysis are shown at the top. Orange bars represent residues whose 1H–15N cross-peak intensities progressively decreased during NMR titration, indicating intermediate exchange on the NMR time scale. (C) Overlaid sections of 1H,15N-HSQC spectra of Siglec-8 showing the representative Arg109 side chain NεHε cross-peak, acquired before and after stepwise addition of indicated carbohydrate ligands (Top), and the corresponding NMR-binding isotherms (Bottom). Reported Kd values are the mean ± SD from separate fitting of binding curves of 9–21 individual residues. The full set of NMR titration curves used for Kd determination is shown in Fig. S2. (D) ITC data obtained by injecting 6′S sLex into a solution of Siglec-8. N, stoichiometry (carbohydrate/protein); ΔH, change in enthalpy; −TΔS, change in entropy. (E) Polyacrylamide-based competitive binding assay for determination of IC50 for Siglec-8 binding to 6′S sLex. Immobilized lectin domains were incubated with the carbohydrate ligand together with a streptavidin-horseradish peroxidase (HRP) conjugated polyacrylamide glycopolymer whose concentration was assessed by colorimetric detection of HRP activity.
Fig. S1.
Siglec-8 binding to nonsulfated (sLex), monosulfated (6S sLex and 6′S sLex), and disulfated (6,6′S sLex) sialyl Lewisx variants, monitored by solution NMR titration experiments. (A) Superimposed 1H,15N-HSQC spectra of the Siglec-8 lectin domain acquired before and after stepwise addition of indicated carbohydrate ligands. Spectra are color-coded according to the protein:carbohydrate molar ratio (folded cross-peaks of free Siglec-8 are colored in gray). Strongly perturbed cross-peaks are labeled with residue name and number. Black arrows indicate directions of peak movements, and black crosses indicate unperturbed peaks. (B) Enlarged sections of the spectra in A are depicted for residue-specific comparison of chemical shift changes induced upon binding of different sLex variants. In all four titrations, essentially the same set of amide resonances was affected, with most of them moving into similar directions, suggesting similar ligand–protein contact surfaces. The most prominent differences between the four titrations were seen for the amide peaks of Tyr58 and Gln59 (backbone NH and side chain NH2), whose perturbation patterns were highly sensitive to the presence or absence of the sulfate group at the C6 position of the Gal, indicating a proximity of these residues to the sulfate recognition site. All spectra were recorded at 20 °C, at 1H frequency of 500 MHz, using 15N-labeled Siglec-8 lectin domain at 200 μM in 20 mM potassium phosphate, 40 mM NaCl at pH 7.4 (5% D2O).
NMR titration curves of significantly perturbed and well-resolved resonances were used to extract equilibrium dissociation constants (Kd) (Fig. 2C and Fig. S2), revealing a distinct hierarchy of binding strengths: sLex binds with the weakest affinity (Kd: 8.3 ± 1.9 mM), and the additional presence of a sulfate group at the C6 position of the GlcNAc moiety of 6S sLex resulted in only a modest gain in affinity (Kd: 2.7 ± 0.8 mM; threefold compared with sLex). In contrast, the presence of a single sulfate group at the C6 position of the Gal moiety of 6′S sLex—the presumed Siglec-8 ligand (26, 27)—produced a dramatic increase in affinity (Kd: 295 ± 26 µM; 28-fold compared with sLex). A similar, however, slightly higher, affinity was determined for the disulfated 6,6′S sLex (Kd: 185 ± 18 µM; 1.6-fold compared with 6′S sLex).
Fig. S2.
Determination of dissociation constants (Kd) for Siglec-8 binding to the four sialyl Lewisx variants by NMR titration. Kd values were determined by fitting the combined 1H/15N chemical shift changes (Δδ), observed in the 15N-labeled Siglec-8 lectin domain upon binding of indicated carbohydrate ligands, as described in Materials and Methods. The corresponding 2D 1H,15N-HSQC NMR titration spectra are shown in Fig. S1. Reported Kd values and errors are the mean values ± SD from performing the fit for 9–21 significantly perturbed, nonoverlapping 1H/15N resonance signals.
To quantify further Siglec-8 binding to 6′S sLex and to elucidate the thermodynamics of this interaction, we used isothermal titration calorimetry (ITC) (Fig. 2D) and a competitive binding assay (31) (Fig. 2E). The obtained Kd of 279 μM and IC50 of 303 µM are both in excellent agreement with the results from NMR (Kd: 295 µM). With a ΔH of −7.8 kcal/mol the interaction is driven by favorable enthalpic forces that overcome an entropic cost of −TΔS of ∼3 kcal/mol.
Collectively, these data evidence a significantly stronger binding of Siglec-8 toward Gal-6–sulfated sLex glycan epitopes (6′S sLex and 6,6′S sLex), compared with GlcNAc-6–sulfated (6S sLex) and nonsulfated sLex. The largest affinity enhancement resulted clearly from the addition of the sulfate group at the C6 position of the Gal moiety of 6′S sLex (28-fold compared with sLex). Based on these observations, we concluded that 6′S sLex contains the minimum epitope specifically recognized by Siglec-8.
Structure of the Human Siglec-8 Lectin Domain.
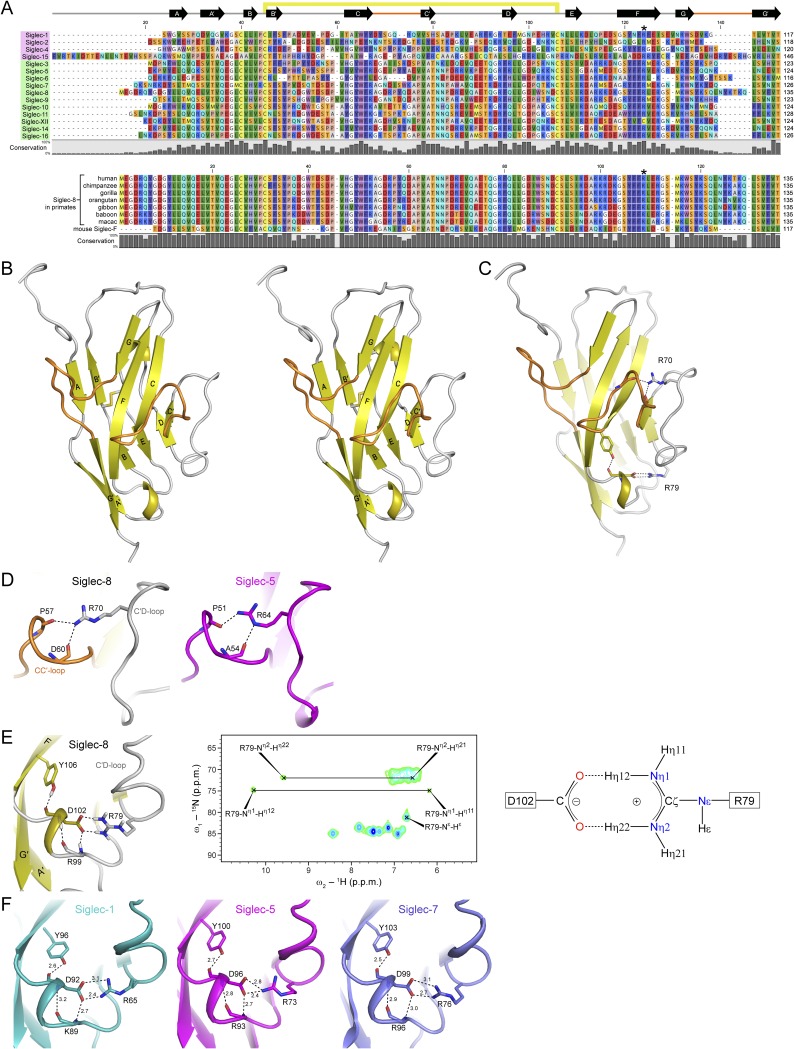
To gain insights into the structural determinants underlying this narrow target specificity, we determined the 3D structure of the human Siglec-8 lectin domain by solution NMR spectroscopy (Fig. 3 A–C, Fig. S3B, and Table S1). The structure revealed a canonical Siglec lectin domain fold, which is a V-set Ig-like β-sandwich of two antiparallel β-sheets formed by β-strands ABED and C′CFG. Characteristic features of Siglec lectin domains were observed: the conserved intrasheet disulfide bond between adjacent β-strands B and E; the strictly conserved essential arginine (Arg109) on β-strand F, known to provide a key salt bridge interaction for sialic acid recognition; and the splitting of the G-strand into two shorter β-strands (G and G′). Structural alignment with available crystal structures of Siglec-1 (32), Siglec-5 (33), and Siglec-7 (34) revealed, besides a nearly invariant core, large conformational differences in the N-terminal segments (before β-strand A) and the B′C intersheet loops, as well as the CC′ and GG′ interstrand loops (Fig. 3D). These four regions show the highest sequence diversity between the lectin domains of the different human Siglec family members, while being highly conserved among primate Siglec-8 orthologs (sequence alignment shown in Fig. S3A). Remarkably, the GG′ loop of Siglec-8, consisting of 11 residues, is substantially extended compared with the typically 5 residue-spanning GG′ loops of most Siglecs. Notably, in Siglec-7 and Siglec-9, the CC′ and GG′ loops had been found to contribute interactions to glycan moieties underlying a terminal sialic acid (35, 36) and are thus the presumed key determinants for the distinct fine carbohydrate specificities of individual Siglecs. Consistently, both loops displayed large chemical shift perturbations upon ligand binding (Fig. 2B). Backbone {1H}-15N-heteronuclear nuclear Overhauser effect (hetNOE) measurements (Fig. 3E) indicated that both loops are well ordered and do not exhibit conformational dynamics on the here probed picosecond-to-nanosecond time scale, which may in part originate from a stabilizing network of hydrogen bonds and salt bridges (Fig. S3 C–F).
