Abstract
Cancer cells propagated in three-dimensional (3D) culture systems exhibit physiologically relevant cell-cell and cell-matrix interactions, gene expression and signaling pathway profiles, heterogeneity and structural complexity that reflect in vivo tumors. In recent years, development of various 3D models have improved the study of host-tumor interaction and use of high-throughput screening platforms for anti-cancer drug discovery and development. This review attempts to summarize the various 3D culture systems, with an emphasis on the most well characterized and widely applied model - multicellular tumor spheroids. This review also highlights the various techniques to generate tumor spheroids, methods to characterize them, and its applicability in cancer research.
Keywords: tumor emboli, apoptosis, high throughput screening, inflammatory breast cancer, oxidative stress, invasion
1. Introduction
Compelling evidence from two decades of research has revealed the critical role of tumor microenvironment (TME) in cancer development and progression (Mbeunkui et al., 2009; D Quail et al., 2013). The cellular components of the TME (transformed epithelial cells, cancer associated fibroblasts (CAFs), tumor infiltrating mesenchymal stem cells (MSCs), tumor infiltrating lymphocytes (TILs), and endothelial cells) interact with tumor cells and impact various biological characteristics such as proliferation, migration, and therapeutic resistance (Wong et al., 2000; Zhu et al., 2009; Joyce et al., 2009; Loebinger et al., 2009; Baker et al., 2012; DF Quail et al., 2013; Kyurkchiev et al., 2014; Smith et al., 2014; Fedorenko et al., 2015; Karakasheva et al., 2015; Yulyana et al., 2015). The non-cellular components of the TME (extracellular matrix (ECM), growth factors, cytokines, and chemokines) play an equally significant role in cancer progression, by presenting cues that affect fundamental aspects of tumor-cell biology (Paszek et al., 2005; Levental et al., 2009; Lu et al., 2012). Dynamic changes in ECM architecture are detected and transduced through transmembrane cell adhesion molecules like integrin, which in turn can activate signaling pathways, causing changes in tumor cell behavior (Fiorilli et al., 2008).
In two-dimensional (2D) culture systems, cells are grown as monolayers on flat solid surface, lacking cell-cell and cell-matrix interactions that are present in native tumors. Additionally, 2D-cultured cells are stretched and undergo cytoskeletal rearrangements acquiring artificial polarity, which in turn causes aberrant gene and protein expression (Cukierman et al., 2001; Nickerson et al., 2001; Kelm et al., 2003; Delarue et al., 2014). In contrast, three-dimensional (3D) culture systems offer the unique opportunity to culture cancer cells alone or with various cell types in a spatially relevant manner, encouraging cell-cell and cell-matrix interactions that closely mimic the native environment of tumors (Baal et al., 2009). These interactions cause the 3D-cultured cells to acquire morphological and cellular characteristics relevant to in vivo tumors (Ma et al., 2012). Some examples include breast cancers cells co-cultured with luminal cells, myoepithelial cells and stromal fibroblasts in 3D exhibit features reflective of ductal carcinoma in situ (Holliday et al., 2009); Ewing tumor MCTS closely resemble patient tumors in context of ERK1/2 MAPK and PI3K ± AKT pathway activation, cell–cell junctions and proliferative index (Lawlor et al., 2002). Comparison of gene and protein expression reveal that metabolic, cell stress-response, structural, signal transduction, and cellular transport proteins are expressed at elevated levels in spheroids compared to 2D-cultured cells (Hickey et al., 2008; Weigelt et al., 2008). Moreover, cell adhesion and junction proteins that influence cell aggregation and compaction can be upregulated in spheroids compared to cells in monolayer (Kang et al., 2007; Oktem et al., 2014). Taken together these studies demonstrate the advantages of using 3D culture systems for in vitro oncology studies, as they allow evaluation of TME’s effect on tumor, bridging the gap between 2D culture models and in vivo whole animal systems.
2. Various 3D culture models of tumor
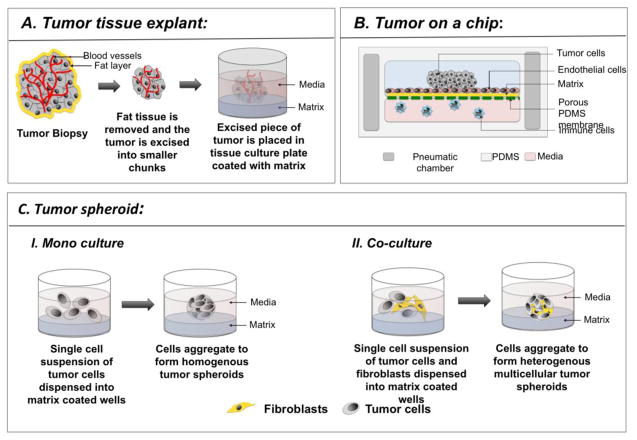
The predominant 3D culture models of cancer include: a) tumor tissue explant, b) “tumor on a chip”, and c) multicellular tumor spheroids (MCTS) (Figure 1, Table 1).
Figure 1.
Schematic representing the various 3D models of cancer. A. Excised tumor biopsy is processed to remove the excess fat and necrotic cells, and cut into small pieces. After washing the tumor in PBS, it is placed on a tissue culture plate that has been coated with a matrix, such as Matrigel of methylcellulose, to which the tumor sits atop firmly or is embedded. Media is added and the tumor is cultured for the duration of the experiment. B. “Tumor on a chip” represents a vasculature mimicking microfluidic device consisting of PDMS chambers with highly organized microchannels and pneumatic chamber (dark grey) on either sides. The microchannels (pink) contain media, in which immune cells and circulating tumor cells navigate. The top chamber contains matrix coated (yellow) porous membrane (green), with a monolayer of endothelial cells on top. The tumor cells are loaded through an inlet into the top chamber. Cells that have been genetically modified to express fluorescent protein can be observed in real time to monitor their functional changes, such as invasion, and migration. C. Schematic depicting tumor spheroid formation where tumor spheroids have been generated by culturing tumor cells alone or in combination with fibroblasts.
