Significance
The listerin (Ltn1) E3 ubiquitin ligase ubiquitylates and promotes degradation of aberrant nascent chains that become stalled on ribosomal 60S subunits. Ltn1-dependent nascent chain ubiquitylation was reconstituted in vitro using extracts of genetically manipulated Neurospora strains. Such extracts, supplemented or not with recombinant factors (such as Ltn1 from Saccharomyces cerevisiae), represent a new system to study ribosome-associated protein quality control. Utilizing this system, we show that mutations in Ltn1’s conserved N-terminal domain result in defective 60S binding and nascent chain ubiquitylation, without affecting Ltn1’s intrinsic E3 activity. Furthermore, we have solved the crystal structure of Ltn1’s N-terminal domain, which provides detailed information and insights into how Ltn1 interacts with stalled 60S subunits. Our observations shed light on how cells handle protein quality control substrates.
Keywords: Listerin, Ltn1, RQC, ribosome, structure
Abstract
The Ltn1 E3 ligase (listerin in mammals) has emerged as a paradigm for understanding ribosome-associated ubiquitylation. Ltn1 binds to 60S ribosomal subunits to ubiquitylate nascent polypeptides that become stalled during synthesis; among Ltn1’s substrates are aberrant products of mRNA lacking stop codons [nonstop translation products (NSPs)]. Here, we report the reconstitution of NSP ubiquitylation in Neurospora crassa cell extracts. Upon translation in vitro, ribosome-stalled NSPs were ubiquitylated in an Ltn1-dependent manner, while still ribosome-associated. Furthermore, we provide biochemical evidence that the conserved N-terminal domain (NTD) plays a significant role in the binding of Ltn1 to 60S ribosomal subunits and that NTD mutations causing defective 60S binding also lead to defective NSP ubiquitylation, without affecting Ltn1’s intrinsic E3 ligase activity. Finally, we report the crystal structure of the Ltn1 NTD at 2.4-Å resolution. The structure, combined with additional mutational studies, provides insight to NTD’s role in binding stalled 60S subunits. Our findings show that Neurospora extracts can be used as a tool to dissect mechanisms underlying ribosome-associated protein quality control and are consistent with a model in which Ltn1 uses 60S subunits as adapters, at least in part via its NTD, to target stalled NSPs for ubiquitylation.
The conservation of the Ltn1 E3 ligase among eukaryotic organisms suggests that it has an important function; accordingly, mutations of the Ltn1 mouse ortholog can cause embryonic lethality or neurodegeneration (1). Using Saccharomyces cerevisiae as a model to elucidate Ltn1’s function, we uncovered a role for yeast Ltn1 (also known as Rkr1) in a pathway of protein quality control (PQC) that is associated with the 60S ribosomal subunit (2). This finding was important because, although it has long been known that PQC mechanisms are critical to maintain protein homeostasis and cellular fitness, only a few pathways mediating the surveillance of aberrant proteins in eukaryotes have been uncovered to date (3–6). In addition, because defective PQC is a hallmark of neurodegenerative diseases (3–6), the above findings suggested that the Ltn1 yeast model might be predictive of basic molecular mechanisms underlying the mouse neurodegenerative phenotype.
Ltn1 orthologs are ∼150- to 180-kDa proteins harboring an E3-catalytic RING domain at the C-terminal end, and an N-terminal domain (NTD) (2). These two conserved regions are separated by a long HEAT repeat domain of ∼85 kDa that is less conserved at the primary amino acid sequence level (2, 7). Our studies in yeast had uncovered Ltn1 as the critical E3 ligase targeting aberrant proteins encoded by mRNA lacking stop codons (“nonstop mRNA”) for ubiquitylation and proteasomal degradation (2). Ribosomes translating nonstop mRNA proceed through the poly(A) tail, which encodes polyLys. Presumably due to electrostatic interactions with the ribosomal nascent chain exit tunnel, nascent polyLys causes translation elongation to halt (8–10), eventually leading to the recruitment of Ltn1, which is vastly substoichiometric with regard to translating ribosomes (2). This phenomenon is not specific to polyLys because fusing a homopolymeric tract of either Lys or Arg to a stop codon-containing reporter protein, as well as internal polybasic tracts in endogenous proteins, is able to halt translation and promote Ltn1-mediated degradation (11–13). Furthermore, it is now known that other mechanisms preventing translational elongation or termination (e.g., mRNA truncation) can also result in Ltn1-mediated ubiquitylation of nascent chains (e.g., refs. 13 and 14).
There is no evidence to suggest that Ltn1 itself can directly sense elongation-halted ribosomes; rather, it has been proposed that such ribosomes are instead primary substrates of ribosome-splitting rescue systems such as Dom34-Hbs1-Rli1 (13, 15–17). Dom34/Pelota and Hbs1 are homologous to the translation termination factors eRF1 and eRF3, respectively. The Hbs1 GTPase loads Dom34 onto translationally halted ribosomes in a stop codon-independent manner, followed by Hbs1 dissociation and Dom34-mediated recruitment of the Rli1/ABCE1 ATPase; Rli1, in turn, mediates splitting of the 60S and 40S subunits (15–17). However, unlike eRF1, Dom34 lacks nascent peptidyl-tRNA hydrolase activity. As a result, among the products of the Dom34-mediated reaction are 60S subunits that remain stalled with tRNA-conjugated nascent chains; it is such an abnormal complex that seems to be targeted by Ltn1 (13, 15–17). Consistent with these findings, Hbs1 was required upstream of Ltn1 for stalled chain ubiquitylation in the rabbit reticulocyte lysate (RRL) system (13), and Ltn1 is predominantly associated with 60S ribosomal subunits in steady state (2, 11, 12).
Ltn1 has been found to function as a subunit of the ribosome-associated protein quality control (RQC) complex (11, 12). In this complex containing stalled 60S subunits, the Rqc2/Tae2 subunit was initially shown to function in stabilizing Ltn1 binding (12). Ltn1’s E3 activity and the Rqc1 subunit of the complex were both required for recruitment of the AAA ATPase Cdc48/p97/VCP and its cofactors Npl4 and Ufd1 (11, 12, 18). Finally, Cdc48 was proposed to extract the ubiquitylated nascent polypeptide from the stalled 60S subunit and deliver it to the proteasome (18). Thus, Ltn1-mediated ubiquitylation seems to play a role both in extracting stalled chains and in targeting them for elimination.
More recently, cryo-EM structures of Ltn1/listerin in complex with Rqc2/NEMF and stalled 60S subunits have been published (19–21). The structures suggest that Rqc2 recognizes the stalled 60S subunit by simultaneously binding to the tRNA moiety of the stalled peptidyl-tRNA and the 60S intersubunit surface. The Ltn1 NTD and C-terminal domain (CTD) both provide contacts within the RQC; the NTD binds both Rqc2 and the 60S in the vicinity of the sarcin-ricin loop (SRL) such that it would clash with the 40S subunit if that subunit were present whereas the RWD domain binds near the opening of the nascent chain exit tunnel (the RWD is a domain structurally related to ubiquitin-conjugating enzymes that is found in certain RING domain- or WD repeat-containing proteins, and in DEAD-like helicases). Ltn1’s HEAT repeat-containing middle region serves as a linker between NTD and CTD bound to distal sites, providing an explanation for size conservation among Ltn1 orthologs. The cryo-EM structures remain incomplete, however, with components still missing and with several regions in which the resolution was insufficient to resolve amino acid side chains.
Although studies with S. cerevisiae have enabled elucidation of Ltn1’s function and the pathways in which it is involved, it is critical to put the models derived from work using that system to test with alternative approaches, such as biochemical reconstitutions in which mechanisms can be examined. Along these lines, a robust RRL-based translation and ubiquitylation system has been developed and used to test the effect of mutations in mammalian listerin on ribosome-associated quality control (20). Here, we present an in vitro system enabling the study of S. cerevisiae Ltn1 function using Neurospora crassa extracts. In addition to exhibiting higher translation and ubiquitylation activities compared with S. cerevisiae extracts, this system offers the advantage that, unlike mammalian systems, Neurospora can also be easily genetically manipulated, thus serving as a source of extracts either cleanly lacking or overexpressing defined components. Using this system, we show that ribosome-stalled nonstop translation products (NSPs) become ubiquitylated in an Ltn1-dependent manner while still ribosome-associated. Importantly, we provide biochemical evidence that Ltn1’s conserved NTD is required for binding to 60S subunits, along with evidence supporting the model that 60S association is required for Ltn1-mediated NSP ubiquitylation. Finally, we provide a structural basis for the observations reported here regarding Ltn1 NTD mutations by determining the crystal structure of the 45-kDa Ltn1 NTD from budding yeast at 2.4-Å resolution. Modeling the Ltn1 NTD into RQC structures revealed a highly conserved, positively charged surface that interacts with the phosphate backbone of the SRL, mutation of which disrupts Ltn1 function. Despite conservation of the Ltn1 NTD among eukaryotes, comparison of yeast and mammalian RQC structures revealed differences in the extreme N terminus that suggest that fungal and metazoan Ltn1 proteins use divergent mechanisms to interact with Rqc2.
Results
Newly Synthesized NSPs Are Ubiquitylated in N. crassa Extracts in an Ltn1-Dependent Manner.
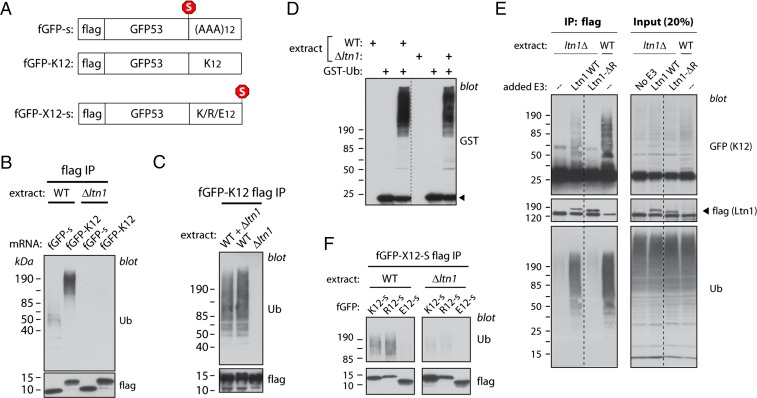
Neurospora extracts were used to study the fate of newly synthesized nonstop translation products (NSPs). We used templates for in vitro translation consisting of sequences encoding a flag tag and the first 53 residues of GFP, followed or not by four stop codons, and by a poly(A) tail of defined length (36 adenines). Thus, translation of the nonstop template resulted in as many as 12 lysines encoded by the poly(A) tail becoming fused to the C terminus of flag-GFP (fGFP-K12) (Fig. 1A).
Fig. 1.