Fig. 3.
Solution structures of the human Siglec-8 lectin domain unliganded and in complex with 6′S sLex. (A) Primary sequence of the human Siglec-8 lectin domain. Sequence numbers correspond to the mature protein. Secondary-structure elements are shown at the top. The intrasheet disulfide bond between Cys31 and Cys91 is indicated by a yellow bar, the C26S mutation (30) is highlighted in cyan, and the essential arginine (Arg109) is in bold. The Siglec-8-unique CC′ and GG′ loops are highlighted in orange. (B) Ensemble of the 20 lowest-energy structures of Siglec-8, showing backbone atoms (N, Cα, and C′) only. (C) Cartoon representation of the lowest-energy structure of Siglec-8, with the disulfide bond depicted as sticks. (D) Superposition of the Siglec-8 solution structure with crystal structures of Siglec-1 (PDB entry: 1QFP), Siglec-5 (2ZG2), and Siglec-7 (1O7V), colored as indicated, with rms deviations ranging from 0.9 to 1.5 Å over 82–112 aligned Cα positions. (E) The {1H}-15N heteronuclear NOE values for Siglec-8 backbone amides plotted versus the residue number, measured at 750 MHz and 293 K. (F) Ensemble of the 20 lowest-energy structures of Siglec-8 in complex with 6′S sLex. (G) Cartoon representation of the lowest-energy structure of the Siglec-8–6′S sLex complex. (H) Molecular surface of the Siglec-8 lectin domain with bound 6′S sLex, colored by electrostatic potential: red, negative; blue, positive. (I) Close-up view of 6′S sLex in the positively charged glycan-binding pocket. Selected residues at the interface are indicated. The carbohydrate is represented as sticks and colored as follows: Neu5Ac (purple), Gal6S (yellow), GlcNAc (violet), Fuc (light red), and the chemical linker (cyan).
Fig. S3.
Sequence alignment of human Siglec lectin domains and NMR solution structure of the human Siglec-8 lectin domain. (A) Multiple amino acid sequence alignment of the N-terminal lectin domains from all known human Siglec family members (Top) and of primate Siglec-8 orthologs together with its proposed murine functional paralog Siglec-F (Bottom), generated by using CLC workbench 8 (www.clcbio.com/) and manually adjusted for having conserved secondary structures aligned. N termini correspond to those of the mature protein sequences [starting after signal peptide, as predicted by SignalP 4.1 (www.cbs.dtu.dk/services/SignalP/)]. Secondary structure elements (derived from the Siglec-8 structure) are schematically shown on the top, with the highly variable CC′ loop and GG′ loop indicated in orange. The conserved intradomain disulfide bond is indicated by a yellow bar, and the strictly conserved essential arginine is indicated by a star. Sequences are grouped into the conserved subgroup (pink-shaded area), which have clear orthologs throughout mammals, and the fast-evolving CD33-related subgroup (light green-shaded area), which have large interspecies differences. Human Siglec-XII was included for completeness, although it lacks the essential arginine residue involved in sialic acid recognition. Residues are colored by type, and the degree of conservation is indicated by the bar plot at the bottom. (B) Stereoview of the NMR solution structure of the Siglec-8 lectin domain, shown in cartoon representation. The intradomain disulfide bond (Cys31–Cys91) is depicted in sticks, the β-strands are labeled with letters, and the Siglec-8-unique CC′ and GG′ loops are colored in orange. (C) Siglec-8 lectin domain is stabilized by intradomain hydrogen bond/salt bridges provided by side chains of Arg70 and Arg79. (D) Arg70 (C′D loop) makes hydrogen bonds from its guanidinium group to backbone carbonyl oxygen atoms of Pro57 and Asp60 (both on CC′ loop) (Left) and may thus contribute to the rigidification of the Siglec-8 CC′ loop conformation. Similar interactions are also apparent in the crystal structure of Siglec-5 (PDB ID code: 2ZG2; Right) but not in structures of Siglec-1 and Siglec-7 (not shown), which have no arginine at this sequence position. (E) Arg79 (C′D loop) forms a salt bridge with Asp102 (EF loop), which is additionally stabilized by hydrogen bonds with backbone amide and carbonyl atoms of Arg99 and the side chain hydroxyl group of Tyr106. These interactions have been identified by NMR from (i) detection of the Arg99 and Asp102 1H–15N backbone amide resonances in a 2D 1H,15N-HSQC spectra measured in D2O, which indicated protection from hydrogen–deuterium exchange; (ii) detection of the Tyr106 1Hη resonance in an 13C/15N F2-filtered NOESY measured in NMR buffer (95% H2O/5% D2O) as well as in 15N- and 13C-edited 3D NOESY spectra, which indicated protection from hydrogen–hydrogen exchange; and (iii) the observation of four dispersed NMR signals from the two NH2 groups of the Arg79 guanidinium in a 2D 1H,15N-HSQC measured in NMR buffer (95% H2O/5% D2O) at 283 K (Middle), a chemical shift pattern which is characteristic for such a guanidinium-carboxylate end-to-end symmetric salt bridge interaction (Right), according to ref. 61. (F) The identified hydrogen bond network (shown in E) is also apparent in crystal structures of Siglec-1, Siglec-5, and Siglec-7 (PDB ID codes: 1QFP, 2ZG2, and 1O7V, respectively) and, as judged from sequence conservation, appears to be well-conserved among all human Siglecs (except for Siglec-2, Siglec-4, and Siglec-15).
Table S1.
NMR and refinement statistics for Siglec-8 and the Siglec-8–6′S sLex complex
| Experimental constraints and structure statistics | Siglec-8 | Siglec-8–6′S sLex | |
| Siglec-8 | 6′S sLex | ||
| NMR distance and dihedral constraints | |||
| Distance constraints | |||
| Total NOE | 3,231 | 3,938 | 104 |
| Intraresidue | 578 | 746 | 61 |
| Interresidue | 2,653 | 3,192 | 43 |
| Sequential (|i – j| = 1) | 825 | 984 | 30 |
| Medium-range (1 < |i – j| < 5) | 482 | 570 | 13 |
| Long-range (|i – j| ≥ 5) | 1,346 | 1,638 | |
| Protein–carbohydrate intermolecular | 113 | ||
| Hydrogen bonds | 41 | 42 | 0 |
| Total dihedral angle restraints | |||
| ϕ | 91 | 75 | 0 |
| ψ | 91 | 75 | 0 |
| Stereospecific assignments of Val/Leu prochiral methyls | 46 | 46 | 0 |
| Structure statistics | |||
| Violations (mean and SD) | |||
| Number of violated distance constraints >0.3 Å | 0.15 ± 0.37 | 0.00 ± 0.00 | |
| Number of violated dihedral angle constraints >5° | 0.00 ± 0.00 | 0.05 ± 0.22 | |
| Max. dihedral angle violation (°) | 2.20 ± 1.08 | 1.67 ± 1.13 | |
| Max. distance constraint violation (Å) | 0.23 ± 0.05 | 0.27 ± 0.02 | |
| Deviations from idealized geometry | |||
| Bond lengths (Å) | 0.004 | 0.004 | |
| Bond angles (°) | 1.57 | 1.55 | |
| Average pairwise rms deviation* (Å) | |||
| Protein† | |||
| Heavy | 0.85 ± 0.08 | 0.64 ± 0.07 | |
| Backbone | 0.37 ± 0.07 | 0.25 ± 0.06 | |
| Carbohydrate‡ | |||
| All carbohydrate heavy | 0.80 ± 0.19 | ||
| Complex†,‡ | |||
| All complex heavy (C, N, O, S) | 0.66 ± 0.07 | ||
Pairwise rms deviation was calculated among 20 refined structures of the protein or the protein–carbohydrate complex.
Values for the protein were derived from the structured region (residues 7–136), excluding unstructured regions (residues 1–7 and 137–145).
Values for the carbohydrate ligand were derived from the whole tetrasaccharide, excluding the aglycon.
Structure of the Human Siglec-8 Lectin Domain in Complex with 6′S sLex.
To understand the structural basis for glycan recognition and discrimination by Siglec-8, we next determined the solution structure of the human Siglec-8 lectin domain in complex with 6′S sLex by NMR spectroscopy. Despite the weak binding affinity (Kd in the high micromolar range), 113 intermolecular NOEs—a large number for a tetrasaccharide ligand—could be unambiguously identified and converted into distance restraints (Fig. S4 A and B). Based on these, plus 104 intracarbohydrate and 3,938 intraprotein NOE-derived distance restraints, we determined a precise structural ensemble of the complex (Fig. 3F and Table S1). Overall, the structure of Siglec-8 in the complex is virtually identical to that of the unliganded Siglec-8 (Cα rms deviation of 0.8 ± 0.1 Å for residues 7–135), indicating that carbohydrate recognition is mediated by a largely preformed binding site, involving only minor side chain rearrangements. The carbohydrate lies embedded in a highly positively charged cleft formed by β-strands C, F, and G encompassed by the Siglec-8-unique CC′ and GG′ loops (Fig. 3 G–I). About 678 Å2 or 46% of its solvent-accessible surface is buried upon binding to Siglec-8. The entire tetrasaccharide is well-ordered, showing a single major conformation throughout the ensemble with tightly clustered glycosidic torsion angles for the core Lex trisaccharide: for the Galβ1–4GlcNAc linkage, 47° < φ < 54° and 12° < ψ < 19°; for Fucα1–3GlcNAc linkage, 47° < φ < 51° and 16° < ψ < 20°, and there was a slightly wider spread of angles for the terminal Neu5Acα2–3Gal linkage, −94° < φ < −66° and 18° < ψ < 25° (see Materials and Methods for angle definitions).
