Significance
IL-7 and IL-15 have been shown to play crucial roles in maintaining the population of memory T cells, which are important in fighting against secondary infections, but how effector and memory T cells receive these cytokines is incompletely understood. We investigated the role of C-C receptor 7 (CCR7) in the development of memory T cells using CCR7-deficient mice. CCR7-deficient memory T cells showed an abnormal migratory pattern and increased survival, especially in the lungs and bone marrow, in an IL-15–dependent fashion compared with wild type memory cells. We conclude that the localization of memory T cells is important to “see” these cytokines for homeostasis.
Keywords: CCR7, memory T cell, IL-7, IL-15, homeostasis
Abstract
C-C receptor 7 (CCR7) is important to allow T cells and dendritic cells to migrate toward CCL19- and CCL21-producing cells in the T-cell zone of the spleen and lymph nodes. The role of this chemokine receptor in regulating the homeostasis of effector and memory T cells during acute viral infection is poorly defined, however. In this study, we show that CCR7 expression alters memory CD8 T-cell homeostasis following lymphocytic choriomeningitis virus infection. Greater numbers of CCR7-deficient memory T cells were formed and maintained compared with CCR7-sufficient memory T cells, especially in the lung and bone marrow. The CCR7-deficient memory T cells also displayed enhanced rates of homeostatic turnover, which may stem from increased exposure to IL-15 as a consequence of reduced exposure to IL-7, because removal of IL-15, but not of IL-7, normalized the numbers of CCR7-sufficient and CCR7-deficient memory CD8 T cells. This result suggests that IL-15 is the predominant cytokine supporting augmentation of the CCR7−/− memory CD8 T-cell pool. Taken together, these data suggest that CCR7 biases memory CD8 T cells toward IL-7–dependent niches over IL-15–dependent niches, which provides insight into the homeostatic regulation of different memory T-cell subsets.
During an immune response to acute infection, pathogen-specific naïve CD8 T cells greatly expand and develop into cytotoxic effector T lymphocytes (1). After viral clearance, most effector T cells die, but a small proportion survive and persist as memory T cells. In general, virus-specific memory CD8 T cells are long-lived, and periodically, some of the cells divide to sustain the population via self-renewal. Memory T cells help protect against secondary infections because of their increased frequency and ability to proliferate and mount effector functions more rapidly than naïve T cells.
The memory CD8 T-cell population is heterogeneous, consisting of subsets that vary in longevity, function, and anatomic location. These subsets can be distinguished based on CD27, CD28, CD62L, CCR7, and CD103 expression (2–4). The well-defined subsets include “central” memory T (TCM) and “effector” memory T (TEM) cells. In general, TCM cells express increased amounts of CD62L, CCR7, CD27, and CD28 and preferentially circulate to the spleen and lymph nodes (LNs). TCM cells tend to produce more IL-2 and have robust proliferative responses on secondary infection. TCM cells also have a greater ability to self-renew and undergo homeostatic proliferation, also known as basal proliferation. In comparison, TEM cells express lower amounts of LN homing molecules and costimulatory receptors and increased amounts of cytotoxic proteins. Consequently, TEM cells are found primarily in the blood and nonlymphoid tissues, such as the liver and lung. When separated, TEM memory T cells may be the most effective in fighting certain types of infections that require immediate effector functions, whereas TCM cells may be most protective against rapidly replicating pathogens that require large clonal bursts (5–7). It is likely that optimal immune defense relies on the cooperative and complementary functions of both memory T-cell subsets. In addition, “resident” memory (TRM) cells have been found in the skin, brain, lung, thymus, salivary gland, vaginal mucosa, and intestine and appear to persist in these tissues without apparent recirculation and to protect against reinfection at these sites (8–12). Understanding the heterogeneity of memory CD8 T-cell populations in more detail will be important for identifying the relative functional contributions of different subsets to short-term and long-term protective immunity.
The dominant cytokines involved in generating and maintaining memory T cells are IL-7 and IL-15 (13). IL-7 is critical for memory T-cell survival and is principally produced by nonhematopoietic cell types (13–16). In the spleen and LNs, IL-7 is produced by gp38+ stromal cells known as fibroblastic reticular cells (FRCs) in the T-cell zone, which also produce the chemokines CCL19 and CCL21 to recruit CCR7+ T cells to that site (17). Therefore, it is plausible that CCR7 may play a crucial role in enabling T cells to “see” IL-7. In contrast, IL-15 is produced by cells of the hematopoietic system, such as dendritic cells (DCs) and macrophages, and is transpresented to T cells via binding to the high-affinity IL-15 receptor α chain (IL-15Rα) expressed on conventional DCs (cDCs) and macrophages (13, 18–20). IL-15 can be induced by type I IFNs during infection (21, 22), but under resting conditions, basal levels of IL-15 aid memory T-cell survival and help replenish the pool of memory CD8 T cells by driving a slow but steady rate of turnover (13). In addition, it has been shown that memory T cells proliferate preferentially in the bone marrow (BM) in an IL-15–dependent fashion (23).
Additional work using conditional deletion of IL-15Rα in cDCs or macrophages has shown that TCM cells are more reliant on production of IL-15 from cDCs, even though both cDCs and macrophages support the homeostasis of memory T cells by presenting IL-15 (18). In the BM, the most memory T cells show a TEM phenotype, and their longevity is largely supported by the IL-15 presentation of macrophages. These results indicate that TEM and TCM engage in different cell–cell interactions that regulate their homeostasis. Moreover, in previous work we showed that CD62L+ TCM and CD62L− TEM cells localized predominantly to the T-cell zone and red pulp (RP) of the spleen, respectively, and thus occupy distinct anatomic niches in lymphoid tissues (24).
In light of these findings, it remains to be better studied how memory T cells localize to these specialized microenvironments, and what signals they receive within that regulate their survival and self-renewal. Furthermore, little information exists on the factors that sustain memory T cells in nonlymphoid organs, and whether there are distinct anatomic niches in which memory T cells reside in these tissues as well.
In the present study, we aimed to better understand the role of CCR7 in developing and maintaining effector and memory T cells in vivo by studying the homeostasis of CCR7-deficient lymphocytic choriomeningitis virus (LCMV)-specific memory CD8 T cells. The prevailing hypothesis was that CCR7 deficiency would interfere with the ability of memory CD8 T cells to gain access to IL-7-niches in the lymphoid organs, which would negatively impact memory CD8 T-cell survival and homeostasis. Although our results do not fully support this hypothesis, they do provide insight into the anatomic factors involved in of memory T-cell homeostasis. Compared with wild type (WT) T cells, CCR7 KO T cells expanded normally, but survived longer and formed greater numbers of memory T cells. As expected, fewer CCR7 KO memory CD8 T cells were found in the LNs and the T-cell zone of spleen, but unexpectedly, greater numbers were found in the lung and BM relative to WT memory T cells. Closer analysis showed that the elevated number of CCR7 KO relative to WT memory CD8 T cells was associated with increased rates of homeostatic turnover and a greater dependency on IL-15 over IL-7 for their persistence in certain tissues. Taken together, these results suggest that memory CD8 T cells lacking CCR7 have augmented exposure to IL-15, possibly because their trafficking toward IL-7–rich niches in lymphoid organs is impaired and compensated for by greater occupancy in IL-15–rich niches in tissues such as the BM and lung. These findings suggest that memory T-cell homeostasis is flexible, and that the types of cellular interactions directed by the various chemokine receptors they express are integral to their normal homeostasis.