Newly synthesized NSPs are ubiquitylated in Neurospora extracts in an Ltn1-dependent manner. (A) Diagram of templates used for in vitro translation, encoding flag-GFP53 (fGFP-s), the equivalent nonstop protein (fGFP-K12), equivalent proteins fused to homopolymeric tracts of 12 lysines (fGFP-K12-s), arginines (fGFP-R12-s), or glutamates (fGFP-E12-s); “s” indicates that the encoding mRNA harbor four in-frame stop codons. (B) Nonstop proteins synthesized in Neurospora extracts become ubiquitylated in an Ltn1-dependent manner. Three hundred nanograms of mRNA encoding fGFP-s or fGFP-K12 reporters were used to program translation reactions (30 μL) prepared from WT or Ltn1-deficient (Δltn1) strains for 30 min. Reaction products denatured with 1% SDS were diluted 10-fold and used for immunoprecipitation (IP) using anti-flag antibody. IPed proteins were used for immunoblots against ubiquitin (Ub) or flag. (C) The NSP ubiquitylation defect of Ltn1-deficient extracts is not due to the presence of trans-acting inhibitors. fGFP-K12 was synthesized in WT or Δltn1 extracts, or in a 1:1 WT:Δltn1 extract mixture. Reaction products were analyzed for ubiquitylation as in B. (D) Ltn1-deficient extracts are competent for de novo ubiquitylation. WT and Δltn1 extracts were incubated with GST-tagged ubiquitin (GST-Ub; 10 μM) in the presence of 1 mM ATP for 10 min at 26 °C as indicated, and the ligation of GST-Ub to proteins in the extract (as revealed by the formation of a smear) was examined by anti-GST immunoblot. The arrowhead indicates free GST-Ub. Dashed lines indicate that lanes were removed from the original image. (E) Recombinant Ltn1 restored NSP ubiquitylation activity to Δltn1 extracts. Recombinant, flag-tagged WT yeast Ltn1 or a mutant lacking the catalytic RING domain (Ltn1-ΔR) was added at 40 nM to Δltn1 extract, as indicated, before translation using 300 ng of fGFP-K12-encoding mRNA (full-length GFP used for this experiment). Ubiquitylation of fGFP-K12 was measured as in B. Dashed lines indicate that lanes were removed from the original image. (F) Proteins encoded by stop codon-containing mRNA, but harboring polybasic tracts, are targeted by Ltn1. WT or Δltn1 extracts were programmed with 300-ng templates encoding fGFP appended with a C-terminal tract of 12 Lys, Arg, or Glu, as represented in A. Reaction products were analyzed for ubiquitylation as in B.
The control reporter fGFP-s (“s” denoting the presence of stop codons) and fGFP-K12 were synthesized at comparable levels in the extracts (Fig. 1B). The anti-flag tag blot failed to reveal a slower migrating ladder or smear associated with fGFP-K12, which might be indicative of ubiquitylation. The inability to detect such an obvious ubiquitylation signature could have been due to inefficient NSP ubiquitylation, or to NSP deubiquitylation by ubiquitin proteases in the extract. We thus used a more sensitive and commonly used method to verify whether NSPs became ubiquitylated in Neurospora extracts. The flag-tagged translational products of fGFP-K12 and FGFP-s were immunoprecipitated (IPed) with flag antibody under denaturing conditions and analyzed by Western blot with anti-ubiquitin antibody. The results in Fig. 1B show that the NSP reporter fGFP-K12 was indeed ubiquitylated and that it was ubiquitylated to a greater extent than the reporter containing stop codons.
To test whether ubiquitylation of fGFP-K12 was Ltn1-dependent, translation was carried out in extracts prepared from an Ltn1-deficient isogenic strain (Δltn1; N. crassa Ltn1 is NCU06534). Although reporter proteins were synthesized normally in N. crassa extracts lacking Ltn1, ubiquitylation of NSPs was defective (Fig. 1B). Similar results were observed with two independently prepared batches of extracts. We note that, under these conditions, we have not observed a smear running immediately above the fGFP-K12 reporter band in either WT or Ltn1-deficient extracts, which might be attributed to Rqc2-mediated C-terminal extension with alanines and threonines (“CAT tails”) (21). Likewise, we have not observed high molecular weight aggregates that could have been formed in a CAT tail-dependent manner (22, 23) (e.g., see Fig. 1B).
The inability of Δltn1 extracts to mediate NSP ubiquitylation might have been due to the presence of a trans-acting inhibitor; however, arguing against this possibility, mixing Δltn1 and WT extracts at a 1:1 ratio did not prevent fGFP-K12 ubiquitylation (Fig. 1C). Importantly, Δltn1 extracts were verified to be fully competent for de novo ubiquitylation, as measured by the ability of exogenously supplied GST-tagged ubiquitin to be ligated to unknown proteins in the extract (Fig. 1D). Finally, to more decisively confirm that the inability of Δltn1 extracts to promote ubiquitylation of fGFP-K12 was due to the absence of Ltn1, we tested whether addition of Escherichia coli-expressed, recombinant budding yeast (S. cerevisiae) Ltn1 to the reaction would suffice to restore fGFP-K12 ubiquitylation activity. The results in Fig. 1E show that full-length Ltn1 did in fact stimulate ubiquitylation of fGFP-K12 in Δltn1 extracts; on the other hand, a mutated Ltn1 lacking the catalytic RING domain (Ltn1-ΔR) that is deficient in E3 activity but competent to bind to 60S subunits (2) failed to restore fGFP-K12 ubiquitylation under these conditions. Therefore, recapitulating the in vivo results with budding yeast, Ltn1 acts as the critical E3 mediating NSP ubiquitylation in Neurospora extracts.
Proteins encoded by normal, stop codon-containing mRNA, but harboring sufficiently long internal polybasic tracts, have also been characterized as Ltn1 substrates (11, 13). Thus, the ubiquitylation of proteins harboring polybasic tracts was also examined in the Neurospora system. Extracts were programmed with templates encoding fGFP appended with a C-terminal tract of 12 Lys or Arg (or Glu as negative control), followed by four stop codons (“s”). As predicted, and further demonstrating the specificity of this in vitro system, fGFP-K12-s and fGFP-R12-s—but not fGFP-E12-s—became ubiquitylated in an Ltn1-dependent manner (Fig. 1F).
Ltn1-Mediated NSP Ubiquitylation Occurs on Ribosomes.
To provide further evidence that Ltn1-mediated NSP ubiquitylation begins on ribosomes, in one set of experiments, fGFP-K12 was translated in WT or Δltn1 extracts and subjected to sucrose gradient sedimentation. Analysis of the fractions (Fig. 2A, Lower, “Input”) indicated the greater presence of unmodified fGFP-K12 in ribosomal fractions in Δltn1 compared with WT extracts. Unmodified fGFP-K12 in ribosomal fractions was also observed in Δltn1 extracts supplemented with the recombinant, E3 ligase-deficient Ltn1-ΔR, but not with Ltn1 WT (Fig. 2B). Moreover, these results correlated with the observation that greater amounts of tRNA-linked fGFP-K12 were present in ribosome-containing pellets after sucrose cushion centrifugation of Δltn1 compared with WT extracts (Fig. 2C).
Fig. 2.
Ltn1-mediated ubiquitylation of NSPs occurs on ribosomes. (A) Evidence for NSP ubiquitylation on ribosomes. Five hundred nanograms of fGFP-K12-encoding mRNA was translated for 30 min in 50 μL of WT or Δltn1 extracts. Reaction products were separated by centrifugation in 15–40% (wt/vol) sucrose gradient; 20% of each fraction was analyzed by immunoblot against ubiquitin (Ub), flag, or the 60S ribosomal protein, Rpl3 (“Input”). The remaining material from each fraction was used for denaturing flag IPs (as in Fig. 1B) and analyzed by immunoblot against Ub or flag. (B) Five hundred nanograms of fGFP-K12–encoding mRNA was translated for 30 min in 50 μL of WT extract or in Δltn1 extract supplemented or not with 40 nM recombinant yeast Ltn1 (WT or Ltn1-ΔR mutant, as indicated). Reaction products were separated by centrifugation in 15–40% (wt/vol) sucrose gradient. Fractions were analyzed by immunoblot against flag or Rpl3. (C) Evidence for tRNA-conjugated fGFP-K12 in ribosomal pellets. Two hundred nanograms of mRNA encoding fGFP-s or fGFP-K12 proteins were incubated with WT or Δltn1 extracts (20 μL) for 30 min. Reaction products were separated by centrifugation on sucrose cushion; 40% of recovered supernatant (“S”) and 100% of resuspended pellet (“P”) were separated by electrophoresis in Bis-Tris gel containing Mes buffer, pH 6.8 (conditions under which peptidyl-tRNA is more stable) and analyzed by immunoblot against flag or Rpl3. Dashed lines indicate that lanes were removed from the original image. (D) Ribosome-stalled but not free NSPs are modified by Ltn1. Three hundred nanograms of fGFP-K12–encoding mRNA were translated in 30 μL of Δltn1 extract for 30 min, followed by cycloheximide addition. To one set of reactions (lanes 1 and 2), fresh WT or Δltn1 extract was added (1:1) and incubated for another 30 min before denaturing flag IP. In another set of reactions, products of the fGFP-K12 translation reaction in Δltn1 extract were separated by centrifugation on sucrose cushion. Supernatant and the resuspended ribosome-containing pellet were then incubated with fresh WT or Δltn1 extracts in the presence of cycloheximide, for 30 min before denaturing flag IP. The same amount of second extract was added to supernatant and pellet fractions. All reactions (lanes 1–6) were equalized for ∼20% (wt/vol) sucrose concentration. The IPed material was analyzed by immunoblot against Ub or flag.
Individual fractions of the gradient shown in Fig. 2A, Lower were also analyzed for fGFP-K12 ubiquitylation by carrying out denaturing flag IP, followed by anti-ubiquitin blot (Fig. 2A, Upper). The results revealed that ribosome-cofractionating fGFP-K12 (fractions 5–8) was ubiquitin-modified in WT but not the Δltn1 extract (Fig. 2A, Upper) [this finding is in contrast to the total ubiquitin signal in the input fractions (Fig. 2A, Lower), which was unaffected by the absence of Ltn1]; this finding supports the model that Ltn1-mediated NSP ubiquitylation occurs on ribosomes.
Notably, the majority of the ubiquitylated fGFP-K12 signal was present in ribosome-free fractions at the top of the gradient (Fig. 2A, Top Left, fractions 1 and 2). Although this result is consistent with the model that ubiquitylation stimulates the release of stalled nascent chains from ribosomes (e.g., refs. 2, 11, 12, and 18), these results could not rule out the alternative possibility that much of fGFP-K12 became ubiquitylated subsequently to being released from ribosomes. To distinguish these possibilities, we developed a different assay. We first verified that fGFP-K12 synthesized in Δltn1 extract could become ubiquitylated upon subsequent mixing with WT extract providing Ltn1 (in the presence of cycloheximide to prevent new translation) (Fig. 2D, lanes 1 and 2). Next, fGFP-K12 synthesized in the Δltn1 extract was subjected to centrifugation through a sucrose cushion, and then the supernatant (containing ribosome-free fGFP-K12) and resuspended pellet (containing ribosome-bound fGFP-K12) were collected and incubated with WT extract for ubiquitylation to proceed. The results show that ribosome-associated fGFP-K12 was readily ubiquitylated upon subsequent mixing with WT extract (Fig. 2D, lane 5); in contrast, free (and more abundant) fGFP-K12 present in the supernatant was not ubiquitylated (Fig. 2D, lane 3). These results provide further evidence that Ltn1-mediated NSP ubiquitylation requires NSP presentation on ribosomes and that it takes place on ribosomes as well.
Ltn1 Requires an Intact N Terminus for NSP Ubiquitylation.