Fig. S4.
Intermolecular NOEs between Siglec-8 and 6′S sLex and arginine residues involved in Siglec-8-glycan recognition, detected by solution NMR spectroscopy. (A) (Left) The 3D 13C F1-edited F3-filtered NOESY spectrum of 13C/15N-labeled Siglec-8 in complex with unlabeled 6′S sLex, recorded at 1H frequency of 700 MHz, at 303 K. Depicted are exemplary sections of indicated Siglec-8 resonances at ω2 (13C edited/selected) showing clear intermolecular NOEs to indicated carbohydrate resonances, which appeared well-dispersed in the direct dimension ω3 (13C filtered/suppressed). (Right) Close-up views of the recognition of Neu5Ac (Top) and the Gal6S (Bottom) in the Siglec-8–6′S sLex complex structure, displayed in cartoon representation. Side chains of key protein residues and the carbohydrate residues are represented as sticks and labeled. Positions of the NMR-observable carbohydrate 1H atoms are indicated with labels and colored by residue type. The black arrow points to the anomeric carbon (C2) atom of Neu5Ac to which the underlying carbohydrate moieties (omitted for visual clarity) are linked. (B) Graphical summary of intermolecular NOEs observed between 13C/15N-labeled Siglec-8 and unlabeled 6′S sLex, detected in 2D 13C/15N F2-filtered NOESY and 3D 13C F1-edited F3-filtered NOESY spectra as described in Materials and Methods, schematically illustrated by red arrows onto the chemical structure of 6′S sLex. (C) Chemical shift differences between free Siglec-8 and Siglec-8 in complex with 6′S sLex (at molar ratio of 1:1), plotted as a function of residue number. (Top) Combined 1H/15N backbone amide chemical shift differences derived from 2D 1H,15N-HSQC spectra. (Bottom) The 13C backbone carbonyl chemical shift differences derived from 3D HNCO spectra. The orange bar indicates that the Ser118 amide resonance peak was invisible in the complex as a result of severe line broadening, due to intermediate exchange effects. (D) (Left) The 2D 1H,15N-HSQC spectra of 15N-labeled Siglec-8 wild type (S8_WT) in its free form (blue contours), overlaid with spectra of indicated Siglec-8/glycan ligand complexes (red contours), showing the spectral regions of the arginine side chain Nε-Hε resonances. The used Siglec-8 lectin domain expression construct contains in total 11 arginine residues (including the nonnatural C-terminal Arg145). In the 2D 1H,15N-HSQC spectrum of free Siglec-8 wild type only 10 Nε–Hε cross-peaks are visible/resolved. Nε-Hε cross-peaks that could be sequence-specifically assigned are labeled accordingly, whereas unassigned resonances, corresponding to those of Arg5, Arg96, Arg99, Arg101, Arg145, are numbered arbitrarily by 1–6. The Nε-Hε cross-peaks of the conserved essential Arg109 and the one of Arg70 show chemical shift perturbations in all four complexes. In contrast, the Arg56-Nε-Hε cross-peak, which is invisible (or overlapped) in the 1H,15N-HSQC spectrum of free Siglec-8, appears only in complexes of Siglec-8 wild type with 6′-sulfated-sLex variants (sulfo-group at the C6 position of Gal) but remains invisible/unaffected in complexes with 6-sulfated (at the C6 position of GlcNAc) sLex variants, as well as in the complex of the Siglec-8 R56A mutant with 6′S sLex. These observations strongly indicate that the guanidinium group of Arg56 is directly involved in the recognition of the sulfate group at the Gal C6, present only in the 6′S sLex and 6,6′S sLex tetrasaccharides. All spectra were recorded at 10 °C, in NMR buffer at pH 7.4 (5% D2O). The observed pattern of arginine Nε-Hε chemical shift changes can be explained by the NMR solution structure of the Siglec-8:6′S sLex complex, as shown in Right, which shows a cartoon representation of the Siglec-8: 6′S sLex complex, showing the side chains of Arg56, Arg70, and Arg109 as sticks, and their Nε-Hε amide atoms are marked by red circles: the essential Arg109 forms a salt bridge (end-on) with the carboxyl group of the terminal Neu5Ac. Arg70 (C′D loop) makes no direct contacts to the carbohydrate ligand but forms intraprotein hydrogen bonds to the backbone carbonyl oxygens of Pro57 and Asp60 on the CC′ loop (shown in Fig. S3 C and D). Arg56 (CC′ loop) forms a salt bridge (side-on) with the sulfate group at the C6 position of the Gal moiety. NH2 resonances were degenerated or not visible and could not be assigned except for Arg79 (see Fig. S3E.)
Intermolecular Contacts at the Complex Interface.
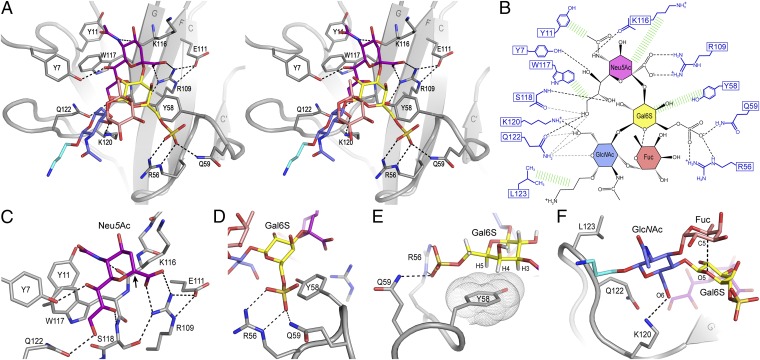
Closer inspection of the binding interface (Fig. 4) revealed that specificity is achieved by two major hot spots: a primary motif for recognition of the terminal Neu5Ac, extended by a secondary motif recognizing the subterminal galactose-6-sulfate (Gal6S). A summary of observed intermolecular contacts in the Siglec-8–6′S sLex complex is given in Table 1. The Neu5Ac is located at the edge of the G-strand, such that its carboxyl group makes a salt bridge with the guanidinium group of the strictly conserved essential Arg109 (on the adjacent F-strand), whose orientation itself is stabilized by salt bridge/hydrogen bond interactions with the side chains of Glu111 (F-strand) and Ser118 (G-strand). The Neu5Ac pyranose ring packs tightly with its hydrophobic A-face (Materials and Methods) against the aliphatic portion of Lys116, which protrudes from the upper part of the G-strand, likely providing an upper boundary for the Neu5Ac recognition site. The N-acetyl amide and the O8 and O9 hydroxyl groups of the glycerol chain form a network of hydrogen bonds with backbone atoms of the G-strand residues Lys116 and Ser118. In addition, the N-acetyl methyl group, together with the H7 and the H91/H92-methylene atoms, makes intimate hydrophobic contacts with the surrounding aromatic rings of Tyr11 and Trp117 (Fig. 4 A–C). Notably, the interactions engaging Neu5Ac and its orientation on the Siglec-8 surface are nearly identical to those seen in structures of other Siglec family members (32, 33, 36), as was expected from sequence comparisons. However, an additional hydrogen bond is formed between the O7 hydroxyl group of Neu5Ac and the side chain hydroxyl group of Tyr7 located in the unique N-terminal extension of Siglec-8 (Fig. 4 A–C and Fig. S3A).
Fig. 4.
Structural basis for 6′S sLex recognition by human Siglec-8 illustrated by a representative structure (lowest energy) of the NMR ensemble. (A) Stereoview of the Siglec-8–6′S sLex interface. The carbohydrate and key interacting amino acids are shown as sticks. Hydrogen bonds are represented by dashed lines. (B) Schematic illustration of the Siglec-8–6′S sLex interaction network. Black dashed lines indicate hydrogen bonds in the depicted structure; gray dashed lines indicate hydrogen bonds abundantly observed in other structures of the ensemble. Hydrophobic contacts are shown in green. (C) Close-up view of the recognition of the terminal Neu5Ac. The arrow points to the anomeric carbon atom (C2) of Neu5Ac to which the underlying carbohydrate moieties (omitted, for visual clarity) are linked. (D) Recognition of the Gal6S moiety involving a salt bridge and hydrogen bonds between the sulfate and the Arg56 and Gln59 side chains and (E) CH/π-stacking interactions between the apolar B-face of Gal6S and the aromatic ring of Tyr58, for which the van der Waals surface is displayed as dotted spheres. (F) Contacts between Siglec-8 and the GlcNAc moiety, also depicted is the intracarbohydrate hydrogen bond between the Fuc C5-H5 and the O5 pyranose ring oxygen of Gal6S.
Table 1.