Materials and Methods
Mice and Infections.
C57BL/6 (B6) and IL-7 KO mice were obtained from the National Cancer Institute and Schering-Plough Corporation, respectively. IL-15 and CCR7 KO mice were obtained from The Jackson Laboratory. The Ly5.1+ P14 TCR transgenic mice and generation of P14 chimeric mice via transfer of 25,000 naive P14 CD8+ T cells into B6 mice have been described previously (25). Mice were infected with 2 × 105 pfu LCMV-Armstrong i.p. All animal experiments were carried out in accordance with approved Institutional Animal Care and Use Committee protocols.
Flow Cytometry.
All antibodies used for flow cytometry were purchased from eBioscience. KLRG1 (2F1) hybridoma was obtained from D. Raulet (University of California Berkeley) and was conjugated to Alexa Fluor 647 (Invitrogen) according to the manufacturer's instructions. Cells were examined by flow cytometry with an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Immunofluorescence Microscopy.
At indicated time points postinfection (pi), spleens were isolated and embedded in OCT compound (Sakura). Tissue blocks were frozen in isopentane (Sigma-Aldrich) chilled with dry ice. Sections (8 μm) were cut with a cryostat, air-dried, and fixed with cold acetone. Sections were stained with 1–5 µg/mL fluorochrome- or biotin-labeled Abs specific for CD4, B220, F4/80, Ly5.1, KLRG1, and IL-7R (eBioscience). Biotinylated Abs were visualized with Alexa Fluor 568-conjugated streptavidin (Invitrogen). Images were captured on an Olympus BX-40 microscope with a SPOT RT Slider digital camera (Scanalytics). To quantitate the number and phenotypes of LCMV-specific CD8 T cells within different splenic regions, equal-sized boxed regions were applied to the T-cell zone or RP for each tissue section, and then P14 cells were enumerated and scored with ImageJ software (https://rsb.info.nih.gov/ij/index.html).
Carboxyfluorescein Succinimidyl Ester Labeling and Adoptive Transfer.
Splenocytes containing CCR7 WT or KO P14 CD8 T cells were isolated from LCMV-infected mice on day 35 pi and then depleted of non-CD8 T cells by magnetic separation before adoptive transfer. In all experiments, equal numbers of CCR7 KO and WT P14 CD8 T cells were transferred i.v. The cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) as described previously (26).
Statistical Analysis.
Standard two-tailed t tests were used for all statistical calculations. All error bars and variances represent SEM.
Results
CCR7 KO Memory T Cells Have Impaired Homing to the T-Cell Zone of the Spleen.
We recently reported that KLRG1loIL-7Rhi memory precursor cells are found in both the T-cell zone and the RP of the spleen, whereas KLRG1hiIL-7Rlo terminal effector T cells are localized exclusively in the RD (24). In that study, we concluded that the longevity of effector and memory T cells is linked to their homing ability to the T-cell zone of the spleen to interact with IL-7–producing stromal cells. To test this hypothesis, we generated “P14 chimeric mice” by transferring small numbers of Ly5.1+ CCR7 WT or CCR7 KO P14 transgenic CD8 T cells, which recognize the DbGP33–41 epitope of LCMV, into Ly5.2+ recipient mice, and then infected these mice with LCMV. We collected the spleens of these mice on days 8 and 35 pi and froze them. These frozen tissues were cut, fixed, and serially stained to analyze the localization of P14 cells using four-color immunofluorescence microscopy.
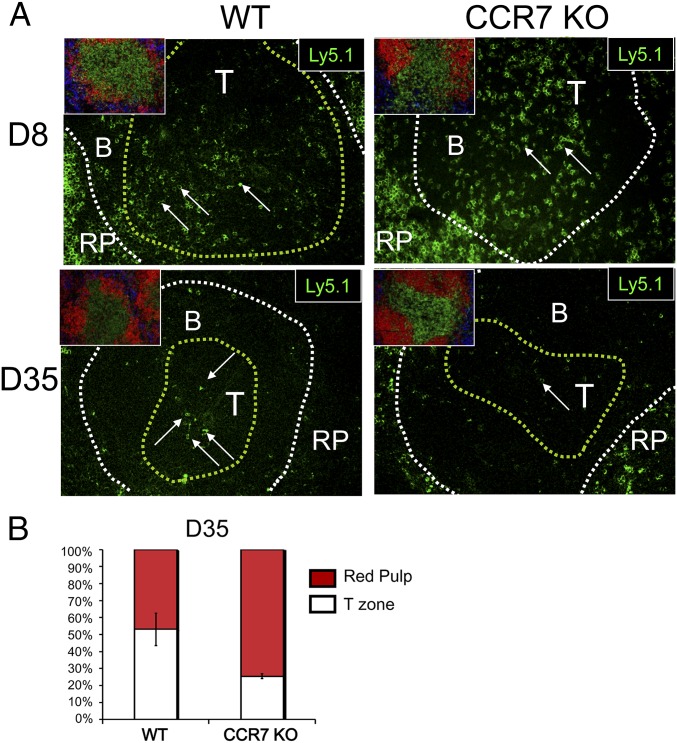
To identify regions of interest, the first serial section was stained with CD4 (T-cell zone), B220 (B-cell zone), and F4/80 (RP) (Fig. 1 A and B). The second section was stained with B220, Ly5.1 (P14 cells), to identify the location of the LCMV-specific effector and memory CD8 T cells (Fig. 1 A and B). At day 8 pi in both the CCR7 WT and KO chimeras, ∼30–40% of the LCMV-specific effector T cells were found in the T-cell zone, with the remainder located in the RP (Fig. 1A). As effector cells contracted and memory CD8 T cells formed on day 35 pi, the localization of CCR7 WT memory cells remained similar to that seen on day 8 pi (Fig. 1A). In contrast, significantly fewer CCR7 KO memory cells were found in the T-cell zone. Based on these findings, we conclude that CCR7 plays a central role in localizing memory, but not necessarily effector, CD8 T cells to the T-cell zones of lymphoid organs. The CCR7-independent trafficking of effector CD8 T cells is likely due to the redundancy with other inflammatory chemokine/chemokine receptor interactions produced during live viral infection.
Fig. 1.
Differential localization of CCR7 WT and KO memory T-cell in the spleen. Ly5.1+ P14 chimeric mice were infected with LCMV, and spleens were harvested on days 8 and 35 pi. Spleens were cut in half; one-half was dissociated for flow cytometry to analyze the Ly5.1+ P14 CD8 T cells, and the other half was frozen and analyzed by serial sections and immunofluorescent microscopy. (A) Representative three-color immunofluorescent images showing the microstructure of the spleen from the first serial sections, stained with CD4 (green), B220 (red), and F4/80 (blue) to outline the T-cell zone (yellow line) and WP (white line) (Insets). A second set of sections was stained with Ly5.1 (green). Arrows indicate Ly5.1+ cells. (B) Mean ± SEM frequency of Ly5.1+ memory T cells located in the T-cell zone and the RP. Data presented are representative of six to eight mice, and multiple frozen sections per mouse were analyzed at each time point.
The Number of CCR7-Deficient Memory T Cells Is Increased After LCMV Infection with Normal Recall Ability.
