Abstract
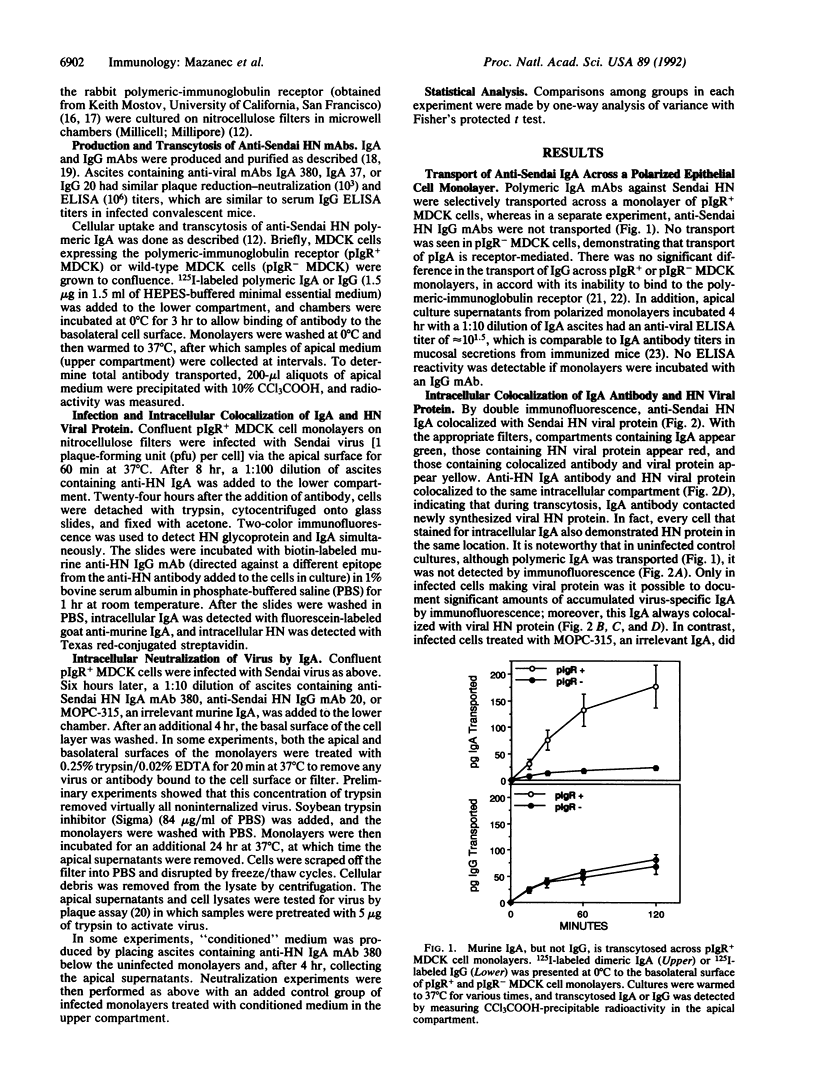
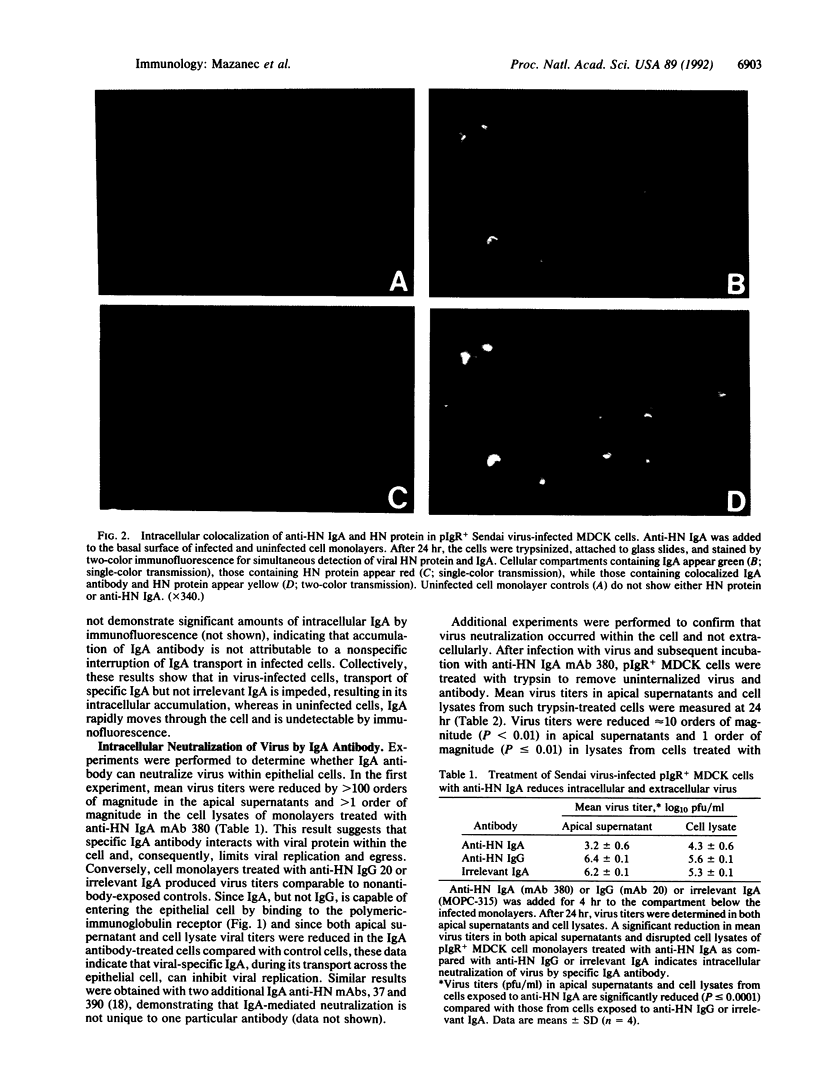
IgA is thought to neutralize viruses at the epithelial surface of mucous membranes by preventing their attachment. Since IgA, a polymeric immunoglobulin, is transported through the lining of epithelial cells by the polymeric-immunoglobulin receptor and since viruses are obligate intracellular parasites, we hypothesized that IgA antibodies may also interfere with viral replication by binding to newly synthesized viral proteins within infected cells. Polarized monolayers of Madin-Darby canine kidney epithelial cells expressing the polymeric-immunoglobulin receptor were infected on the apical surface with Sendai virus. Anti-Sendai virus IgA monoclonal antibody delivered from the basolateral surface colocalized with viral protein within the cell, as documented by immunofluorescence. More importantly, anti-viral IgA reduced virus titers greater than 1000-fold (P less than 0.0001) in apical supernatants and greater than 10-fold (P less than 0.0001) in cell lysates from monolayers treated with anti-viral IgA compared with those treated with either anti-viral IgG or an irrelevant IgA monoclonal antibody. We believe that the differences in viral titers between cell layers treated with specific IgA, which enters the epithelial cell by binding to the polymeric-immunoglobulin receptor, and those treated with specific IgG, which does not enter the cells, or irrelevant IgA indicate that specific intracellular IgA antibodies can inhibit viral replication. Thus, in addition to the classical role of humoral antibodies in extracellular defense, IgA antibody may be able to neutralize microbial pathogens intracellularly, giving IgA a role in host defense that has traditionally been reserved for cell-mediated immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breitfeld P. P., Casanova J. E., Harris J. M., Simister N. E., Mostov K. E. Expression and analysis of the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells using retroviral vectors. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Childers N. K., Bruce M. G., McGhee J. R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- Eichelberger M., Allan W., Zijlstra M., Jaenisch R., Doherty P. C. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991 Oct 1;174(4):875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M. A., Kaetzel C. S., Davis P. B. Sustained production of secretory component by human tracheal epithelial cells in primary culture. Am J Physiol. 1991 Oct;261(4 Pt 1):L255–L261. doi: 10.1152/ajplung.1991.261.4.L255. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel C. S., Robinson J. K., Chintalacharuvu K. R., Vaerman J. P., Lamm M. E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris R. M., Yetter R. A., Cogliano R., Ramphal R., Small P. A. Passive serum antibody causes temporary recovery from influenza virus infection of the nose, trachea and lung of nude mice. Immunology. 