Contacts between Siglec-8 and 6′S sLex observed in the NMR ensemble
| 6′S sLex | Siglec-8 | Type* | Occ.† | Supporting NMR data |
| Neu5Ac O1A/O1B | Arg109 Nη1 | SB | 16/20 | Large NεHε chemical shift changes‡ |
| Neu5Ac O1A/O1B | Arg109 Nη2 | SB | 20/20 | Large NεHε chemical shift changes‡ |
| Neu5Ac H3eq,H4 | Lys116 | HC | 20/20 | Intermolecular NOEs§ |
| Neu5Ac N5 | Lys116 O | HB | 20/20 | Large C′ chemical shift change§ |
| Neu5Ac CH3 | Tyr11 | HC | 15/20 | Intermolecular NOEs§ |
| Neu5Ac H6 | Trp117 Hα | HC | 20/20 | Intermolecular NOEs§ |
| Neu5Ac O7 | Tyr7 Oγ | HB | 18/20 | Intermolecular NOEs§ |
| Neu5Ac O8 | Ser118 N | HB | 20/20 | NH chemical shift disappeared upon binding‡ |
| Ser118 Oγ | HB | 5/20 | ||
| Neu5Ac H7,H91/H92 | Trp117 | CH/π | 20/20 | Intermolecular NOEs§ |
| Neu5Ac O9 | Ser118 O | HB | 17/20 | Largest C′ chemical shift change§ |
| Lys120 Nζ | HB | 9/20 | ||
| Gln122 Oε1 | HB | 7/20 | ||
| Gln122 Nε2 | HB | 2/20 | Large Nε2Hε21/22 chemical shift changes‡ | |
| Gal6S SO−3 | Arg56 Nη1 | SB | 6/20 | |
| Arg56 Nη2 | SB | 6/20 | ||
| Arg56 Nε | SB | 6/20 | NεHε chemical shift changes§ | |
| Gln59 Nε2 | HB | 16/20 | Large Nε2Hε21/22 chemical shift changes‡ | |
| Gal6S H3,H4,H5 | Tyr58 | CH/π | 20/20 | Intermolecular NOEs§ |
| GlcNAc O5 | Gln122 Nε2 | HB | 4/20 | Large Nε2Hε21/22 chemical shift changes‡ |
| GlcNAc O6 | Lys120 Nζ | HB | 13/20 | Intermolecular NOEs§ |
| Gln122 Nε2 | HB | 5/20 | Large Nε2Hε21/22 chemical shift changes‡ |
Hydrogen bond (HB) and salt bridge (SB) interactions are listed for heavy atom distances of 3.2 Å or less. HC, hydrophobic contacts; CH/π, CH/π-stacking interactions.
Occ., occurrence in the NMR ensemble of the 20 lowest-energy structures of the Siglec-8–6′S sLex complex. Note that all intermolecular restraints used for structure calculation were derived from intermolecular NOE data, and none of the listed interactions was used as artificial input.
Shown in Fig. S1.
Shown in Fig. S4.
The most striking and distinguishing feature of this complex is the recognition of the Gal6S, which is mediated exclusively by the side chains of three residues (Arg56, Tyr58, and Gln59) on the Siglec-8–unique CC′ loop (Fig. 4 D and E). The aromatic ring of Tyr58 lies flat and exposed on top of the CC′ loop, providing a platform for CH/π-stacking interactions with the apolar B-face of the galactopyranose ring, particularly with the plane formed by H3, H4, and H5. The exocyclic moiety of Gal6S approaches the edge of the CC′ loop and positions the negatively charged sulfate group in the center of the flanking Arg56 and Gln59 side chains, thereby forming a salt bridge with the guanidinium group of Arg56 and/or a hydrogen bond with the NH2 of Gln59. Remarkably, in 9 of the 20 refined structures, both side chains embrace the sulfate from opposite sides in a clamp-like configuration, thus contributing simultaneously to its recognition. These interactions are confirmed by the chemical shift changes of the Arg56 and Gln59 side chain amides observed only upon titration with sLex variants containing a Gal-6-sulfate modification (Figs. S1B and S4D).
Only few intermolecular contacts were found with the GlcNAc: its apolar B-face and N-acetyl group are exposed to solvent, whereas its exocyclic hydroxymethylene group (directed toward the Neu5Ac glycerol chain) is interacting with the long and flexible side chains of Lys120 and Gln122 on the GG′ loop. In most structures of the ensemble, the GlcNAc O6 is hydrogen bonded by the positively charged NH3+ of Lys120 (Fig. 4F), which otherwise donates a hydrogen bond to the nearby Neu5Ac O9. In few structures, the GlcNAc O6 is hydrogen bonded by the NH2 of Gln122, which in turn forms in some structures a hydrogen bond with the O9 of Neu5Ac (Fig. 4 B and C and Table 1).
The 3-amino-propyl linker, attached to the reducing end of the tetrasaccharide, exits the binding site toward the tip of the GG′ loop (close to Gln122 and Leu123; Fig. 4F) and appears disordered. No intermolecular contacts were found to Fuc. Its B-face is fully solvent-exposed, whereas its A-face stacks onto the A-face of the Gal6S, stabilizing the Lex conformation by donating an intracarbohydrate C–H⋅⋅⋅O hydrogen bond from its C5–H5 to the O5 ring oxygen of the Gal6S (Fig. 4F), as has been found previously in structures of Lex (37) and sLex (38) oligosaccharides. The presence of this hydrogen bond is indicated by the distance between Fuc-H5 and Gal-O5 of 2.2–2.3 Å within the ensemble, which is significantly shorter than the sum of the corresponding van der Waals radii (2.6 Å), and further supported by the characteristic NMR chemical shift of Fuc-H5 (∼4.8 ppm) (37, 38).
Mutations of Key Interface Residues Impair Siglec-8 Binding to 6′S sLex.
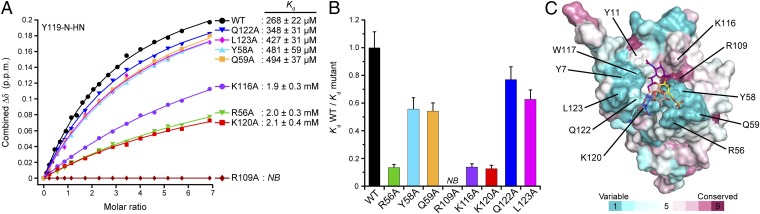
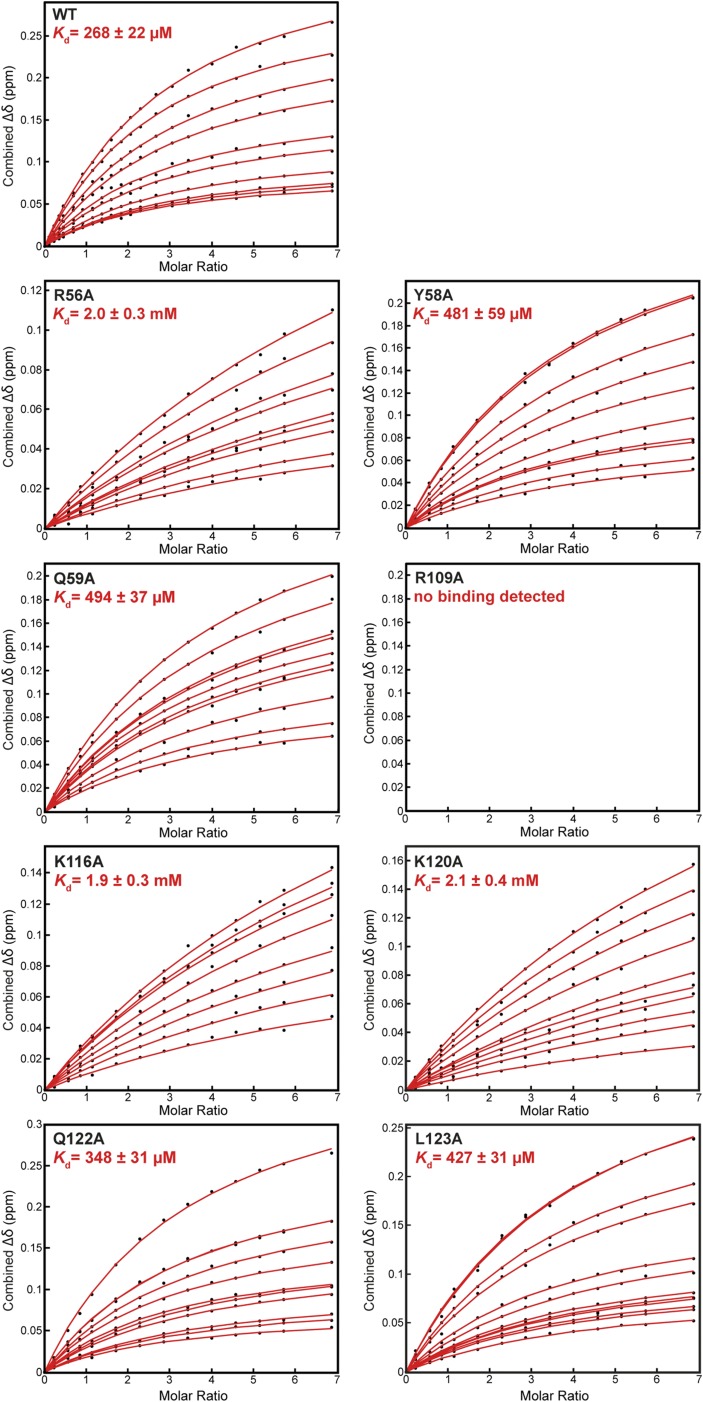
To dissect quantitatively the contributions of individual amino acid side chains to carbohydrate recognition, we introduced several single alanine substitutions at the interface and measured their effects on binding to 6′S sLex by using NMR titration experiments (Fig. 5 A and B and Fig. S5). None of these mutations affected the overall structure of Siglec-8, as indicated by very similar 2D 1H,15N-HSQC fingerprint spectra compared with the wild-type protein. As expected, substitution of the strictly conserved essential Arg109, which eliminated the salt bridge with the Neu5Ac carboxyl group, completely abrogated binding, confirming its indispensable role in carbohydrate recognition. Substitution of Lys116, which participates in hydrophobic contacts with the Neu5Ac pyranose ring, strongly impaired binding (sevenfold loss in affinity). A similar drastic drop in affinity (eightfold) resulted from substitution of Arg56 on the CC′ loop, whereas substitutions of the adjacent Tyr58 or Gln59 only modestly affected binding (twofold affinity decrease for each mutant). Hence, a major contribution to the binding energy must originate from the salt bridge formation between the Arg56 guanidinium group and the sulfate. Substitution of the GG′ loop residue Lys120, which forms hydrogen bonds with the GlcNAc O6 and/or the Neu5Ac O9, had a large effect on binding (eightfold drop in affinity). In contrast, substitutions of its neighboring GG′ loop residues Gln122 or Leu123 had only minor effects on binding (less than twofold affinity decrease for each mutant).