Table 1.
3D culture systems of tumor
2.1. Tumor tissue explant
“Tumor tissue explant” is one of the earliest 3D models of cancer and involves culturing excised human tumors in tissue culture plates (Ritter et al., 2007). This model has been used mainly for in vitro testing of drug efficacy. In this method, tumor tissue collected after biopsy is cleared of necrotic tissue and is placed on collagen-coated surface, where it adheres to or gets embedded within the collagen (Figure 1A). Media is added and the tumor is cultured for a desired period of time, followed by intratumoral injection with test compounds (Freeman et al., 1986). Preservation of the original tumor tissue architecture, including the cellular and non-cellular components of the TME, is one of the advantages of this technique. However, the major drawback of this model is lack of reproducibility owing to natural heterogeneity of donor tissues. Additional limitations of tumor tissue explants include difficulty in application of investigative techniques like imaging and flow cytometry, and maintenance of culture for more than 3 weeks without tissue degeneration.
2.2. “Tumor on a chip”
“Tumor on a chip” is a revolutionary microengineered biomimetic model that involves fabrication of a functional unit of tumor on a microfluidic device. The device allows co-culture of tumor cells with other cell types in a spatially relevant manner replicating the tumor microenvironment (Albanese et al., 2013; Esch et al., 2015). The microfluidic device consists of microwells (250 μm – 450 μm), connected by vasculature mimicking microfluidic channels, the geometries of which can vary from simple and straight to a complex array of micro-channels. An array of micro-channels are etched or molded onto surfaces of inert materials, such as glass, silicon, and polydimethylsiloxane (PDMS). The tumor cells grow above underlying layers of matrix coated porous membrane and endothelial cells, while immune cells and circulating tumor cells (CTC) navigate through the micro-channels (Figure 1B). Custom microfabrication of the chip and real time data recording are some of the advantages of this technique. Thus, “Tumor on a chip” model provides new avenues for genomic and drug screenings, in addition to detection of circulating tumor cells (CTC) (Alessandri et al., 2013).
2.3. Multicellular tumor spheroids (MCTS)
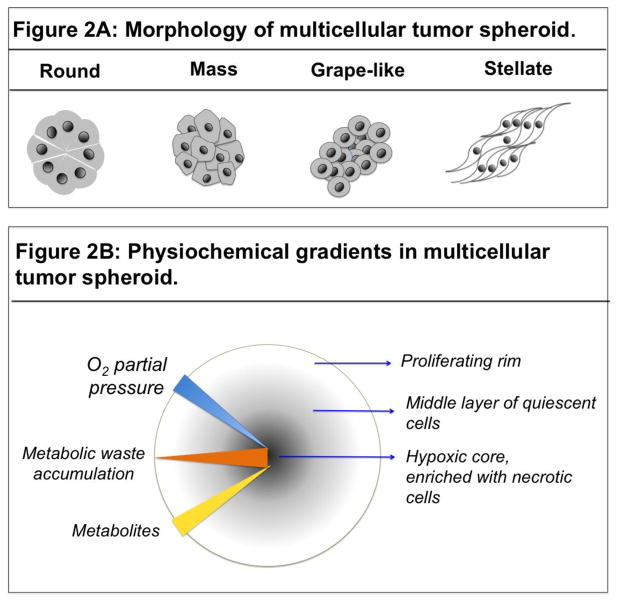
MCTS is the most well characterized organotypic model of cancer. MCTS are constructed from tumor cells alone or in combination with other cell types with or without scaffolds (Baal et al., 2009) (Figure 1C). Tumor spheroids display various morphologies depending on the inherent nature of the cell and the culture conditions (Figure 2A).
Figure 2.
A: Schematic representing the various morphologies of tumor spheroids. Tumor spheroids adapt various shapes depending upon the culture conditions and inherent nature of the tumor cells. B. Schematic representing the presence of physiochemical gradients in a spheroid and the resulting complexity in spheroid composition. Availability of the O2 (blue triangle) and nutrients (yellow triangle) diminishes with increasing depth of the spheroid. Whereas, metabolic waste accumulation (red triangle) is highest at the core compared to the peripheral layer of the spheroid. Hypoxia at the core (blue arrow) of the spheroid triggers necrosis (black circle), which precedes a layer of apoptotic cells. A middle layer of quiescent cells is sandwiched between the necrotic core and the peripheral layer of proliferating cells.
MCTS is an attractive model as it recapitulates the in vivo tumor cell characteristics with respect to growth kinetics, cellular heterogeneity, signal pathway activity, and gene expression (Table 2) (Friedrich et al., 2009). Large MCTS (>500μm in diameter) display physiochemical gradients similar to micrometastases and avascular tumors of size 0.5–1mm3, caused due to limited diffusion of O2, nutrients, metabolic waste, and soluble factors (cytokines, growth factors, and chemokines), making them an ideal model for studying the effects of these physiochemical gradients on tumor cell characteristics (Groebe et al., 1991; Mehta et al., 2012). Hypoxia induced by O2 deficiency triggers changes in gene expression, promoting aerobic glycolysis and lactic acid production, lowering pH (0.6 pH units) of the inner layer of cells (Alvarez-Pérez et al., 2005). Additionally, metabolic waste buildup triggers necrotic death of cells at the core. Cross-section of MCTS reveals concentric rings of heterogeneous cell populations, comprising of an innermost layer of necrotic cells with apoptotic cells in the peri-necrotic zone, surrounded by a middle layer of quiescent viable cells, and an outermost layer of highly proliferative and migratory cells (Figure 2B) (Bell et al., 2001; Hirschhaeuser et al., 2010). A large number of cancer cells have been cultured using MCTS model (Table 3). This model is of particular significance for studying cancers that are characterized by pathological presence of a closely packed tumor cell cluster called a “tumor embolus”. Inflammatory breast cancer (IBC), a lethal subtype of breast cancer, is an example of such a cancer. IBC is marked by the presence of dermal and stromal tumor emboli in breast tissue, a hallmark of the disease. Upon obstructing lymphatic vessels, tumor emboli prevent proper drainage of the lymph fluid, causing skin reddening and painful swelling of breast tissue (Vermeulen et al., 2010; Lehman et al., 2013). IBC cells display high ALDH positivity, express high levels of E-cadherin, and interestingly, continue to express elevated levels of epithelial cell markers like E-cadherin, while gaining mesenchymal and stem-like characteristics (Nguyen et al., 2006; Charafe-jauffret et al., 2010; Cohen et al., 2015). In addition, activation of anti-apoptotic and antioxidant signaling cascades allow the IBC cells to survive in the presence of various cell death signals, leading to therapeutic resistance (Thomas et al., 2011; Allensworth et al., 2013; Williams et al., 2013; Price et al., 2015; Evans, 2016). In particular, the MCTS model has been identified to possess features that are more suitable for high throughput screening assays (Kunz-Schughart et al., 2004).