1988 Mar;63(3):349–353. [PMC free article] [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- Mazanec M. B., Nedrud J. G., Lamm M. E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987 Aug;61(8):2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec M. B., Nedrud J. G., Liang X. P., Lamm M. E. Transport of serum IgA into murine respiratory secretions and its implications for immunization strategies. J Immunol. 1989 Jun 15;142(12):4275–4281. [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mills J., 5th, Van Kirk J. E., Wright P. F., Chanock R. M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J Immunol. 1971 Jul;107(1):123–130. [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Outlaw M. C., Dimmock N. J. Mechanisms of neutralization of influenza virus on mouse tracheal epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the viral haemagglutinin. J Gen Virol. 1990 Jan;71(Pt 1):69–76. doi: 10.1099/0022-1317-71-1-69. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach G., Lamm M. E., Gigli I. Activation of the guinea pig alternative complement pathway by mouse IgA immune complexes. J Exp Med. 1982 Jan 1;155(1):231–247. doi: 10.1084/jem.155.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. O., Russell M. W., Brown T. A., Mestecky J. Selective hepatobiliary transport of human polymeric IgA in mice. Mol Immunol. 1984 Oct;21(10):907–914. doi: 10.1016/0161-5890(84)90147-0. [DOI] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991 Apr;65(4):2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Palladino G., Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992 Jan 1;148(1):212–217. [PubMed] [Google Scholar]
- Schwartz A. L. Cell biology of intracellular protein trafficking. Annu Rev Immunol. 1990;8:195–229. doi: 10.1146/annurev.iy.08.040190.001211. [DOI] [PubMed] [Google Scholar]
- Snider M. D. Remodeling of glycoprotein oligosaccharides after endocytosis: a measure of transport into compartments of the secretory apparatus. Methods Cell Biol. 1989;32:339–350. doi: 10.1016/s0091-679x(08)61179-6. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W., Geuze H. J., Griffith J. M., Strous G. J. The pathways of endocytosed transferrin and secretory protein are connected in the trans-Golgi reticulum. J Cell Biol. 1988 Jun;106(6):1821–1829. doi: 10.1083/jcb.106.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K., Maru M., Sato K. A sensitive plaque assay for Sendai virus in an established line of monkey kidney cells. Jpn J Microbiol. 1974 May;18(3):262–264. doi: 10.1111/j.1348-0421.1974.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Colombatti M. Hybridoma cells containing intracellular anti-ricin antibodies show ricin meets secretory antibody before entering the cytosol. J Biol Chem. 1987 Apr 5;262(10):4676–4682. [PubMed] [Google Scholar]