Fig. 5.
Binding affinities of Siglec-8 mutants for 6′S sLex determined by NMR-titration, and conservation surface mapping. (A) Combined 1H–15N chemical shift changes (Δδ) of the representative Tyr119 amide resonance detected on 15N-labeled Siglec-8 wild-type (WT) and various alanine-substituted mutants plotted as a function of the molar ratio (carbohydrate/protein). Dissociation constants (Kd) are presented as mean ± SD from separate fitting of the NMR-binding isotherms of 9–12 individual residues, as shown in Fig. S5. NB, no binding detected. (B) Kd values for binding of Siglec-8 wild-type and mutants to 6′S sLex, normalized with respect to that of Siglec-8 wild-type (set to 1.0). Error bars represent propagation of errors. (C) Conservation analysis among the human Siglec family members mapped onto the molecular surface of Siglec-8 in complex with 6′S sLex. Amino acid residues are color-coded according to sequence conservation scores calculated by ConSurf (39). Side chains of selected residues within the binding site are shown as sticks and labeled.
Fig. S5.
Determination of dissociation constants (Kd) for binding of Siglec-8 wild type and different alanine point mutants to 6′-sulfo sialyl Lewisx by NMR titration. Kd values were determined by fitting the combined 1H/15N chemical shift changes (Δδ) of 15N-labeled Siglec-8 observed in 2D 1H,15N-HSQC spectra upon titration with 6′S sLex, as described in Materials and Methods. Reported Kd values and errors are the mean values ± SD from performing the fit for 9–12 significantly perturbed, nonoverlapping 1H/15N resonance signals. All spectra were recorded at 20 °C, at a 1H frequency of 500 MHz, using 15N-labeled Siglec-8 concentrated at 100 µM in 20 mM potassium phosphate, 40 mM NaCl at pH 7.4 (5% D2O).
Overall, substitutions of the positively charged residues at the binding site (Arg56, Arg109, Lys116, and Lys120) caused the largest reductions in binding affinity. Besides providing specific hydrogen-bonding contacts, these tightly clustered positive charges (Fig. 3 H and I) may contribute to a substantially enhanced association rate (kon) owing to attractive Coulomb interactions (40) with the doubly negatively charged carbohydrate ligand. Electrostatic enhancement of complex formation has been described for protein–RNA interactions, where binding can be several orders of magnitude faster than in a purely diffusion-limited process (41), and is particularly conceivable for the present interaction characterized by fast-exchanging free and bound states. It is plausible that this effect is further amplified when clustered receptors on cell surfaces avidly interact with multivalently presented glycan epitopes on large polysaccharide or mucin-type glycoprotein ligands, leading to markedly higher in vivo binding affinities, compared with the here investigated monovalent lectin–glycan interaction.
Discussion
Here we have elucidated the molecular basis of how human Siglec-8 selectively recognizes its principal carbohydrate target 6′S sLex. Our comparative analysis, combined with previous functional studies (26, 27), provides clear evidence that Siglec-8 preferentially recognizes sLex epitopes, which display a sulfate group at the C6 of the Gal moiety, and demonstrates that both the presence and position of this modification are critical determinants of Siglec-8 specificity. Quantification revealed the sulfate group to account for a 28-fold greater affinity for binding to 6′S sLex (Kd ≃ 300 µM), compared with nonsulfated sLex (Kd ≃ 8.3 mM). In contrast, a sulfate modification at the C6 of the GlcNAc moiety was found to make only a minor contribution to binding affinity (Fig. 2 B and C). The solution structure of the Siglec-8 lectin domain in complex with 6′S sLex explains these differences in affinity, revealing that tight specificity results from the unique combination of a primary recognition motif for terminal Neu5Ac, which is largely canonical among Siglecs, and a secondary motif for recognition of the underlying Gal6S, which is exclusive to Siglec-8. Conservation analysis of the Siglec-8 binding interface across the human Siglec family (Fig. 5C) shows that only residues engaging Neu5Ac have elevated levels of conservation, whereas all interactions to the underlying glycan moieties are solely mediated through nonconserved side chains. High selectivity for Gal6S is conferred by the cooperative action of three residues on the Siglec-8–unique CC′ loop (Arg56, Tyr58, and Gln59), and mutational dissection revealed this interaction to be dominated by a salt bridge between the Arg56 guanidinium and the sulfate group of Gal6S (Figs. 4 D and E and 5). Although GlcNAc makes direct protein contacts that contribute to affinity, the low number and high variability of interactions observed in the NMR ensemble (Table 1) imply only a minor role for this residue in specificity. Notably, the O6 of GlcNAc is anchored in the interior of the binding groove by hydrogen bonds with the side chains of Lys120 and/or Gln122 (Fig. 4F and Table 1), thus suggesting how the GlcNAc-6-sulfate group in 6S sLex and 6,6′S sLex ligands may be contacted by Siglec-8. However, molecular modeling of the Siglec-8–6,6′S sLex complex (Fig. S6) indicates that binding of a bulkier sulfate would interfere with the architecture of the binding pocket, implying that the small net gain in affinity produced by a GlcNAc-6-sulfate modification may arise from additional favorable electrostatic and hydrogen bonding interactions, largely counteracted by less favorable steric complementarity. The lack of contacts to Fuc, which projects away from the Siglec-8 surface, is consistent with a recent glycan microarray analysis (42) where a Siglec-8-IgFc fusion protein was found to bind to 6′S sLacNAc (6′-sulfo 3′-sialyl N-acetyllactosamine; corresponding to 6′S sLex, minus α1,3-linked Fuc), in addition to 6′S sLex, suggesting this monosaccharide to be dispensable for Siglec-8 recognition. However, Fuc stabilizes the core Lex trisaccharide in a binding-competent conformation by forming an intracarbohydrate C–H⋅⋅⋅O hydrogen bond to Gal6S (Fig. 4F), together with hydrophobic interactions (37, 38, 43), and may thus indirectly contribute to affinity by decreasing the entropic penalty of fixing multiple conformational degrees of freedom upon binding.
Fig. S6.
Molecular modeling of 6,6′S sLex recognition by Siglec-8. (A) Stereoview of a structural model of the complex between the Siglec-8 lectin domain and the disulfated 6,6′S sLex. The carbohydrate and key amino acid residues are shown as sticks. Potential hydrogen bonds are indicated by black dashed lines. The model was generated using the NMR structure of the Siglec-8–6′S sLex complex as a template, modified by addition of a sulfate group at the GlcNAc-O6. This resulted in steric clashes with the side chains of Lys120 and Gln122, which were subsequently relieved by energy minimization and molecular dynamics (MD) simulation, as described in Materials and Methods. (B) Superposition of the modeled Siglec-8–6,6′S sLex complex (light blue/magenta) and the lowest-energy NMR structure of the Siglec-8–6′S sLex complex (gray/cyan), shown in stereoview. Potential hydrogen bonds in each complex are indicated by dashed lines in green and black, respectively. In our model, the GlcNAc-6-sulfate group is placed in a position to interact directly with the side chains of Lys120 and Gln122; the Arg56 side chain is reoriented, allowing it to form additional salt bridges with the GlcNAc-6-sulfate group, while simultaneously maintaining a salt bridge with the Gal-6-sulfate group. These additional hydrogen bond and salt bridge interactions, together with favorable Coulomb interactions, may account for the increase in affinity caused by a GlcNAc-6-sulfate modification. Although the similar chemical shift changes observed for Gln122 (backbone and side chain amides) upon binding 6′S sLex and 6,6′S sLex ligands do not unambiguously support this model, the distinct trajectories of chemical shift changes for Arg56 and Lys120 backbone amides (Fig. S1B) may reflect direct involvement of these residues in the GlcNAc-6-sulfate recognition; however, the lack of NMR-observable resonances for Lys120 NH3+ and Arg56 NH2 groups hampers further validation of the model by the available NMR titration data. Notably, superposition of our model with the NMR structure of the Siglec-8–6′S sLex complex suggests that accommodation of the bulkier GlcNAc-6-sulfate group requires reorientation of the entire Lex moiety, as seen most markedly by the displacement of the GlcNAc moiety by more than 3 Å from the initial position [a black arrow indicates the distance between the anomeric carbon (C1) atoms of the GlcNAc moieties in each complex]. It is reasonable to assume that this reflects a sterically less favorable overall interaction, which would explain the much smaller gain in affinity associated with a GlcNAc-6-sulfate compared with a Gal-6-sulfate modification (Fig. 2C).
