Significance
Mycobacterium abscessus is currently the most frequently isolated rapid-growing mycobacterium in human pathology and is responsible for devastating pulmonary infections in cystic fibrosis patients. It commutes from a nonvirulent smooth to a virulent rough morphotype. The latter produces characteristic serpentine cords that often associate with severe infections, but the molecular basis and contribution of cording in the physiopathology of the infection remain obscure. Herein, we characterized a dehydratase and found it to be required for cording. We demonstrate that the absence of this dehydratase correlates with an extremely attenuated phenotype in immunocompetent and immunocompromised zebrafish. Therefore, targeting the dehydratase may open the way to antivirulence strategies to control M. abscessus, notorious for being one of the most drug-resistant mycobacterial species.
Keywords: M. abscessus, zebrafish, cording, dehydratase, virulence
Abstract
Mycobacterium abscessus (Mabs) is a rapidly growing Mycobacterium and an emerging pathogen in humans. Transitioning from a smooth (S) high-glycopeptidolipid (GPL) producer to a rough (R) low-GPL producer is associated with increased virulence in zebrafish, which involves the formation of massive serpentine cords, abscesses, and rapid larval death. Generating a cord-deficient Mabs mutant would allow us to address the contribution of cording in the physiopathological signs of the R variant. Herein, a deletion mutant of MAB_4780, encoding a dehydratase, distinct from the β-hydroxyacyl-ACP dehydratase HadABC complex, was constructed in the R morphotype. This mutant exhibited an alteration of the mycolic acid composition and a pronounced defect in cording. This correlated with an extremely attenuated phenotype not only in wild-type but also in immunocompromised zebrafish embryos lacking either macrophages or neutrophils. The abolition of granuloma formation in embryos infected with the dehydratase mutant was associated with a failure to replicate in macrophages, presumably due to limited inhibition of the phagolysosomal fusion. Overall, these results indicate that MAB_4780 is required for Mabs to successfully establish acute and lethal infections. Therefore, targeting MAB_4780 may represent an attractive antivirulence strategy to control Mabs infections, refractory to most standard chemotherapeutic interventions. The combination of a dehydratase assay with a high-resolution crystal structure of MAB_4780 opens the way to identify such specific inhibitors.
Microscopic cords, a phenotypic characteristic of Mycobacterium tuberculosis, were originally observed by Robert Koch in 1882, but their potential significance in virulence was reported in 1947 by Middlebrook et al. (1). They are made of tight bundles formed by parallel alignments of bacilli and are historically considered as a distinctive trait of M. tuberculosis. Their presence in smears from liquid cultures is a reliable criterion for the rapid presumptive identification of M. tuberculosis isolates in many laboratories (2, 3). Cord formation correlates with extracellular replication of mycobacteria and can be observed in sputum smears from tuberculosis patients with severe lung cavitation. However, while focusing on the complex network of interactions between intracellular mycobacteria and host cells, most investigators have neglected or underestimated the role of extracellular mycobacteria in pathogenesis (4). Recent studies demonstrated the occurrence of cording in Mycobacterium marinum (5) and in several nontuberculous mycobacteria (NTM) distantly related to M. tuberculosis, including Mycobacterium abscessus (Mabs) (6).
Mabs is one of the most pathogenic and drug-resistant rapid-growing mycobacterial species (7, 8) and accounts for 10% of the NTM-induced lung infections in several countries (9). It infects lungs of patients with underlying lung disorders such as cystic fibrosis (10) but also causes skin and soft tissue infections in immunocompetent individuals (11). Mabs can exhibit a rough (R) or a smooth (S) morphotype, depending on the absence or presence of surface-associated glycopeptidolipids (GPLs) (12, 13). R strains possess mutations in the gpl locus that lead to a defect in GPL production/transport and are also associated with the ability to produce cords in vitro, as recently demonstrated in clinical isolates of Mabs (14) as well as in Mycobacterium spp. bolletii (15), a subspecies of the Mabs complex. We recently used the zebrafish embryo as a tractable infection model to study, at a spatiotemporal level, the physiopathology of Mabs infection and demonstrated the high propensity of virulent R variant Mabs to produce cords in vivo (16, 17). We showed that extracellular cording is an example of morphological plasticity allowing bacteria to escape the host immune system. Therefore, interfering with cording could preclude the establishment of a lethal infection and could be viewed as a therapeutic or prophylactic strategy for Mabs (8). If identified, the determinants controlling cording may represent drug targets for innovative therapeutic approaches and would undoubtedly add new insights into the pathogenicity of Mabs-driven infections. However, whereas the search for genetic determinants of cording has mainly focused on M. tuberculosis (18), those involved in cording in NTM remain unknown.
Mutations in hadC, encoding a component of the HadBC dehydratase complex of the mycolic acid biosynthesis machinery, have been associated with high resistance levels to the antitubercular drug thiacetazone (TAC) in M. tuberculosis (19–21). Moreover, deletion of hadC in M. tuberculosis correlates with a loss of cording and attenuation of virulence in infected mice (22), thus emphasizing the intimate interplay between TAC resistance, mycolic acid biosynthesis, cording, and virulence. We have previously shown that Mycobacterium smegmatis possesses a gene, MSMEG_6754, responsible for its remarkable resistance to TAC and involved in mycolic acid metabolism and that disruption of MSMEG_6754 in M. smegmatis was associated with impaired growth in Acanthamoeba castellanii (23). Interestingly, MSMEG_6754 encodes a putative dehydratase and is structurally related to the HadABC complex (23). Blast analyses revealed the presence of genes homologous to MSMEG_6754 in several NTMs, all of which possess intact hadABC genes (23). The presence of such a homolog in Mabs, designated MAB_4780, prompted us to perform the biochemical/structural characterization of MAB_4780 to investigate its biological function with respect to mycolic acid biosynthesis. We found important roles for this protein in cording and Mabs pathogenicity in the zebrafish model.
Results
Enzymatic and Structural Characterization of a Dehydratase from Mabs.
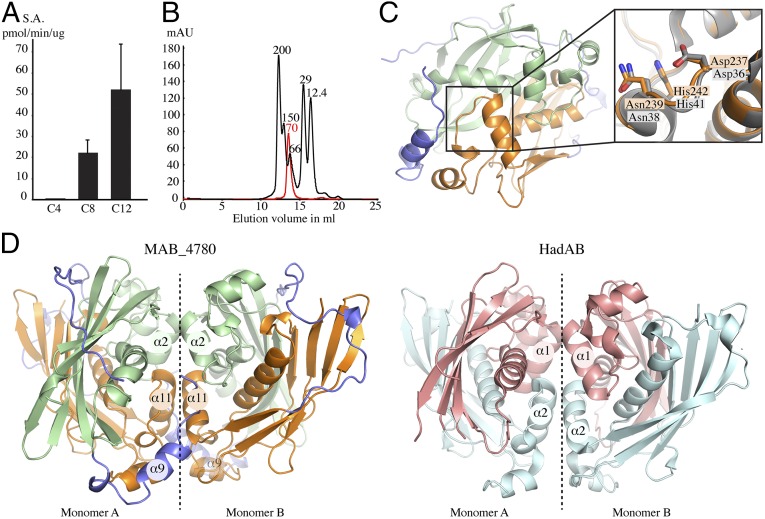
MAB_4780 shares high identity levels with the M. tuberculosis HadA, HadB, and HadC dehydratase subunits. To get functional insights into the enzymatic activity of MAB_4780, the recombinant protein was used in a functional assay. Because the hydratase/dehydratase family members are usually more efficient at catalyzing the hydration reaction when isolated from their complex (24), a hydratase-based assay was set up with trans-2-enoyl-CoA substrates exhibiting various chain lengths. Whereas no activity was detected with crotonoyl-CoA (C4), MAB_4780 exhibited a measurable activity in the presence of octanoyl-CoA (C8), which was further increased with dodecenoyl-CoA (C12) (Fig. 1A). The specific activities with C8 and C12 were similar to those reported previously with the M. tuberculosis HadBC dehydratase of the type II fatty acid synthase (25), suggesting that MAB_4780, like HadBC, is a dehydratase with a substrate preference for long fatty acyl chains.
Fig. 1.
Enzymatic and structural characterization of MAB_4780. (A) The specific activity of MAB_4780 was determined in the presence of trans-2-enoyl-CoAs with various fatty acyl chain lengths and expressed in picomole of product formed per minute per microgram of enzyme. C4, C8, and C12 indicate crotonoyl-CoA, trans-2-octenoyl-CoA, and trans-2-dodecenoyl-CoA, respectively. Error bars represent SDs. (B) Oligomeric state of MAB_4780 determined by size exclusion chromatography. The elution profile of the proteins used for the calibration is displayed as a black line, and the elution profile of MAB_4780 is shown in red. Calibration was established using β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome C (12.4 kDa), eluting with estimated volumes of 16.1, 15.2, 13.4, 12.6, and 11.9 mL, respectively. The elution volume of MAB_4780 was estimated to 12.7 mL, corresponding to a calculated molecular weight of 70 kDa. (C) Schematic representation of the overall monomeric crystal structure of MAB_4780. The N-terminal domain is represented in pale green, the C-terminal domain in orange, and the linker domain surrounding the protein and acting as a belt in blue. The enlarged panel on the side highlights the MAB_4780 catalytic residues (in orange) superposed to the ones of M. smegmatis HadAB (in gray; PDB ID code 4rv2). (D) Structure of the MAB_4780 homodimer and comparison with the HadAB heterodimer structure. Shown is a representation of one biological unit (homodimer, monomers A and B) of MAB_4780 (with the same color code as in C) and one biological unit (heterodimer, monomer A and B) of HadAB (HadB in pale cyan and HadA in salmon). Dashed lines indicate the dimeric interface. The designations of the helices involved in the interface of dimerization are indicated in the circles.
Because most dehydratases have been found to form homo- or heterodimers (26, 27), we next evaluated the oligomeric state of MAB_4780. The size exclusion chromatography elution profile attests to the presence of a protein of around 70 kDa (Fig. 1B). According to the primary sequence, the predicted molecular weight of one monomer is 36.5 kDa, implying that MAB_4780 is very likely to form a dimer in solution. Additionally, we crystallized and solved the structure of MAB_4780 at very high resolution (1.65 Å) (Table S1), in which most residues could be rebuilt, with the exception of the first nine residues of the N terminus and residues 172–180 of the flexible linker. This structure is very similar to the one of the homologous protein in M. smegmatis (MSMEG_6754) (23) and to HadAB from M. smegmatis (28) (Fig. 1C) and M. tuberculosis (29). The superposition of the MAB_4780 and HadAB crystal structures indicates that the N-terminal and C-terminal domains of MAB_4780 correspond to the HadA and HadB proteins, respectively. A notable structural difference relies on the existence of a long flexible linker in MAB_4780, absent in HadAB from M. smegmatis (Fig. 1C). Analysis of the MAB_4780 crystal packing using the proteins, interfaces, structures and assemblies (PISA) server (30) predicts the existence of a stable homodimer. The dimer interface is formed by interactions between helices α2 (R28-A37), α9 (T158-A165) and α11 (R225-A235) of the first monomer and the equivalent helices of the second monomer (Fig. 1D). This dimer interface is also conserved in HadAB, where the helix α1 (G21-A31) of HadA and helix α2 (T23-G35) of HadB interact with their corresponding helices of the second monomer. Overall, this indicates that MAB_4780 is a homodimer sharing similar biochemical and structural features with the HadAB/HadBC dehydratases.
Table S1.
Data collection and refinement statistics
| PDB ID code | 5I7N |
| Beamline | ESRF-BM30 |
| Wavelength, Å | 0.979 |
| Space group | P 41 21 2 |
| Cell dimensions | 83.32, 83.32, 124.39, 90, 90, 90 |
| Resolution, Å | 42.77–1.65 (1.709–1.65) |
| Rmeas, % | 9.46 (101.7) |
| <I/σ(I)> | 15.91 (2.18) |
| Completeness, % | 98.64 (97.56) |
| Redundancy | 6.4 (6.6) |
| Rwork/Rfree, % | 15.99 (22.65)/18.51 (26.02) |
| No. atoms | |
| Protein | 2,558 |
| Water | 529 |
| Average B values, Å2 | |
| Protein overall | 24.1 |
| Water | 35.5 |
| rmsd | |
| Bond lengths, Å | 0.014 |
| Bond angles, ° | 1.42 |
| Ramachandran favored, % | 98 |
| Ramachandran allowed, % | 2 |
The values in parentheses are for the last resolution shell.