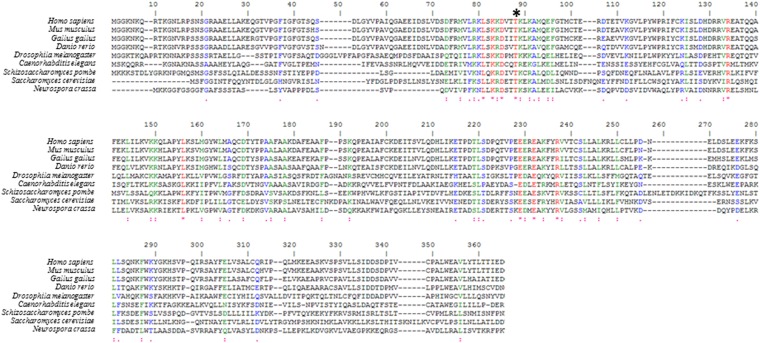
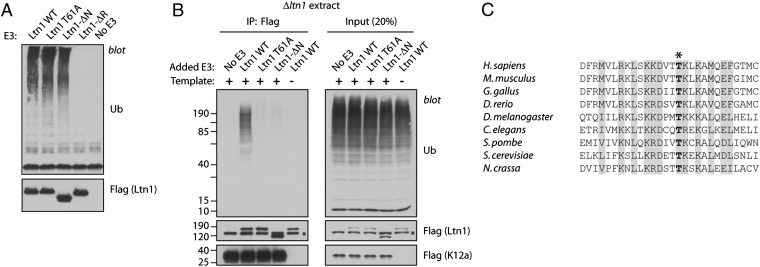
We next began a structure–function analysis of Ltn1. In addition to the C-terminal RWD and RING domains, Ltn1’s NTD is conserved from yeast to humans (Fig. S1 and ref. 2). The requirement of an intact NTD for Ltn1-mediated NSP ubiquitylation was therefore tested in the Neurospora system. For this purpose, we used flag-tagged WT yeast Ltn1 (amino acids 1–1562) and an NTD-deleted mutant (Ltn1-ΔN; amino acids 477–1562) that were both expressed and purified from E. coli. Previous structural characterization had revealed no obvious differences between Ltn1 WT and Ltn1-ΔN other than the absence of the deleted N-terminal region (7). Ltn1-ΔN was as competent as the WT protein in mediating ubiquitylation in the absence of added ribosomes or NSPs (Fig. 3A) (these reaction products presumably result from autoubiquitylation, based on observations with several other E3 ligases under similar reaction conditions, containing few pure components and no canonical substrate). As shown in Fig. 3B (and in Fig. 1E), addition of recombinant WT yeast Ltn1 to a Δltn1 extract restored fGFP-K12 ubiquitylation, which required Ltn1’s RING domain. In contrast, Ltn1-ΔN failed to restore fGFP-K12 ubiquitylation under these conditions (Fig. 3B), despite the fact that the Ltn1-ΔN mutant has an E3-active RING domain (Fig. 3A).
Fig. S1.
Alignment of the N terminus of Ltn1 orthologs. Clustal W alignment of the N-terminal domain in selected Ltn1 orthologs (residues 1–322 in S. cerevisiae). The residues equivalent to S. cerevisiae Thr61 are shown under the black asterisk.
Fig. 3.
Ltn1 requires an intact N terminus for NSP ubiquitylation. (A) The conserved Ltn1 N-terminal domain (NTD) is not required for autoubiquitylation activity. In vitro ubiquitylation assay containing E. coli-expressed E1, E2 (Ubc4), GST-Ub, and C-terminal flag-tagged yeast Ltn1. The Ltn1 proteins used were WT (amino acids 1–1562), NTD-deleted mutant (Ltn1-ΔN; amino acids 477–1562), T61A mutant, and RING domain-deleted mutant (Ltn1-ΔR; amino acids 1–1502). (B) An intact NTD is required for Ltn1 function in NSP ubiquitylation. fGFP-K12–encoding mRNA (500 ng; full-length GFP used) was translated in Δltn1 extract [50 μL supplemented with 40 nM recombinant Ltn1 (WT or mutants, as in “A”) for 30 min] and analyzed for ubiquitylation by denaturing flag IP as in Fig. 1B. The control reaction in lanes 5 lacked fGFP-K12 template mRNA. (C) Alignment showing evolutionary conservation of Thr61 (bold, asterisk) and neighboring residues (46–73 in yeast) in selected Ltn1 orthologs.
We extended these analyses by testing the effects of mutating an Ltn1 NTD highly conserved residue (Ltn1 T61A) (Fig. 3C). We selected Thr61 (in the yeast numbering), based on the rationale that a hydrophilic residue would be more likely to be surface-exposed (an expectation confirmed by the NTD structure presented further below) and thus less prone to affect the domain’s overall structure when mutated. Like the WT protein and the mutant lacking the entire NTD, Ltn1 T61A was competent for autoubiquitylation (Fig. 3A). Furthermore, like Ltn1-ΔN, Ltn1 T61A was less efficient in restoring fGFP-K12 ubiquitylation in a Δltn1 extract (Fig. 3B). Thus, an intact NTD is required for Ltn1 function in NSP ubiquitylation.
Ltn1 NTD Mutations Interfere with 60S Binding.
Ltn1 ubiquitylates ribosome-stalled NSPs in an NTD-dependent manner (Fig. 3 and ref. 24). Although predicted by the RQC structures, the NTD requirement for Ltn1 binding to the complex has not been experimentally tested. Because Ltn1 NTD mutants exhibited intact autoubiquitylation activity, we tested whether their failure to ubiquitylate NSPs was a consequence of defective 60S binding.
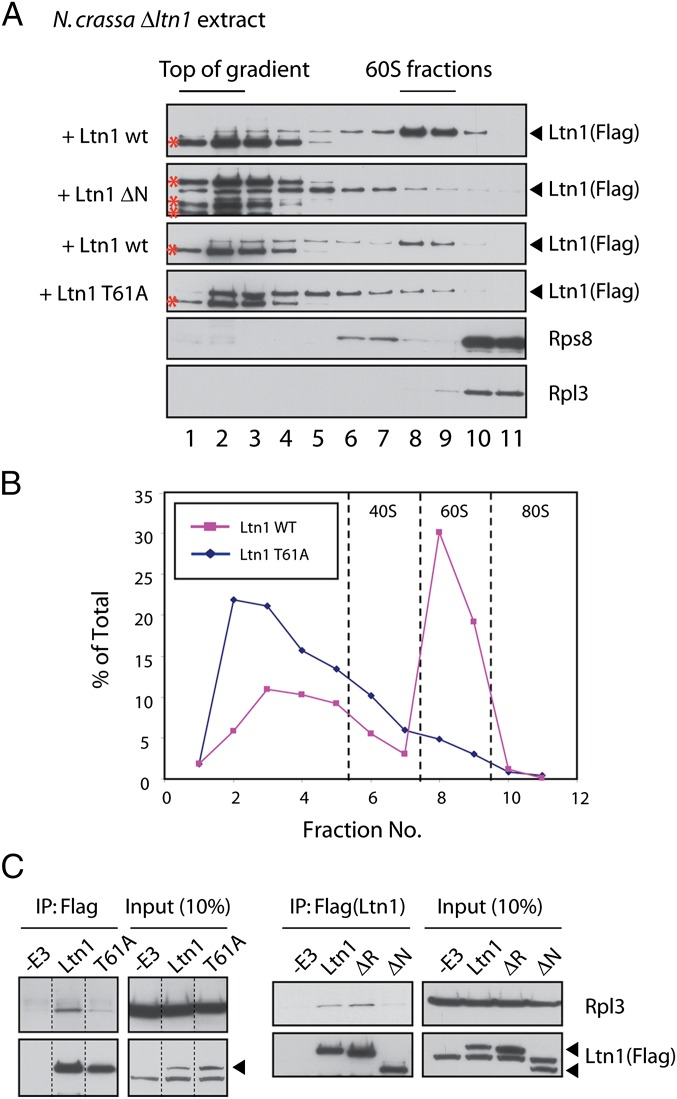
Flag-tagged WT or NTD mutant Ltn1 proteins were incubated with Δltn1 Neurospora extracts and subjected to sucrose gradient centrifugation. Fractions were collected and analyzed by anti-flag immunoblot (Fig. 4A). Ltn1-independent, flag cross-reacting bands, present in Fig. 4A, fractions 1–4, are marked with asterisks (see also Fig. S2). As expected, Ltn1 added to the Δltn1 extract was predominantly found in the 60S fractions (Fig. 4A and, e.g., refs. 2 and 11–13). Importantly, compared with Ltn1 WT, both Ltn1 T61A and Ltn1-ΔN mutants exhibited decreased association with the 60S fraction (quantitation for Ltn1 WT and T61A presented in Fig. 4B).
Fig. 4.
Ltn1 NTD mutations interfere with ribosome binding. (A) An intact NTD domain is required for Ltn1 to cofractionate with 60S ribosomes as measured by sucrose gradient centrifugation. Flag-tagged Ltn1 WT or NTD mutants (40 nM) were incubated with 50 μL of Δltn1 extracts for 15 min and subjected to centrifugation on 10–30% (wt/vol) sucrose gradient. Fractions were analyzed by immunoblot against flag, the 60S ribosomal protein Rpl3, or the 40S ribosomal protein Rps8. Ltn1-independent, flag cross-reacting bands present mostly in fractions 1–4 are marked with asterisks. (B) Graphic representation of results in the Lower panels of A. (C) An intact NTD domain is required for Ltn1 to co-IP with the 60S ribosomal protein Rpl3. Fifty microliters of Δltn1 extract were incubated with flag-tagged Ltn1 WT or NTD mutants (40 nM) for 15 min. Ltn1 was IPed with antibody against flag and IP products analyzed by immunoblot against flag (Ltn1) or Rpl3. Dashed lines indicate that lanes were removed from the original image.
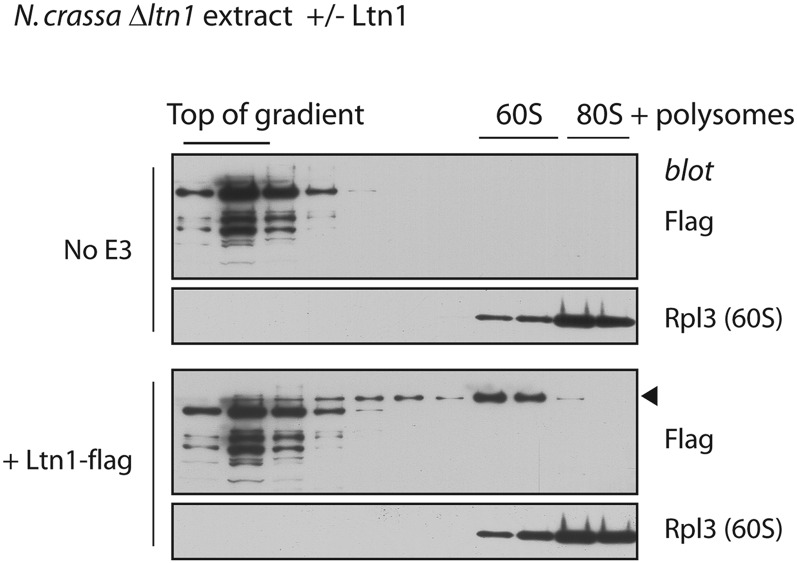
Fig. S2.