Disruption of the salt bridge to the Neu5Ac carboxylate by mutation of the essential Arg109 leads to a complete loss of binding, and similarly, without Gal-6-sulfation the binding affinity is decreased to values that are likely well below the threshold needed for Siglec-8 signaling activation. Together this demonstrates that simultaneous recognition of Neu5Ac and the underlying Gal6S is absolutely required for effective Siglec-8 engagement. Considering that no other human Siglec, nor any other known endogenous receptor, shares this carbohydrate specificity, this unique binding mode may ensure a maximum control of the Siglec-8 antiinflammatory signaling pathway, while safeguarding against cross-activation by other sialylated glycans omnipresent on vertebrate cell surfaces or by hijacking pathogens decorated with sialic acids to evade host immune surveillance.
Of note, murine Siglec-F has been considered, albeit controversially, to be a functional paralog of Siglec-8, owing to a similar (although less restricted) expression pattern, glycan specificity, and ligation-induced proapoptotic effects, and was therefore proposed as an in vivo model for exploring Siglec-8 function in mice (3, 27). In light of our results, sequence analysis reveals that key residues of Siglec-8 responsible for the sulfate recognition are maintained only across its primate orthologs but are absent in Siglec-F (Fig. S3A), suggesting either that Siglec-F recognizes 6′S sLex by a distinct mode or, in support of recent findings (44, 45), that Gal C6 sulfation is not required for Siglec-F ligands.
Our findings also established that a single sulfate modification at the Gal C6 renders the poor-affinity sLex glycan epitope into the preferred ligand for Siglec-8 and thus into a potential antiinflammatory signal. By contrast, nonsulfated sLex and variants carrying a sulfate group at GlcNAc C6 are known ligands for the selectin family of cell adhesion receptors (E-, P-, and L-selectin) (28) that mediate the recruitment of leukocytes (including eosinophils and mast cells) to inflammatory sites. Given that selectins play key roles in the initiation of eosinophil inflammation, whereas Siglec-8 appears to be implicated in resolving eosinophil inflammatory responses, it is tempting to speculate that site-specific sulfation of the sLex epitope acts as one of the molecular switches that dictate whether eosinophil inflammation is initiated or terminated.
Our results provide unprecedented atomic-level insight on how site-specific glycan sulfation can potently modulate glycan affinity and specificity to prime its selective recognition by its cognate receptor. This type of glycan modification adds, in addition to the nonlinearity and multitude of linkage sites per monosaccharide, yet another layer of complexity to the coding capacity of glycan structures and hence to the processes controlled by their recognition. Future studies identifying and characterizing the involved glycan-editing sulfotransferases and sulfatases will be crucial to understand further the intricate role of glycan sulfation in immune regulation and inflammation, as well as for developing new strategies for therapeutic intervention.
Collectively, we report here the complete molecular and structural description of how a Siglec receptor specifically recognizes its entire glycan ligand epitope, revealing the key determinants of glycan specificity and discrimination. Whereas the conserved sialic acid recognition motif governs general specificity, local sequence diversity in the variable loop regions defines the fine specificity and translates into unique binding modes, enabling individual Siglec family members to participate in distinct and discrete immune-regulatory functions. This work will help elucidate the precise role and mechanism of Siglec-8 in immune cell homeostasis and inflammation resolution and provides a structural template for the rational design of Siglec-8 agonists to harness its potent signaling capacity in novel antiinflammatory therapies for asthma.
Materials and Methods
Carbohydrates.
The 6-sulfo sialyl Lewisx, 6′-sulfo sialyl Lewisx, and 6,6′-disulfo sialyl Lewisx were chemically synthesized as described in SI Appendix. Methyl sialyl Lewisx was purchased from Carbosynth. Identity and purity of all carbohydrates was assessed by 2D NMR spectroscopy.
Protein Expression and Purification.
Proteins were expressed recombinantly and purified as described elsewhere (30). In brief, human Siglec-8 lectin domain (Met1-His139, containing a C26S point mutation to facilitate soluble expression; herein referred to as wild type), C-terminally fused to a thrombin cleavage-site and a His6-tag, was natively expressed in the oxidative cytoplasm of Escherichia coli Rosetta-gami B (trxB−/gor−) (Novagen), using LB medium or M9 minimal medium supplemented with 1 g/L 15NH4Cl and 4 g/L d-glucose (13C-labeled for 13C/15N-labeled proteins). Proteins were purified by Ni-NTA affinity chromatography, followed by thrombin cleavage for His6-tag removal and size exclusion chromatography (Superdex 75; GE Healthcare). Protein purity and uniform presence of the intradomain disulfide bond were confirmed by SDS/PAGE (reducing vs. nonreducing) and ESI-TOF mass spectrometry, and the oxidized state of the disulfide bond was additionally confirmed by NMR spectroscopy (details are shown in Fig. S7). Mutants were constructed by PCR-mediated site-directed mutagenesis, confirmed by DNA sequencing and expressed and purified as the wild-type protein. The proper fold of all recombinant proteins was confirmed by the observation of well-dispersed 2D 1H,15N-HSQC NMR spectra.
Fig. S7.
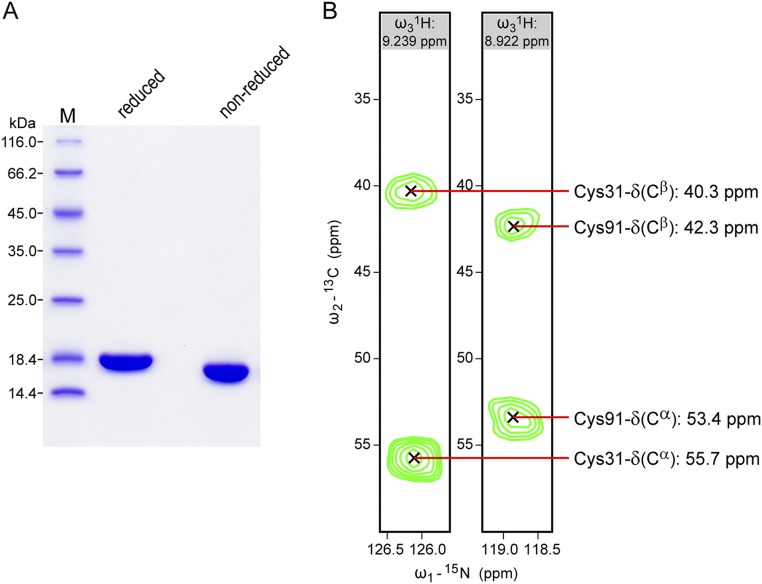
Purity of recombinant Siglec-8 lectin domain and presence of the intradomain disulfide bond (between Cys31 and Cys91) assessed by SDS/PAGE and NMR spectroscopy. (A) Coomassie blue-stained 15%-SDS/PAGE of purified Siglec-8 (natural isotopic composition) under reduced and nonreduced conditions. The slightly faster migration of nonreduced versus reduced protein, and the appearance of the nonreduced protein as a single band clearly indicate the uniform presence of the intradomain disulfide-bond. M, molecular weight marker. (B) Strips of a 3D CBCA(CO)NH spectrum of 13C/15N-labeled Siglec-8 at the 1H-15N resonances of Ser32 and Ser92 reveal the 13C chemical shifts of the Cα and Cβ atoms of their preceding residues Cys31 and Cys91, respectively. The observed 13C chemical shift frequencies are within the characteristic ranges for oxidized cysteines (62), confirming their involvement in a disulfide bond.
NMR Spectroscopy.