Disruption of MAB_4780 Alters Resistance to TAC and Affects the Mycolic Acid Composition.
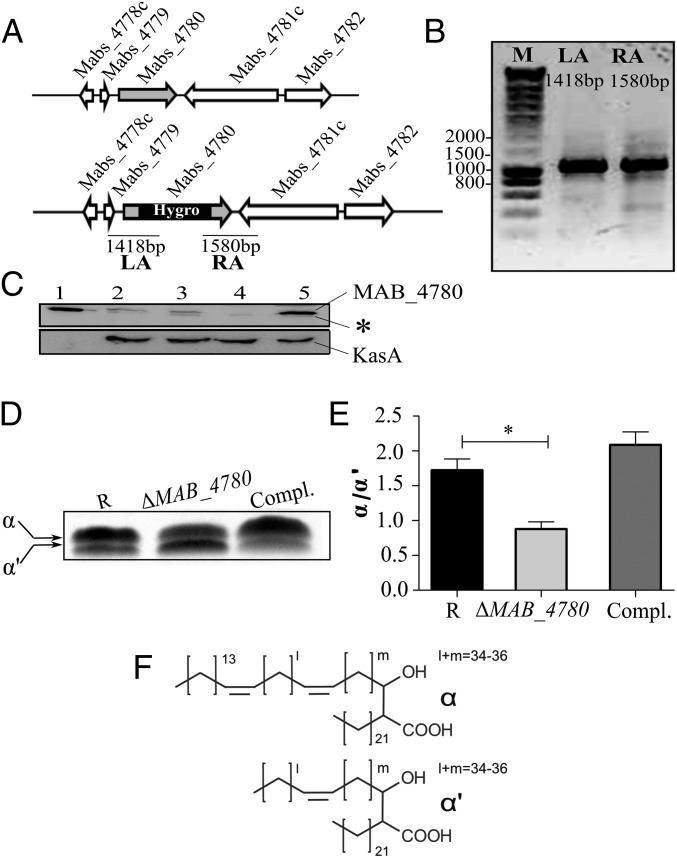
To decipher the in vivo functions of MAB_4780, the corresponding gene was deleted in the R strain Mabs 104536T. The recombineering method (31) was used to create a mutant with a hygromycin resistance cassette disrupting MAB_4780 (Fig. 2A). A hygromycin-resistant colony was PCR analyzed for correct allelic exchange at the MAB_4780 locus (Fig. 2B). Western blotting using anti-MAB_4780 antibodies highlights the absence of a specific band in the ΔMAB_4780 strain and the correct complementation using pMV306_4780 (Fig. 2C). As reported earlier for the M. smegmatis MSMEG_6754 mutant (23), the MAB_4780 mutant displays increased susceptibility to TAC both on agar plates and broth medium (Table 1). Introduction of pMV306_4780 partially restored TAC resistance in ΔMAB_4780 and in ΔMSMEG_6754 (Table 1), supporting the overlapping functions of MAB_4780 and MSMEG_6754 in TAC resistance.
Fig. 2.
Interruption of MAB_4780 in Mabs R leads to changes in the mycolic acid profile. (A) Genomic organization of the MAB_4780 locus in Mabs CIP104536T (Upper) and schematic representation of the MAB_4780 locus after disruption with a hygromycin cassette (Lower). The primers used for PCR amplifications are indicated by small arrows, and the expected sizes of the left (LA) and right (RA) arms are indicated. (B) Agarose gel electrophoresis of the PCR amplified LA and RA products using the genomic DNA from the ΔMAB_4780 mutant. Identity of the amplicons was verified by DNA sequencing. (C) Proper inactivation of MAB_4780 was further confirmed by Western blotting using mouse anti-MAB_4780 antibodies. The same lysates were also probed with rat anti-KasA antibodies used as loading controls. Lane 1, purified His-tagged MAB_4780 (10 ng); lane 2, crude lysate of Mabs S; lane 3, crude lysate of Mabs R; lane 4, crude lysate of Mabs R ΔMAB_4780; lane 5, ΔMAB_4780 carrying pMV306_4780 (complemented strain) (35 µg in each lane). An asterisk indicates a cross-reacting band. (D) TLC analysis of mycolic acid methyl esters (MAMEs) isolated from logarithmically growing cultures following metabolic labeling with 14C-acetate. After extraction, equal counts (100, 000 cpm) were loaded in each lane, and radiolabeled lipids were separated using petroleum ether/acetone (95:5, vol/vol) before exposure to a film. (E) Histograms representing the α/α′-mycolic acid ratio determined from the TLC densitometric analysis. Results are expressed as the means and their SE obtained from three independent experiments. The mean values of the wild-type and mutant strains were compared using a one-tailed Mann–Whitney test. *P < 0.05. (F) Structure of α and α′-mycolic acids based on MS, NMR, and GC–MS analyses.
Table 1.
MICs of TAC in Middlebrook 7H10/OADC agar and cation-adjusted Mueller–Hinton (CaMH) broth
| Strain | MIC, µg·mL–1 | |
| 7H10 | CaMH | |
| Mabs 104536T (R) | 40 | 150 |
| ΔMAB_4780 | 10–20 | 37 |
| ΔMAB_4780 (pMV306_4780) | 40 | 83 |
| M. smegmatis mc2155 | >50 | 75 |
| ΔMSMEG_6754 | <10 | 0.4 |
| ΔMSMEG_6754 (pMV306_4780) | >50 | 37 |
MICs were determined according to the Clinical and Laboratory Standards Institute guidelines (51) used for antimycobacterial susceptibility testing of rapidly growing mycobacteria. The broth microdilution method was used in media with an inoculum of 5 × 105 cfu/mL in the exponential growth phase. We added 100 µL of drug dilutions to 100 µL of bacterial suspension and incubated the mixture at 37 °C for 3 d. MICs were recorded by visual inspection and by absorbance at 560 nm to confirm visual recording.
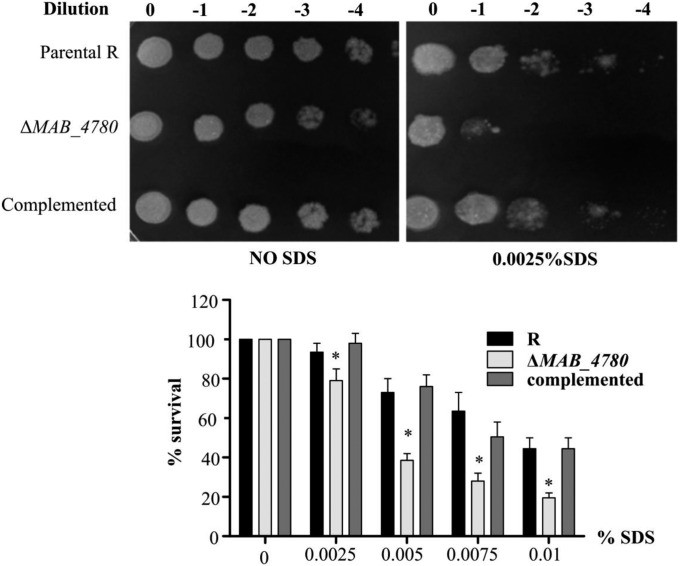
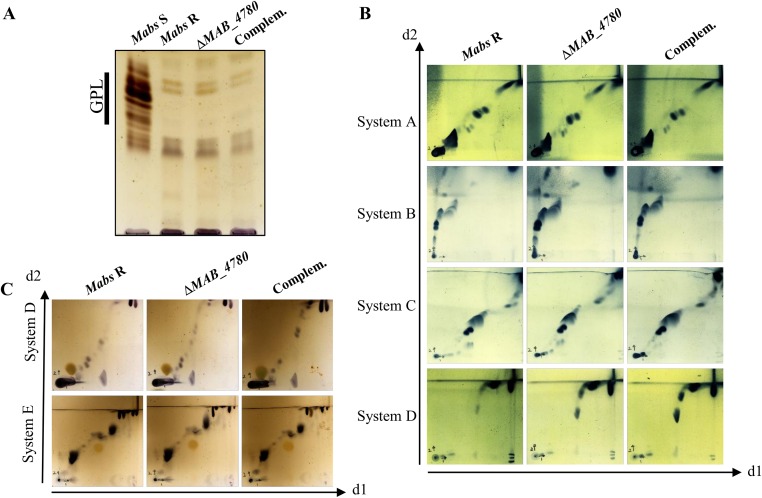
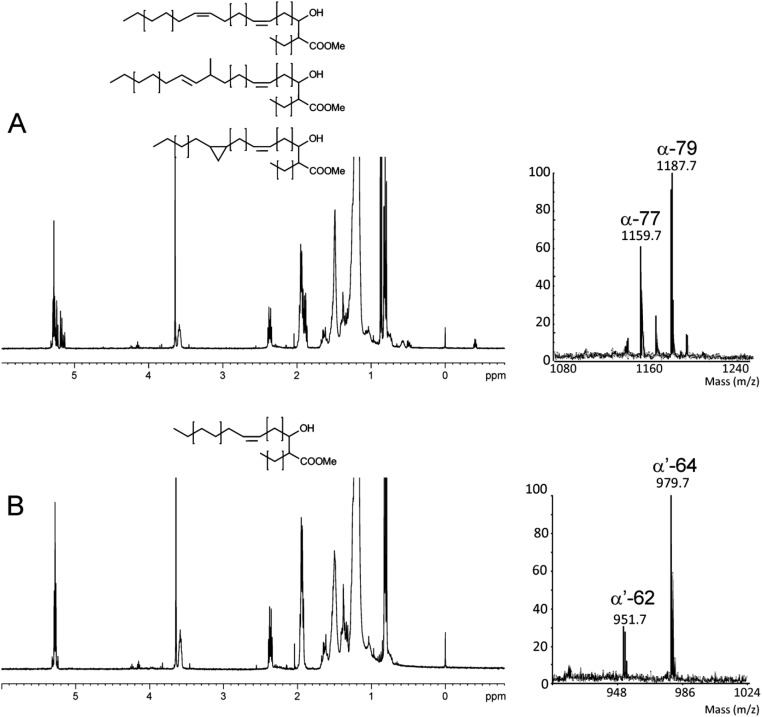
Susceptibility of ΔMAB_4780 to detergent was tested on agar plates and in liquid cultures with various SDS concentrations (Fig. S1). Complementation with pMV306_4780 reversed the susceptibility to the parental levels. This suggests that MAB_4780 plays an important role on the envelope integrity. To confirm the possible involvement of the dehydratase in cell wall-associated lipid biosynthesis, a TLC-based systematic analysis of apolar and polar lipids was performed. Neither the (glyco)lipid nor the GPL patterns of the ΔMAB_4780 mutant showed major alterations compared with that of the parental and complemented strains (Fig. S2). GC–MS analyses also failed to show important differences in the total fatty acid profiles (Fig. S3). We next determined and compared the mycolic acid composition in both the parental and ΔMAB_4780 strains. Mass spectrometry and NMR analyses revealed the presence of α- and α′-mycolic acids sharing identical structures in both strains (Figs. S4 and S5). However, labeling the cultures with 14C-acetate, followed by TLC/autoradiography analysis, unraveled a substantial change in the typical α-/α′-mycolic acid ratio (Fig. 2D). Whereas α-mycolic acids appeared as the major mycolic acid subclass in the parental R strain, α′ -mycolic acids represented a more prominent mycolic acid subclass in ΔMAB_4780 and this change was fully restored upon complementation (Fig. 2 D and E). Because α- and α′-mycolic acids correspond to long-chain (C77–79) and short-chain (C62–64) mycolic acids, respectively (Fig. 2F), an alteration of the α-/α′-mycolic acid ratio in ΔMAB_4780 may directly impact on the overall cell wall morphology, permeability, and virulence properties of this strain.
Fig. S1.
Interruption of MAB_4780 in Mabs R leads to increased susceptibility to SDS. Bacterial cells were serially diluted and dropped on LB plates in the absence or presence of SDS and incubated at 37 °C for 4 d (Upper). The percentage of bacterial survival was assessed after addition of increasing amounts of SDS to liquid cultures and incubation at 37 °C for 24 h by plating serial bacterial dilutions on LB plates and cfu enumerations (Lower). The ΔMAB_4780 mutant is significantly more susceptible to SDS than the R variant (Mann–Whitney test; *P < 0.05). Error bars represent the SD. Results are representative of two independent experiments.