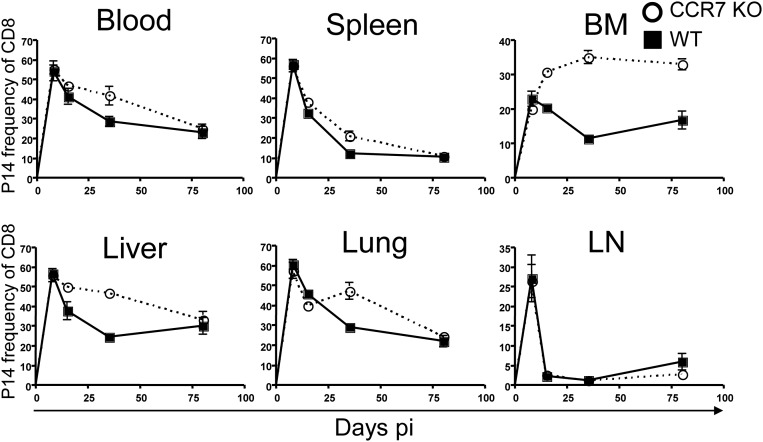
To examine the effects of CCR7 deficiency in memory CD8 T-cell maintenance and homeostasis, we infected chimeric mice containing P14 CCR7 WT or KO cells with LCMV and analyzed the donor cells over the course of LCMV infection in multiple tissues, including the spleen, liver, lung, LNs, blood, and BM. The frequencies of P14 cells among CD8 T cells on day 8 pi were comparable in CCR7 WT and KO cells in all tissues analyzed, even the LNs (Fig. S1). Although this LN homing might be unexpected because naïve T cells home into the LNs in a CCR7-dependent manner, it supports our data indicating that CCR7 KO cells were localized in the T-cell zone at day 8 pi. As effector T cells developed and became memory T cells, the frequencies of CCR7 KO memory cells were significantly higher in the BM, liver, and lung compared with WT cells at the early memory time point (day 30 pi).
Fig. S1.
Enhanced survival of CCR7 KO memory CD8 T cells. P14 chimeric mice were infected, and the tissues were analyzed. Line graphs show CCR7 WT (squares) and KO (circles) P14 CD8 T-cell frequencies. Data are representative of three independent experiments including at least two animals per time point.
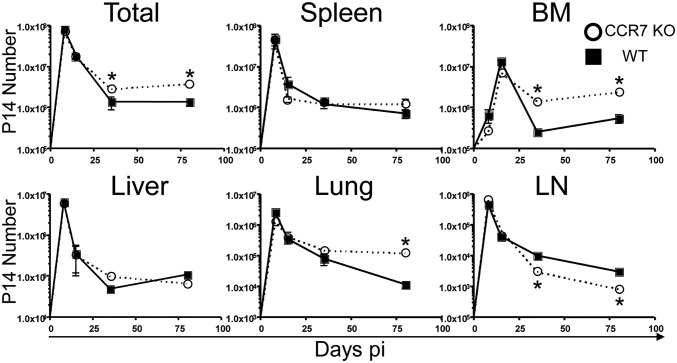
Although the frequency of CCR7 KO memory cells in the liver and lung declined over time and became similar to that of WT cells, the frequency of CCR7 KO cells remained significantly higher than that of WT cells in the BM on day 80 pi. We also calculated the number of memory T cells found in these tissues. Similar to the frequency, the number of P14 effector cells was similar in CCR7-deficient and -sufficient cells on day 8 pi (Fig. 2); however, the total combined numbers of memory T cells in all tissues that we analyzed were substantially higher in CCR7 KO memory T cells compared with WT memory T cells on days 35 and 80 pi. Although there were fewer CCR7 KO memory T cells than WT cells in the LNs, these cells survived better in the BM on days 35 and 80 pi. In addition, although the frequency of memory T cells was comparable, there were more CCR7 KO T cells than CCR7 WT T cells in the lung on day 80 pi. We confirmed that this discrepancy was from the number of CCR7 WT and KO T cells in the parenchyma of the lung and BM using i.v. CD8 staining (Fig. S2). The numbers of CCR7 WT and KO memory T cells in other tissues, including the spleen and liver, were similar.
Fig. 2.
Increased survival of CCR7-deficient memory CD8 T cells. P14 chimeric mice were infected, and the tissues were analyzed on various days pi, as indicated. Line graphs show CCR7 WT (squares) and KO (circles) P14 CD8 T-cell numbers in the spleen, liver, lung, inguinal LNs, BM, and total from all tissues. Data are representative of at least three independent experiments including at least two animals per time point. *P < 0.05.
Fig. S2.
Increased survival of CCR7-deficient memory T cells in the parenchyma of the lung and BM. Mice containing P14 cells were infected. On days pi as indicated, mAb recognizing CD8α was injected i.v., and the tissues were dissected 5 min later to calculate the number of P14 cells. (A) Line graphs showing CCR7 WT (squares) and KO (circles) P14 CD8 T-cell numbers in spleen, lung, LN, and BM. (B) P14 cells in the parenchyma were gated based on i.v. staining of CD8α on day 80 pi. Shown are the accumulated data from two to six animals per time point. *P < 0.05.
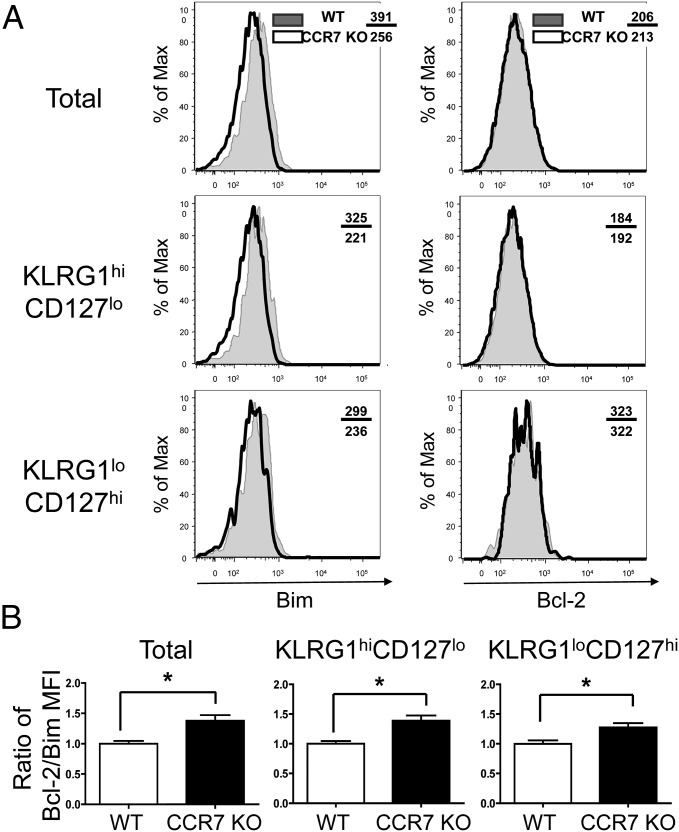
Given the differing numbers of CCR7 WT and KO memory T cells on day 30 pi, we examined whether the expression of proapoptotic or antiapoptotic molecules differed between CCR7 WT and KO effector cells. Although the bcl-2 expression levels were comparable, CCR7 KO effector T cells had significantly lower levels of Bim compared with WT effector T cells on day 8 pi (Fig. 3 A and B). These data may indicate the importance of Bim expression in the improved survival of CCR7 KO effector T cells.
Fig. 3.