NMR spectra were acquired on Bruker AVIII 500-, 600-, 700-, 750-, and 900-MHz spectrometers (all equipped with a cryogenic probe, except for AVIII 750 MHz) at 293 K, unless mentioned otherwise. Samples were measured in 20 mM potassium phosphate, 40 mM NaCl at pH 7.0 (free protein) or pH 7.4 (complex), and at protein concentrations between 0.3–1.2 mM, containing either 5% or 100% (vol/vol) D2O. Protein–carbohydrate complexes were prepared by titrating carbohydrate solution of typically 7–17 mM into a 0.7–1.2 mM protein solution until a molar stoichiometry of 1:1, 1:1.2, or 1:2 was reached. Sequence-specific assignment of protein backbone and side-chain resonances was achieved through 2D 1H,15N-HSQC, 2D 1H,13C-HSQC, 3D HNCA, 3D HNCACB, 3D CBCA(CO)NH, 3D HNCO, 3D HN(CO)CA, 3D (H)CCH-TOCSY, 3D 15N-edited NOESY-HSQC, 2D 1H,1H-NOESY, and two 3D 13C-edited NOESY-HSQC, optimized for the observation of protons attached to aliphatic carbons and to aromatic carbons, respectively. Stereospecific assignments of Val and Leu methyl groups were obtained from 2D 1H,13C-HSQC spectra by using a 10% 13C-labeled sample as described (46). The 2D 1H,1H-TOCSY and 2D 1H,13C-HSQC spectra in D2O aided the assignment of aromatic side chain resonances. For the 2D 1H,1H-TOCSY experiments, mixing times of 13 and 60 ms were used, and for the 3D (H)CCH-TOCSY spectrum a mixing time of 21.7 ms was used. All NOESY experiments were recorded using a mixing time of 120 ms, unless otherwise stated. Resonance assignment of the carbohydrate was achieved by using natural abundance 2D 1H,13C-HSQC, 2D 1H,13C-HMBC, 2D 1H,13C-HMQC-COSY, and 2D 1H,1H-TOCSY spectra. Resonance assignments of the carbohydrate in complex with the Siglec-8 lectin domain were performed using natural abundance 2D 1H,13C-HSQC, 2D 13C/15N F2-filtered 1H,1H-TOCSY, and 2D 13C/15N F1-filtered F2-filtered NOESY spectra. Intracarbohydrate NOEs were assigned from 2D 13C/15N F1-filtered F2-filtered NOESY experiments. Protein–carbohydrate intermolecular NOEs were obtained from 2D 13C/15N F2-filtered NOESY and 3D 13C F1-edited F3-filtered HSQC-NOESY (47) spectra, recorded at 293 K or 303 K, with mixing times of 60–150 ms. Spectra were processed in TopSpin 3.0 (Bruker) and analyzed in Sparky (T. D. Goddard and D. G. Kneller, SPARKY 3, University of California, San Francisco). The 1H chemical shifts are referenced to 2,2-dimethyl-2-silapentane-5-sulfonic acid (DSS). The 13C and 15N chemical shifts are indirectly referenced using scaling factors of 0.251449530 and 0.101329118, respectively (48).
Structure Calculation and Refinement.
The software package ATNOS CANDID (49, 50) was used for picking of initial cross-peaks of NOESY spectra. The resulting peak lists were assigned using a combination of manual and automated assignments within the NOEASSIGN module of CYANA 3.0 (51). For the complex, an extended CYANA library including the carbohydrate residues was used, for which the topology was derived from a structural model of sLex that was generated in SWEET2 (www.glycosciences.de) (52) and modified for addition of the 3-aminopropyl-aglycon at C1 of GlcNAc and the sulfate group at C6 of Gal within ChemBio 3D Ultra 12.0 (Cambridge Soft). Structure calculations with torsion-angle dynamics were conducted within CYANA 3.0 (51), using additional restraints for backbone torsion angles derived from chemical shifts by using the program TALOS+ (53), stereospecific assignments of Val and Leu prochiral methyl groups, restraints for the disulfide bond generated with the CYANA macro ssbond, and distance restraints for intraprotein hydrogen bonds, based on the observation of slow-exchanging amide hydrogens in D2O. Hydrogen bond acceptors were identified from inspection of initial structures. Two hydrogen bonds involving Tyr hydroxyl groups (Tyr106 and Tyr119) were identified from detection of their Hη resonances in an F2-filtered NOESY measured in H2O and also in 15N- and 13C-edited 3D NOESY spectra, indicating protection from proton exchange, which typically prevents the observation of such signals. Intracarbohydrate and intermolecular (protein–carbohydrate) NOEs were assigned manually and converted into distance restraints based on cross-peak signal-to-noise (S/N) ratios. From 500 structures calculated in CYANA 3.0 (51) the 50 lowest-energy conformers were refined within AMBER 12 (54), using the ff12SB force field for the protein and simultaneously the GLYCAM_06h force field (55) for the protein–carbohydrate complex, in implicit solvent. For the 3-aminopropyl-linker the topology and parameters files were computed within Antechamber (56), using the General Amber Force Field (57) and the AM1-BCC (58) model for assignment of partial atomic charges. The 20 best structures were selected and analyzed with PROCHECK-NMR (59). The Ramachandran statistics for Siglec-8 (residues 7–136) are 89.7% in most favored and 10.3% in additionally favored regions for the free protein and 90.4% in most favored and 9.6% in additionally favored regions for the protein in the complex. The NMR and refinement statistics are provided in Table S1. Carbohydrate conformations in the NMR ensemble of the complex were compared by glycosidic torsion angles (φ, ψ), defined as φ = H1-C1-OX-CX and ψ = C1-OX-CX-HX, where X is the number of the carbon atom of the second monosaccharide involved in the glycosidic linkage. For the Neu5Ac(α2–3)Gal linkage, angles are defined as φ = C1-C2-OX-CX and ψ = C2-OX-CX-HX. A-face and B-face of the described monosaccharides are defined as the faces on which the numbering of the carbon atoms increases in a clockwise and anticlockwise direction, respectively. Structural figures were prepared using PyMOL (Schrödinger, LLC).
NMR Titration Experiments.
NMR titrations were carried out by recording a series of 2D 1H,15N-HSQC spectra of 15N-labeled Siglec-8 lectin domain at 200 µM in 20 mM potassium phosphate, 40 mM NaCl, pH 7.4 (5% D2O), with the addition of increasing amounts of carbohydrate ligands. Titrations for comparison of Siglec-8 wild type and mutants binding to 6′S sLex were performed by a similar procedure, except that the starting protein concentrations were 100 µM. All NMR spectra were recorded at 20 °C and at 500 MHz. Quantifications of NMR-binding isotherms were carried out only for nonoverlapping 1H-15N protein resonances showing significant chemical shift changes in fast chemical exchange. Equilibrium dissociation constants (Kd) were obtained from nonlinear least-squares fit in MATLAB (MathWorks) to the equation (60)
where [P]t and [L]t are total concentrations of protein and ligand, respectively, which were calculated for each titration point with [P]t = ([P]iVi/(Vi + Vad)) and [L]t = ([L]sVad/(Vi + Vad)), where [P]i and Vi are the initial concentrations and initial volume of the protein sample, Vad is the total volume of added ligand, and [L]s is the concentration of the ligand stock solution. Δδ is the observed chemical shift change, and Δδmax is the chemical shift change at saturation, both calculated as a combination of 1H and 15N chemical shift changes according to
where Δδ1H and Δδ15N denote the chemical shift differences in parts per million (ppm) of amide hydrogen and nitrogen atoms, respectively, between the free and the carbohydrate-bound states of the protein.
Isothermal Titration Calorimetry.
ITC was performed using a VP-ITC microcalorimeter (MicroCal). Prior the experiment the carbohydrate was dialyzed against 20 mM potassium phosphate, 40 mM NaCl, pH 7.4 (same buffer as used for the protein), using a Micro DispoDIALYZER (100 Da MWCO; Harvard Apparatus). The microsyringe was loaded with a solution of carbohydrate (7 mM), and the sample cell was loaded with a solution of protein (200 µM). Titration was conducted at 20 °C using one initial injection of 2 µL with a duration of 4 s and 400 s spacing, followed by 34 identical injections of 4 µL with a duration of 8 s per injection and 10 min spacing between injections. Data were analyzed using the MicroCal ITC module of Origin 7.0 (OriginLab), applying a single-site binding model.
Competitive Binding Assay.
Polyacrylamide-based competitive binding assay (31) was used to evaluate the binding affinity of Siglec-8 for 6′S sLex. Microtiter plates (F96 MaxiSorp, Nunc) were coated with 100 µL per well of a 10 µg/mL solution of Siglec-8 lectin domain in assay buffer (20 mM Hepes, pH 7.4; 150 mM NaCl; and 1 mM CaCl2), overnight at 4 °C. The coating solution was discarded, and the wells were blocked with 150 µL per well of 3% BSA in assay buffer for 2 h at 4 °C. After three washing steps with assay buffer (150 µL per well), a threefold serial dilution of the 6′S sLex ligand (50 µL per well) in assay buffer and streptavidin-peroxidase coupled polyacrylamide glycopolymer [6′S sLex-PAA (Lectinity), 50 µL per well of a 0.3 µg/mL solution] were added. The plate was incubated on a thermoshaker (PHMP-4, Grant Instruments) for 3 h at 25 °C and shaking speed 350 rpm and then carefully washed four times with 150 µL per well assay buffer. After the addition of 100 µL per well of the horseradish peroxidase substrate 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonic acid), the colorimetric reaction was allowed to develop for 3 min, then stopped by the addition of 2% aqueous oxalic acid, before the optical density was measured at 415 nm on a microplate-reader (Spectramax 190, Molecular Devices). The IC50 value of 6′S sLex was calculated with the Prism software (GraphPad Software, Inc.). The IC50 defines the molar concentration of the test compound that reduces the maximal specific binding of 6′S sLex-PAA polymer to Siglec-8 by 50%.
Modeling of Human Siglec-8–6,6′S sLex Complex.
A structural model of the Siglec-8–6,6′S sLex interaction was generated by using the Siglec-8–6′S sLex complex NMR structure as a template. The bound 6′S sLex was modified by addition of a sulfate group to the GlcNAc-6 position in ChemBio 3D Ultra 12.0 (Cambridge Soft). To eliminate steric clashes with the inserted sulfate moiety, Lys120 and Gln122 side chains were adjusted with Pymol (Schrödinger, LLC). For further refinement, the model was subjected to energy minimization, followed by a 100-ps simulated annealing molecular dynamics in AMBER 12 (54), with the ff12SB force field and the GLYCAM_06h parameters (55) for the carbohydrate, during which the terminal Neu5Ac (only heavy atoms) was held rigid by imposing a weak positional force constant of 2 kcal/mol·Å2, without using any additional restraints.