Table 2.
Key features of tumor cells in monolayer, spheroids and in-vivo tumors.
| Features | Cells in monolayer | Spheroids | In-vivo tumor |
|---|---|---|---|
| Spatial restriction of cells | ✓ |
|
|
| Concentration gradient of O2, nutrients, and metabolic wastes |
|
✓ | ✓ |
| Heterogenous clonal subpopulations | ✓ | ✓ | ✓ |
| Hypoxic core |
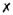
|
✓ | ✓ |
| Biological zones – proliferative, quiescent and necrotic zones |
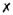
|
✓ | ✓ |
| Cancer stem cell niche |
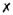
|
✓ | ✓ |
| Glucose flux rate | low | high | high |
| Gene expression profile | Different | Similar | Similar |
Table 3.
List of cancer cells that have been cultured in 3D systems.
| Tumor type | Cell Line | Biomaterial | Applications | Refrence |
|---|---|---|---|---|
| Breast Cancer | BT-20, MCF-7 | Polydimethylsiloxane (PDMS) | Findings suggested that the 3D spheroids are more resistant than 2D cultured cells to TRAIL mediated apoptosis and have stem like characteristics (CD44hi, CD24lo, ALDHhi caused due to activation of COX-2/PGE-2 signaling pathways. | Chandrasekaran, PlosOne, Oct 2014, volume 9, Issue 10 |
| 25 cell lines of Luminal, Basal A and B subtyes. | Matrigel | Comparative analysis of gene expression and signaling was performed between 25 B C cells grown in 2D and 3D cultures. | Paraic A Kenny, Molecular Oncology, 2007 | |
| Lung cancer | H1437, H356, H2170, A549, Chago Kl, H23, H1703 | Laminin rich ECM | Lung cancer cells grown on laminin rich ECM can be differentiated into different subtypes based on their growth patterns as smooth and branched structures. | Magdelena A cinchona, Intergrated Biol 2012, volume 4, issue 4. |
| Prostate Cancer | RWPE-2 LNCaP |
Polydimethylsiloxane (PDMS) | Findings suggested that the 3D spheroids are more resistant that the 2D cultured cells to chemotherapeutic drugs | Karen F Chambers et al PlosONe, Nov 2014, Volume 9, Issue 11. |
| PC-3, PC-3M, PrCa, MDA-Pca-2b, NCI-H660 | Matrigel | PrCa did not form spheroids whereas, PC-3 and PC-3M form well differentiated spheroids which transform into invasive cells. Targeting PI3K pathway blocked growth of the invasive cells in spheroids. | Harma V. Virtanen, PlosOne, 2010, Volumes, Issues 5. | |
| Colorectal Cancer | SW-480, HT-29, DLD-1, LOVO, CACO-2, CACO-205, COLO-206F, | Laminin rich ECM | The migratory, invasive or proliferative capacity of the cells did not change on forming spheroids. However, the gene expression profile of the spheroids altered significantly compared to 2D cultures. Also inhibition of EGFR is less effective in spheroids. | Anna C Luca, Plos One, 2013, Volume 8, Issue 3. |
| Ovarian cancer | MLS | Agar | Growth and radiation sensitivity was measured in 3D spheroids of l00 uM diameter consisting of 20 cell cluster. | R.M. Sutherland, British Journal of Cancer, 1989 |
| HEYA8, SKOV3, HEY, | Ultra-low attachment plate | Differential viral oncolytic efficacy was measured in 3D models of epithelial ovarian cancer | Trevor G Shepherd, Molecular Therapy-Oncolytics, 2015 |
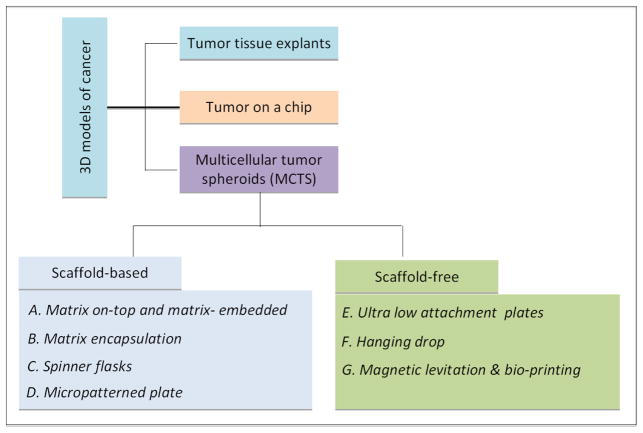
3. Techniques for generating MCTS
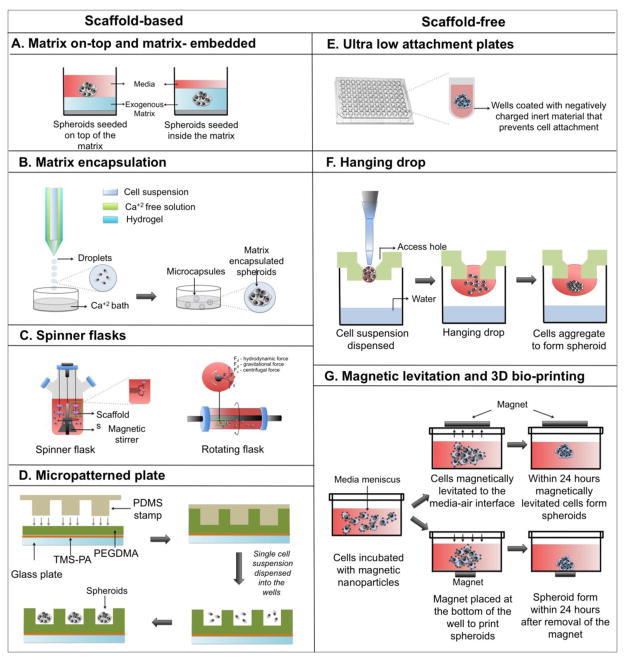
Several methods have been developed over the years to generate spheroids, such as matrix-on top, matrix embedded, matrix-encapsulation, spinner flasks, micropatterned plates, ultra-low attachment plates, hanging drop, magnetic levitation, and magnetic 3D printing (Figure 3). Each technique possesses certain advantages and limitations, as summarized in Table 4. Spheroid size and complexity depend on the growth kinetics of individual cell types, cell density during seeding, duration of culture, and spatial limitations, such as the diameter of culture wells. Since spheroid size and heterogeneity can influence robustness of endpoint assays, it is critical to generate spheroids of uniform size and complexity for biochemical assays and high throughput screening. Typically 48-hour long cultures generate small spheroids (200μm in diameter) of uniform size and homogeneity (Winters et al., 2006), whereas long-term cultures (>4 days) generate large (>500μm in diameter) heterogeneous spheroids with hypoxic core and cells of different proliferation kinetics, thereby making them suitable for pathophysiological studies.