Low expression of Bim in CCR7 effector T cells. (A) P14 CCR7 WT (shaded) and KO (open) effector T cells were analyzed for expression of Bim and Bcl-2 on day 8 pi by intracellular staining and flow cytometry. The mean fluorescence intensity (MFI) of Bim and Bcl-2 was compared between CCR7 WT and KO T cells as indicated. (B) Bar graphs showing the mean ± SEM ratio of Bcl-2 and Bim MFI from three independent experiments. *P < 0.05.
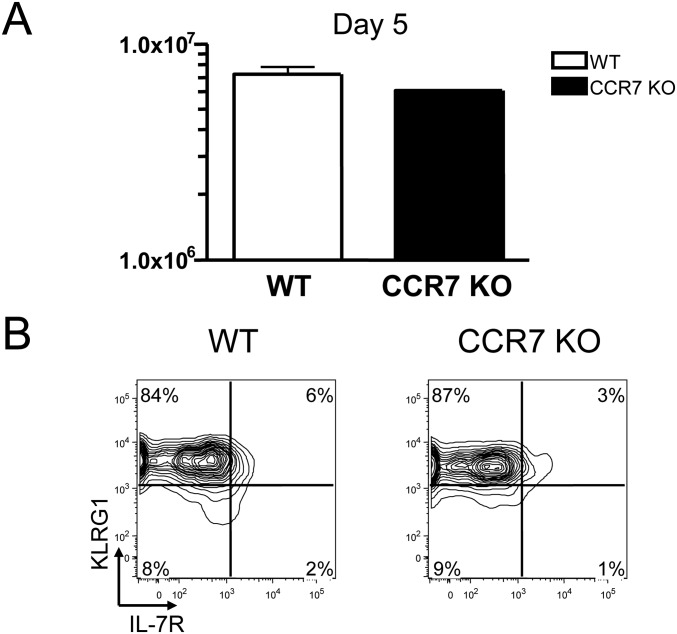
It has been shown that unlike WT memory cells, CCR7 KO memory T cells are not able to mount a proper recall response against local infections (27). Whether CCR7 KO memory T cells can respond normally to systemic secondary infections is not clear, however. To answer this question, we prepared P14 CCR7 WT or KO memory T cells from mice previously infected with LCMV and transferred these cells to naïve mice. These animals were subsequently infected with a Listeria strain genetically engineered to produce LCMV epitope gp33 (LM-33). These mice were killed, and single-cell suspensions were prepared from their spleens on day 5 pi. These splenocytes were stained with mAbs against CD8, Ly5.1, IL-7R, and KLRG1 and then analyzed by flow cytometry. The numbers of P14 cells from each spleen were counted and compared. As shown in Fig. S3A, the numbers of P14 WT and KO T cells were comparable in these spleens.
Fig. S3.
Normal recall response of CCR7 KO memory T cells. Here 50,000 P14 CD8 T cells from CCR7 WT (white bar) or KO (black bar) mice infected with LCMV ∼60 d earlier were transferred into naive recipients that were subsequently infected with LM-33. (A) Mean ± SEM number of secondary effector cells in the spleen was calculated at 5 d pi. (B) The expression of IL-7R and KLRG1 on secondary memory T cells was analyzed by flow cytometry. Data are representative of two independent experiments.
We also tested whether the phenotype of CCR7 KO effector T cells in response to the secondary infection differed from that of WT effector T cells based on the expression of IL-7R and KLRG1. In agreement with previous reports (28), most WT secondary effector T cells were KLRG1hiIL-7Rlo (Fig. S3B). CCR7 KO effector cells also showed the same phenotype as WT effector cells.
Overall, antigen-specific CCR7 KO CD8 T cells expanded normally during systemic viral infection, but they survived and developed memory T cells better than CCR7 WT T cells, especially in the BM and lung. This enhanced survival of CCR7 KO T cells may be related, at least in part, to the low expression of Bim. In addition, CCR7 KO memory T cells can mount a recall response to systemic secondary infections similar to that of WT cells.
CCR7 KO KLRG1loIL-7Rhi Memory Cells Preferentially Survive and Form Memory T Cells.
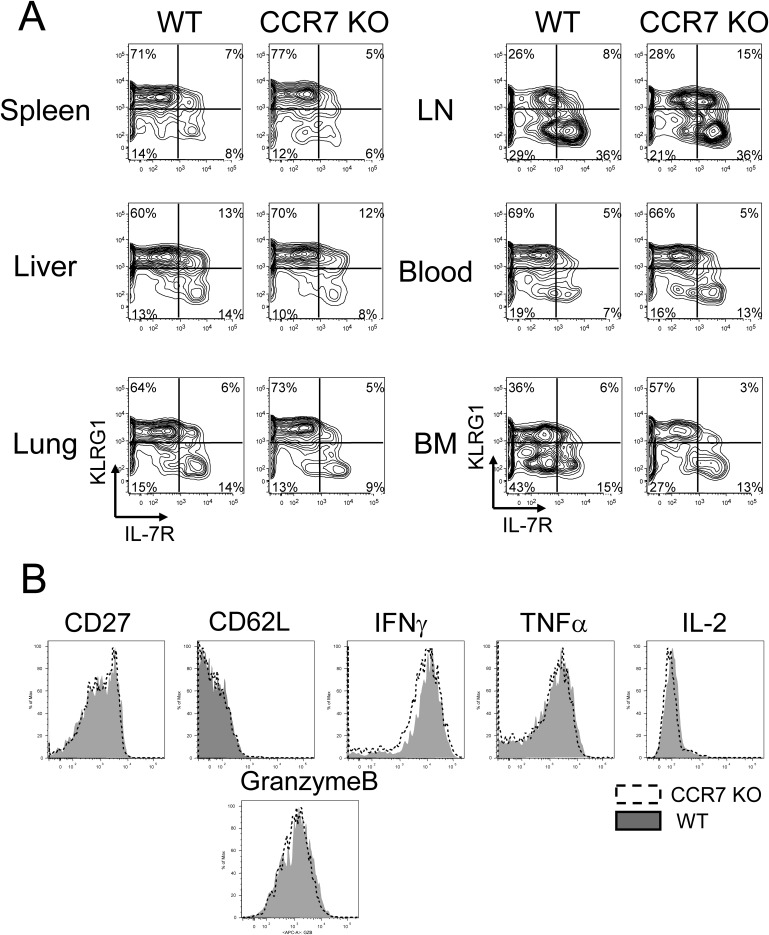
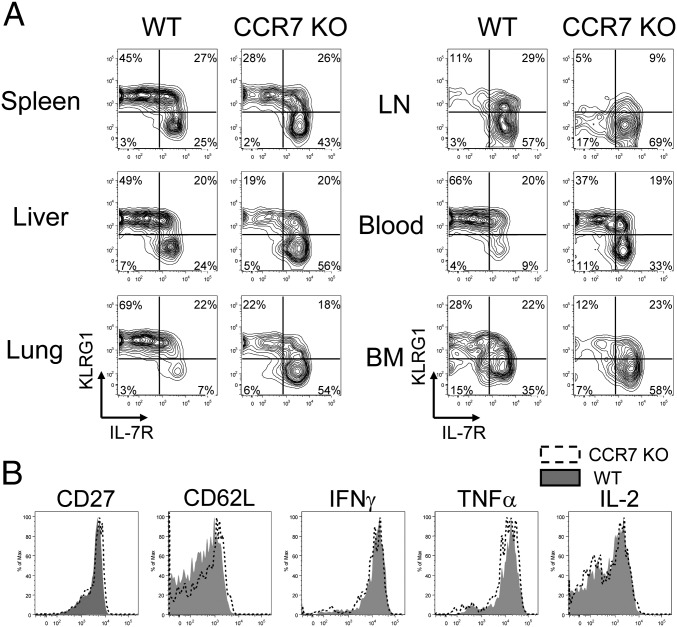
Given our finding of an increased number of CCR7 KO memory T cells, we hypothesized that the frequency of KLRG1loIL-7Rhi memory precursor and memory T cells is greater than that of WT cells. To address this question, P14 chimeric mice were infected with LCMV, and single-cell suspensions were prepared on days 8 and 35 pi as shown previously. In agreement with previous reports, the majority of CCR7 WT effector T cells on day 8 pi were KLRG1hiIL-7Rlo terminally differentiated cells in all tissues analyzed (Fig. S4A). CCR7 KO effector cells were mostly KLRG1hiIL-7Rlo, similar to WT cells. In addition, CCR7 deficiency did not affect the surface expression of CD27 and CD62L or the ex vivo production of TNFα, IFNγ, granzyme B, and IL-2 by effector cells in the spleen (Fig. S4B). These data correlate the localization of KLRG1loIL-7Rhi memory precursor cells to the T-cell zone. In contrast, P14 CCR7 KO memory T cells showed increased frequencies of KLRG1loIL-7Rhi subsets compared with WT memory cells (Fig. 4A). The most striking differences were found in the lung (7% vs. 54%; P < 0.05) and blood (9% vs. 33%; P < 0.05). We also compared the phenotype of these cells in the spleen by analyzing the expression of CD27 and CD62L along with the production of TNFα, IFNγ, and IL-2 (Fig. 4B). These splenic memory T cells showed minimal or no difference in phenotype or function between CCR7 WT and KO cells. These data indicate that subsets of effector T cells developed normally without the expression of CCR7, whereas CCR7 KO KLRG1loIL-7Rhi memory T cells persisted better than WT KLRG1loIL-7Rhi WT T cells.