Sucrose gradient sedimentation analysis of Ltn1 binding to Neurospora ribosomes. Fifty microliters of ltn1Δ extracts were incubated or not with flag-tagged Ltn1 WT (40 nM) for 15 min and subjected to centrifugation on a 10–30% (wt/vol) sucrose gradient. Fractions were analyzed by immunoblot against flag or the 60S ribosomal protein Rpl3. The Ltn1 band is indicated by an arrowhead, in the “+Ltn1-flag” blot. Note that Ltn1-independent, flag cross-reacting bands appear in the “No E3” anti-flag blot, mostly in fractions 1–4.
Defective cofractionation of Ltn1 NTD mutants with 60S subunits was independently confirmed by assessing Ltn1’s ability to co-IP with Rpl3 (Fig. 4C). Thus, these results suggest that Ltn1’s conserved N terminus is important for RQC binding and, together with the results in Fig. 3 that NTD mutants do not ubiquitylate stalled NSPs, substantiate the model that NSP ubiquitylation is mediated by Ltn1 binding to stalled 60S subunits.
Structure of the Ltn1 N-Terminal Domain.
Having found that an intact Ltn1 NTD was required for binding to 60S subunits and stalled nascent chain ubiquitylation, we set out to further characterize this function by determining the structure of the NTD to elucidate positions for its conserved residues, including Thr61, and how it interacts with the RQC complex. Limited proteolysis and N-terminal sequencing of full-length Ltn1 and an Ltn1 construct consisting of residues 1–440 enabled the identification of a stable domain that includes residues 13–424. Crystals of Ltn113–424 were obtained, and a structure was determined using single-wavelength anomalous dispersion (SAD) with selenomethionine-substituted (SeMet) protein (SI Experimental Procedures). SeMet Ltn113–424 crystallized in space group P3121 and contained one molecule in the asymmetric unit. The structure included amino acid residues 14–419 and was refined at 2.5-Å resolution to an Rwork/Rfree of 0.20/0.24 and with good stereochemistry (Table 1). Native Ltn113–424 was solved by molecular replacement using SeMet Ltn1 coordinates as a search model in PHASER (25). Native Ltn113–424 crystallized in space group P21, contained two molecules in the asymmetric unit, and refined at 2.4-Å resolution to an Rwork/Rfree of 0.18/0.21 and with good stereochemistry (Table 1). Amino acids 14–420 were observed for both protomers in the asymmetric unit.
Table 1.
Crystallographic data and refinement statistics
| Dataset | Native | SeMet |
| Data collection* | ||
| Source | APS 24IDC | APS 24IDC |
| Wavelength, Å | 0.979 | 0.979 |
| No. of crystals | 1 | 1 |
| Space group | P21 | P3121 |
| Cell dimensions | ||
| a,b,c, Å | 88.51, 80.35, 97.33 | 58.1, 58.1, 347.1 |
| α,β,γ, ° | 90, 116.58, 90 | 90, 90, 120 |
| Resolution, Å | 44.3–2.41 (2.49–2.41) | 49.8–2.55 (2.64–2.55) |
| Completeness, % | 98.0 (91.0) | 99.0 (100.0) |
| Total reflections | 150,619 (12,207) | 221,836 (18,312) |
| Unique reflections | 46,438 (4,285) | 23,371 (2,261) |
| Wilson B-factor | 47.3 | 58.8 |
| Redundancy | 3.2 (2.8) | 9.5 (7.9) |
| Rmerge, % | 6.1 (41.6) | 7.9 (56.8) |
| CC1/2, % | 99.8 (81.0) | 99.9 (94.5) |
| CC*, % | 100.0 (94.6) | 100.0 (98.6) |
| <I>/σ(I) | 11.6 (2.3) | 21.5 (3.5) |
| FOM (Phaser – 4 Se) | 0.19 | |
| FOM (Resolve) | 0.53 | |
| Refinement* | ||
| Resolution, Å | 44.3–2.41 (2.49–2.41) | 49.8–2.55 (2.64–2.55) |
| No. of reflections (work/free) | 44,530/1,880 | 22,174/1,152 |
| Rwork/Rfree, % | 17.6 (24.5)/21.2 (29.4) | 20.2 (28.6)/24.3 (28.4) |
| No. of atoms | 6,770 | 3,333 |
| Protein | 6,587 | 3,301 |
| Ligand | 14 | 1 |
| Water | 169 | 31 |
| Average B-factors, Å2 | 58.6 | 89.0 |
| Protein | 58.9 | 81.1 |
| Ligand | 58.4 | 75.7 |
| Water | 47.8 | 65.9 |
| rmsd | ||
| Bond lengths, Å | 0.002 | 0.004 |
| Bond angles, ° | 0.61 | 0.70 |
| Molprobity† | ||
| Favored, % | 96.1 (778) | 95.3 (385) |
| Allowed, % | 100.0 (810) | 100.0 (404) |
| Outliers, % | 0.0 (0) | 0.0 (0) |
| Clash score | 100th percentile | 100th percentile |
| Molprobity score | 100th percentile | 100th percentile |
| PDB code | 5FG0 | 5FG1 |
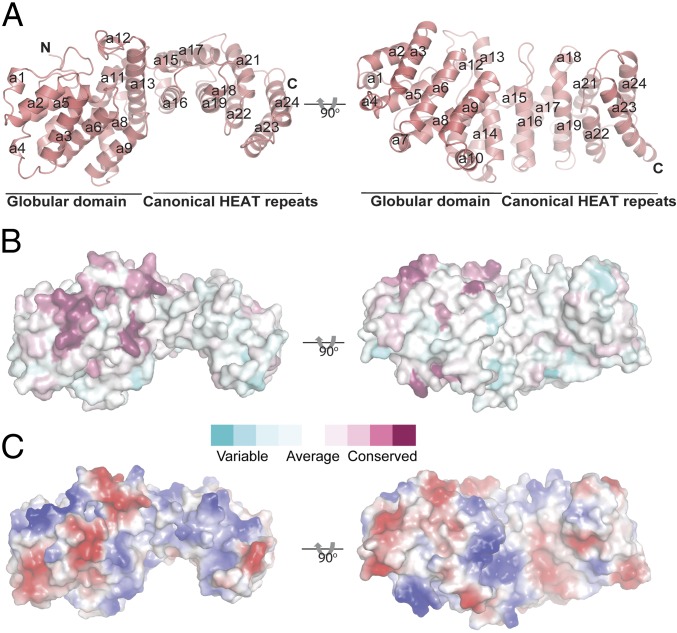
The Ltn1 NTD is predominantly composed of α-helices that are arranged into HEAT repeats (Fig. 5). HEAT repeats consist of two antiparallel α-helices that pack against each other in a repeating array. Overall, the Ltn1 NTD adopts a slightly curved topology with an outer and inner surface, an architecture frequently observed for other HEAT repeat-containing proteins (26). Residues 14–242 form a globular subdomain containing an N-terminal region that lacks a defined secondary structure, followed by four pairs of HEAT repeats (Fig. 5). The most N-terminal residues in the structure (residues 14–35) adopt a loop configuration that packs against helices a5, a7, a8, a11, and a12 through primarily hydrophobic interactions mediated by Leu18, Val23, Ile25, Leu27, and Tyr29. This globular subdomain is followed by an extended subdomain that includes four canonical HEAT repeat pairs. Although the primary sequence of the globular subdomain is conserved across evolution, the sequence of the extended HEAT repeats is only weakly conserved (7).
Fig. 5.
Structure of the N-terminal domain of Ltn1. (A) The structure of the Ltn1 NTD depicted as a schematic model in two orientations. Helices are labeled from a1 to a24, N and C termini are labeled N and C, respectively. The N-terminal globular domain and C-terminal canonical HEAT repeat regions are indicated. (B) Surface representation of Ltn1 with the sequence conservation mapped onto the surface. (C) Surface representation of Ltn1 with the electrostatic potential mapped onto the surface, with blue and red indicating positive and negative potential, respectively.
Budding yeast Ltn1 NTD adopts an overall fold similar to the partial poly-alanine model of human listerin NTD (Fig. S3) that was modeled based on electron density maps of the mammalian RQC EM structure (20). Although the primary amino acid sequence was not assigned in the structure of the human protein due to the relatively low resolution of the map in the NTD region, budding yeast Ltn1 NTD from residues 34–347 aligned well with its human counterpart, with an rmsd of 2.4 Å, enabling our tentative assignment of amino acid positions within the human Ltn1 structure (Fig. S3). Human and yeast Ltn1 aligned best within the conserved globular domain whereas the extended HEAT repeats diverged somewhat. Despite the similarities between human and yeast Ltn1 NTD structures, fungal Ltn1 proteins differed in sequence and structure at their extreme N termini compared with higher eukaryotes (Fig. S3B). Human listerin has an additional 18 unique residues at its N terminus, and, in the human Ltn1 structure, residues 13–27 adopt a loop-α-helix structure that is not observed in the overall fold of yeast Ltn1 NTD (Fig. S3A). Conversely, although the loop made up of residues 14–35 in yeast Ltn1 seems part of the globular subdomain, this loop conformation is not observed in the human NTD structure.
Fig. S3.
Comparison of Ltn1 from budding yeast and human. (A) Superimposition of the coordinates of the NTDs of yeast Ltn1 and human Ltn1 (PDB ID code 3J92) highlighting the conservation of the overall fold of Ltn1. Yeast Ltn1 is colored salmon, and hLtn1 is colored gray. The divergent extreme N termini of yeast and human Ltn1 are indicated by arrows. The N terminus of yeast Ltn1 (residues 14–39) is colored in red. (B) Sequence alignment of Ltn1 from fungi and metazoans. The secondary structure of yeast Ltn1 is displayed above the sequences in salmon, and that of human Ltn1 is displayed below in gray.
Ltn1 Recognition of the 60S Component of the RQC.
The NTD of human and yeast Ltn1 orthologs directly contact components of the RQC complex (19, 20, 24) and has been shown to be important for proper function of human Ltn1 with respect to stalled polypeptide ubiquitylation (20). The data in Fig. 4 show that the yeast Ltn1 NTD is important for association with ribosomes. To obtain higher resolution details on how Ltn1 binds to the RQC complex, we placed coordinates from the EM structure of the yeast 60S ribosomal subunit (PDB ID code 4V8T) and the Ltn1 NTD crystal structure generated here into EM maps of the yeast RQC by rigid body fitting. The Ltn1 NTD and the 60S ribosome fit well into the electron density and superposed with the orthologous proteins from the mammalian RQC structure (Fig. 6A and Fig. S4). As observed with the mammalian RQC (20), the Ltn1 NTD globular subdomain interacts with the SRL of the 23S rRNA (Fig. 6 B and C). The remaining NTD HEAT repeats extend away from the 60S ribosomal surface and terminate at the hinge point between the NTD and the elongated middle domain of Ltn1 (Fig. 6A).
Fig. 6.