Figure 3.
Schematic explains the various methods to generate tumor spheroids. Figures AD represent scaffold-based methods, whereas figures E–F represent scaffold-free methods.
Table 4.
Advantages and disadvantages of the different techniques employed to generate spheroids.
| Techniques | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Matrix on-top and matrix-embedded |
|
|
|
| Matrix encapsulation (microfluidic device) |
|
|
|
| Micropatterned plates |
|
|
|
| Hanging drop |
|
|
|
| Ultra low attachment plates |
|
|
|
| Magnetic levitation and Magnetic Bio-printing |
|
|
3.1. Scaffold-based MCTS
In scaffold-based MCTS, the biologically active scaffolds not only support 3D organization of cancer cells but also act as a source of external cues that promote cell-cell and cell–matrix interactions and influence tumor cell functions. The scaffolds that are commonly used in 3D culture systems include ECM-based natural hydrogels, synthetic hydrogels, and engineered hydrogels that mimic native ECM (synthetic hydrogel with integrin binding motif).
Hydrogels are water-insoluble, extensive network of cross-linked synthetic or natural polymers with tissue-like elastic properties. Hydrogels possess high water retaining capacity due to interconnected microscopic pores (Tibbitt et al., 2009), which facilitates easy transport of O2, nutrients, metabolic wastes, growth and other soluble factors through the porous channels (Nguyen et al., 2002). Hydrogels are derived from natural, synthetic or semi-synthetic, engineered polymers, and each of which possess certain advantages and disadvantages (Table 5A). Matrigel is a popular commercially available ECM-based natural hydrogel derived from secreted basement membrane extracts of Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells. Matrigel is rich in ECM components, such as laminin, collagen, heparin sulfate proteoglycans, entactin, and several soluble factors. Hydrogels constructed from natural polymers have endogenous chemokines and growth factors, which contribute to the viability and growth-promoting properties of natural hydrogels. However, the endogenous soluble factors add variability to the culture conditions making it difficult to obtain reproducible assay results. The presence of integrin-binding ligands on the natural polymers allows signal transduction through the transmembrane proteins and enable cells to respond to changes in the microenvironment. In addition, the hydrogels constructed from natural polymers have low tensile strength, increasing the likelihood of rapid degradation upon manipulation of their physical properties. In contrast, hydrogels constructed from synthetic polymers [poly (ethyl glycol), poly (vinyl alcohol), poly (2hydroxy methacrylate)], poly-2-hydroxyethyl-methacrylate are biologically inert but have high tensile strength, tunable mechanical properties, and give reproducible assay results. Swelling properties and permeability of synthetic hydrogels can be adjusted with external stimuli (Ahmed, 2015), such as changes in pH (Gupta et al., 2002), temperature (Klouda et al., 2008), light (Tomatsu et al., 2011), and electric field (Murdan, 2003) (Table 5B). This greater control of hydrogel swelling facilitates controlled release of biological factors (cytokines, chemokines, growth or angiogenic factors) and drugs with distinct kinetics (Richardson et al., 2001; Ehrbar et al., 2008). Semi-synthetic bioengineered hydrogels (PEGylated fibrinogen) (Mironi-Harpaz et al., 2014) are becoming increasingly popular nowadays, where motifs or active peptide sequences, such as integrin-binding sites Arg-Gly-Asp (RGD), (found within fibronectin), Tyr-Ile-Gly-Ser-Arg (YIGSR), and Ile-Lys-Val-Ala-Val (IKVAV) (found within laminin) are incorporated within the polymeric backbone. Magnetic hydrogels are infused with magnetic nanoparticles, which allow for greater control of the swelling and collapsing properties of the hydrogels using an external magnetic field (Jaganathan et al., 2014; Bumpers et al., 2015). Nano magnetic particles, such as magnetite (Fe3O4) (Souza et al., 2010), ferric oxide (Fe2O3) (Liu et al., 2010), cobalt ferrite (CoFe2O4) (Giani et al., 2012), iron platinum (FePt) are incorporated into hydrogels by crosslinking, blending, and in situ precipitation (Li et al., 2013).