Fig. S4.
Comparable phenotype between CCR7 WT and KO effector CD8 T cells. (A) P14 CCR7 WT and KO effector T cells on day 8 pi were analyzed for the expression of IL-7R and KLRG1 by flow cytometry. (B) Effector T cells from the spleen were also analyzed for expression of CD27, CD62L (l-selectin), TNFα, IFNγ, IL-2, and granzyme B. Histogram plots show expression of CD27, CD62L, and granzyme B on CCR7 WT (shaded) and KO (open) cells directly ex vivo. The production of IFNγ, TNFα, and IL-2 by CCR7 WT (shaded) and KO (open) cells was assessed by 5-h GP33–41 peptide stimulation in vitro. All plots are gated on donor Ly5.1+ P14 CD8 T cells.
Fig. 4.
Phenotype differs between CCR7 WT and KO memory T cells. (A) On day 35 pi, P14 CCR7 WT and KO memory T cells were analyzed for the expression of IL-7R and KLRG1 by flow cytometry. (B) Memory T cells from the spleen were also analyzed for expression of CD27, CD62L (l-selectin), TNFα, IFNγ, and IL-2. Histogram plots show the expression of CD27 and CD62L on CCR7 WT (shaded) and KO (open), directly ex vivo. The production of IFNγ, TNFα, and IL-2 by CCR7 WT (shaded) and KO (open) cells, was assessed by 5-h GP33–41 peptide stimulation in vitro. All plots are gated on donor Ly5.1+ P14 CD8 T cells. Similar results were obtained from four independent experiments containing at least two mice per group.
CCR7 KO Memory T Cells Showed Faster Homeostatic Proliferation Than WT Cells.
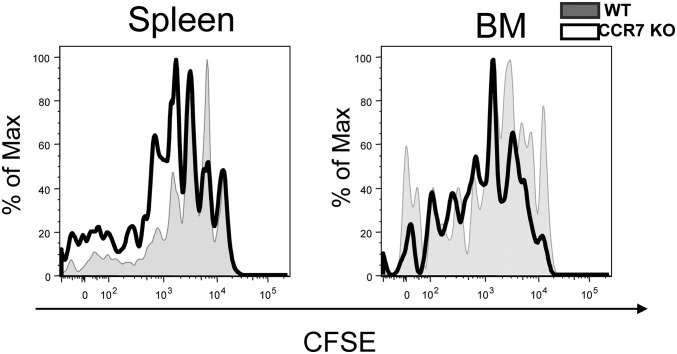
Because CCR7 KO memory T cells persist better than WT cells, we hypothesized that CCR7 KO memory T cells may proliferate homeostatically faster than WT cells. This hypothesis is also supported by the finding of a greater number of CCR7 memory T cells than WT memory cells in the BM and the fact that the BM is the tissue of greatest memory T cell turnover in an IL-15–dependent fashion (23). To test this hypothesis, we prepared splenocytes containing either P14 CCR7 WT or KO memory T cells from mice on day 80 pi and labeled them with CFSE. These cells were transferred into naïve B6 mice and analyzed 8 wk later by flow cytometry (Fig. 5). Both CCR7 WT and KO cells underwent homeostatic turnover, but CCR7 KO cells proliferated more than WT cells in the spleen and BM. These data suggest that the number of CCR7 KO memory T cells was increased due, at least in part, to their faster homeostatic turnover compared with WT memory T cells.
Fig. 5.
Enhanced homeostatic turnover of CCR7 KO memory T cells. CFSE-labeled CCR7 WT (shaded) and KO (open) P14 memory CD8+ T cells from day ∼60 pi were transferred into naive mice. These cells were harvested from the spleen and BM, and then analyzed for CFSE 6–8 wk later. These data were reproduced in two additional experiments with one or two mice per experiment.
Survival of CCR7 KO Cells Was Enhanced by IL-15 in the Liver, Lung, and BM, Whereas the Homeostasis of CCR7 KO Cells Was Little Changed in the Spleen and LNs of IL-7 KO Mice.