Model for interactions between the Ltn1 NTD and 60S component of the RQC. (A) Schematic representation of the yeast RQC structure depicting the region where yeast Ltn1 (salmon) contacts the 60S ribosome. The sarcin-ricin loop (SRL) is colored orange, and Rpl8A is colored light blue. The EM map of the yeast RQC (EMD-6170) contoured to 1.5σ is displayed in blue showing the fit of the yeast 60S coordinates (PDB ID code 4V8T) and yeast Ltn1 NTD into the maps. The same model is displayed in the Right panel oriented 90° from the view shown in the Left panel. (B) Close-up of the interface of the SRL (orange) and Rpl8A (blue) with Ltn1. Ltn1 is shown as a diagram colored from teal to purple by the degree of sequence conservation (low to high). Ltn1 residues that contact the SRL and Rpl8 are shown as sticks, and the position of Rqc2 is shown as a diagram in green. (C) Same as in B but with the electrostatic potential mapped onto the surface of Ltn1.
Fig. S4.
Comparison of Ltn1 interactions with the RQC in budding yeast and human. Superimposition of the yeast and mammalian RQCs. The yeast Ltn1 NTD coordinates (salmon) and a model of the yeast Rqc2 (green) were fit into the EM maps of the yeast RQC, and the coordinates of the mammalian RQC (PDB ID code 3J92) were aligned to this model using the 60S subunit. The yeast 60S is colored in blue, and the mammalian 60S is colored in gray. (A) Comparison of the Ltn1 NTD interaction with the SRL and RPL8A components of the 60S ribosome. (B) Comparison of the interaction between Ltn1 and Rqc2 at the SRL.
Alignment of the primary sequence of Ltn1 orthologs reveals at least two main regions of high conservation on the Ltn1 surface between residues 49–64 (helices a2–a3) and 202–211 (helix a13) (Fig. 5B and Fig. S3B). In the model, conserved residues from Glu202 to Val211 are not involved in direct interactions with the 60S subunit whereas the other conserved region that includes residues Leu49 to Lys64 forms an α-helix-loop-α-helix element that directly contacts the SRL (Fig. 6B). The surface formed by the latter residues includes a positively charged patch in addition to other residues that could provide hydrogen bond donor/acceptor pairs and that are in suitable positions to make contacts with the phosphate backbone moieties of SRL nucleotides 3022–3028 (Fig. 6 B and C). The ε-amino group of Ltn1 Lys52 is within hydrogen-bonding distance to the phosphate backbone of A3024. Lys56 is also proximal to this position although it could also contact the phosphate backbones of U3023 and G3028. The Arg57 guanidinium group is buttressed by Tyr198 and is proximal to the G3028 backbone phosphate whereas Asp58 is proximal to the G3028 ribose. Supporting the functional analysis described above, we observed that the hydroxyl groups of Thr60 and Thr61 and the Lys64 side chain are proximal to the backbone phosphate of G3022 whereas Leu49 is within van der Waals (VDW) distance of His96 of the RPL8 ribosomal subunit. These residues are highly conserved from yeast to mammals, indicating that yeast and mammalian Ltn1 use a similar mechanism to directly engage the 60S ribosome.
To probe the importance of the aforementioned contacts in the NTD, we generated structure-guided NTD mutations within the context of recombinant, full-length Ltn1 harboring a Flag tag at the C terminus and examined their effect both on ribosome binding and on ubiquitylation of a stalled nascent chain reporter substrate using the in vitro N. crassa system as described in Newly Synthesized NSPs Are Ubiquitylated in N. crassa Extracts in an Ltn1-Dependent Manner.
The relative efficiency of ribosome binding by Ltn1 mutants was evaluated using limiting amounts of recombinant Ltn1 proteins mixed with Δltn1 extracts and centrifuged over a sucrose cushion. The results in Fig. 7A show that, in this assay, the R57A and T61A mutants exhibited decreased binding to ribosomes, as revealed by the presence of a higher fraction of those proteins in the postcentrifugation supernatant compared with WT Ltn1. The effect of mutating Arg57 was more modest than mutating Thr61, perhaps due to the presence of several other basic residues in this SRL-interacting patch.
Fig. 7.
Effect of mutations in Ltn1’s SRL-binding surface on ribosome binding and nascent chain ubiquitylation. Dashed lines indicate that lanes were removed from the original image. (A) Mutations in Ltn1’s SRL-binding surface affect ribosome binding as measured by sucrose gradient centrifugation. Flag-tagged Ltn1 WT or NTD mutants (R57A or T61A) were incubated with Δltn1 Neurospora extract and subjected to centrifugation on sucrose cushion, as described in SI Experimental Procedures. Input, supernatant, and pellet were analyzed by immunoblot against Flag (Ltn1), the 60S ribosomal protein Rpl3, or the cytosolic protein Pgk1. Ltn1-independent, anti-Flag antibody cross-reacting bands are marked with asterisks. (B) Mutations in Ltn1’s SRL-binding surface affect stalled nascent chain ubiquitylation. Stalling reporter-encoding mRNA was translated in Δltn1 extract supplemented or not with recombinant Ltn1 (WT, R57A mutant, or T61A mutant) and analyzed for ubiquitylation by denaturing IP (see also Fig. S5). Immunoblots were against Flag (Ltn1), Ubiquitin (Ub), and GFP (stalling reporter). (C) As in A, except that Ltn1 R57A and D68A mutants were analyzed. (D) As in B, except that Ltn1 R57A and D68A mutants were analyzed, the “no template mRNA” control is shown, and the WT extract was also used as positive control.
We next investigated the extent to which the effect of Ltn1 mutations on ribosome binding correlated with defects in ubiquitylation of ribosome-stalled nascent chains. For this purpose, Δltn1 Neurospora extracts were mixed with Ltn1 WT or mutant proteins and programmed to translate the fGFP-K12 mRNA template (nonstop reporter). As in the experiments described in Figs. 1 and 2, in WT extract, but not Δltn1 extract, nonstop reporter protein was ubiquitylated (see also Fig. S5, lanes 1 and 2). Reporter ubiquitylation could be restored in the Δltn1 extract by addition of recombinant WT yeast Ltn1, but not by a Ltn1 mutant lacking the RING domain (Ltn1 ΔRING) (Fig. S5, lanes 3 and 4). Ubiquitylation defects were apparent for the Ltn1 R57A and, to a greater extent, Ltn1 T61A mutants (Fig. 7B), consistent with the importance of these conserved residues for mediating direct interactions with the 60S subunit (an additional mutation, K64A, yielded more modest and variable defects in this assay). We also tested the effect of mutating Asp68, whose negative charge is conserved among Ltn1 orthologs. Surprisingly, the Ltn1 D68A mutant seemed to exhibit improved binding to ribosomes compared with WT Ltn1 and was fully competent for reporter ubiquitylation (Fig. 7 C and D, respectively). One possibility to explain this observation is that the negative charge helps modulate Ltn1-60S binding toward an optimal, rather than maximal, extent by neutralizing the positive charge in the neighboring basic patch. For comparison, substitution of additional selected conserved residues that are exposed elsewhere on the NTD surface (Ltn1 K130A, E202A, and E205A mutants) revealed no obvious consequence in these assays (Fig. S5, lanes 5–7).
Fig. S5.
Effect of Ltn1 mutations on nascent chain ubiquitylation (as in Fig. 7 B and D). Stalling reporter-encoding mRNA was translated in WT or Δltn1 extract, supplemented or not with recombinant Ltn1 (WT or mutants) (as indicated), and analyzed for ubiquitylation by denaturing IP. Lane 2 shows the “no Ltn1” control, and lane 8 shows the “no template” control reaction incubated with Ltn1 WT.
Ltn1 Interaction with Rqc2.
Rqc2 increases association of Ltn1 to the RQC complex and enhances ubiquitylation of stalled nascent chains (11, 12). In the RQC, Ltn1 and Rqc2 interact with each other near the SRL in a position such that surfaces important for 40S ribosomal subunit association become sterically occluded (19, 21). We placed a model of S. cerevisiae Rqc2 [obtained using the coordinates of human Rqc2 (NEMF)] into the yeast RQC maps using rigid body fitting. S. cerevisiae Rqc2 adopts a position similar to that observed for human Rqc2 in the mammalian RQC structure (Fig. 8A and Fig. S4B). Based on the cryo-EM structures, Rqc2 consists of an N-terminal NFACT-N domain that is believed to recruit Ala- or Thr-tRNA to the A site for CAT tail synthesis, and an additional density (that may include NFACT-R and perhaps also NFACT-C domains) that contacts the stalled P site tRNA (20, 21). Coiled-coils extend from these domains and connect them to a globular middle domain (M-domain) that makes multiple interactions with Ltn1, the SRL, and the P stalk of the 60S ribosome (20).
Fig. 8.
Model for interactions between Ltn1 and Rqc2. (A) Schematic representation of the interaction of yeast Ltn1 (salmon) with a model of yeast Rqc2 (green) and the SRL. The EM map of the yeast RQC (EMD-6170) contoured to 1.5σ is displayed in blue showing the fit of the proteins in the maps. (B and C) Comparison of the Ltn1-Rqc2 interface and the Ltn1 N termini in yeast versus mammals. (B) Schematic representation of yeast Ltn1 (salmon) and Rqc2 (green). The EM map of the yeast RQC contoured to 1.5σ is displayed in blue, and Ltn1 residues 14–35 are colored red, highlighting the presence of electron density for this region. The position of the extreme N-terminal helix (orange) of the human Ltn1 NTD based on an alignment with the yeast RQC is indicated by an arrow demonstrating the lack of electron density in this region of the map. (C) Schematic representation of the human Ltn1 (orange) and Rqc2 (teal) interface (PDB ID code 3J92) with the EM map from the mammalian RQC (EMD-2832) contoured at 1.5σ. The position of the extreme N terminus from yeast Ltn1 is shown in red, and the human Ltn1 extreme N terminus is indicated by an arrow. (D) Secondary structure-based sequence alignment of the N-terminal residues from yeast and metazoans. Conserved residues are highlighted in black (highly conserved) and gray (moderately conserved). The secondary structure of yeast Ltn1 is shown above the sequence alignment whereas that of the human structure is shown below. Ltn1 residues in the interface with Rqc2 in yeast are indicated by pink dots above the residues.
Although it is clear that the ability of Ltn1 to interact with the 60S ribosome and Rqc2 components of the RQC is shared among eukaryotes, the Ltn1–Rqc2 interaction surface does not seem to be fully conserved. S. cerevisiae Ltn1 residues 30–36, which form an interaction interface for Rqc2, are strongly conserved in fungi and less conserved in higher eukaryotes (Fig. 8D and Fig. S3B). Furthermore, there seems to be no structural equivalent for this interaction when RQC complexes are compared. Electron density is present for the N-terminal yeast Ltn1 residues in the yeast RQC complex (Fig. 8B), but no electron density is evident in this region in the human RQC complex (Fig. 8C). Perhaps in lieu of this interface, metazoan Ltn1 residues 13–27 adopt an α-helix conformation that makes contacts with both Rqc2 and ribosomal subunit eL40, on the opposite surface of Rqc2 from where Ltn1 contacts Rqc2 in the yeast complex (Fig. 8C). This Ltn1 helix has been shown to be important for function in mammals but seems to be absent in yeast because there is neither sequence conservation nor electron density in the same position in the yeast RQC EM maps (Fig. 8B). Therefore, metazoan listerin seems to have evolved a unique manner to interact with its Rqc2 ortholog.
