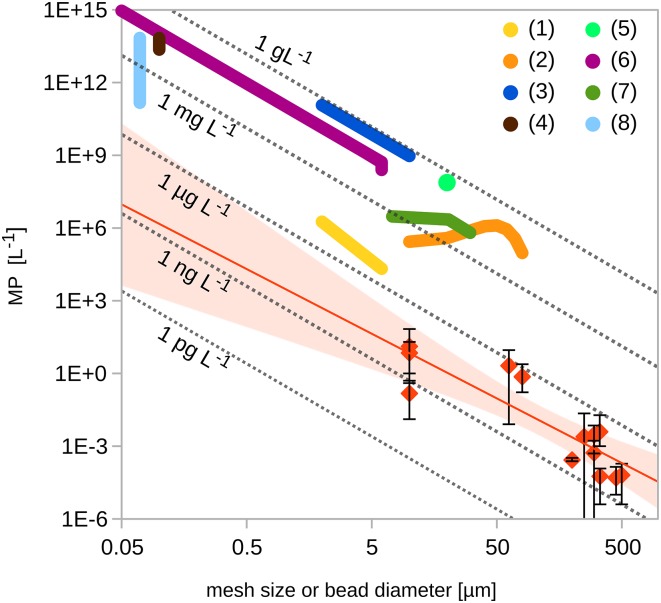
To understand the impact of microplastic (MP) pollution to aquatic ecosystems, it is important to identify the mechanisms of interaction with organisms. Exposure experiments, like the study of Sussarellu et al. (1) recently published in PNAS, may provide such insights. However, the results of dose–response experiments must always be interpreted in light of environmental concentrations, and the experimental concentrations examined by Sussarellu et al. (1) and several others (2–8) are orders-of-magnitude higher than those reported from field studies (Fig. 1).
Fig. 1.
Comparison between MP concentrations used in exposure studies (fat colored lines) and observed environmental levels (red diamonds: average concentrations; error bars: minimum and maximum concentration). The red line extrapolates the field data with best fit using a power law regression (y = 3,188 ⋅ x−2.67; 95% confidence intervals as pale red areas). The dotted gray isolines show equal mass concentrations for particles density = 1.04 g ⋅ cm−3. The x-axis scale is the diameter of the micro beads in exposure experiments and the mesh size used in environmental studies, respectively.
Experimental studies on effects of MP on mussels (2, 3), lugworms (4), copepods (5–7), and oysters (1) have documented reduced feeding, survival, and fecundity, as well as promoted polychlorinated biphenyl bioaccumulation linked to MP uptake. In addition, nano-sized plastic particles may reduce body size and lead to reproductive malfunctioning in Daphnia (8), and to reduced CO2 uptake and enhanced production of reactive oxygen species in algae (9).
All of these experimental studies use MP concentrations far above the levels documented in the marine environment (Fig. 1). Many of those studies used MP beads smaller than those reported from the field, but even if one extrapolates field-measured concentrations of MP particles to the size range used in experiments, experimental and expected field concentrations are very far apart. Studies that determined concentrations for micro-bead exposures from field measurements of larger-sized MP assumed a mass-conserving fragmentation (Fig. 1), which compares to a theoretical 3D fragmentation process, where numbers of particles will scale inversely with the particle radius to the power of 3. In contrast, the environmental concentrations documented in studies appear to follow a slightly lower exponent (2.67) (Fig. 1), possibly caused by size-dependent removal processes, lower dimensional breakdown (i.e., of flakes, sheets, and fibers), or considerable influence of new MP input of larger sizes. Additionally, decreased detection accuracy of applied sampling methods in their respective lower size ranges might play a role.
Experimental exposure concentrations tend to be between two to seven orders-of-magnitude higher than environmental levels. The most recent study (1) used concentrations closest to those found in nature. However, Sussarellu et al. calculate their MP concentrations based on sediment data from a pollution hot-spot area close to a ship-breaking yard in India (8, 10); thus, these data are unlikely to be representative of general concentrations beyond the local area.
Microplastic research is an emerging field, and there is a lot of misunderstanding and in some cases overreaction or misinterpretation of results from MP science in the public. We therefore strongly suggest that future studies of MP impact on marine ecosystems should also include concentrations that have been documented in the environment to yield more realistic estimates of sublethal effects.
Acknowledgments
The authors thank Thomas Kiørboe and Mary Wisz for helpful comments on this letter.
Footnotes
The authors declare no conflict of interest.
Data deposition: A fully referenced version of the data review has been deposited at figshare.com, accessible under: https://figshare.com/articles/Size_and_concentration_comparison_for_microplastics_between_reported_environmental_levels_and_laboratory_exposure_studies/3206449.
References
- 1.Sussarellu R, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci USA. 2016;113(9):2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Moos N, Burkhardt-Holm P, Koehler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46(20):11327–11335. doi: 10.1021/es302332w. [DOI] [PubMed] [Google Scholar]
- 3.Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L) Environ Sci Technol. 2008;42(13):5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 4.Besseling E, Wegner A, Foekema EM, van den Heuvel-Greve MJ, Koelmans AA. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.) Environ Sci Technol. 2012;47(1):593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- 5.Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol. 2015;49(2):1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 6.Lee KW, Shim WJ, Kwon OY, Kang JH. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ Sci Technol. 2013;47(19):11278–11283. doi: 10.1021/es401932b. [DOI] [PubMed] [Google Scholar]
- 7.Cole M, et al. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013;47(12):6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- 8.Besseling E, Wang B, Lürling M, Koelmans AA. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol. 2014;48(20):12336–12343. doi: 10.1021/es503001d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya P, Lin S, Turner JP, Ke PC. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C. 2010;114(39):16556–16561. [Google Scholar]
- 10.Reddy MS, Basha S, Adimurthy S, Ramachandraiah G. Description of the small plastics fragments in marine sediments along the Alang-Sosiya ship-breaking yard, India. Estuar Coast Shelf Sci. 2006;68(3-4):656–660. [Google Scholar]