Fig. S2.
Analysis of the lipid profiles of Mabs R, ΔMAB_4780, and complemented strains. Bacteria (late exponential growth phase) were cultured without agitation in Sauton’s medium lacking tyloxapol before extraction of lipids. (A) The GPL profile. (B) The apolar lipid profiles. System A: d1, petroleum ether/ethyl acetate (98:2) ×3; d2, petroleum ether/acetone (98:2). System B: d1, petroleum ether/acetone (92:8) ×3; d2, toluene/acetone (95:5). System C: d1, chloroform/methanol (96:4); d2, toluene/acetone (80:20). System D: d1, chloroform/methanol/water (100:14:0.8); d2, chloroform/acetone/methanol/water (50:60:2.5:3). (C) The polar lipid profiles. System D: d1, chloroform/methanol/water (100:14:0.8); d2, chloroform/acetone/methanol/water (50:60:2.5:3). System E: d1, chloroform/methanol/water (60:30:6); d2, chloroform/acetic acid/methanol/water (40:25:3:6).
Fig. S3.
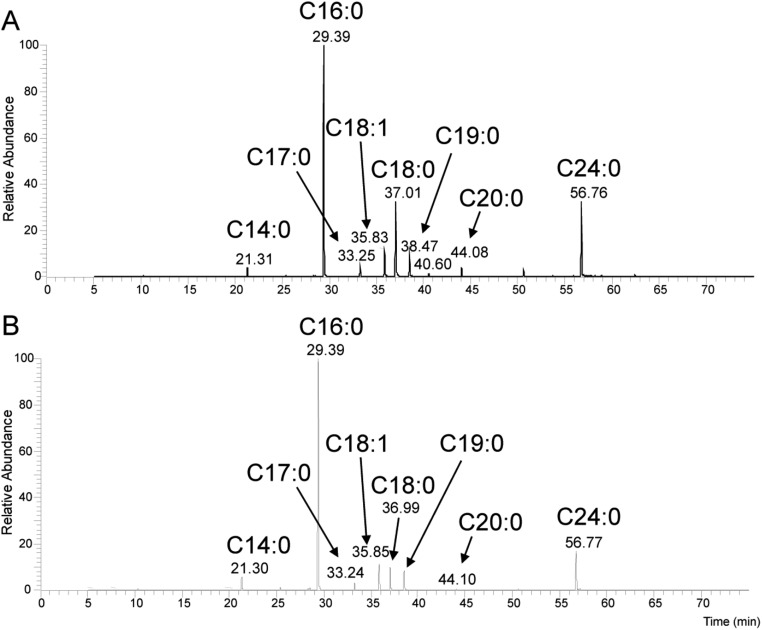
Fatty acid profiles of Mabs R and ΔMAB_4780 strains. Total fatty acids from washed and dried mycobacterial pellets were saponified and carboxymethylated in 0.5 M methanolic HCl at 80 °C overnight. Fatty acid methyl esters (FAMEs) were extracted with chloroform, dried, and analyzed by GC–MS. Representative total ion chromatograms of total FAMEs from (A) Mabs R and (B) ΔMAB_4780 strains, showing a family of compounds ranging from C14:0 to C24:0, with a major signal identified as C16:0. The structure of all compounds was assigned based on retention time of standard molecules and individual electronic impact fragmentation patterns. Comparison of the FAME patterns did not show important variations, except for a reduction of the C18:0 ratio in ΔMAB_4780.
Fig. S4.
Analysis of the mycolic acids in Mabs. Mycolic acids were extracted and purified from a preparative TLC plate (petroleum ether/acetone; 95:5; vol/vol) before analysis. Analysis of (A) α-MAMEs and (B) α′-MAMEs by 1D 1H NMR (Left) and MALDI-TOF–MS as [M+Na]+ adducts (Right). Altogether, these analyses establish that α-mycolic acids were made of a mixture of C76 to C80 (with major C77 and C79) mycolates containing cis-double bonds, trans-double bonds, and cis-cyclopropane functional groups. The nature of the functional groups was confirmed by detailed structural analyses, as shown in Fig. S5. The α′-mycolic acid fraction contained C62 and C64 mycolic acids, exclusively substituted by cis-double bonds. Comparison of α- and α′-MAMEs strongly suggests that the proximal position of α-mycolates is exclusively functionalized by cis-double bonds, whereas the distal position is functionalized by a combination of cis-double bonds, trans-double bonds, and cis-cyclopropanes.
Fig. S5.
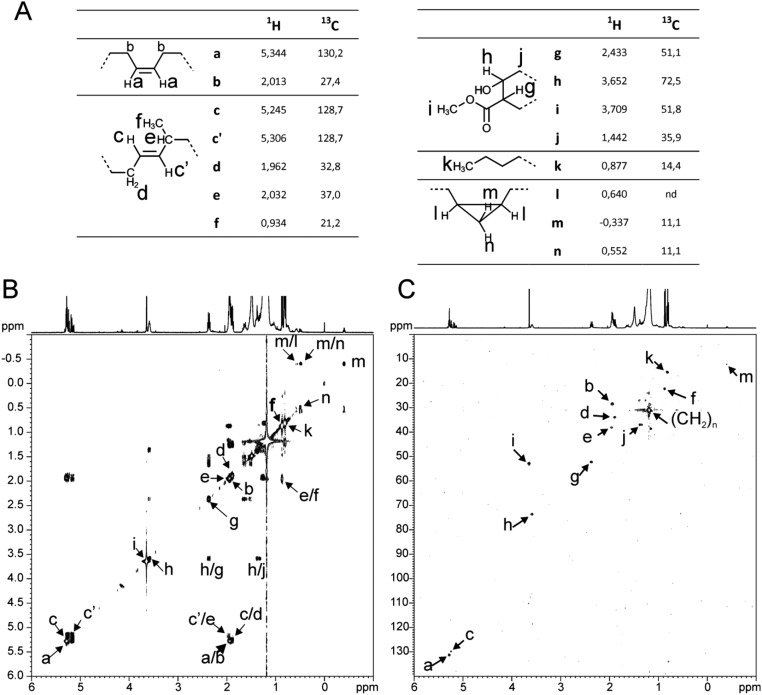
Identification of functional groups associated with α-mycolic acids of Mabs R. The functional groups of α-mycolic acids were identified owing to the 1H and 13C chemical shifts (A) observed from 1D NMR experiments (Fig. S4) as well as from (B) 3JH,H connectivities on 1H-1H COSY NMR and (C) 1JH,C connectivities on 1H-13C HSQC NMR experiments acquired in per-deuterated chloroform.
MAB_4780 Is Critical for Cording in R Mabs.
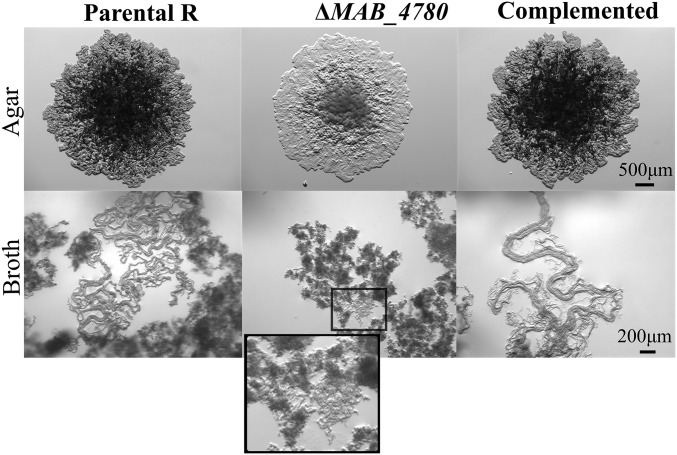
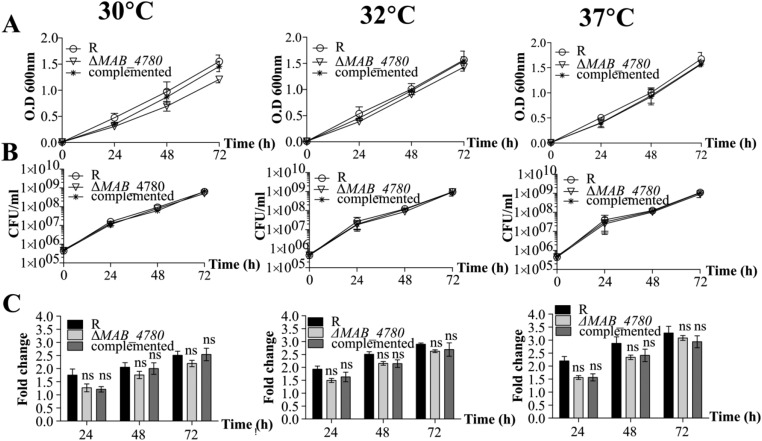
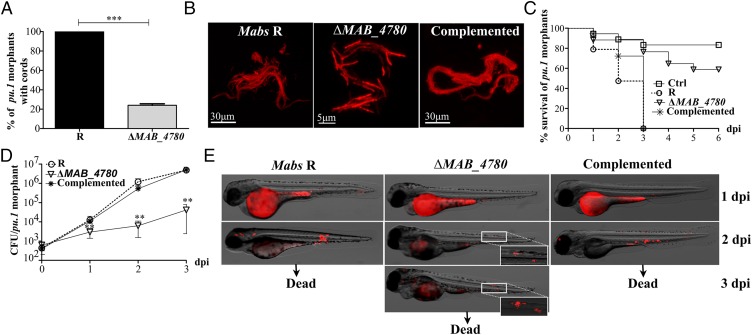
Mabs R grows optimally at temperatures ranging from 30 to 37 °C and is characterized by a rough and dry texture on agar plates with prominent cords made of numerous irregular and long serpentine filaments that can be easily observed in liquid medium (Fig. 3) (16). The ΔMAB_4780 mutant failed to produce these typical cell wall-associated traits, indicating that MAB_4780 plays a central role in Mabs cording at 32 °C (Fig. 3). Complementation of the mutant restored cording. All three strains displayed comparable planktonic growth rates in 7H9 broth at different temperatures based on OD600 measurements (Fig. S6A), on the determination of cfu/mL (Fig. S6B), or on the fluorescence intensity of bacilli expressing tdTomato (Fig. S6C).
Fig. 3.
Disruption of MAB_4780 correlates with loss of cording in Mabs R. Colony morphology of the parental (R strain), ΔMAB_4780 mutant, and complemented strains grown on LB medium at 37 °C (Upper) and cording aspect of all three strains grown in 7H9 broth at 32 °C and observed under the microscope (Lower). All images were taken at the same magnification, and an enlarged view of the boxed inset is shown for the mutant.
Fig. S6.
In vitro growth profile of the Mabs R, ΔMAB_4780 mutant, and complemented strains. Bacteria were grown in 7H9 broth supplemented with ADC and 0.05% Tween 80 at different temperatures. Growth was monitored by following the OD600 (A), by determining the cfu/mL at different time points (B) or by determining the fold change in fluorescence intensity of bacteria expressing TdTomato at different time points compared with 0 h (C). Results are the average of three independent experiments. Error bars represent the SEM. ns, nonsignificant difference compared with the parental R strain (Kruskal–Wallis with Dunn’s multiple test).
Acute Infection Is Highly Compromised in Zebrafish Infected with ΔMAB_4780.