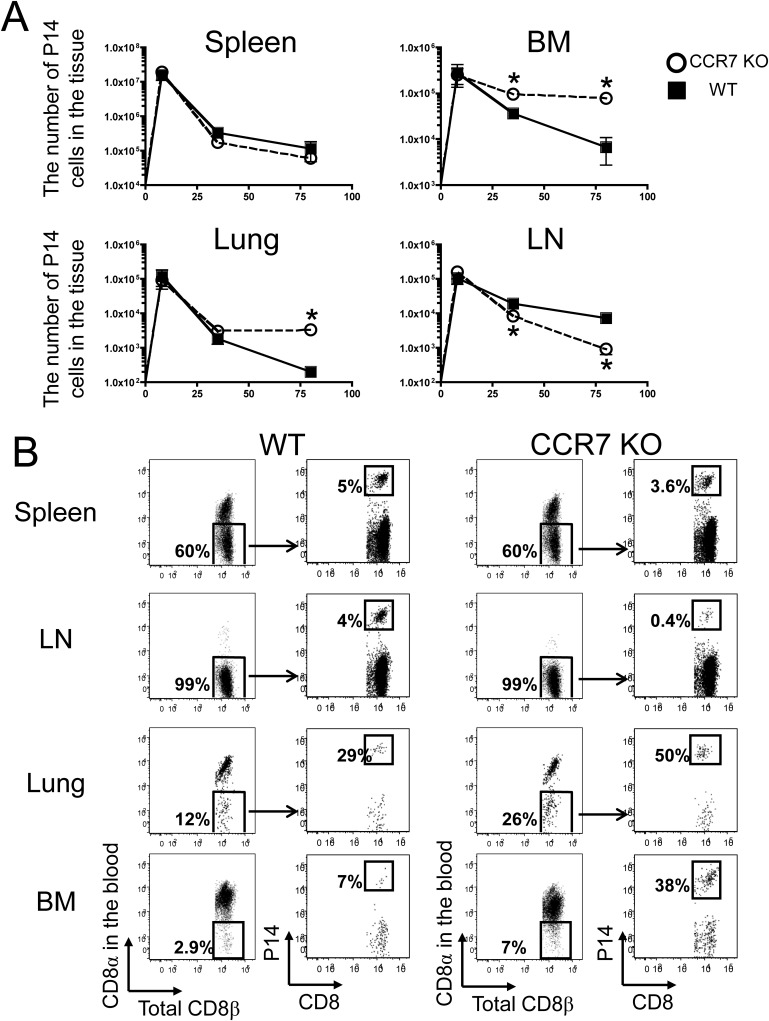
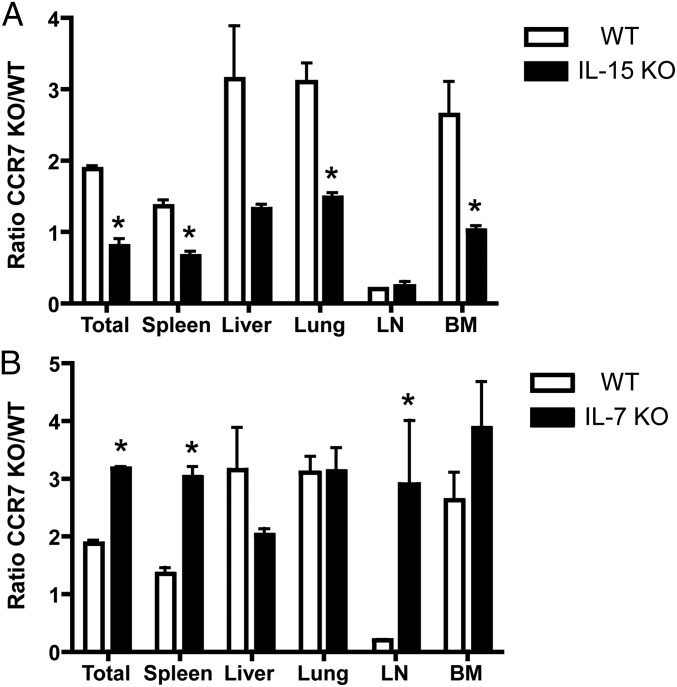
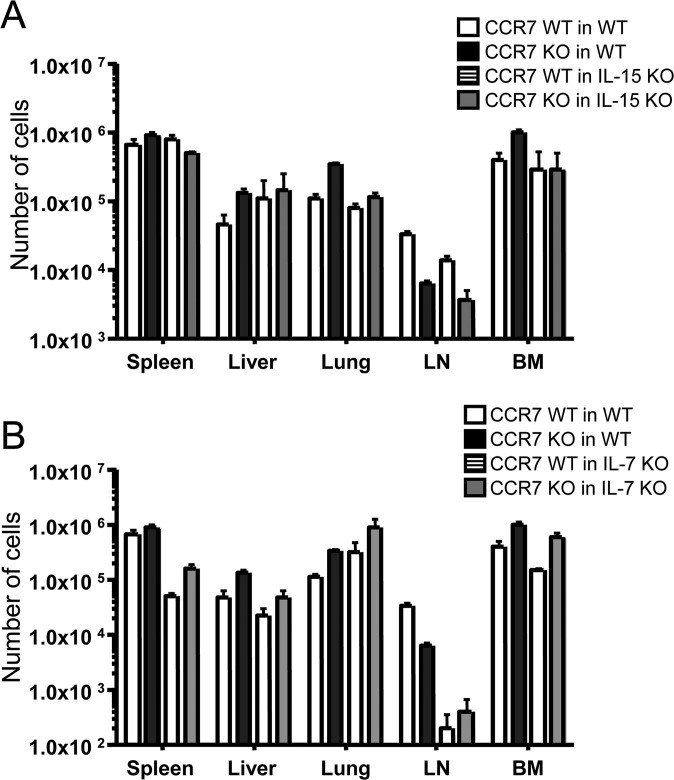
It has been shown that memory T cells in the BM survive and proliferate in an IL-15–dependent manner (23). Therefore, we hypothesized that CCR7 KO virus-specific memory T cells in the BM survive better than WT memory cells in an IL-15–dependent fashion. To test this hypothesis, we transferred naïve P14 CCR7 WT or KO cells into WT B6 and IL-15 KO mice and infected the mice with LCMV. On day 8 pi, we bled the mice and confirmed that these P14 cells had properly expanded. The mice were killed on day 35 pi, and the number of P14 cells from each tissue was counted (Fig. 6A). Although the numbers of P14 CCR7 KO T cells were increased compared with P14 CCR7 WT in the liver, lung, and BM of WT B6 mice similar to the data reported above, the total numbers of these cells in the IL-15 KO were comparable to the numbers of P14 CCR7 WT cells (Fig. S5A).
Fig. 6.
Decreased survival of CCR7 KO memory cells in IL-15 KO mice, but not in IL-7 KO mice. (A) P14 CCR7 WT or KO memory cells were formed in WT (open bar) and IL-15 KO (closed bar) mice and analyzed on day 35 pi. (B) P14 CCR7 WT or KO memory cells were formed in WT (open bar) and IL-7 KO (closed bar) mice and analyzed on day 35 pi. Bar graphs show the mean ± SEM ratios of P14 CCR7 WT and KO cells in the spleen, liver, lung, LN, and BM. Data are representative of three similar experiments. *P < 0.05.
Fig. S5.
Decreased numbers of CCR7 KO memory cells in IL-15 KO mice, but not in IL-7 KO mice. (A) P14 CCR7 WT or KO memory cells were formed in WT (open bar) and IL-15 KO (closed bar) mice and analyzed on day 35 pi. (B) P14 CCR7 WT or KO memory cells were formed in WT (open bar) and IL-7 KO (closed bar) mice and analyzed on day 35 pi. Bar graphs show the mean ± SEM number of P14 CCR7 WT and KO cells in the spleen, liver, lung, LN, and BM. Data are representative of three similar experiments.
We next tested whether IL-7 plays a crucial role in developing virus-specific CCR7 KO memory T cells better than WT cells, because IL-7 has been shown to be important for the survival of effector and memory T cells and also because IL-7 is also produced in the BM (14, 29). It is also notable that IL-7 is expressed by FRCs in the T-cell zone of the spleen and LNs (17), and that CCR7 is required for memory T cells to home into these microenvironments (30). Chimeric mice were created by transferring P14 CCR7 WT or KO T cells into WT or IL-7 KO mice, followed by LCMV infection. On day 35 pi, the numbers of P14 cells in each tissue were counted (Fig. 6B). Unlike IL-15 KO hosts, the formation of CCR7-deficient memory cells was substantially greater in the spleen, LN, and BM of IL-7 KO animals compared with CCR7-sufficient cells (Fig. S5B).