Table 5A.
Hydrogel subtypes based on their source:
| Subtypes | Advantages | Disadvantages |
|---|---|---|
| Polymeric natural hydrogels |
|
|
| Polymeric synthetic hydrogels |
|
|
| Semisynthetic hydiogels |
|
|
| Molecular peptide hydrogels |
|
|
Table 5B.
Hydrogel subtypes based on their tunable properties:
| Types of hydrogels | Advantages | Disadvantage |
|---|---|---|
| Thermo-responsive | The swelling properties can be controlled by adjusting temperature | High gelling temperature adjustments if too harsh can damage cells |
| pH-responsive | The swelling properties can be controlled by adjusting pH | pH adjustments if too harsh can damage cells |
| Electo-responsive | The swelling properties can be controlled by application of mild electric field | Hydrogels generally shrink, affecting culture conditions |
| Magnetic hydrogels | The swelling properties can be controlled by subjecting them under magnetic field | Overnight pretreatment required to coat cells with the magnetic hydrogel |
The hydrogelation process of some natural and synthetic hydrogels involves pH or temperature adjustments, which if exceedingly harsh can destroy cells. In contrast, self-assembling peptide-based molecular hydrogels (h9e, RADA16-I) require mild hydrogelation conditions and allow cells to remain viable during culture (Huang et al., 2012; Cormier et al., 2013). Some of the advantages of peptide-based hydrogels include easy recovery of cells post culture (Huang et al., 2013), engineering of hydrogels with enhanced gel-strength, biocompatibility and biodegradability via manipulation of peptide composition, length, and stereochemistry.
3.1.1 Matrix- on-top and matrix embedded
In this technique, cells are either seeded on top of a solidified layer of matrix or seeded along with liquid matrix such that the cells get embedded within the matrix upon gelation (Figure 3A). In both techniques, wells of the tissue culture plate are first pre-coated with a matrix, such as Matrigel, Methylcellulose, etc. Chilled Matrigel is dispensed onto the pre-chilled surface of the well and placed at 37°C to allow gelation. Alternatively, warm 1% agarose solution is dispensed into the wells with a hot micropipette tip, which undergoes gelation at room temperature. In matrix-on-top method, cells in single cell suspension are seeded on top of solidified matrix, followed by gentle agitation during incubation at 37°C. The cells spontaneously aggregate to form spheroids while remaining attached to the matrix (Allensworth et al., 2015; Ingeson-carlsson et al., 2015). In matrix embedded technique, cells suspended in liquefied matrix (chilled Matrigel) are dispensed onto the matrix pre-coated well and incubated at 37°C during which cells get embedded within the matrix upon gelation (Lee et al., 2007). This technique is commonly used to generate mammospheres. Post-culture processing and imaging of spheroids are relatively easy with the matrix-on-top method.
3.1.2. Matrix encapsulation
Matrix-encapsulated tumor spheroids are created using microfluidic devices and hydrogels. Although microfluidic devices with various designs are available, the basic principal remains the same, which involves enclosing droplets of cell suspension in a hydrogel shell forming microcapsules. One such microfluidic device comprises of three glass capillary tubes, which are filled with the cell suspension in the innermost capillary tube, calcium-free solution in the middle capillary tube, and a hydrogel solution in the outermost capillary tube (Figure 3B) (Alessandri et al., 2013). When droplets of cell suspension mixed with hydrogel falls into the calcium bath, the hydrogel undergoes gelation forming cellular microcapsules. The tumor cells within microcapsule assemble to form matrix-encapsulated spheroids. The calcium-free solution serves as a barrier, preventing diffusion of intracellular calcium to the hydrogel contained in outermost tube, which upon premature gelation may choke up the device. Self-assembling peptide- hydrogels minimize risk of damaging the microfluidic device as they do not require calcium for gelation (Mendes et al., 2012). To encapsulate cells, peptide solution (lyophilized powder of in-vitro synthesized peptides dissolved in sodium bicarbonate) is mixed with cell suspension followed by gelation at physiological temperature. Typically, the capsules are 200–300 μm in diameter and the shell thickness ranges from 5–35 μm. One of the advantages of this technique is that it allows fabrication of uniform sized microcarriers, thereby yielding homogenous spheroids. However, compaction induces slower growth rate and increased necrosis at the core.
3.1.3. Spinner flasks
Spinner flasks or bioreactors are more common for large-scale production of tumor spheroids, where cells are grown as multicellular spherical aggregates in stirred suspension culture (Kunz-Schughart et al., 1998). Two of the most commonly used bioreactors are spinner flasks and rotating flasks. The spinner flasks contain a magnetic stirrer at the center of the flask that ensures continuous distribution of O2 and nutrients throughout the medium. Stationary scaffolds are placed inside the flask suspended through a rod and the cells flow across the surface of the scaffolds through the moving fluid (Figure 3C). However, cells experience sheer force generated by continuous motion of the stirring bar, which adversely affects cellular physiology (Lin et al., 2008). Rotating flasks function similarly to the spinner flasks, but instead of using a stirring bar to keep the cells in suspension, the culture flask itself is rotated causing the cells to experience less force (Goodwin et al., 1993; Muhitch et al., 2000). Moreover, rotational speed of the flasks can be adjusted to obtain spheroids of desired sizes. One of the advantages of this technique is uncomplicated nutrient and waste product exchange. However, a homogenous distribution of cells throughout the scaffold is not achieved as most cells are predominantly found towards the periphery. The rotating flasks consist of a cylindrical vessel that is rotated at a constant speed such that the downward gravitational force is counterbalanced by upward hydrodynamic force, resulting in maintenance of the cells and scaffold in suspension (Figure 3C). The rotating fluid allows thorough mixing of nutrients and O2 throughout the media while remaining gentle to cells.