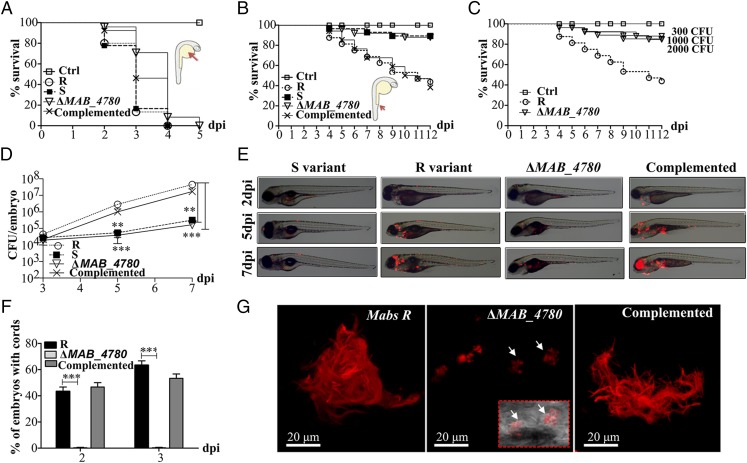
The virulence of the Mabs R strain, compared with that of the S strain, could be due to the loss of GPL or to the massive production of serpentine cords. To distinguish between these two possibilities, we tested the virulence of the cording-defective ΔMAB_4780 (R background). First, the various strains were inoculated in the yolk sac of zebrafish embryos, previously used as a site of injection of several fast-growing bacteria and resulting in intense bacteraemia and early death of embryos (32, 33). All strains, including the otherwise attenuated Mabs S variant, induced a robust infection with unrestricted proliferation, leading to rapid larval death (Fig. 4A). ΔMAB_4780 behaved similarly to its parental and complemented counterparts.
Fig. 4.
The ΔMAB_4780 mutant is extremely attenuated in infected zebrafish embryos. (A) Survival curves of embryos injected in the yolk sac (∼100–200 cfu) of tdTomato-expressing Mabs S, Mabs R, ΔMAB_4780 mutant, or the complemented strain (n = 20). There are no significant differences between the different infected strains. Data are representative of three independent experiments. The Inset represents an embryo at 30 hpf, and the arrow indicates the injection site in the yolk. Ctrl, noninfected control embryos. (B) Survival curve for zebrafish embryos (n = 20) injected i.v. at 30 hpf with 100–200 cfu of the different Mabs strains compared with control embryos. Larvae infected with the ΔMAB_4780 mutant showed a significant increase in survival compared with larvae infected with the parental strain (P = 0.0008, log-rank test), whereas the complemented strain behaved like the wild-type R variant and restored virulence (no statistically significant differences were seen in the survival of embryos infected with the R or complemented strains; log-rank test). Shown are representative data of three independent experiments. The Inset represents an embryo at 30 hpf, and the arrow indicates the caudal vein injection site. (C) Survival curve of embryos infected with increasing doses of the ΔMAB_4780 mutant. Embryos injected with the ΔMAB_4780 mutant showed no statistically significant difference with the control group in terms of survival, regardless of the dose. Data shown are representative of two independent experiments (n = 20). (D) In vivo growth of the ΔMAB_4780 mutant. Enumeration was performed by plating homogenates of whole individual larvae at different time points on selective agar plates and subsequent counting of bacterial colonies. A significant reduction in bacterial burden was observed with the ΔMAB_4780 mutant (Kruskal–Wallis with Dunn’s multiple test; **P < 0.01; ***P < 0.001). Data are representative of three independent experiments. (E) Bright field/fluorescence overlay images of tdTomato-labeled Mabs (∼150 cfu). Each larva was followed and imaged at 2, 5, and 7 dpi. (F) Cords were recorded in i.v.-infected embryos with either tdTomato-expressing Mabs R, ΔMAB_4780 mutant, or complemented strains at 2 and 3 dpi. Cords were never observed in ΔMAB_4780-infected embryos. Histograms represent means calculated from three independent experiments (n = 10 per experiment), and the statistical test used was Fisher’s exact test; ***P < 0.001. Error bars represent the SEM. (G) Maximum intensity projection of confocal images showing representative pathological events in 3 dpi embryos i.v.-infected with Mabs R, ΔMAB_4780, or complemented strains expressing tdTomato (100–200 cfu). Arrows indicate infected cells containing the tdTomato-labeled ΔMAB_4780 mutant. Only Mabs R and complemented strains formed cords.
We next investigated whether circulation-injected embryos, which, in contrast to the yolk-injected embryos, possess a myeloid cell-dependent immunity, can resist ΔMAB_4780 lethal infection. Bacterial suspensions (100–200 cfu) were injected into the caudal vein of embryos at 30 h postfertilization (hpf). As reported earlier, the R variant induced a robust and lethal infection with about 50% mortality of embryos at 9 d post infection (dpi), whereas the S bacteria failed to induce larval mortality (Fig. 4B) (16). ΔMAB_4780 was found to be highly attenuated at levels comparable to the S variant, and complementation fully restored the R virulence phenotype (Fig. 4B). Injection of large amounts of ΔMAB_4780 (up to 2,000 cfu) did not result in increased larval mortality, further emphasizing the extreme attenuated phenotype of ΔMAB_4780 (Fig. 4C). The bacterial loads in Mabs S and ΔMAB_4780-infected embryos increased only slightly after infection, whereas those in the R variant and complemented infected embryos increased exponentially from 3 dpi to 7 dpi (Fig. 4D). Consistent with the very low bacterial burden in the ΔMAB_4780-infected embryos, time-lapse imaging revealed the presence of mainly isolated bacteria and very rare infection foci (Fig. 4E). In contrast, embryos infected with the parental R and complemented strains harbored massive abscesses and cords, mainly located within the brain (Fig. 4 E and F) (16). Representative confocal microscopy images revealed highly organized serpentine cord structures of variable sizes, often larger than 100 µm, in fish infected with the R or the complemented strains (Fig. 4G). In sharp contrast, only small bacterial aggregates found within macrophages were observed with ΔMAB_4780 (Fig. 4G, Inset).
MAB_4780 Is Required for Intracellular Growth and Granuloma Formation.
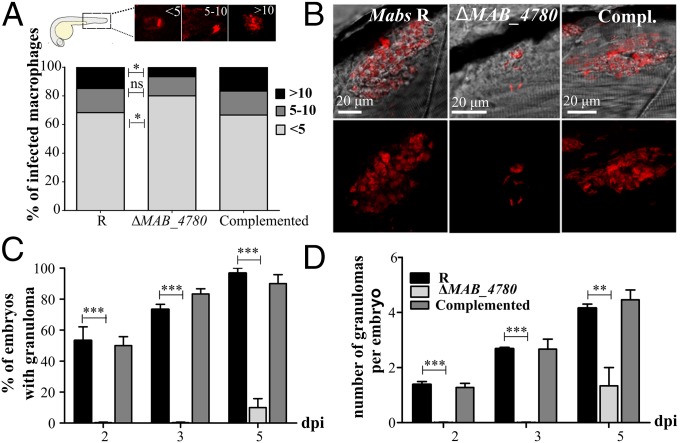
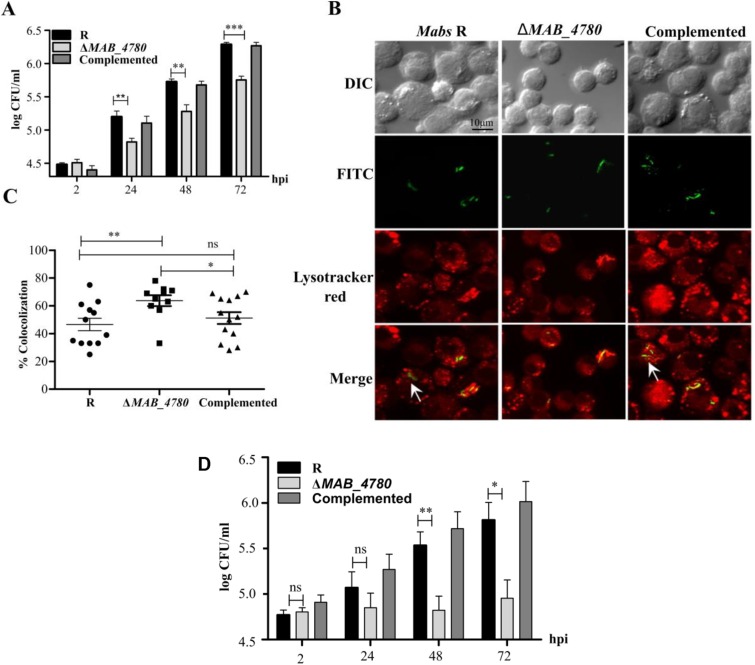
We next determined the proportion of mildly infected (<5 bacteria), moderately infected (5–10 bacteria), and highly infected (>10 bacteria) macrophages in zebrafish. Significantly more macrophages were heavily infected in embryos with the R or the complemented strains, whereas embryos with ΔMAB_4780 contained more macrophages of the lower infected class (Fig. 5A). This intramacrophage growth defect of ΔMAB_4780 was subsequently confirmed in infected J774 macrophages (Fig. S7A). Moreover, the reduced intracellular growth of ΔMAB_4780 was also found in infected A. castellanii (Fig. S7D), posited to be a natural environmental host for several NTMs (34). Because inhibition of phagolysosome fusion represents a key strategy used by pathogenic mycobacteria to survive and replicate intracellularly (35), we tested whether the reduced ability of the mutant to grow intracellularly was linked to lysosome-mediated degradation. FITC-labeled bacteria were used to study the localization of the pathogen in acidic compartments in infected J774 macrophages labeled with LysoTracker Red Dye (Fig. S7B). Although 47% of the parental bacteria colocalized to acidic vesicles, 64% of ΔMAB_4780 colocalized with LysoTracker red-positive compartments (Fig. S7C) and the complemented strain showed similar colocalization rates to the parental strain.
Fig. 5.
The intracellular growth defect of ΔMAB_4780 is associated with impaired granuloma formation in zebrafish. (A) Average proportion of infected macrophages classified as mildly, moderately, or highly infected (containing <5, 5–10, and >10 bacteria, respectively) at 24 hpi. Significance was assessed by a Kruskal–Wallis test with Dunn’s multiple posttest (*P < 0.05). Top enclosed panel shows representative infected macrophage of each class. (B) Maximum intensity projection of confocal images showing representative granuloma-like structures in 3 dpi larvae i.v.-infected with Mabs R, ΔMAB_4780, or the complemented strain expressing tdTomato. (C) Kinetics of granuloma formation in intravenously-infected embryos (∼150 cfu; n = 30). Histograms represent means calculated from three independent experiments. Overall, ΔMAB_4780 mutant-infected embryos developed significantly less granuloma compared with the R and complemented strains. (n = 30; Fisher’s exact test; ***P < 0.001). Error bars represent the SEM. (D) Number of granulomas per embryo harboring granuloma. A significant reduction in the number of granulomas per embryo is found in embryos infected with ΔMAB_4780 compared with R-infected embryos. The statistical test used was the Kruskal–Wallis test with Dunn’s multiple posttest (n = 30); **P < 0.01; ***P < 0.001. Error bars represent the SEM.
Fig. S7.
Intramacrophage and intra-amoebal growth of ΔMAB_4780. (A) J774 cells were infected with Mabs R, ΔMAB_4780, or the complemented strain at an MOI of 1:1. Histograms and error bars represent mean ± SEM (SE mean), respectively, calculated from three independent experiments (Kruskal–Wallis test with Dunn’s multiple comparisons posttest; **P < 0.01; ***P < 0.001). (B) Colocalization of the different Mabs strains with the acidotropic dye LysoTracker Red DND-99 in infected J774 macrophages. Arrows indicate mycobacteria inside host cells but not colocalizing with Lysotracker red. ΔMAB_4780 bacilli were mainly associated with the acidic Lysotracker red-positive vacuoles. (C) Percentage of bacilli colocalized with lysosomes in J774 macrophages for the parental, the mutant, and the complemented strains. Mean percentage colocalization (depicted by a horizontal bar) and corresponding SD (depicted by error bars) were calculated from 10 photographs. Data shown are representative of two independent experiments (Mann–Whitney test; *P < 0.05; **P < 0.01; ns, P = 0.2480). (D) A. castellanii was grown and maintained at 30 °C in 150-cm3 culture flasks containing 30 mL of PYG (peptone yeast glucose–712 medium; ATCC). Amoebae suspensions were washed three times with Page's modified Neff's Ameoba Saline (PAS) buffer and dispensed into 12-well plates at a final concentration of 5 × 105 cells per mL. After 1 h at 32 °C, the amoebae monolayer was infected at an MOI of 20. After an additional 3 h of incubation at 32 °C, the medium was aspirated and the monolayer was washed three times in PAS buffer and treated with 250 μg/mL amikacin for 2 h to kill the remaining extracellular mycobacteria. Infected amoebae were washed, and fresh PAS buffer was added. Each day, 50 μL of a suspension of 107 heat-inactivated E. coli were added to each well to reduce the formation of cysts. The number of intra-amoebal bacteria was determined after lysis of the A. castellanii monolayer with 0.5% SDS for 30 min at 32 °C, plating, and enumerating the number of cfu. Histograms and error bars represent means and SD, respectively. Significant survival difference of bacteria was found between ΔMAB_4780 and the R variant (Mann–Whitney test; *P < 0.05; **P < 0.01; ns, P = 0.3619 at 2 hpi and P = 0.1449 at 24 hpi).