SI Experimental Procedures
Materials.
Anti-flagM2 (F3165) and anti-GST (G1160) mouse monoclonal antibodies and anti-flagM2 conjugated magnetic beads (M8823) were obtained from Sigma. Anti-HA (12CA5) and anti-GFP (11814460001) antibodies and protease inhibitor mixture were from Roche. Protein-G Dynabeads were from Life Tech. To detect ubiquitin, DAKO rabbit polyclonal antibody Z0458 was used. Mouse monoclonal anti-Rpl3 antibodies were a kind gift of J. Warner, Albert Einstein College of Medicine, New York. Rabbit polyclonal anti-yeast Rps8 antibody was a kind gift of G. Dieci, Università degli Studi di Parma, Parma, Italy (27).
Preparation of Neurospora Extracts.
Neurospora conidia from both WT [Oak Ridge 74-OR23-IVA; Fungal Genetics Stock Center (FGSC) 2489] and Δltn1 (NCU06534; FGSC 11923) strains were cultured and harvested using the same procedure as previously described for the preparation of Neurospora cell-free extract (28). Freshly prepared buffer A [30 mM Hepes (pH 7.6), 100 mM KOAc, 3 mM Mg(OAc)2, 2 mM DTT; 1:1 volume/cell weight ratio] was dripped directly into liquid nitrogen by using a sterile Pasteur pipette to form small ice beads. Frozen buffer beads and cells were transferred into prechilled grinding vials of a SPEX SamplePrep 6850 Freezer/Mill and powdered in the liquid nitrogen-filled tub using a setting consisting of 10 min of precooling followed by three 2-min grind cycles with 1-min recooling between each cycle. The powdered cells were transferred to prechilled 50-mL polycarbonate centrifuge tubes, allowed to thaw on ice, and then centrifuged at 4 °C for 15 min at 16,000 rpm in an SS34 fixed-angle rotor (Thermo Fisher Scientific). The supernatant was carefully collected with a sterile Pasteur pipette, avoiding both the pellet and the fatty upper layer, and transferred in a second prechilled polycarbonate centrifuge tube and centrifuged again with the same settings. The supernatant was carefully collected and placed in a fresh 50-mL conical tube that was maintained on ice. Small molecules were removed from the extract by chromatography through Zeba spin desalting columns (Pierce). The purified extract was then treated with protease inhibitor mixture as described (28). For storage, aliquots of extract (100 µL) were put into 0.6-mL Eppendorf tubes, frozen with liquid nitrogen, and stored at –80 °C.
Generation of DNA Templates for in Vitro Transcription.
DNA fragments for in vitro transcription were generated by PCR, with the pEGFP-N1 vector as the source of GFP coding sequence. The forward primer used to generate all templates encoded a T7 RNA polymerase promoter, a 5′ flag tag, and the 5′ portion of the GFP coding sequence: 5′-TAATACGACTCA CTATAGGGAGAGCCACCATGGACTACAAAGACGATGACGACAAGGTGAGCAAGGGCGAG-3′.
The various reverse primers used provided the 3′ portion of the GFP coding sequence (until codon 53 or full length), and encoded or not homopolymeric tracts of 12 amino acids as well as quadruple stop codons, as follows: fGFP-s, 5′-(TTT)11TTTATCATCATTACAGCTTGCCGGTGGT’-3′; fGFP-K12, 5′-(TTT)12CAGCTTGCCGGTGGT-3′; fGFP-K12-s, 5′-TTTATCATCATTA(CTT)12 CAGCTTGCCGGTGGT-3′; fGFP-E12-s, 5′-TTTATCATCATTA(TTCCTC)6CAGCTTGCCGGTGGT-3′; fGFP-R12-s, 5′-TTTATCATCATTA(CCT)12 CAGCTTGCCGGTGGT-3′; fGFP(FL)-s, 5′-(TTT)11 TTTATCATCATTACTTGTACAGCTCGTCC-3′; and fGFP(FL)-K12, 5′-(CTT)12 GTACAGCTCGTCCATG.
PCR products were separated in agarose gel, and the DNA in bands of the expected size was eluted from gel slices using a gel extraction kit (Qiagen).
In Vitro Transcription.
Purified PCR products were used as DNA templates for in vitro transcription with T7 RNA polymerase for 1 h at 37 °C, using the Message Machine kit (AM1344M; Ambion). This kit allows coupled capping of the newly synthesized mRNA. Approximately 260-nt mRNA products were purified with a High-Pure miRNA isolation kit (Roche).
In Vitro Translation with Neurospora Extracts.
Capped RNA was incubated with 10–50 μL of Neurospora extract-containing reaction mixtures for 10–30 min (as indicated) at 26 °C. Reaction mixtures contained 50% of extract (vol/vol; final protein concentration typically 50–70 mg/mL), mRNA (10 ng/μL), 80 mM KOAc, 20 mM Hepes–KOH (pH 7.5), 2.4 mM Mg(OAc)2, 10 μM each amino acid, 20 mM creatine phosphate, 0.12 U/μL creatine phosphokinase, 2 mM DTT, 1 mM ATP, and 0.1 mM GTP. The concentration of cycloheximide (Sigma), when used, was 1 mM.
In Vitro Ubiquitylation Assay with Recombinant Proteins.
For in vitro autoubiquitylation assays, 100 nM E1, 1 μM Ubc4, 1 μM recombinant yeast Ltn1 (WT or mutated), and 5 μM GST-Ub were mixed with 2 mM ATP in ubiquitylation buffer (50 mM Tris⋅HCl, pH 7.5, 2.5 mM MgCl2, 0.5 mM DTT) and incubated at 30 °C for 1 h. The reaction mixture was boiled with sample buffer and run in SDS/PAGE.
In Vitro Reconstitution of Ltn1-Dependent Ubiquitylation.
To analyze the relative ubiquitylation activity of recombinant Ltn1 variants, a reaction mixture containing 40 nM recombinant Ltn1, 40 nM mRNA (flag-GFP-K12-nonstop), 50% (vol/vol) of N. crassa extract, 20 U RNasin (Promega), 80 mM KOAc, 2.4 mM Mg(OAc)2, 10 µM each amino acid, 20 mM creatine phosphate, 6 U creatine phosphokinase, 2 mM DTT, 1 mM ATP, and 0.1 mM GTP was incubated at 26 °C for 30 min. The reaction was stopped by adding an equal volume of SDS buffer (50 mM Tris⋅HCl, pH 7.4, 5 mM EDTA, 5 mM N-ethylmaleimide, 1% SDS, and protease inhibitors) and boiling for 5 min. The resulting mixture was diluted 10-fold and incubated overnight with anti-flag M2 magnetic beads (SIGMA) in ice-cold IP buffer (50 mM Tris-Cl, pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40). After three washes with IP buffer, the magnetic beads were boiled in sample buffer, and eluates were used for immunoblotting.
Sucrose Density Gradient and Sucrose Cushion Centrifugation.
The 15–40% (wt/vol) sucrose gradient sedimentation analyses were performed as described (2) with minor modifications. Reaction products (50 μL) were mixed with 150 μL of sucrose buffer solution (buffer S: 10 mM Tris-Cl, pH 7.0, 140 mM NaCl, 1.5 mM MgCl2) and overlaid onto discontinuous gradient prepared in buffer S (330 μL each of 15–40% (wt/vol) sucrose solutions at 5% increments). The samples were centrifuged for 80 min at 4 °C in a TLS-55 rotor at 215,000 × g. Twelve 180-μL fractions were collected.
The 10–30% (wt/vol) sucrose gradient sedimentation analyses were performed as above. Reaction products (50 μL) were mixed with 150 μL of sucrose buffer solution (buffer H: 50 mM Hepes-KOH, pH 7.4, 100 mM KOAc, 5 mM MgCl2) and overlaid onto a discontinuous gradient prepared in buffer H. The samples were centrifuged for 80 min at 4 °C in a TLS-55 rotor at 215,000 × g. Twelve 165-μL fractions were collected.
For sucrose cushion centrifugation, reaction products (20 μL) were overlaid onto 60 μL of 30% (wt/vol) sucrose in buffer S. Samples were centrifuged for 30 min at 4 °C in a TLA-100 fixed-angle rotor (Beckman) at 100,000 rpm. Pellets were resuspended by repeated pipetting in 15 μL of either extract or sample buffer, depending on the downstream application.
Coimmunoprecipitation.
To analyze Ltn1-Rpl3 coimmunoprecipitation, 1 μg of recombinant protein was incubated with 50 μL of Δltn1 extract mixture containing 1 mM ATP and 0.1 mM GTP at 26 °C for 10 min. After diluting samples 10-fold in IP buffer (50 mM Hepes-KOH, pH 7.4, 100 mM KOAc, 5 mM MgCl2, 15% (vol/vol) glycerol, 0.1% IGEPAL CA-630, protease inhibitors), anti-Flag M2 antibody was added for 17 h at 4 °C. Prewashed protein-G Dynabeads (10003D; Life Tech) were then added to the protein-antibody mixture for 4 h at 4 °C. After three washes in IP buffer, proteins were eluted by boiling in reducing gel loading buffer.
Ltn1-Ribosome Binding Assay (Sucrose Gradient).
To analyze Ltn1 binding to ribosomes, recombinant protein (40 nM) was incubated with 50 μL of Δltn1 extract mixture containing reconstitution buffer (80 mM KOAc, 20 mM Hepes–KOH, pH 7.5, 2.4 mM Mg(OAc)2, 20 mM creatine phosphate, 0.12 U/μL creatine phosphokinase, 2 mM DTT, 1 mM ATP, and 0.1 mM GTP) for 15 min at 26 °C, and subsequently loaded on top of a sucrose gradient. Samples were centrifuged for 80 min at 4 °C in a TLS-55 rotor at 215,000 × g. Twelve 165-μL fractions were collected, and 15 μL of each fraction were analyzed by Western blot.
Ltn1-Ribosome Binding Assay (Sucrose Cushion).
To reconstitute the association of Ltn1 to ribosomes in vitro, recombinant Ltn1 (40 nM) was incubated for 10 min at 26 °C in a Neurospora extract mixture containing 80 mM KOAc and 2.4 mM Mg(OAc)2. Then, 20 µL of the reaction mixture was overlaid on top of a 60-µL sucrose cushion (1 M sucrose in 10 mM Tris⋅HCl, pH 7.0, 140 mM NaCl, 1.5 mM MgCl2). After ultracentrifugation at 353,000 × g for 45 min (Optima TLX microultracentrifuge, TLA-100 rotor), the supernatant was subsequently separated from the pellet, and Ltn1 levels in both fractions were determined by immunoblotting.
Denaturing IP.
For denaturing IP, in vitro translation reactions were boiled in 1% SDS buffer (50 mM Tris⋅HCl, pH 7.4, 5 mM EDTA, 5 mM NEM, 1% SDS, and protease inhibitors). Denatured mixtures were diluted 10-fold in IP buffer (50 mM Tris⋅HCl, pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40) and incubated with 20 μL of magnetic bead-conjugated anti-flag M2 antibody for 4 h overnight at 4 °C. After three washes with IP buffer, the beads were boiled with sample buffer, and eluates were used for immunoblot.
Gel Electrophoresis.