Supplementary Material
Acknowledgments
We thank M. Blatter and P. Barraud for advice and help with structure calculations; J. Boudet for support concerning the ITC instrument and helpful discussions; F. Damberger, G. Wider, T. Suter-Stahel, and C. Maris for ensuring the best performance of the NMR infrastructure; M. Aebi, S. Campagne, G. Dorn, and K. Fowler for helpful discussions and critically reading the manuscript; and R. Woods for providing GLYCAM. This work was supported by the Swiss National Science Foundation Sinergia Grant CRSII3_127333 (to M.S., F.H-T.A., B.E., and Markus Aebi).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates, NMR chemical shifts, and restraints of the herein reported structures have been deposited in the Protein Data Bank (PDB), www.rscb.org, and the Biological Magnetic Resonance Data Bank (BMRB), www.bmrb.wisc.edu, with the following accession codes: ligand-free Siglec-8 (PDB, 2N7A; BMRB, 25798) and Siglec-8-6′S sLex complex (PDB, 2N7B; BMRB, 25799).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602214113/-/DCSupplemental.
References
- 1.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 3.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14(10):653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kletter D, Singh S, Bern M, Haab BB. Global comparisons of lectin-glycan interactions using a database of analyzed glycan array data. Mol Cell Proteomics. 2013;12(4):1026–1035. doi: 10.1074/mcp.M112.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A, Angata T. Siglecs--The major subfamily of I-type lectins. Glycobiology. 2006;16(1):1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 6.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 7.von Gunten S, Simon HU. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. FASEB J. 2006;20(6):601–605. doi: 10.1096/fj.05-5401hyp. [DOI] [PubMed] [Google Scholar]
- 8.Floyd H, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275(2):861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 9.Kikly KK, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105(6 Pt 1):1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 10.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: A selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101(12):5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 11.Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6′-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 2009;330(2):608–612. doi: 10.1124/jpet.109.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi H, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121(2):499–505.e1. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38(1):121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2012;57(1):169–174. doi: 10.1016/j.cyto.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: Role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132(2):437–445. doi: 10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Gunten S, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119(4):1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Jia Y, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135(3):799–810.e7. doi: 10.1016/j.jaci.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiwamoto T, Katoh T, Tiemeyer M, Bochner BS. The role of lung epithelial ligands for Siglec-8 and Siglec-F in eosinophilic inflammation. Curr Opin Allergy Clin Immunol. 2013;13(1):106–111. doi: 10.1097/ACI.0b013e32835b594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: Phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11(7):577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic Clin Pharmacol Toxicol. 2014;114(1):109–117. doi: 10.1111/bcpt.12163. [DOI] [PubMed] [Google Scholar]
- 21.Gao PS, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18(6):713–719. doi: 10.1038/ejhg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30(5):240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135(3):327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8(3):464–475. doi: 10.1038/mi.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochner BS, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280(6):4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 27.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15(11):1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 28.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8(11):874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campanero-Rhodes MA, et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun. 2006;344(4):1141–1146. doi: 10.1016/j.bbrc.2006.03.223. [DOI] [PubMed] [Google Scholar]
- 30.Pröpster JM, Yang F, Ernst B, Allain FH, Schubert M. Functional Siglec lectin domains from soluble expression in the cytoplasm of Escherichia coli. Protein Expr Purif. 2015;109:14–22. doi: 10.1016/j.pep.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Rabbani S, Jiang X, Schwardt O, Ernst B. Expression of the carbohydrate recognition domain of FimH and development of a competitive binding assay. Anal Biochem. 2010;407(2):188–195. doi: 10.1016/j.ab.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 32.May AP, Robinson RC, Vinson M, Crocker PR, Jones EY. Crystal structure of the N-terminal domain of sialoadhesin in complex with 3′ sialyllactose at 1.85 A resolution. Mol Cell. 1998;1(5):719–728. doi: 10.1016/s1097-2765(00)80071-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhuravleva MA, Trandem K, Sun PD. Structural implications of Siglec-5-mediated sialoglycan recognition. J Mol Biol. 2008;375(2):437–447. doi: 10.1016/j.jmb.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alphey MS, Attrill H, Crocker PR, van Aalten DM. High resolution crystal structures of Siglec-7. Insights into ligand specificity in the Siglec family. J Biol Chem. 2003;278(5):3372–3377. doi: 10.1074/jbc.M210602200. [DOI] [PubMed] [Google Scholar]
- 35.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J Biol Chem. 2002;277(8):6324–6332. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 36.Attrill H, et al. Siglec-7 undergoes a major conformational change when complexed with the alpha(2,8)-disialylganglioside GT1b. J Biol Chem. 2006;281(43):32774–32783. doi: 10.1074/jbc.M601714200. [DOI] [PubMed] [Google Scholar]
- 37.Zierke M, et al. Stabilization of branched oligosaccharides: Lewis(x) benefits from a nonconventional C-H···O hydrogen bond. J Am Chem Soc. 2013;135(36):13464–13472. doi: 10.1021/ja4054702. [DOI] [PubMed] [Google Scholar]
- 38.Battistel MD, Azurmendi HF, Frank M, Freedberg DI. Uncovering nonconventional and conventional hydrogen bonds in oligosaccharides through NMR experiments and molecular modeling: Application to sialyl Lewis-X. J Am Chem Soc. 2015;137(42):13444–13447. doi: 10.1021/jacs.5b03824. [DOI] [PubMed] [Google Scholar]
- 39.Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38(Web Server issue):W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selzer T, Albeck S, Schreiber G. Rational design of faster associating and tighter binding protein complexes. Nat Struct Biol. 2000;7(7):537–541. doi: 10.1038/76744. [DOI] [PubMed] [Google Scholar]
- 41.Auweter SD, et al. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 2006;25(1):163–173. doi: 10.1038/sj.emboj.7600918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiwamoto T, et al. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133(1):240–247.e3. doi: 10.1016/j.jaci.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor ME, Drickamer K. Structural insights into what glycan arrays tell us about how glycan-binding proteins interact with their ligands. Glycobiology. 2009;19(11):1155–1162. doi: 10.1093/glycob/cwp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patnode ML, et al. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 2013;288(37):26533–26545. doi: 10.1074/jbc.M113.485409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiwamoto T, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135(5):1329–1340.e9. doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neri D, Szyperski T, Otting G, Senn H, Wüthrich K. Stereospecific nuclear magnetic resonance assignments of the methyl groups of valine and leucine in the DNA-binding domain of the 434 repressor by biosynthetically directed fractional 13C labeling. Biochemistry. 1989;28(19):7510–7516. doi: 10.1021/bi00445a003. [DOI] [PubMed] [Google Scholar]
- 47.Dominguez C, Schubert M, Duss O, Ravindranathan S, Allain FH. Structure determination and dynamics of protein-RNA complexes by NMR spectroscopy. Prog Nucl Magn Reson Spectrosc. 2011;58(1-2):1–61. doi: 10.1016/j.pnmrs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Markley JL, et al. IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy Recommendations for the presentation of NMR structures of proteins and nucleic acids. J Biomol NMR. 1998;12(1):1–23. doi: 10.1023/a:1008290618449. [DOI] [PubMed] [Google Scholar]
- 49.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J Mol Biol. 2002;319(1):209–227. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 50.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE-identification in the NOESY spectra using the new software ATNOS. J Biomol NMR. 2002;24(3):171–189. doi: 10.1023/a:1021614115432. [DOI] [PubMed] [Google Scholar]
- 51.Güntert P. Automated NMR structure calculation with CYANA. Methods Mol Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 52.Bohne A, Lang E, von der Lieth CW. SWEET - WWW-based rapid 3D construction of oligo- and polysaccharides. Bioinformatics. 1999;15(9):767–768. doi: 10.1093/bioinformatics/15.9.767. [DOI] [PubMed] [Google Scholar]
- 53.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Case DA, et al. 2012. AMBER 12 (University of California, San Francisco)
- 55.Kirschner KN, et al. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J Comput Chem. 2008;29(4):622–655. doi: 10.1002/jcc.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006;25(2):247–260. doi: 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem. 2004;25(9):1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 58.Jakalian A, Jack DB, Bayly CI. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem. 2002;23(16):1623–1641. doi: 10.1002/jcc.10128. [DOI] [PubMed] [Google Scholar]
- 59.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8(4):477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 60.Williamson MP. Using chemical shift perturbation to characterise ligand binding. Prog Nucl Magn Reson Spectrosc. 2013;73:1–16. doi: 10.1016/j.pnmrs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Gargaro AR, et al. NMR detection of arginine-ligand interactions in complexes of Lactobacillus casei dihydrofolate reductase. Eur J Biochem. 1996;238(2):435–439. doi: 10.1111/j.1432-1033.1996.0435z.x. [DOI] [PubMed] [Google Scholar]
- 62.Sharma D, Rajarathnam K. 13C NMR chemical shifts can predict disulfide bond formation. J Biomol NMR. 2000;18(2):165–171. doi: 10.1023/a:1008398416292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.