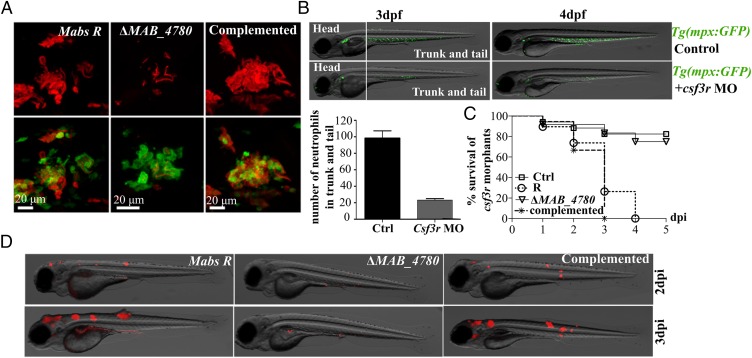
We next evaluated the impact of defective intracellular replication of ΔMAB_4780 on its ability to stimulate granuloma formation. Large granuloma-like aggregates containing Mabs R could be imaged (Fig. 5B), as reported earlier (16). In contrast, ΔMAB_4780 failed to induce typical structured granulomas (Fig. 5B). At 5 dpi, granulomas were present in more than 80% of embryos infected with the R and complemented strains (Fig. 5C). In ΔMAB_4780-infected embryos, no granulomas were present at 3 dpi but eventually started to appear at 5 dpi in very few numbers and in a very limited proportion of embryos (Fig. 5 C and D). These results suggest that MAB_4780 plays an important role to avoid lysosome-mediated degradation in macrophages, which compromises survival of ΔMAB_4780 in macrophages and, as a consequence, granuloma formation.
Cording Is the Hallmark of M. abscesssus-Induced Lethal Infection.
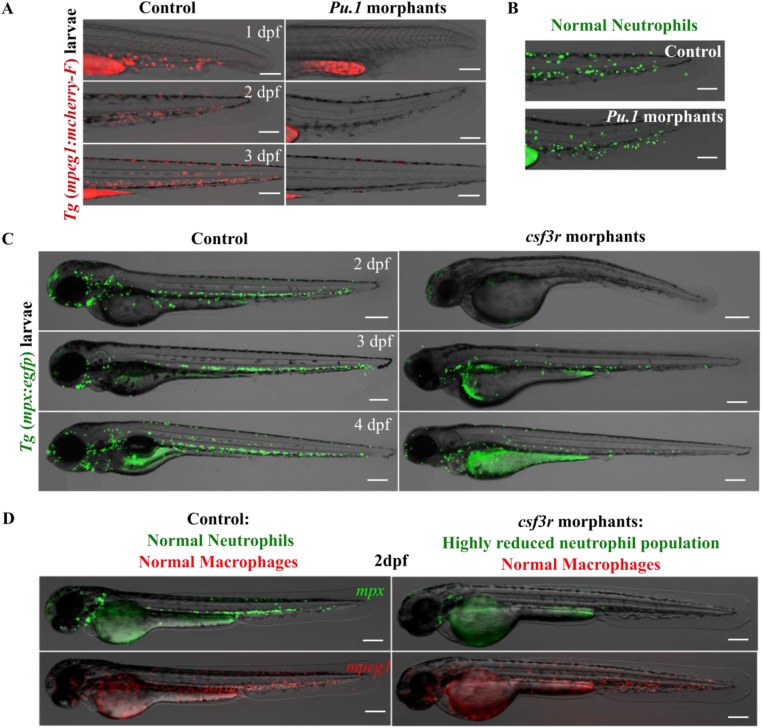
We next investigated the propensity of ΔMAB_4780 to produce cords in embryos in the absence of macrophages. Injection of antisense morpholinos against the myeloid transcription factor gene pu.1 (36) leads to macrophage-depleted embryos without affecting the neutrophils (Fig. S8 A and B). Following infection and as shown previously (16), 100% of the morphants were heavily infected with the R variant and developed serpentine cords (Fig. 6A). Only 23% of the pu.1 morphants infected with ΔMAB_4780 exhibited extracellular bacterial structures (Fig. 6A), which did not ressemble organized cords and were much smaller in size (Fig. 6B). Whereas R infection resulted in 100% of larval killing at 3 dpi, ΔMAB_4780 infection led to only 20% of larval death at 6 dpi compared with the uninfected embryos (Fig. 6C). The cfu plating indicated although the R bacterial counts increased drastically in the pu.1 morphants, the ΔMAB_4780 loads increased much slower with a 2 Log reduction in the cfu compared with Mabs R (Fig. 6D), consistent with whole embryo imaging (Fig. 6E). This suggests that the lack of macrophages could not rescue growth and virulence of ΔMAB_4780. Importantly, these in vivo conditions stimulating hypercording of the R strain and resulting in acute and lethal infection were not sufficient to promote cording of the mutant, further substantiating a direct correlation between cording and acute infection of Mabs R.
Fig. S8.
Efficacy of the macrophage and neutrophil depletion strategy. (A) The double transgenic zebrafish line [tg(mpx:egfp)/(mpeg1:mCherry-F)] was injected at the one-cell stage with the pu.1 morpholino to specifically deplete macrophages. Representative overlays of transmission and red fluorescence stereomicroscope time-lapse images showing the pu.1 morpholino effect on the macrophage population in the tail region. (Scale bars, 200 μm.) (B) Representative overlays of transmission and green fluorescence stereomicroscope images showing the absence of effect of the pu.1 morpholino on the neutrophil population in the tail region at 48 hpf. (Scale bars, 200 μm.) This resulted in a total depletion of macrophages, whereas neutrophils remained unaffected. The first macrophages start to renew at 4 dpf. (C) The double transgenic zebrafish line [tg(mpx:egfp)/(mpeg1:mCherry-F)] exhibiting green neutrophils and red macrophages was injected at the one-cell stage with the csf3r morpholino to specifically deplete neutrophils. Representative overlays of transmission and green fluorescence stereomicroscope time-lapse images show the csf3r morpholino effect in neutrophil population in whole embryo. (Scale bars, 200 μm.) This resulted in a highly reduced neutrophil population. (D) Shown are the overlays of transmission and green/red fluorescence stereomicroscope images of representative 2 dpf larvae showing that the macrophage population remained unaffected in the csf3r morphants.
Fig. 6.
MABS_4780 is required for acute and lethal zebrafish infection. (A) Cords were recorded in pu.1 embryos infected i.v. with ∼100–200 cfu of either tdTomato-expressing Mabs R, ΔMAB_4780, or the complemented strain at 2 and 3 dpi (n = 20). Percentage of pu.1 morphants harboring mycobacterial cords after infection with either the R variant or the ΔMAB_4780 mutant (n = 20) at 3 dpi. Data are expressed as means calculated from three independent experiments (n = 10 per experiment). The statistical test was Fisher’s exact test; ***P < 0.001. Error bars represent the SEM. (B) Maximum intensity projection of confocal images showing representative cords in pu.1 morphants i.v. infected with the Mabs R, ΔMAB_4780, and complemented strains expressing tdTomato at 2 dpi (∼150 cfu). Only Mabs R and the complemented strain exhibited cords made of structured networks of multiple tight bundles, consisting of end-to-end and side-to-side parallel-aligned bacilli along the long axis of the cord, whereas extracellular growth of ΔMAB_4780 resulted essentially in size-limited and very thin structures. (C) Survival of macrophage-depleted embryos infected with 150 cfu at 30 hpf. Embryos are significantly more susceptible to R infection (P < 0.0001, log-rank test) than to ΔMAB_4780 infection (statistically not different from pu.1 morphant control). Shown are representative data of three independent experiments. (D) Growth kinetic of ΔMAB_4780 in pu.1 morphants (∼100–200 cfu). The cfu enumeration showed reduced ΔMAB_4780 loads compared with R loads (**P < 0.01; Mann–Whitney test). Symbols represent means calculated from three independent experiments. Error bars represent the SEM. (E) Real-time imaging of pu.1 morphants infected as in B, with special emphasis on cording. Autofluorescence of the yolk is seen at 1 dpi.
ΔMAB_4780 Infection Is Controlled in Partially Depleted Neutrophil Embryos.
That ΔMAB_4780 remained attenuated even in the absence of macrophages may be explained by the possible role of neutrophils in restricting growth of the mutant. Macrophage-depleted Tg(mpx:egpf) embryos, harboring green fluorescent neutrophils, were therefore infected with the tdTomato-expressing bacteria and analyzed by confocal microscopy. Both Mabs R and the complemented strain produced cords that were much larger in size than neutrophils and never found to be contained within these cells (Fig. 7A). In contrast, ΔMAB_4780 was mostly present as individual bacilli and essentially contained within neutrophils (Fig. 7A), suggesting that, in the absence of macrophages, neutrophils may contribute to the clearance of ΔMAB_4780. Consistent with a previous report (37), csf3r knockdown clearly reduced the neutrophil population (Fig. 7B and Fig. S8C) but not the population of macrophages (Fig. S8D). Indeed, at 2 dpf and 3 dpf, the csf3r morphants displayed a significant reduction of mpx-positive neutrophils (about 5 times less in the csf3r morphants compared with the wild-type embryos in the trunk and tail) (Fig. 7B). Csf3r morphants were highly susceptible to Mabs R infection with a rapid larval killing (Fig. 7C) and exhibited important pathological signs such as abscesses in the central nervous system (Fig. 7D), further emphasizing the key role of neutrophils in the control of Mabs infection. However, as for the pu.1 morphants, depletion of the vast majority of neutrophils in the csf3r morphants failed to rescue virulence of ΔMAB_4780 (Fig. 7 C and D), presumably because in the absence of these cells, the sole macrophage population remained efficient in controlling infection of the mutant. Overall, these results indicate that ΔMAB_4780 is highly attenuated, even in immunocompromized embryos.
Fig. 7.
Virulence of ΔMAB_4780 is attenuated in a neutrophil-depleted host. (A) Maximum intensity projection of confocal images showing representative neutrophil activity (at 2 dpi) in pu.1 Tg(mpx:egpf) morphants i.v.-infected with Mabs R, ΔMAB_4780, or complemented strain expressing tdTomato. (B) Csf3r Tg(mpx:egpf) morphants showed highly reduced neutrophil numbers at 3 and 4 dpf, confirming the proper activity of the morpholino. The numbers of mpx-positive neutrophils counted in the trunk and tail at 3 dpf are depicted in the graph. (C) Survival curve of neutrophil-depleted larvae i.v.-infected with the Mabs R, ΔMAB_4780, and complemented strains (∼150 cfu, n = 20 per group). These larvae were hypersusceptible to Mabs R and to the complemented strain (P < 0.001, log-rank test) but not to ΔMAB_4780 (statistically not different from PBS-injected controls). Shown are representative data from two independent experiments. (D) Representative fluorescence and DIC overlays of infected csf3r morphants at 2 and 3 dpi.
Discussion
Analysis of the pathogenicity of Mabs has long been hampered by the lack of genetic tools and the restricted panel of cellular/animal models. Making targeted genetic deletions are particularly challenging in the R variant due to its extremely high aggregative properties. Herein, we combined the larval zebrafish as a tractable host to study the innate immune response to Mabs (16, 17) and the successful achievement of the first deletion mutant in the R background. We demonstrate that the recombineering method (31) can be successfully applied to inactivate MAB_4780 in the R variant, leading to multiple and remarkable in vitro and in vivo phenotypes.