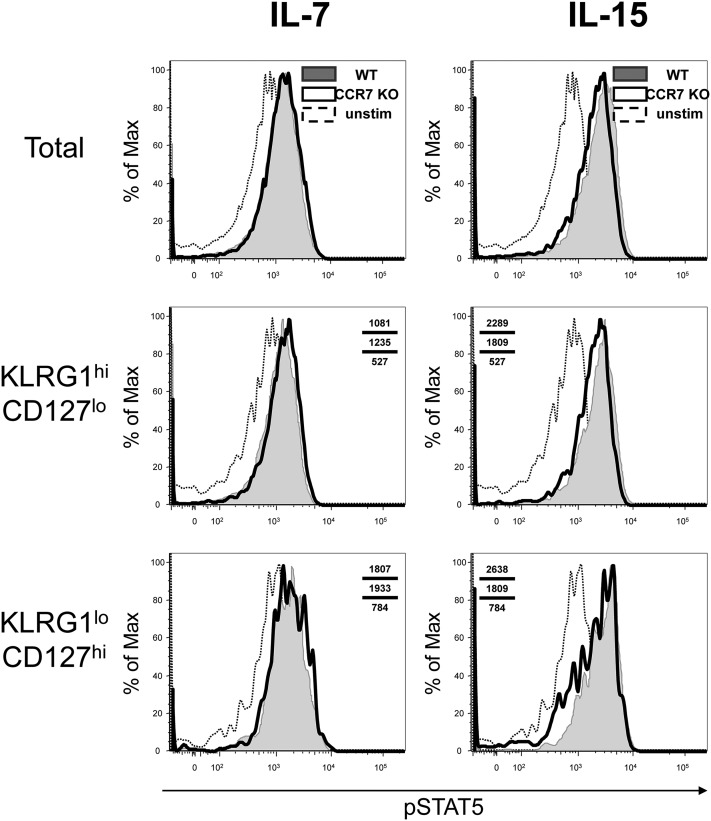
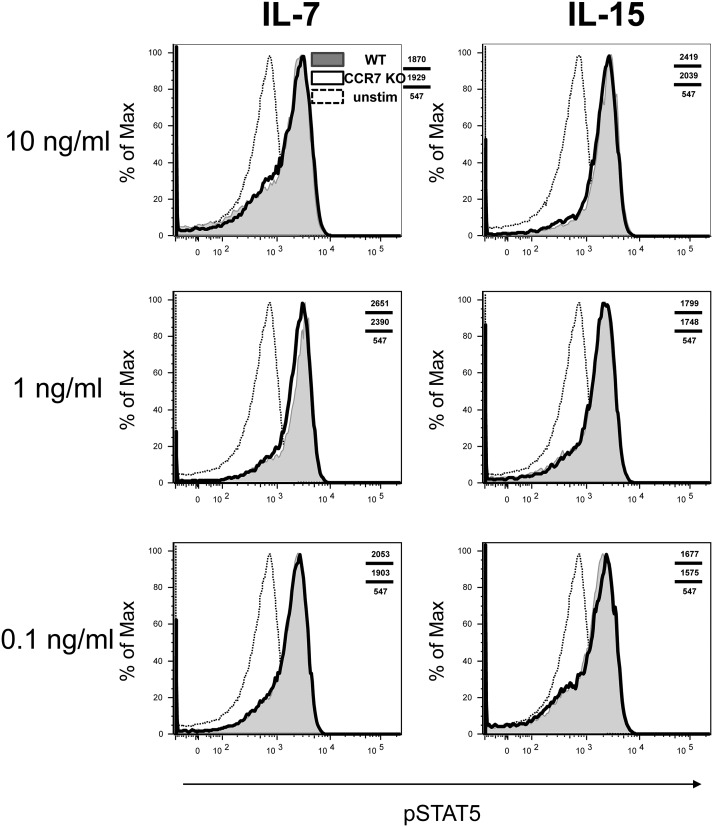
Because we could not rule out the possibility that CCR7 KO effector T cells are more sensitive to IL-15 and less sensitive to IL-7 compared with CCR7 WT effector T cells, we evaluated the signaling abilities of these effector and memory T cells. On day 8 or 35 pi, CCR7 WT and KO T cells were prepared from the spleen and treated with either IL-7 or IL-15 in vitro. These cells were stained intracellularly for phospho-STAT5 (pSTAT5), followed by FACS analysis. The levels of pSTAT5 after these treatments were comparable in the CCR7 WT and KO T cells during both effector and memory phases, suggesting that CCR7 KO T cells can transduce IL-7 and IL-15 signals normally (Figs. S6 and S7).
Fig. S6.
Normal STAT5 phosphorylation of CCR7 KO effector T cells stimulated by either IL-7 or IL-15. P14 CCR7 WT (shaded) and KO (open) T cells were prepared and stimulated by the indicated cytokines at 10 ng/mL on day 8 pi. These cells were analyzed for the phosphorylation of STAT5 by flow cytometry. Unstimulated cells (dotted line) served as negative controls. The data were reproduced three times with three animals per experiment.
Fig. S7.
Comparable STAT5 phosphorylation of CCR7 WT and KO memory T cells after treatment with IL-7 or IL-15. P14 CCR7 WT (shaded) and KO (open) T cells were prepared and stimulated by indicated cytokines at 10, 1, or, 0.1 ng/mL on day 35 pi. The phosphorylation of STAT5 was determined by flow cytometry. Unstimulated cells (dotted line) served as negative controls. The data are representative of two independent experiments with three animals per experiment.
Taken together, these data suggest that the increased formation of CCR7 KO memory T cells is dependent on IL-15, and that CCR7 plays a role in the ability of memory T cells to see IL-7. We also conclude that memory T cells use IL-7 in the spleen and LNs for their longevity, whereas IL-15 is a major survival cytokine in the liver, lung, and BM.
Discussion
CCR7 plays a key role in the homing of naïve and central memory T cells into the T-cell zone of the spleen and LNs. Using CCR7 KO mice, we examined the role of CCR7 in developing and maintaining memory CD8 T cells in response to acute viral infections. In response to systemic LCMV infection, these CCR7 KO cells expanded and developed into effector cells normally, but CCR7 KO memory T cells survived and were maintained better than WT cells, especially in the BM and lung. The increased number of CCR7 KO memory cells in the BM and lung was dependent on IL-15, not on IL-7, and due at least in part to reduced Bim expression and enhanced homeostatic proliferation. A deficiency of IL-7 has a greater effect on the longevity of CCR7 WT T cells compared with CCR7 KO T cells. The majority of these CCR7 KO memory T cells were found in the RP of the spleen, whereas the effector T cells were able to home into the T-cell zone of the spleen. In addition, these memory T cells showed increased numbers of KLRG1loIL-7Rhi subsets and a normal recall response to secondary infection compared with WT memory T cells. Taken together, these data show that the deficiency of CCR7 expression on CD8 T cells can induce the homing of effector and memory T cells into the BM and lung, and that in these tissues, memory T cells can survive and proliferate homeostatically in an IL-15–dependent, not an IL-7–dependent, manner.
The importance of CCR7 in recruiting naïve T cells into the T-cell zone of the spleen and LNs has been described previously (30), and it has been proposed that this receptor plays an important role in the generation of memory T cells in response to viral infections. One study used P14 cells that constitutively express CCR7 and can mount a normal immune response and form the proper memory T cells; however, owing to the overexpression of CCR7 in memory T cells in this system, they cannot be recalled properly following the footpad injection of antigen (31). Another group used CCR7 KO mice and showed a delayed immune response to viral infection, resulting in the persistence of virus as described above. Perhaps viral persistence was why the formation of CCR7 KO memory T cells was approximately eightfold lower than that of WT cells in the spleen (27). Another study used CCL21-tg mice in which CCL21 was expressed in a ubiquitous fashion. These mice showed normal number of effector T cells in the spleen in response to systemic infections (32). Our further investigation demonstrates that CCR7 KO memory T cells survive better than WT cells especially in the BM, when viral clearance is normal. These data provide in-depth information regarding CCR7 function in the development of memory T cells.