3.1.4. Micropatterned plates
Micropatterned plates consist of hydrogel-coated microwells of uniform size (150 – 600 μm). These plates yield large number of spheroids of uniform dimensions, making them ideal for high-throughput screening. In this technique, a glass plate is first coated with a layer of 3-trimethoxysilyl polymethacrylate (TMS-PA) followed by an even layer of hydrogel, such as polyethylene glycol dimethacrylate (PEGDMA). Polydimethylsiloxane (PDMS) stamp with micropillars are photocrosslinked to PEGDMA to construct microwells. The TMS-PA pre-coating ensures covalent attachment of the hydrogel microwells onto the glass plate (Figure 3D) (Singh et al., 2015). In direct photo patterning, UV exposure produces reactive oxygen species (ROS), which in turn makes the protein-repellent part of a molecule that has been grafted on the substrate to detach, allowing ECM protein to further bind onto the substrate (Théry, 2010). One advantage of direct photo patterning is that it does not require etching, like micro-contact printing (Monjaret et al., 2015). Some of the drawbacks of micropatterning include unequal protein transfer onto the substrate during stamping, requirement of dedicated chemistry to engineer photosensitive materials, and use of bio-incompatible photosensitizers.
3.2. Scaffold-free MCTS
Scaffold-free methods are suitable for culturing tumor cells, particularly inflammatory breast cancer cells that secrete ECM proteins and undergo self-aggregation into highly organized three-dimensional tissue-like structures. Compared to scaffold-based MCTS that comprise of compactly arranged cells with fibronectin expression localized to the peripheral rim, scaffold-free MCTS is comprised of loosely arranged cells with even distribution of fibronectin throughout the spheroid (Alessandri et al., 2013).
3.2.1. Ultra-low attachment plates
In ultra-low attachment plates (ULA) the wells are coated with an inert substrate (polystyrene), which blocks cell attachment and causes cells in suspension to aggregate into visible spheroids (Figure 3E)(Kelm et al., 2003; Vinci et al., 2012). In forced aggregation a mixture of multiple cell types of arbitrary numbers are seeded into an ultra-low attachment plate. The cells are briefly centrifuged at 200g for 5 minutes, allowing them to aggregate into multicellular heterogeneous spheroids (Baraniak et al., 2012; Zimmermann et al., 2014).
3.2.2. Hanging drop
Hanging drop is a scaffold-free technique. In this technique, droplets of cell suspension are dispensed onto the underside of a petri dish lid from which they hang due to surface tension. The cells in suspension spontaneously aggregate into spheroids under gravity. The petri dish contains phosphate buffered saline (PBS), which prevents dehydration of the droplets (Jørgensen et al., 2014) (Figure 3F). More recently, spheroid culture array plates have replaced the use of petri dishes. These array plates consist of an upper compartment with tiny holes to deploy hanging drops and a bottom compartment to hold PBS (Tung et al., 2011)(Torisawa et al., 2007). Although spheroids of defined size are obtained by this technique, it is one of the most labor-intensive methods to culture MCTS. Another drawback of this technique, which affects cell viability, is elevated osmolarity caused by evaporation of media from the droplets. To circumvent rapid evaporation of media relatively large volumes of droplets (e.g. 15–30 μL) are dispensed. However, this limits the number of number of spheroids that can be obtained in a given area.
3.2.3. Magnetic levitation and bioprinting
Magnetic levitation and 3D bioprinting have similar working principles. Both these techniques employ super paramagnetic iron oxide nanoparticles (SPIONs) that act as patterning agents to guide self-assembly of cells into spheroids under magnetic forces. Semi-confluent adherent cells are incubated overnight with SPIONS to allow cellular uptake (Whatley et al., 2014). Excess SPIONS are washed off and the magnetically labeled cells are trypsinized, counted, and re-seeded in low attachment plates. Immediately afterwards, a magnet is placed on top of the plate lid (in magnetic levitation) or beneath the plate (in magnetic bioprinting), during which the SPION labeled cells are pulled up or down respectively under magnetic forces. The cells self-aggregate into spheroids within few hours (Tseng et al., 2015; Leonard et al., 2016) (Figure 3G).
4. Tools for characterization of MCTS
Biochemical assays, microscopy, and flow cytometry are commonly applied for phenotypic and morphological analysis of tumor spheroids, to evaluate efficacy of anti-cancer agents, disrupt spheroid formation, and change tumor cell characteristics in pre-formed spheroids.
4.1. Microscopy
To measure the total cell count in MCTS, the spheroids are usually enzymatically disintegrated into single cell suspension, stained with trypan blue, and viewed under bright field microscope. However, this technique is incompatible with endpoint analysis that requires viable spheroids, as cell viability is poorly affected following destruction of spheroid integrity. High Content Assay (HCA) is a nondestructive live-cell imaging technique that allows simultaneous quantitative analysis of total cell count, density, dimensions, growth kinetics, nuclear mass, and mitochondrial membrane potential of live MCTS (Sirenko et al., 2015). HCA also allows high throughput screening of anti-cancer drug candidates (Arora et al., 2014).
Scanning electron microscopy generates high-resolution images of the superficial topography of MCTS, whereas transmission electron microscopy and multiphoton microscopy generates high-resolution 3D images of the internal structures of large MCTS (Ma et al., 2012). Confocal images of spheroids provide insightful information about cytoskeletal organization and in situ protein expression (Weiswald et al., 2010). The different biological zones (the outer proliferative rim, middle quiescent zone, and the dark necrotic core) can be visualized by staining MCTS cryosections with hematoxylin and eosin (Ma et al., 2012). Confocal and fluorescent microscope images of MCTS that are immunostained with antibodies against fibronectin, laminin, collagen IV, tenascin and other ECM proteins, are able to show ECM deposition (Correa de Sampaio et al., 2012). Staining with Hoechst or DAPI, phalloidin, Ki-67, caspases, Annexin V, Propidium iodide, and TUNEL can provide additional information about the morphology, cytoskeletal arrangement, proliferation, and live/dead status of the cells in spheroid (Bell et al., 2001; Ingeson-carlsson et al., 2015). Ultra-structural changes that occur during apoptosis and necrosis can be further visualized by electron microscopy (Bell et al., 2001; Uroukov et al., 2008).