Just like in M. smegmatis, inactivation of MAB_4780 led to a higher susceptibility of Mabs to TAC. Genetic complementation with either MAB_4780 or MSMEG_6754 restored resistance, suggesting a functional redundancy between the two proteins. TAC affects mycolic acid biosynthesis (19–21, 38, 39), suggesting that MAB_4780 may participate in mycolic acid metabolism, despite the fact that all enzymatic steps required for the mycolic acid elongation machinery had been previously identified (40, 41). Compelling evidence indicates that MAB_4780 is a mycolic acid-associated dehydratase: (i) Its specific activity is similar to that reported previously for M. tuberculosis HadBC dehydratase when using trans-2-enoyl-CoA substrates (25); (ii) the crystal structure of MAB_4780 is highly similar to the HadAB dehydratases from M. smegmatis (28) and M. tuberculosis (29); and (iii) inactivation of MAB_4780 correlates with a reduction of the pool of long-chain α-mycolic acids. Our structural mycolic acid analysis indicates that in Mabs α-mycolic acids contain mainly 79 carbon atoms, whereas α′-mycolic acids contain 64 carbon atoms. Although α′-mycolic acids can be considered as precursors of the α-mycolic acids, it is not known why these precursors accumulate in some mycobacterial species. A possible explanation could be that HadBC activity may be deficient in these species due to a defect in gene expression or regulation. A mutation or deletion of hadC affects the biosynthesis of oxygenated mycolic acids in M. tuberculosis, substantially reducing their production and demonstrating the involvement of HadBC in the late elongation steps (22). Combined with our findings that, like HadBC, MAB_4780 exhibits a substrate preference for long fatty acyl chains (C12 but not C4) and that a change in the α/α′-mycolic acid ratio occurs in ΔMAB_4780, it can be inferred that MAB_4780 participates also in the last elongation steps of meromycolic acid biosynthesis. However, the presence of these two redundant dehydratases (MAB_4780 and HadBC) in Mabs remains puzzling. Perhaps MAB_4780 participates in the elongation step under conditions where HadBC is expressed in limiting concentrations.
Subtle changes in the composition and/or fine structure of the mycolic acids, such as those affecting either the chain length or nature of the chemical functions, can condition their biological activities (42). This may have important consequences like (i) the molecular arrangement and fluidity of the mycomembrane and (ii) the biological activities of numerous mycolylated cell wall components, usually considered as pathogenic determinants. Among them, trehalose-dimycolate (TDM; also known as cord factor) is implicated in various biological functions, such as pathogenesis, immunomodulation, inflammation, granuloma formation, or cording (43, 44). Strikingly, the cord-deficient phenotype of ΔMAB_4780 that may directly result from the changes in the mycolic acid pattern provides an ideal tool to unravel the role of cording in pathogenesis of Mabs R. Indeed, disruption of MAB_4780 abrogated all pathophysiological traits of the R strain—virulence, neurotropism, intramacrophage survival, granuloma formation, and cording—emphasizing the critical role of MAB_4780 in pathogenicity of Mabs R. It is worth mentioning that mutation/deletion of hadC also impacts cording and virulence of M. tuberculosis in mice (22), further underlining the redundant functions between MAB_4780 and HadBC. Interestingly, whereas MAB_4780 is needed for cording in Mabs, a functional ortholog (MSMEG_6754) is present in the noncording M. smegmatis and no orthologs are apparently present in M. tuberculosis, a known cording species. This suggests that cording is a complex process that involves a set of genes that are species-specific.
Pathogenic mycobacteria survive within macrophages by residing in phagosomes, which they prevent from maturing and fusing with lysosomes. Mycolic acids are among the bacterial components found to modulate phagosome processing, and recent work has highlighted the importance of PcaA-mediated cyclopropanation of mycolates in prevention of phagosome maturation and intracellular survival of Mycobacterium bovis bacillus Calmette–Guérin in human monocyte-derived macrophages (45). Other studies have shown the ability of the surface-exposed TDM to inhibit fusion events between phospholipid vesicles inside host macrophages. Moreover, macrophages incubated with beads coated with a synthetic analog of TDM containing shorter fatty acid chains demonstrated increased colocalization with LysoTracker (46). This implies that the fatty acyl chain length of TDM contributes to the relatively nonfusogenic activity of TDM and may explain the higher proportion of ΔMAB_4780 that colocalized with acidic vesicles in macrophages. Indeed, the shorter chain length of the ΔMAB_4780 TDM pool, resulting from the decreased production of long chain α-mycolic acids, may directly affect its capacity to prevent phagolysosomal fusion. This finding, in turn, may explain the poor intramacrophage and intraamoebal survival of the mutant compared with the parental strain, thereby compromising its ability to stimulate early granuloma formation. Alternatively, because TDM has also been proposed as a potent granulomatogenic component of the cell wall (47), one cannot rule out that the subtle structural modifications affecting TDM in ΔMAB_4780 may negatively impact granuloma formation.
Another unanticipated finding arising from this work relies on the innate phagocyte populations required to control infection of ΔMAB_4780. Depletion of either macrophages or neutrophils rendered host larvae susceptible to invasive disease by the R variant but resulted in an attenuated disease following challenge with the less virulent ΔMAB_4780 strain. We hypothesize that early interactions between ΔMAB_4780 and neutrophils in pu.1 morphants or with macrophages in csf3r morphants may contribute to the specific virulence defect for ΔMAB_4780, especially considering that this strain has decreased levels of α-mycolic acids, leading to a defect in the shielding hydrophobic cell envelope composition/architecture. However, either macrophages or neutrophils are required to control the infection of ΔMAB_4780, as the mutant regains full virulence when injected in the yolk consisting of a single giant cell encapsulated by a plasma membrane and where chemotaxis of phagocytes is impaired. We have previously shown that bacteria released from apoptotic macrophages grow extracellularly and form cords, which subsequently prevent Mabs R from being phagocytozed by macrophages and neutrophils (16). The present results suggest that ΔMAB_4780, because it is defective in cording, grows as more homogenous nonclustered bacilli, which are rapidly phagocytozed and destroyed by macrophages and neutrophils.
In summary, this study clearly demonstrates that inactivation of the dehydratase MAB_4780 strongly affects Mabs R pathogenicity and that attenuation is very likely to result from both cord deficiency and intracellular growth impairment. Because interfering with MAB_4780 results in an extremely attenuated phenotype in multiple cellular and animal hosts, its selective inhibition may represent an original and alternative approach to standard chemotherapy to control invasive and acute Mabs infections. Chemical inhibition of MAB_4780 may represent an attractive way to attenuate cording. In this context, the in vitro dehydratase assay combined with our high-resolution crystal structure will be particularly helpful in the process of screening and selecting high-affinity inhibitors. Once obtained, the selected molecules could then be assessed in the Mabs/zebrafish system, which has recently been validated as a preclinical animal model to test the in vivo therapeutic efficacy of drugs active against Mabs (48, 49). Such advanced strategies are particularly warranted as Mabs is refractory to most antibiotics treatments and often associated with high therapeutic failure.
Methods
Bacterial Strains and Growth Conditions.
All bacterial strains used in this study are listed in Table S2. For cloning, Escherichia coli strains were grown at 37 °C in LB medium with kanamycin (25 μg/mL), hygromycin (25 μg/mL), or ampicillin (200 μg/mL) when required. Mycobacteria were routinely maintained at 32 °C in Middlebrook 7H9 broth supplemented with 10% (vol/vol) oleic acid–albumin–dextrose–catalase (OADC) enrichment and 0.05% Tween 80 or grown on Middlebrook 7H10 agar supplemented with 10% (vol/vol) OADC and appropriate antibiotics. In vitro growth was examined by inoculating the midlog phase culture into fresh 7H9 at an OD600 of ∼0.01, followed by regular measurements at different time points, along with enumeration of the cfu on plates. Due to the tendency of Mabs to clump, resulting in heterogenous suspensions that may prevent accurate growth determinations, a third method was adopted by monitoring the red fluorescence emitted by the strains carrying pTEC27 (expressing tdTomato).
Table S2.
Mycobacterial strains and plasmids used in this study
| Strain/plasmid | Description | Marker | Source |
| Strain | |||
| M. smegmatis | mc2155 | — | (58) |
| M. smegmatis | mc2155 ΔMSMEG_6754 | Hyg | (23) |
| M. smegmatis | mc2155 ΔMSMEG6754::pMV306_4780 | Hyg, Kan | This study |
| Mabs S | Mabs strain CIP104536T S | — | ATCC19977T |
| Mabs R | Mabs strain CIP104536T R | — | ATCC19977T |
| Mabs R | Mabs ΔMABS_4780 | Hyg | This study |
| Mabs R | ΔMABS_4780::pMV306_4780 | Hyg, Kan | This study |
| Plasmid | |||
| pSMT3_eGFP | Multicopy shuttle plasmid in which eGFP is expressed under the control of the hsp60 promoter | Hyg | (59) |
| pMV261 | Multicopy E. coli/mycobacterial shuttle vector; cloned genes are under the control of the constitutive hsp60 promoter | Kan | (60) |
| pMV306 | Plasmid that allows single copy integration of the cloned gene at the attB site on the genome | Kan | (60) |
| pMV306_4780 | MAB_4780 cloned into pMV306, under the hsp60 promoter | Kan | This study |
| pJSC347 | To clone left and right arms flanking a hyg resistance cassette | Hyg | W.R. Jacobs (AECOM) |
| pJV53-Zeo | pJV53 with the Kan cassette replaced by a Zeo cassette | Kan | (13) |
| pTEC27 | Multicopy E. coli/mycobacterial shuttle vector to express tdTomato under the control of a strong mycobacterial promoter | Hyg | Addgene (plasmid 30182) |
| pMV206_Apra | pMV206 in which the Kan has been replaced by an apramycin resistance cassette | Apra | W.R. Jacobs (AECOM) |
| pMV206_Apra_tdTomato | tdTomato gene is cloned into pMV206_Apra | Apra | This study |
| pET32a | Bacterial vector for high-level expression of peptide sequence fused with the 109 aa thioredoxin protein | Amp | Novagen |
Amp, ampicillin; Apra, apramycin; Hyg, hygromycin; Kan, kanamycin; Zeo, zeocin.
Plasmids and DNA Manipulation.
All plasmids used in this study are listed in Table S2. The pMV306_4780 construct was made by amplifying MAB_4780 from Mabs R genomic DNA using forward primer (5′-CCCTGGCCATGACTGCACCAGTAGACGGGTCG-3′; MscI) and reverse primer (5′-CGGGATCCTCAGCTGAAGCGCACGCTCATCG-3′; BamHI). The amplified product was then ligated into the MscI/BamHI-restricted pMV261. The MAB_4780 gene along with the hsp60 promoter was then restricted with XbaI and HindIII and subcloned into the XbaI/HindIII-digested pMV306, yielding pMV306_4780. To generate pMV206_Apra_tdTomato, the tdTomato gene was excised from pTEC27 using MfeI/NheI and directly ligated into the MfeI/XbaI-restricted pMV206_Apra. Constructs were verified by DNA sequencing.
Construction of ∆MAB_4780 Deletion Mutant.
All genetic manipulations in Mabs R were carried out using the recombineering technique (31). Briefly, an allelic exchange substrate for the MAB_4780 gene deletion was prepared by amplifying the left and right flanking arms and cloning them on either side of the hygromycin-resistant gene in the shuttle vector pJSC347. The left arm was directly cloned using SpeI and HindIII sites and the right arm using XbaI and KpnI. The allelic exchange substrate was then excised from the vector backbone by digesting with SpeI and AflII and directly electroporated into freshly prepared electrocompetent Mabs R cells carrying pJV53-Zeo (31). Plates were incubated at 37 °C for 1 week, and colonies resistant to zeocin and hygromycin were screened by PCR for correct gene replacement. Primers used to amplify the left and right arms are listed in Table S3.
Table S3.
Primers used in this study
| Primer names | Gene | 5′ to 3′ sequence |
| Cloning primers | ||
| Cloning in pMV261 | ||
| Fow | MABS_4780 | CCCTGGCCATGACTGCACCAGTAGACGGGTCG |
| Rev | MABS_4780 | CGGGATCCTCAGCTGAAGCGCACGCTCATCG |
| Cloning in pET32a | ||
| Fow | MABS_4780 | CGGGATCCgagaatctgtacttccagggaATGACTGCACCAGTAGACGGGTCG (tev site in lowercase) |
| Rev | MABS_4780 | CCCTCGAGTCAGCTGAAGCGCACGCTCATCG |
| KO primers | ||
| Cloning in pJSC347 | ||
| KO4780 left fow SpeI | MABS_4780 | GACTAGTCGCGGTGCGGGATGGCGTAGAGAG |
| KO4780 left rev HindIII | MABS_4780 | CCCAAGCTTACAGGCCATGGCGGGGGTCGAAGTG |
| KO4780 right fow XbaI | MABS_4780 | GCTCTAGATGTGATCGCACACGGGATGCTCAC |
| KO4780 right rev KpnI | MABS_4780 | GGGGTACCCACGTTCTCGAATTGGACCTGGC |
| KO checking primers | ||
| pJSC347 L3′ | GACGACCCTAGAGTCCTGTCC | |
| pJSC347 R5′ | GACACCGCCCCCGGCGCCTGA | |
| KO4780 left up | MABS_4780 | AGCCATCGCGAGCGCGTCGGGCTCG |
| KO4780 right down | MABS_4780 | TCGCGGTTCGACGTCGTGTTACTCG |
Fow, forward; Rev, reverse.