Proteins in samples were separated by electrophoresis in 4–20% (wt/vol) acrylamide Tris-Glycine gel (Invitrogen) run at pH 8.7.
When analyzing peptidyl-tRNA conjugates, for better preserving the labile ester bond that links amino acids to tRNA during SDS/PAGE, we used sample preparation and gel running conditions with more acidic pH compared with the more commonly used Tris-Glycine–based systems: Samples were mixed with Laemmli sample buffer (pH 6.8) and run on NuPAGE 10% (wt/vol) acrylamide Bis-Tris gels (pH 6.4) with Mes buffer (50 mM Mes, 50 mM Tris-Base, 0.1% SDS, 1 mM EDTA, pH 7.3).
Immunoblot.
Proteins and protein conjugates separated by SDS/PAGE were transferred to PVDF membranes using a semidry transfer kit. Antigens were visualized by sequential treatment with specific antibodies, HRP-conjugated secondary antibodies, and an enhanced chemiluminescence substrate kit (Pierce). Semiquantitation of band signal intensity was performed with Image J.
Ltn1 Expression and Purification.
DNA encoding full-length Ltn1 (FL-Ltn1) was inserted into the pET28b vector containing an in-frame N-terminal His6-Smt3 tag (29) and a C-terminal Flag tag. To generate mutants in the context of full-length Ltn1, mutations were first introduced into pSMT3–Ltn11–440 plasmids with a QuikChange kit (Stratagene), and the DNA containing the verified sequences were subcloned into the full-length pSMT3-Ltn1-flag constructs. His6-Smt3-Ltn1-flag was expressed by transforming the expression plasmids into E. coli strain BL21 (DE3) codon plus RIL (Stratagene). Ltn1-Flag was expressed and purified as described for FL-Ltn1 (7). Cultures of E. coli containing the expression plasmids were grown in super broth in baffled flasks at 37 °C to an A600 of 2.0, after which cells were cold-shocked by placing flasks on ice for 30 min. Isopropyl-β-d-thiogalactoside (IPTG) was added to a final concentration of 0.05 mM, and the cultures were incubated at 18 °C overnight with constant shaking. Cells were harvested by centrifugation at 16,000 × g and resuspended in buffer containing 50 mM Tris⋅HCl, pH 8.0, and 20% (wt/vol) sucrose. Cells were disrupted by resuspending cells in lysis buffer with a final composition of 500 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, 20 mM imidazole, 1 mM β-mercaptoethanol (BME), 0.1% IGEPAL (Fluka), and 1 mM phenylmethanesulfonylfluoride (PMSF), followed by sonication. Lysates were clarified by centrifugation at 45,000 × g, and the resulting supernatants were applied to an Ni-NTA superflow resin (Qiagen) previously equilibrated in buffer containing 350 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, 1 mM BME, and 20 mM imidazole. FL-Ltn1 was eluted with buffer containing 350 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, 1 mM BME, and 250 mM imidazole, and His6-Smt3 tags were removed with Ulp1 (29) at a protein:Ulp1 ratio of 1,000:1 (wt/wt) overnight at 4 °C. FL-Ltn1 was purified with size-exclusion chromatography (Superdex 200; GE Healthcare) in buffer containing 350 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, and 1 mM BME. After gel filtration, FL-Ltn1 was buffer exchanged into 100 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, and 1 mM BME during concentration. FL-Ltn1 was further purified with cation-exchange chromatography (MonoS 5/5; GE Healthcare), and peak fractions of the bound material, as judged by SDS/PAGE and Coomassie blue staining, were pooled and concentrated to 1–2 mg/mL in 200 mM NaCl, 20 mM Tris⋅HCl, pH 8.0, and 1 mM BME.
For the sets of experiments involving Ltn1-ΔN-flag, Ltn1-T61A-flag, or Ltn1-ΔR-flag (and the Ltn1-flag control), the respective Ltn1 coding sequences were subcloned into pSmt3 vector to generate constructs harboring N-terminal His6-Smt3 and C-terminal flag tags. Expression of the recombinant Ltn1 proteins was performed at 23 °C using E. coli BL21 codon plus (DE3)-RIL (Agilent Tech) grown in Terrific broth supplemented with kanamycin and chloramphenicol. Cells were harvested and resuspended in 20 mM Tris⋅HCl, pH 8.0, 500 mM NaCl, 1 mM β-mercaptoethanol, 20 mM imidazole, 0.1% IGEPAL, and 1 mM PMSF. Cells were lysed by sonication, and His-tagged proteins were purified using Ni-NTA agarose (Qiagen) in Bio-Rad disposable columns. Beads were washed with 20 mL of equilibration buffer and eluted with 5× 0.5 mL of 250 mM imidazole in 20 mM Tris⋅HCl, pH 8.0, 350 mM NaCl, 1 mM β-mercaptoethanol. The eluate (normally pooled fractions 2 and 3) was dialyzed against buffer containing 20 mM Tris⋅HCl, pH 8.0, 350 mM NaCl, 1 mM β-mercaptoethanol, 0.2% IGEPAL, and 15% (vol/vol) glycerol. One thousand units of His-tagged SUMO protease (MCLAB) was added to 500 μg of purified protein to cleave the Smt3 tag and incubated for 17 h at 4 °C. The reaction products were again exposed to Ni-NTA beads for removal of both His-SUMO protease, cleaved His-Smt3, and uncleaved protein. The unbound, cleaved protein in dialysis buffer was stored in small aliquots at −80 °C.
For crystallization, DNA encoding Ltn1 residues 13–424 (Ltn113–424) was inserted into the pSMT3 vector and transformed into BL21 (DE3) codon plus RIL. Ltn113–424 was expressed and harvested in the same way as FL-Ltn1, except cells were induced with 0.5 mM IPTG. Selenomethionine-labeled Ltn113–424 was expressed by transforming the Ltn113–424 containing pSMT3 expression vector into E. coli strain B834 (DE3) (Novagen). Cultures of B834 (DE3) containing the expression plasmids were grown in LeMaster medium (30) containing 50 mg/L selenomethionine in baffled flasks at 37 °C to an A600 of 0.8. Cells were then cold-shocked by placing flasks on ice for 30 min. IPTG was added to a final concentration of 0.5 mM, and the cultures were then incubated at 18 °C overnight with constant shaking. Cells were harvested by centrifugation at 16,000 × g and resuspended in buffer containing 50 mM Tris⋅HCl, pH 8.0, and 20% (wt/vol) sucrose.
Cells expressing Ltn113–424 were disrupted in lysis buffer with a final composition of 500 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, 20 mM imidazole, 5 mM BME, 0.1% IGEPAL (Fluka), and 1 mM PMSF, followed by sonication. Lysates were clarified by centrifugation at 45,000 × g, and the resulting supernatants were applied to an Ni-NTA superflow resin (Qiagen) previously equilibrated in buffer containing 350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, 5 mM BME, and 20 mM imidazole. The resin was washed with 2 column volumes of wash buffer (350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, 5 mM BME, and 20 mM imidazole) supplemented with 50 mM KCl, 10 mM MgSO4, and 2 mM ATP, followed by a further 5 column volumes of wash buffer. Proteins were eluted with buffer containing 350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, 5 mM BME, and 250 mM imidazole, and His6-Smt3 tags were cleaved by incubation with Ulp1 (29) at a protein:Ulp1 ratio of 1,000:1 (wt/wt) overnight at 4 °C while dialyzing in buffer containing 350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, 5 mM BME. Proteins were reapplied to an Ni-NTA superflow resin to remove free His6-Smt3, and the flow-through was collected. Proteins were filtered (Superdex 75; GE Healthcare) in buffer containing 350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, and 5 mM BME to separate Ltn1 from any remaining His6-Smt3. After gel filtration, Ltn113–424 was concentrated to 8–10 mg/mL in a final buffer composition of 350 mM NaCl, 20 mM Hepes-NaOH, pH 7.0, and 5 mM BME.
Crystallization and Data Collection.
Selenomethionine-labeled Ltn1 (13-424) crystallized in 7.5% (wt/vol) PEG8000, 0.1 M potassium chloride, and 6% (vol/vol) glycerol in hanging drop by vapor diffusion and was cryo-protected in buffer containing 9% (wt/vol) PEG8000, 0.1 M potassium chloride, 20% (vol/vol) glycerol, and 5 mM Tris(2-carboxy-ethyl)phosphine (TCEP). Data were collected using a Dectris Pilatus-6M detector at the Advanced Photon Source, NE-CAT beamline 24-IDC. Data were indexed, integrated, and scaled using HKL2000 (31). The crystal belongs to space group P3121 and contains 1 protomer in the asymmetric unit. Six selenium atoms were identified using SHELXD (32), four of which were later confirmed as authentic using PHASER (28) heavy atom refinement and phasing. Phases from PHASER were input to RESOLVE for density modification, followed by autobuilding using PHENIX (25), resulting in a model that contained 332 residues. The remainder of the molecule was rebuilt by hand using COOT (33). The structure was refined at 2.55-Å resolution to an Rwork/Rfree of 0.202/0.243 using PHENIX (25). The final model contained Ltn1 residues 14–419. The model had excellent geometry, with 95.3%, 100%, and 0% in the most favored, allowed, and disallowed regions, respectively, of Ramachandran space as calculated by MolProbity (26).
Native Ltn1 (13-424) crystallized in 7% (wt/vol) PEG8000, 0.1 M potassium chloride, and 2.5% (vol/vol) glycerol in hanging drop by vapor diffusion and was cryo-protected in buffer containing 9% (wt/vol) PEG8000, 0.1 M potassium chloride, 2.5% (vol/vol) glycerol, and 20% (vol/vol) ethylene glycol. Data were collected using a Dectris Pilatus-6M detector at the Advanced Photon Source, NE-CAT beamline 24-IDC. Data were indexed, integrated, and scaled using HKL2000 (31). Molecular replacement was performed using PHASER (28), with the coordinates from selenomethionine-labeled Ltn113–424. The crystal indexed as space group P21, with two molecules of Ltn1 in the asymmetric unit. The model was inspected and rebuilt using COOT (33) and refined at 2.41-Å resolution to an Rwork/Rfree of 0.176/0.212 with PHENIX (25). Both molecules of Ltn1 included residues 14–420. The model had good geometry, with 96.1%, 100%, and 0% in the most favored, allowed, and disallowed regions, respectively, of Ramachandran space as calculated by MolProbity (26).
Discussion
Here, we present genetically modified Neurospora extracts as a system to study RQC and use this system to provide biochemical evidence that Ltn1's conserved NTD is required for binding to 60S subunits. Importantly, we determined the 2.4-Å X-ray crystal structure of the Ltn1 NTD; by modeling the NTD structure onto available lower resolution RQC complex structures, we now provide more detailed information on how Ltn1 interacts both with the 60S subunit and with its RQC complex cofactor, Rqc2. Finally, we present evidence supporting the model that 60S association is required for Ltn1-mediated ubiquitylation of stalled nascent chains, which we also show as taking place on ribosomes.
A New System to Study Ribosome-Associated Quality Control.