Imaging large spheroids (>150 μm) by confocal microscopy is extremely challenging, mostly due to poor light and antibody penetration, and attenuation of fluorescent signal by light scattering. Additionally, mobility of spheroids in suspension makes imaging of live spheroids challenging. To stabilize the spheroids, a thermo-reversible cell-mounting agent called CyGEL is used. CyGEL is an inert, optically clear liquid with low auto-fluorescence, which rapidly reverts to a gel state above 21°C. CyGEL restricts spheroid movement without compromising the viability, morphology, and protein expression in spheroids (Robertson et al., 2010). Additionally, the small size and fragile nature of spheroids require special fixing and sectioning techniques (Olsen et al., 2014). High quality confocal images are obtained by culturing spheroids on clear glass bottom plates and using wet immersion objective (Roux et al., 2008). Compared to confocal microscopy, selective plane illumination microscopy (SPIM) creates high contrast images of large spheroids and allows monitoring of live cell division dynamics in spheroids. In SPIM, the specimen is illuminated perpendicularly to the axis of the microscope objective. This allows sequential focal sectioning of the specimen, resulting in high-resolution images (Verveer et al., 2007; Lorenzo et al., 2011). SPIM is specifically useful for obtaining high-resolution images of the hypoxic core in spheroids, which is difficult to image by conventional light microscopy. However, spatial variations in refractive index caused due to heterogeneity in spheroids may cause major shift in the light path, resulting in obscure images.
4.2. Biochemical assays
Poor O2 delivery coupled with metabolic waste accumulation affects viability of MCTS. Acid phosphatase (AP) and Resazurin are sensitive and high-throughput-compatible assays that measure cell viability (Vinci et al., 2012; Wen et al., 2013; Ivanov et al., 2014). Acid phosphatase released from the MCTS catalyzes dephosphorylation of the phosphate group of p-nitrophenyl phosphate (AP substrate) yielding a yellow colored product, which can be read by a colorimeter. The intensity of yellow color is an indication of the acid phosphatase activity and is therefore an indirect measurement of the number of live cells within MCTS. Resazurin reduction assay is a fluorescent assay that measures viability based on the metabolic activity of live cells. Dehydrogenase enzymes released by the metabolically active cells reduce the non-fluorescent blue substrate resazurin to fluorescent resorufin, the intensity of which can be read by a fluorescent plate reader. The relative fluorescence units are proportional to the number of metabolically live cells in MCTS.
4.3. Flow cytometry
Flow cytometry allows quantitative measurement of cell viability, proliferation kinetics, apoptosis and CSC phenotype analysis in MCTS. For live/dead cell analysis single cells are stained with calcein-AM and ethidium homodimer, which live cells and cells with damaged membrane respectively. Staining patterns enable quantification of viable cells and identification of the distinct subpopulations in MCTS – calcein stained proliferating cells; Calcein and ethidium-1 stained quiescent cells; and ethidium-1 stained cells of the necrotic core (Ma et al., 2012). However, spheroids are trypsinized into single-cell suspension for flow analysis, which destroys spheroid integrity, affecting cell viability in MCTS. In contrast, COPAS flow cytometers, which specially engineered fluidic system enabling measurement of particles ranging in size from 20–1500 μm, is a non-destructive technique that allows analysis of intact MCTS. Thus, COPAS flow cytometers allow accurate determination of viability and provide insightful knowledge about the cancer stem cells niche in MCTS.
5. Applications of tumor spheroids in cancer research
5.1. Hypoxia, oxidative stress, and cancer-cell metabolism
In large MCTS (>500 μm) O2 and ATP deficiency induces hypoxia (Bertuzzi et al., 2010). Increased ROS accumulation during oxidative stress stabilizes the transcription factor hypoxia inducible factor-1α (HIF-1α). HIF-1α has been shown to cause metabolic switch in tumor cells by modulating expression of genes involved in glucose uptake, glycolytic pathway, and glutamine consumption (Chandel et al., 2000; Sulkowska et al., 2009). This metabolic transition from mitochondrial oxidative phosphorylation to aerobic glycolysis and lactic acid fermentation, known as Warburg effect, generates ATP independently of oxygen. Increased glycolysis and lactate production causes slower cell cycle, acidification of the TME, and increased secretion of pro-angiogenic factors and ECM constituents. Lactic acid modulates activation and antigen expression abilities of dendritic cells (Gottfried et al., 2016). Interestingly, upregulation of glucose uptake in itself can activate oncogenic signaling pathways, such as EGFR, β1 integrin, MEK, and AKT, leading to loss of tissue polarity and increased growth (Onodera et al., 2014). Studies have indicated that tumor spheroids display significantly increased glucose consumption and lactate production compared to 2D-cultured cancer cells (Khaitan et al., 2006; Liao et al., 2014). Changes in the expression and activity of metabolic enzymes and substrate transporters also contribute to metabolic shift in cancer cells. For example, expression and activity of glucose transporter 1 (GLUT1) and several glycolytic enzymes, such as hexokinase, phosphofructokinase-1, pyruvate kinase and lactate dehydrogenase, glucose-6-phosphate dehydrogenase, and malate dehydrogenase increase during log phase of tumor spheroids (Longati et al., 2013; Bloch et al., 2014). Additionally, genes that are involved in lipid metabolism and de-novo lipogenesis are significantly upregulated in spheroids, which helps cancer cells to survive in low exogenous fat environment as commonly found in tumors (Smans et al., 2014; Takahashi et al., 2015).