Susceptibility to SDS and TAC.
Susceptibility of Mabs to SDS or TAC was assessed visually on Middlebrook 7H10/OADC plates containing increasing detergent or drug concentrations, as reported earlier (50). Tenfold serial dilutions of actively growing culture were plated and incubated at 37 °C for 4–5 d. The minimal inhibitory concentration (MIC) was defined as the minimum concentration required to inhibit 99% of the bacterial growth. Drug susceptibility testing was also determined using the microdilution method, in cation-adjusted Mueller–Hinton broth, according to the Clinical and Laboratory Standards Institute guidelines (51).
Mycobacterium Preparations for Infection Studies.
Cultures were grown to midlog phase, pelleted, washed twice, and resuspended in PBS supplemented with 0.05% Tween-80 (PBST). Bacterial clumps were disrupted by 10 successive passages through a 26-gauge needle and sonicated three times for 10 s each to disperse bacteria. Remaining clumps were allowed to sediment by centrifugation at 150 × g for 1 min. The OD was then measured at 600 nm to adjust the final concentration of the bacteria.
Macrophage Infections.
Murine J774 macrophages were grown at 37 °C in a 5% CO2 incubator in DMEM supplemented with 10% (vol/vol) FBS. Cells were seeded 24 h before infection at 1 × 105 cells/mL. Bacteria were added at a multiplicity of infection (MOI) of 1. After 3 h of infection, extracellular mycobacteria were removed by washing before adding fresh medium containing 250 µg/mL of amikacin for 1 h. Fresh medium containing 50 µg/mL of amikacin was then added. For cfu determination, cells were lysed with 1% Triton X-100 in PBS, and bacteria were quantified after plating serial dilutions of lysates on 7H10 plates and incubation at 37 °C.
Zebrafish Manipulation and Infection Studies.
All zebrafish experiments were approved by the Direction Sanitaire et Vétérinaire de l'Hérault and Comité d'Ethique pour l'Expérimentation Animale de la région Languedoc Roussillon under the reference CEEA-LR- CEEALR36-1145. Experiments were done using the golden zebrafish mutant (52), the Tg(mpeg1:mCherryF) to visualize macrophages (16), or the Tg (mpx:egfp) to visualize neutrophils (53). Bacteria were adjusted to an OD600 of 1 in PBST and mixed with phenol red. Microinjections of ∼2–3 nL of bacterial suspensions, containing around 150 bacteria, were done directly into the bloodstream or into the yolk sac of 30 hpf dechorionated and anesthetized embryos. To determine the survival curves, infected larvae were transferred into individual wells and incubated at 32 °C. Mortality was determined by recording the number of dead embryos daily. To monitor the bacterial loads, groups of three infected embryos were collected at different time points, lysed individually in 2% (vol/vol) Triton X-100, and homogenized though a 26-gauge needle and the homogenates were serially diluted and plated on Middlebrook 7H10/OADC supplemented with BBL MGIT PANTA (BD) (16).
Microinjection of Antisense Morpholinos.
Oligonucleotide morpholinos consisting of splice-blocking 5′-GGTCTTTCTCCTTACCATGCTCTCC-3′ and ATG 5′-CCTCCATTCTGTACGGATGCAGCAT-3′ morpholinos against pu.1 (Gene Tools) were combined to final concentrations of 0.025 mM and 0.375 mM, respectively (36). Transient depletion of the neutrophils was performed in the transgenic Tg(mpeg:mcherry) zebrafish line using the csf3r morpholino 5′-TTTGTCTTTACAGATCCGCCAGTTC-3′ (Gene Tools) at 0.7 mM. Morpholino solutions were injected (2–3 nL) into one-cell stage embryos (37).
Live Imaging and Confocal Microscopy.
For live imaging, tricaine-anesthetized infected embryos were mounted in 1% low-melting point agarose in a 35-mm Petri dish. Direct observations were performed using a Zeiss microscope with a Zeiss Plan Neo Fluor Z 1×/0.25 objective and equipped with an AxioCam 503 monochrom camera (Zeiss) and acquired and processed with ZEN 2 software (blue edition). For confocal microscopy, anesthetized embryos were fixed overnight at 4 °C with 4% (vol/vol) paraformaldehyde (PAF) in PBST, washed twice in PBST, and incubated successively in increasing glycerol concentration up to 50% to preserve the integrity of the tissues. Fixed embryos were then moved onto depressed transparent slides, covered with a coverslip for observation with a 40× Leica Apo oil 1.15 NA objective, and visualized using a Leica DM2500CSQ upright microscope equipped with a Leica TCS SPE confocal scan head as well as fluorescence and differential interference contrast (DIC) optics. Fluorescence and DIC images were acquired and assembled.
Lysotracker Red.
LysoTracker Red DND-99 is an acidotropic fluorescent probe that was used as a marker of phagosomal acidification and maturation (54). J774A cells were seeded onto glass coverslips and infected with Fluorescein isothiocyanate, FITC-labeled Mabs R, ∆MAB_4780, and complemented strains (55). In brief, bacterial cultures were suspended in 7H9 broth and labeled with 100 µg/mL of FITC for 2 h at room temperature (RT) with gentle agitation in the darkness. FITC-labeled bacteria were washed once with 7H9 broth supplemented with 4% (wt/vol) BSA and then twice with 7H9 broth without BSA. The final concentration of the bacteria was assessed by measuring OD600, and infection was done at an MOI of 1:1. After 3 h of infection, cells were washed twice with DMEM to remove extracellular bacteria and incubated with DMEM containing 1 μM LysoTracker Red DND-99 (Molecular Probes) for 30 min at 37 °C. Subsequently, cells were washed three times with PBS and fixed with 4% (vol/vol) PAF for microscopy. The z stacks were acquired using a Zeiss Axio Imager Z2 equipped with an Orca flash 4.0 sCMOS camera (Hamamatsu) and with an Apotome (Zeiss) for optical slicing, using a 100× NA 1.4 Apochromat objective. Images were processed for luminosity–contrast adjustment with Zen Software (Zeiss). Colocalization was counted via Fiji. At least 100 phagosomes were counted for each strain performed in two independent experiments.
Expression and Purification of MAB_4780.
MAB_4780 was cloned into the BamHI/XhoI-restricted pET32a(+) (Novagen) allowing the gene to be in frame with the S-, hexa-histidine, and thioredoxin tags. A Tobacco Etch Virus (TEV) protease cleavage site was added between the tags for subsequent removal of the tags during the purification process. MAB_4780 was expressed in E. coli BL21 Rosetta 2 as follows. An overnight preculture grown in LB supplemented with ampicillin and chloramphenicol was used to inoculate 4 L of LB supplemented with both antibiotics. Cultures were grown at 37 °C to 0.8 OD600, and protein expression was induced with 1 mM IPTG, overnight (ON) at 16 °C. Bacteria were harvested; resuspended in lysis buffer containing 50 mM Tris·HCl, pH 8, 0.4 M NaCl, 5 mM β-mercaptoethanol, 20 mM imidazole, and 1 mM benzamidine; and disrupted by four cycles of 2 min sonication before centrifugation for 35 min at 30,000 × g. The resulting supernatant was loaded onto a 3 mL Sepharose nickel affinity column using gravity. Beads were washed with five volumes of lysis buffer and then with five volumes of 50 mM Tris·HCl, pH 8, and 1 M NaCl. Protein elution was done with three volumes of 50 mM Tris·HCl, pH 8, 0.2 M NaCl, 5 mM β-mercaptoethanol, 400 mM imidazole, and 1 mM benzamidine. The eluted protein was then mixed with TEV in a 1:50 (wt/wt) ratio; dialyzed ON at 4 °C against 50 mM Tris·HCl, pH 8, 0.2 M NaCl, and 5 mM β-mercaptoethanol; and subjected to a second nickel affinity chromatography column to release the tag-free protein in the flow-through. The protein was concentrated to 5 mg/mL and subjected to a size exclusion chromatography onto an ENrichTM SEC 650 column (Biorad). Purity was estimated around 95% by SDS/PAGE.
Size Exclusion Chromatography.
A calibration curve was obtained using the Gel Filtration Markers Kit for Protein Molecular Weights 12,400–200,000 Da (Sigma-Aldrich) by plotting the partition coefficient Kav against the logarithms of the molecular weight of standard proteins. The void volume was determined with blue dextran. Proteins were loaded onto an ENrichTM SEC 650 column (Biorad) and eluted with buffer containing 50 mM Tris·HCl, pH 8, 200 mM NaCl, and 5 mM β-mercaptoethanol at a flow rate of 0.5 mL/min at RT.
Synthesis of 2-Trans-Enoyl-CoA Substrates.
To a solution of 2-trans-octenoic acid or 2-trans-dodecenoic acid (0.55 mmol) in t butyl methyl ether (14 mL) we added diisopropylethylamine (0.1 mL, 0.57 mmol) and ethyl chloroformate (0.1 mL, 0.52 mmol). The suspension was stirred at RT overnight under nitrogen, filtered, and evaporated to dryness. A solution of CoA sodium salt (50 mg, 0.06 mmol) in 0.3 M aqueous sodium bicarbonate solution/95% (vol/vol) EtOH/ethyl acetate (1/1/1; vol/vol/vol; 6 mL) was added drop-wise to the residue. The reaction mixture was stirred for 1 h at RT and quenched with acetic acid (0.1 mL). After three extractions with ethyl acetate, the aqueous phase was lyophilized, and the 2-trans-enoyl-CoAs were purified by HPLC on a C18 reverse phase column. The column was pre-equilibrated before the injection for 10 min with H2O/CH3OH/10% (vol/vol) CH3OH in 0.1 M NaH2PO4 (72/8/20, vol/vol/vol) at a flow rate of 3 mL/min. For purification of the enoyl-CoAs, the flow rate was set at 1 mL/min and the wavelength at 260 nm. The mobile phase was H2O/CH3OH 90/10 (vol/vol) with a linear increase to 30% (vol/vol) CH3OH in 30 min. Crotonoyl-CoA was purchased from Sigma-Aldrich.
Dehydratase Assay.
The dehydratase activity was assayed as the decrease of the OD263 nm corresponding to the disappearance of the double bond, where a loss of 0.67 OD corresponds to a product concentration of 100 μM. The reaction mixture contained 20 mM potassium phosphate buffer at pH 7, 100 μM of enoyl-CoA, and 430 nM of enzyme. The reaction was assayed in a 1 mL quartz cuvette at 25 °C in a NanoDrop 2000c spectrophotometer (Thermo Scientific).
Crystallization, Data Collection, and Structure Refinement.
The protein concentrated to 5 mg/mL and in the presence of 2 mM trans-2-dodecanoyl-CoA was crystallized in 0.1 M Tris·HCl, pH 8.5 ,and 2.8 M AmSO4 in sitting drops at 18 °C. Crystals were cryocooled in liquid nitrogen without any cryoprotecting solution before data collection. A full dataset was collected at the BM30 beamline [European Synchrotron Radiation Facility (ESRF)]. The data collection statistics are reported in Table S1. The structure was solved by molecular replacement using Phaser from the Phenix package (56) and using as search model for the structure of the M. smegmatis MSMEG_6754 protein [Protein Data Bank (PDB) ID code 4v12] (23). Refinement and model quality were done using the Phenix package (56), and the model building was performed with COOT (57). Figures were made using Pymol.
Production of Polyclonal Anti-MAB_4780 Antibodies and Western Blotting.