In addition to the similar kinetics of CCR7 KO T-cell response to viral infections, these effector T cells were localized into the T-cell zone as efficiently as WT cells. Given that the expression of homeostatic cytokines CCL19 and CCL21 has been shown to be down-regulated during viral infection, it has been suggested that effector T cells use other mechanisms to home into the T-cell zone of the spleen (33). These data contradict our previous findings that KLRG1loIL-7Rhi effector T cells show better migration toward CCL19 than KLRG1hiIL-7Rlo cells and are preferentially localized in the T-cell zone of the spleen. It is possible that effector T cells home into the T zone of the spleen in a CCR7-independent manner and that KLRGloIL-7Rhi cells use CCR7 to circulate to other tissues, because nonlymphoid tissues such as the liver and lung also produce CCL21 during the immune response to viral infections (33); however, we cannot exclude the possibility that CCR7 expressed on the surface of effector T cells can recognize unusual chemokines that induce the migration of these cells in the T-cell zone of the spleen.
We found CCR7 KO memory T cells in all tissues that we analyzed. Of particular interest is the BM, in which a significantly increased number of these cells was found compared with WT memory T cells. It has been shown that the survival factors IL-7 and IL-15 are expressed in the BM (23, 34, 35). In addition, memory CD8 T cells are reported to use IL-15 in the BM to turn over (23). Therefore, it is presumed that memory CD8 T cells and IL-15–producing cells, such as DCs and macrophages, may be colocalized in the BM (18). It also has been shown that memory CD4 T cells reside near IL-7–expressing cells in the BM (29). In our study of CD8 memory T cells, IL-7 was not required for the survival of CCR7 KO cells, suggesting that CD8 memory T cells might not share niches with CD4 memory T cells in the BM. In addition to the BM, CCR7 KO memory T cells need IL-15 for their homeostasis in the lung and liver. These tissues also have been shown to produce both IL-7 and IL-15, but there may be separate niches for these two cytokines.
Our results also support the previous finding that memory CD8 T cells can rely on either IL-7 or IL-15 for their maintenance (36). Unlike in the BM, IL-7 appears to be important for the longevity of CCR7 WT memory T cells in the spleen and LNs. These data suggest that CCR7lo memory T cells, such as TEM cells, may rely on IL-15 for their longevity, whereas IL-7 is a crucial cytokine for the homeostasis of CCR7hi memory T cells, including TCM cells. Their dependence on IL-15 also may lead to the low expression of Bim for their survival, as reported previously (37). Furthermore, CCR7 may play a crucial role in migration toward cells expressing IL-7, which include FRCs in the spleen and LNs.
CCR7 KO memory T cells showed increased turnover, possibly due to their homing into the BM, the preferred site for homeostatic proliferation (23); however, this conclusion is contradictory to the observation that CCR7+ TCM cells undergo homeostatic turnover faster than CCR7− TEM (38–41). It is possible that a portion of CCR7 KO memory cells may contain intrinsic factors similar to TCM cells, even though CCR7 KO memory T cells cannot be CCR7+ TCM cells. Indeed, the subset of CCR7 KO KLRG1loIL-7Rhi memory cells was increased in the BM compared with WT cells, and this subset includes CD62L+ memory cells. These data also led us to hypothesize that TCM cells can dynamically regulate the expression of CCR7 to control their migration patterns to see both IL-7 and IL-15. This capability may ensure their survival and turnover by using either cytokine while circulating through multiple tissues.
The present study shows that the expression of CCR7 on memory T cells can greatly influence their homing, especially into the LNs, liver, lung, and BM. As a result, this homing ability can affect their reliance to survival cytokines and longevity. Therefore, this finding increases our understanding of the contribution of migration into different microenvironments to the homeostasis and regulation of memory T cells. Further examination of the cell types and signals provided in these specialized microenvironments will help us better understand how memory T cells develop and persist in vivo following infections, which could ultimately lead to new types of vaccines and treatments that may enhance protection against infectious disease and cancer.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and Technology (NRF-2014R1A2A1A11051066).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602899113/-/DCSupplemental.
References
- 1.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 3.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176(4):2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 4.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 5.Hikono H, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204(7):1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann MF, Wolint P, Schwarz K, Jäger P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175(7):4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac VP, Harty JT. Manipulating the rate of memory CD8+ T cell generation after acute infection. J Immunol. 2007;179(1):53–63. doi: 10.4049/jimmunol.179.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Mueller SN, Zaid A, Carbone FR. Tissue-resident T cells: Dynamic players in skin immunity. Front Immunol. 2014;5:332. doi: 10.3389/fimmu.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 10.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20(5):551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 11.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207(3):553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teijaro JR, et al. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 14.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Alves NL, et al. Characterization of the thymic IL-7 niche in vivo. Proc Natl Acad Sci USA. 2009;106(5):1512–1517. doi: 10.1073/pnas.0809559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heufler C, et al. Interleukin 7 is produced by murine and human keratinocytes. J Exp Med. 1993;178(3):1109–1114. doi: 10.1084/jem.178.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 18.Mortier E, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31(5):811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183(8):4948–4956. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stonier SW, Schluns KS. Trans-presentation: A novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127(2):85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185(12):2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 23.Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174(3):1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 24.Jung YW, Rutishauser RL, Joshi NS, Haberman AM, Kaech SM. Differential localization of effector and memory CD8 T cell subsets in lymphoid organs during acute viral infection. J Immunol. 2010;185(9):5315–5325. doi: 10.4049/jimmunol.1001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 27.Junt T, et al. Impact of CCR7 on priming and distribution of antiviral effector and memory CTL. J Immunol. 2004;173(11):6684–6693. doi: 10.4049/jimmunol.173.11.6684. [DOI] [PubMed] [Google Scholar]
- 28.McMahon CW, et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169(3):1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 29.Tokoyoda K, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30(5):721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Förster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 31.Unsoeld H, Voehringer D, Krautwald S, Pircher H. Constitutive expression of CCR7 directs effector CD8 T cells into the splenic white pulp and impairs functional activity. J Immunol. 2004;173(5):3013–3019. doi: 10.4049/jimmunol.173.5.3013. [DOI] [PubMed] [Google Scholar]
- 32.Unsoeld H, et al. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. Int Immunol. 2007;19(11):1281–1289. doi: 10.1093/intimm/dxm098. [DOI] [PubMed] [Google Scholar]
- 33.Mueller SN, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 34.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176(5):2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 35.Cassese G, et al. Bone marrow CD8 cells down-modulate membrane IL-7Ralpha expression and exhibit increased STAT-5 and p38 MAPK phosphorylation in the organ environment. Blood. 2007;110(6):1960–1969. doi: 10.1182/blood-2006-09-045807. [DOI] [PubMed] [Google Scholar]
- 36.Tan JT, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurtulus S, et al. Bim controls IL-15 availability and limits engagement of multiple BH3-only proteins. Cell Death Differ. 2015;22(1):174–184. doi: 10.1038/cdd.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6(8):793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35(4):1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 40.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166(3):1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 41.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154(11):5870–5875. [PubMed] [Google Scholar]