We describe a system enabling elucidation of mechanisms underlying the fungal Ltn1 pathway using translation- and ubiquitylation-competent Neurospora cell-free extracts. Importantly, Neurospora can be manipulated genetically with relative ease, allowing one to generate custom extracts that are cleanly altered for specific factors of interest. Indeed, using WT and Δltn1 extracts, we were able to reconstitute essential features of the Ltn1 pathway elucidated in previous studies and to biochemically substantiate aspects of the proposed model of Ltn1 function. We first showed that NSP ubiquitylation was defective in Δltn1 extracts. Adding power to the analyses, it was possible to rescue the defect by adding to the reaction recombinant yeast Ltn1 purified from E. coli. Finally, we have also been able to use this system to provide direct physical evidence for an important role for Ltn1’s conserved NTD in 60S binding and substrate targeting.
A Structural Basis for Ltn1 NTD Interactions with the Ribosome.
Cryo-EM structures of Ltn1/listerin in complex with other RQC subunits and the stalled 60S show that Ltn1 is an extended molecule spanning the distance from the vicinity of the intersubunit surface to the opening of the ribosomal exit tunnel (19–21), as we had previously proposed (7). Furthermore, those structures show that Ltn1’s conserved NTD and CTD domains bind to other components of the complex, with the NTD binding directly both to the 60S subunit near the SRL, as well as to the Rqc2/NEMF subunit.
Accordingly, in the work presented here, we observed a requirement for the Ltn1 NTD in 60S association. Furthermore, mutation of the conserved Ltn1 Thr61 residue was sufficient to cause defects in both complex binding and NSP ubiquitylation. At the resolution of the RQC complex cryo-EM structures that have been solved, it had not been possible to assign specific function to the conserved NTD residues, so the exact role of the Ltn1 Thr61 residue was not clear. The crystal structure reported here, analyzed in conjunction with the cryo-EM structures of RQC complexes from human and yeast, sheds additional light on NTD function. Among other findings, it shows that the Thr61 side chain hydroxyl group is indeed proximal to the SRL of the 60S subunit.
The 2.4-Å X-ray crystal structure of the yeast Ltn1 NTD has revealed that Thr61 belongs to a conserved surface. Although interactions with the SRL of the 60S subunit provide a plausible explanation for the conservation of such a surface, the structure revealed additional conserved surfaces on the Ltn1 NTD, which warrant further investigation because they suggest alternative strategies that Ltn1 may use for stable association with the RQC; alternatively, these surfaces may be conserved for other functions.
Comparison of the yeast Ltn1 NTD with the analogous domain of human listerin showed similarity throughout most of the structure although it is clear that the extreme N-terminal segments differ in primary amino acid composition (human listerin includes an additional ∼15 aa), structure, and function. Although human listerin includes an amphipathic helix that tucks under human Rqc2, this element is not apparent in the EM density of the yeast complex, and yeast Ltn1 seems to have a unique N-terminal segment that packs into the Ltn1 globular domain near the interface with yeast Rqc2. Although our structure of S. cerevisiae Ltn1 does not include the N-terminal 12 amino acids, sequence comparison with the human protein does not reveal significant similarity in this region, consistent with the model that yeast Ltn1 does not use an N-terminal helix for interaction with Rqc2. This latter point is also supported by the apparent lack of electron density for this segment in a higher resolution EM structure of yeast Ltn1 in complex with the RQC (21).
In contrast to the results presented here with yeast Ltn1, neither a triple mutation of conserved human listerin NTD residues nor deletion of most of the NTD affected NSP ubiquitylation in the mammalian system; it was only when NTD and CTD mutations were combined that a defect in NSP ubiquitylation was observed (20). It remains to be determined whether the stronger dependency on the Ltn1 NTD that we observed results from innate differences between the fungal and mammalian systems, such as the structural differences described in the preceding paragraph, or whether this dependency is due to differences in the sensitivities of the assay procedures.
Ribosome-Associated Ubiquitylation.
We provide here two lines of evidence supporting the model that stalled nascent chains are presented to Ltn1 and become ubiquitylated while still associated with the 60S subunit. First, we demonstrated a requirement for NSP presentation on ribosomes in order for Ltn1-mediated ubiquitylation to take place because Ltn1 was unable to ubiquitylate NSPs when mixed subsequently with NSPs that had been released from ribosomes. Second, we generated Ltn1 NTD mutants that are defective in 60S binding and showed that these NTD mutations impaired NSP ubiquitylation. Ltn1’s ability to target its protein quality control substrates while the latter are still 60S-associated ensures that aberrant proteins are marked for degradation before being released in the cytosol, where they might escape proteolysis and engage in detrimental interactions. Furthermore, by using 60S subunits as adapters, Ltn1 is able to target a large variety of nascent polypeptides that can become stalled during translation. These findings contribute toward our understanding of how E3s that function in protein quality control are able to recognize heterogeneous substrates—an important, yet unresolved, problem.
Experimental Procedures
In vitro translation with Neurospora extracts, preparation of recombinant yeast Ltn1, Ltn1-ribosome binding assays, denaturing IP, analysis of peptidyl-tRNA conjugates, an in vitro ubiquitylation assay with recombinant proteins, and protein crystallization are described in SI Experimental Procedures.
Acknowledgments
We thank G. Dieci and J. Warner for reagents and the Fungal Genetics Stock Center for providing Neurospora strains. Work in the C.A.P.J. laboratory is supported by R01 Grant NS075719 from the National Institute of Neurological Disorders and Stroke (NINDS) of the NIH and R01 Grant CA152103 from the National Cancer Institute (NCI) of the NIH. Work in the M.S.S. laboratory is supported by P01 Grant GM068087. Work in the C.D.L. laboratory is supported by NIH Grant R01 GM061906 and NIH/NCI Cancer Center Support Grant P30 CA008748. X-ray crystallographic work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (Grant P41 GM103403). The Pilatus 6M detector on the 24-ID-C beamline is funded by NIH-ORIP HEI Grant S10 RR029205. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. C.D.L. is an investigator of the Howard Hughes Medical Institute. This is manuscript 28002 from The Scripps Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Research Collaboratory for Structural Bioinformatics Protein Data Bank, www.pdb.org [PDB ID codes 5FG0 (native Ltn1) and 5FG1 (selenomethionine-substituted Ltn1)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605951113/-/DCSupplemental.
References
- 1.Chu J, et al. A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc Natl Acad Sci USA. 2009;106(7):2097–2103. doi: 10.1073/pnas.0812819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtson MH, Joazeiro CA. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature. 2010;467(7314):470–473. doi: 10.1038/nature09371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F, Canadeo LA, Huibregtse JM. Ubiquitination of newly synthesized proteins at the ribosome. Biochimie. 2015;114(Jul):127–133. doi: 10.1016/j.biochi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff S, Weissman JS, Dillin A. Differential scales of protein quality control. Cell. 2014;157(1):52–64. doi: 10.1016/j.cell.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Lykke-Andersen J, Bennett EJ. Protecting the proteome: Eukaryotic cotranslational quality control pathways. J Cell Biol. 2014;204(4):467–476. doi: 10.1083/jcb.201311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandman O, Hegde RS. Ribosome-associated protein quality control. Nat Struct Mol Biol. 2016;23(1):7–15. doi: 10.1038/nsmb.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyumkis D, et al. Single-particle EM reveals extensive conformational variability of the Ltn1 E3 ligase. Proc Natl Acad Sci USA. 2013;110(5):1702–1707. doi: 10.1073/pnas.1210041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Deutsch C. Electrostatics in the ribosomal tunnel modulate chain elongation rates. J Mol Biol. 2008;384(1):73–86. doi: 10.1016/j.jmb.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charneski CA, Hurst LD. Positively charged residues are the major determinants of ribosomal velocity. PLoS Biol. 2013;11(3):e1001508. doi: 10.1371/journal.pbio.1001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edenberg ER, Downey M, Toczyski D. Polymerase stalling during replication, transcription and translation. Curr Biol. 2014;24(10):R445–R452. doi: 10.1016/j.cub.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 11.Brandman O, et al. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151(5):1042–1054. doi: 10.1016/j.cell.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defenouillère Q, et al. Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. Proc Natl Acad Sci USA. 2013;110(13):5046–5051. doi: 10.1073/pnas.1221724110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao S, von der Malsburg K, Hegde RS. Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol Cell. 2013;50(5):637–648. doi: 10.1016/j.molcel.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letzring DP, Wolf AS, Brule CE, Grayhack EJ. Translation of CGA codon repeats in yeast involves quality control components and ribosomal protein L1. RNA. 2013;19(9):1208–1217. doi: 10.1261/rna.039446.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker T, et al. Structure of the no-go mRNA decay complex Dom34-Hbs1 bound to a stalled 80S ribosome. Nat Struct Mol Biol. 2011;18(6):715–720. doi: 10.1038/nsmb.2057. [DOI] [PubMed] [Google Scholar]
- 16.Shoemaker CJ, Eyler DE, Green R. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science. 2010;330(6002):369–372. doi: 10.1126/science.1192430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisareva VP, Skabkin MA, Hellen CU, Pestova TV, Pisarev AV. Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J. 2011;30(9):1804–1817. doi: 10.1038/emboj.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma R, Oania RS, Kolawa NJ, Deshaies RJ. Cdc48/p97 promotes degradation of aberrant nascent polypeptides bound to the ribosome. eLife. 2013;2:e00308. doi: 10.7554/eLife.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyumkis D, et al. Structural basis for translational surveillance by the large ribosomal subunit-associated protein quality control complex. Proc Natl Acad Sci USA. 2014;111(45):15981–15986. doi: 10.1073/pnas.1413882111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao S, Brown A, Santhanam B, Hegde RS. Structure and assembly pathway of the ribosome quality control complex. Mol Cell. 2015;57(3):433–444. doi: 10.1016/j.molcel.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen PS, et al. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science. 2015;347(6217):75–78. doi: 10.1126/science.1259724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonashiro R, et al. The Rqc2/Tae2 subunit of the ribosome-associated quality control (RQC) complex marks ribosome-stalled nascent polypeptide chains for aggregation. eLife. 2016;5:e11794. doi: 10.7554/eLife.11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe YJ, et al. Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature. 2016;531(7593):191–195. doi: 10.1038/nature16973. [DOI] [PubMed] [Google Scholar]
- 24.Shao S, Hegde RS. Reconstitution of a minimal ribosome-associated ubiquitination pathway with purified factors. Mol Cell. 2014;55(6):880–890. doi: 10.1016/j.molcel.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: Structures, functions, and evolution. J Struct Biol. 2001;134(2-3):117–131. doi: 10.1006/jsbi.2001.4392. [DOI] [PubMed] [Google Scholar]
- 27.Dieci G, Bottarelli L, Ottonello S. A general procedure for the production of antibody reagents against eukaryotic ribosomal proteins. Protein Pept Lett. 2005;12(6):555–560. doi: 10.2174/0929866054395860. [DOI] [PubMed] [Google Scholar]
- 28.Wu C, Amrani N, Jacobson A, Sachs MS. The use of fungal in vitro systems for studying translational regulation. Methods Enzymol. 2007;429:203–225. doi: 10.1016/S0076-6879(07)29010-X. [DOI] [PubMed] [Google Scholar]
- 29.Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell. 2000;5(5):865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson WA, Horton JR, LeMaster DM. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): A vehicle for direct determination of three-dimensional structure. EMBO J. 1990;9(5):1665–1672. doi: 10.1002/j.1460-2075.1990.tb08287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276(Pt A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64(Pt 1):112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12, Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]