Polyclonal antibodies against recombinant MAB_4780 were generated in BALB/c mice (Janvier) according to standards protocols. Antigen (20 µg per mice) diluted in PBS was emulsified at a 1/1 proportion in incomplete Freund’s adjuvant. Mice were s.c. immunized (final volume of 200 µL) at multiple sites, at days 1, 28, and 57. Blood samples were collected 1 wk after the last immunization from the retro-orbital plexus, and sera were stored at −20 °C until use. Animal experiments were performed according to institutional and national ethical guidelines (Agreement no. 92-033-01, Préfecture des Hauts de Seine, Boulogne, France). For Western blotting, bacteria were harvested, resuspended in PBS, and disrupted by bead beating with 1-mm diameter glass beads. Protein concentration for the resulting lysates was determined using the BCA Protein Assay Reagent kit (Pierce), according to the manufacturer’s instructions. Equal amounts of proteins (35 µg) were subjected to SDS/PAGE separation and then transferred to a nitrocellulose membrane. For detection of MAB_4780, the membrane was incubated overnight with the mouse anti-MAB_4780 antibody (dilution 1:1,000), washed, and subsequently incubated with goat anti-mouse antibody conjugated to peroxidase (Jackson ImmunoResearch Laboratories). The signal was revealed using the Luminata Forte Western HRP substrate (Millipore).
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism 5 (Graphpad Software). Comparison between survival curves was performed using the log rank test. The cfu counts were analyzed using the Mann–Whitney t test when comparing two groups or one-way ANOVA (Kruskal–Wallis with Dunn’s multiple test) when comparing more than two groups. For the quantification experiments, Fisher’s exact test was used. Only P values < 0.05 were considered as statistically significant.
Acknowledgments
The authors thank J. P. Levraud for the generous gift of csf3r morpholinos. We also thank the staff at ESRF for their help during X-ray data collection. This study was supported by the French National Research Agency (DIMYVIR ANR-13-BSV3-0007-01), the Labex EpiGenMed (I.H.), the Fondation de France (S.C.-K.), and the InfectioPôle Sud (A.V.). L.K. acknowledges the support of Fondation pour la Recherche Médicale (FRM) Grant DEQ20150331719.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5I7N).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605477113/-/DCSupplemental.
References
- 1.Middlebrook G, Dubos RJ, Pierce C. Virulence and morphological characteristics of mammalian tubercle bacilli. J Exp Med. 1947;86(2):175–184. doi: 10.1084/jem.86.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaminski DA, Hardy DJ. Selective utilization of DNA probes for identification of Mycobacterium species on the basis of cord formation in primary BACTEC 12B cultures. J Clin Microbiol. 1995;33(6):1548–1550. doi: 10.1128/jcm.33.6.1548-1550.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarter YS, Ratkiewicz IN, Robinson A. Cord formation in BACTEC medium is a reliable, rapid method for presumptive identification of Mycobacterium tuberculosis complex. J Clin Microbiol. 1998;36(9):2769–2771. doi: 10.1128/jcm.36.9.2769-2771.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: An underestimated adversary. Antimicrob Agents Chemother. 2003;47(3):833–836. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staropoli JF, Branda JA. Cord formation in a clinical isolate of Mycobacterium marinum. J Clin Microbiol. 2008;46(8):2814–2816. doi: 10.1128/JCM.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Julián E, et al. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J Bacteriol. 2010;192(7):1751–1760. doi: 10.1128/JB.01485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medjahed H, Gaillard J-L, Reyrat J-M. Mycobacterium abscessus: A new player in the mycobacterial field. Trends Microbiol. 2010;18(3):117–123. doi: 10.1016/j.tim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: A new antibiotic nightmare. J Antimicrob Chemother. 2012;67(4):810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 9.Koh W-J, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129(2):341–348. doi: 10.1378/chest.129.2.341. [DOI] [PubMed] [Google Scholar]
- 10.Parkins MD, Floto RA. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros. 2015;14(3):293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Torres-Coy JA, Rodríguez-Castillo BA, Pérez-Alfonzo R, DE Waard JH. Source investigation of two outbreaks of skin and soft tissue infection by Mycobacterium abscessus subsp. abscessus in Venezuela. Epidemiol Infect. 2016;144(5):1117–1120. doi: 10.1017/S0950268815002381. [DOI] [PubMed] [Google Scholar]
- 12.Howard ST, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152(Pt 6):1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 13.Medjahed H, Reyrat J-M. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: comparison of genetic tools. Appl Environ Microbiol. 2009;75(5):1331–1338. doi: 10.1128/AEM.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawlik A, et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol Microbiol. 2013;90(3):612–629. doi: 10.1111/mmi.12387. [DOI] [PubMed] [Google Scholar]
- 15.Bernut A, et al. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol Microbiol. 2016;99(5):866–883. doi: 10.1111/mmi.13283. [DOI] [PubMed] [Google Scholar]
- 16.Bernut A, et al. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc Natl Acad Sci USA. 2014;111(10):E943–E952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernut A, et al. Deciphering and imaging pathogenesis and cording of Mycobacterium abscessus in zebrafish embryos. J Vis Exp. 2015;103(103):e53130. doi: 10.3791/53130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glickman MS, Cox JS, Jacobs WR., Jr A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell. 2000;5(4):717–727. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 19.Belardinelli JM, Morbidoni HR. Mutations in the essential FAS II β-hydroxyacyl ACP dehydratase complex confer resistance to thiacetazone in Mycobacterium tuberculosis and Mycobacterium kansasii. Mol Microbiol. 2012;86(3):568–579. doi: 10.1111/mmi.12005. [DOI] [PubMed] [Google Scholar]
- 20.Grzegorzewicz AE, et al. A common mechanism of inhibition of the Mycobacterium tuberculosis mycolic acid biosynthetic pathway by isoxyl and thiacetazone. J Biol Chem. 2012;287(46):38434–38441. doi: 10.1074/jbc.M112.400994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coxon GD, et al. Synthesis, antitubercular activity and mechanism of resistance of highly effective thiacetazone analogues. PLoS One. 2013;8(1):e53162. doi: 10.1371/journal.pone.0053162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slama N, et al. The changes in mycolic acid structures caused by hadC mutation have a dramatic effect on the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2016;99(4):794–807. doi: 10.1111/mmi.13266. [DOI] [PubMed] [Google Scholar]
- 23.Carrère-Kremer S, et al. A new dehydratase conferring innate resistance to thiacetazone and intra-amoebal survival of Mycobacterium smegmatis. Mol Microbiol. 2015;96(5):1085–1102. doi: 10.1111/mmi.12992. [DOI] [PubMed] [Google Scholar]
- 24.Rock CO, Cronan JE. Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim Biophys Acta. 1996;1302(1):1–16. doi: 10.1016/0005-2760(96)00056-2. [DOI] [PubMed] [Google Scholar]
- 25.Sacco E, et al. The missing piece of the type II fatty acid synthase system from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2007;104(37):14628–14633. doi: 10.1073/pnas.0704132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Guja KE, Thomas ST, Garcia-Diaz M, Sampson NS. A distinct MaoC-like enoyl-CoA hydratase architecture mediates cholesterol catabolism in Mycobacterium tuberculosis. ACS Chem Biol. 2014;9(11):2632–2645. doi: 10.1021/cb500232h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koski KM, Haapalainen AM, Hiltunen JK, Glumoff T. Crystal structure of 2-enoyl-CoA hydratase 2 from human peroxisomal multifunctional enzyme type 2. J Mol Biol. 2005;345(5):1157–1169. doi: 10.1016/j.jmb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Biswas R, et al. Crystal structure of dehydratase component HadAB complex of mycobacterial FAS-II pathway. Biochem Biophys Res Commun. 2015;458(2):369–374. doi: 10.1016/j.bbrc.2015.01.119. [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, et al. Molecular basis for the inhibition of β-hydroxyacyl-ACP dehydratase HadAB complex from Mycobacterium tuberculosis by flavonoid inhibitors. Protein Cell. 2015;6(7):504–517. doi: 10.1007/s13238-015-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krissinel E. Stock-based detection of protein oligomeric states in jsPISA. Nucleic Acids Res. 2015;43(W1):W314–W319. doi: 10.1093/nar/gkv314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis. Nat Methods. 2007;4(2):147–152. doi: 10.1038/nmeth996. [DOI] [PubMed] [Google Scholar]
- 32.Prajsnar TK, Cunliffe VT, Foster SJ, Renshaw SA. A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell Microbiol. 2008;10(11):2312–2325. doi: 10.1111/j.1462-5822.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 33.van der Sar AM, et al. Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell Microbiol. 2003;5(9):601–611. doi: 10.1046/j.1462-5822.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 34.Drancourt M. Looking in amoebae as a source of mycobacteria. Microb Pathog. 2014;77:119–124. doi: 10.1016/j.micpath.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Deretic V, Fratti RA. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31(6):1603–1609. doi: 10.1046/j.1365-2958.1999.01279.x. [DOI] [PubMed] [Google Scholar]
- 36.Clay H, et al. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2(1):29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palha N, et al. Real-time whole-body visualization of Chikungunya Virus infection and host interferon response in zebrafish. PLoS Pathog. 2013;9(9):e1003619. doi: 10.1371/journal.ppat.1003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alahari A, et al. Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria. PLoS One. 2007;2(12):e1343. doi: 10.1371/journal.pone.0001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alahari A, et al. Mycolic acid methyltransferase, MmaA4, is necessary for thiacetazone susceptibility in Mycobacterium tuberculosis. Mol Microbiol. 2009;71(5):1263–1277. doi: 10.1111/j.1365-2958.2009.06604.x. [DOI] [PubMed] [Google Scholar]
- 40.Marrakchi H, Lanéelle M-A, Daffé M. Mycolic acids: Structures, biosynthesis, and beyond. Chem Biol. 2014;21(1):67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Pawełczyk J, Kremer L. 2014. The molecular genetics of mycolic acid biosynthesis. Microbiol Spectr 2(4):MGM2-0003-2013.
- 42.Verschoor JA, Baird MS, Grooten J. Towards understanding the functional diversity of cell wall mycolic acids of Mycobacterium tuberculosis. Prog Lipid Res. 2012;51(4):325–339. doi: 10.1016/j.plipres.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36(4):371–386. [PubMed] [Google Scholar]
- 44.Rao V, Fujiwara N, Porcelli SA, Glickman MS. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J Exp Med. 2005;201(4):535–543. doi: 10.1084/jem.20041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corrales RM, et al. Phosphorylation of mycobacterial PcaA inhibits mycolic acid cyclopropanation: consequences for intracellular survival and for phagosome maturation block. J Biol Chem. 2012;287(31):26187–26199. doi: 10.1074/jbc.M112.373209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Indrigo J, Hunter RL, Jr, Actor JK. Cord factor trehalose 6,6′-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149(Pt 8):2049–2059. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- 47.Geisel RE, Sakamoto K, Russell DG, Rhoades ER. In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guérin is due principally to trehalose mycolates. J Immunol. 2005;174(8):5007–5015. doi: 10.4049/jimmunol.174.8.5007. [DOI] [PubMed] [Google Scholar]
- 48.Bernut A, et al. In vivo assessment of drug efficacy against Mycobacterium abscessus using the embryonic zebrafish test system. Antimicrob Agents Chemother. 2014;58(7):4054–4063. doi: 10.1128/AAC.00142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubée V, et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother. 2015;70(4):1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 50.Dover LG, et al. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob Agents Chemother. 2007;51(3):1055–1063. doi: 10.1128/AAC.01063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods GL, et al. Susceptibility Testing of Mycobacteria, Nocardiae and Other Aerobic Actinomycetes: Approved Standard. 2nd Ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2011. pp. M24–A2. [Google Scholar]
- 52.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310(5755):1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 53.Renshaw SA, et al. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108(13):3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- 54.Toyooka K, Takai S, Kirikae T. Rhodococcus equi can survive a phagolysosomal environment in macrophages by suppressing acidification of the phagolysosome. J Med Microbiol. 2005;54(Pt 11):1007–1015. doi: 10.1099/jmm.0.46086-0. [DOI] [PubMed] [Google Scholar]
- 55.Brzostek A, et al. Either non-homologous ends joining or homologous recombination is required to repair double-strand breaks in the genome of macrophage-internalized Mycobacterium tuberculosis. PLoS One. 2014;9(3):e92799. doi: 10.1371/journal.pone.0092799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 59.Stoop EJM, et al. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis Model Mech. 2011;4(4):526–536. doi: 10.1242/dmm.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stover CK, et al. New use of BCG for recombinant vaccines. Nature. 1991;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]