O2 consumption by spheroids is determined by culturing spheroids in Oxoplates, a specially designed plate coated with a mixture of an oxygen-sensing indicator and reference dyes. Fluorescence intensities of the oxygen-sensing indicator and reference dyes are measured every few minutes for several hours, and total cell number is counted. Oxygen concentration at each time point (pO2) is calculated using the formulae as described in (Cook et al., 2012). Oxygen consumption rate is measured by calculating the ratio of oxygen concentration at each time point, divided by the total cells. The level of intracellular ROS is measured using the fluorescent dye 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA). Multicellular tumor spheroids are incubated with H2DCF-DA, which after cellular uptake is converted into a membrane-impermeable non-fluorescent polar derivative (H2DCF), catalyzed by cellular esterases. H2DCF is rapidly oxidized to fluorescent 2,7-dichlorofluorescein (DCF), and the fluorescence is read by confocal microscopy (Artenberg et al., 2003).
Direct measurement of hypoxia involves insertion of polarographic electrodes into spheroids, a technique restricted by both spatial and temporal dimensions. Some of the non-invasive methods of measuring hypoxia in MCTS include autoradiography, staining with fluorescent probes, immunohistochemistry, magnetic resonance imaging (MRI), and positron emission tomography (PET). Spheroids are incubated with a radiotracer fluoromisonidazole (3H-FMISO) that is taken up by live cells. Autoradiography reveals zones of heavily 3H-FMISO labeled (white silver grains) live proliferating cells, intermediately labeled quiescent cells, and unlabeled (dark) necrotic core (Rasey et al., 1985). Perkin Elmer’s in vivo near-infrared (NIR) agents allow visualization of hypoxic areas and quantification of cancer-associated biomarkers in live tumor microtissue or spheroids (Waschow et al., 2012). Fluorescent-based probes allow detection of hypoxic regions within MCTS by confocal or fluorescent microscopy. Non-fluorescent reductase-based probes are reduced to fluorescent probes by reductases, such as nitro-reductase (NTR), quinone-reductase (QR) and azo-reductase (AzoR), that are abundantly present in hypoxic regions (Hirokazu et al., 2010; Kehua et al., 2013; L Zhao et al., 2013; Sun et al., 2015). Necrotic areas within MCTS are visualized by confocal examination after labeling the cells with the hypoxic marker pimonidazole, a 2-nitroimidazole compound, which forms covalent bonds with cellular macromolecules at oxygen levels below 1.3% (Senkowski et al., 2015).
To measure glucose uptake, MCTS are stained with a fluorophore-labeled variant of 2-deoxy-D-glucose called IRDye800CW-2DG, followed by counter-staining with DRAQ5, an infrared dye that binds stoichiometrically to DNA. Detection of fluorescence using Odyssey infrared imaging system allows measurement of glucose uptake by cells. Another technique called imaging bioluminescence allows mapping and quantitative measurement of ATP, glucose, and lactate concentrations in different regions of the spheroid at high spatial resolution (Walenta et al., 2000). All these metabolites are measured based on an ATP dependent reaction, wherein luciferin is catalyzed by luciferase to oxoluciferin emitting light, the intensity of which is proportional to the tissue content of the metabolites (Tamulevicius et al., 2000). Glucose is avidly consumed by cancer cells and is metabolized into lactate and CO2, resulting in acidification of the interstitial space. Lactate release by spheroids into the culture media can be measured using lactate assay kits or YSI 2700 SELECT™ Biochemistry Analyzer (Longati et al., 2013).
5.2. Cancer cell invasion and migration
The features of cell migration on soft 3D matrix and stiff 2D surfaces are distinct. Migration of cells in 2D surfaces is lamellopodia-driven and accompanied by formation of focal adhesions which are integrin-based structures formed along the contact site of cell with the ECM substrate. In contrast, cells navigating through the matrix release matrix degrading metalloproteases (Zaman et al., 2006) and form invadopodia, which are spindle-like projections that radiate from the spheroid in all directions (Wolf, 2003; Stylli et al., 2008). Both focal adhesions and invadopodia mediate strong cell-substrate adhesion. The traction forces emanating from surfaces of a moving cell are transmitted intracellularly through these cell-adhesion molecules leading to activation of signal pathways and consequent changes in gene expression (Hynes, 1992; Schwartz et al., 1995; Geiger et al., 2011; Creed et al., 2015).
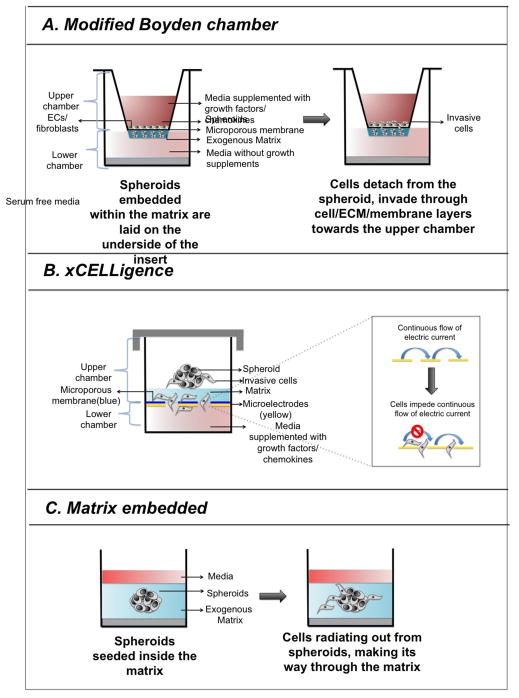
There are several methods to determine invasive potential of cells in spheroid. In modified Boyden chamber-based invasion assay, a thin layer of spheroid and liquid matrix mixture is coated onto the underside of an insert covering the entire surface of the porous membrane. The spheroids get embedded within the matrix upon gelation at room temperature (Figure 4A). Serum free media is added to the bottom chamber and media supplemented with growth factors that act as a chemoattractant is added to the top chamber. Within few hours cells begin to disseminate from the spheroid, proteolytically cleave through the matrix and migrate towards the upper chamber (Lehman et al., 2013). The invasive potential of cells through cellular barrier can be evaluated by further modifying the assay where endothelial cells are plated atop the porous membrane. After 24 hours, media is aspirated from the insert; spheroid-matrix layer is removed from the underside of the insert, and stained with crystal violet to visualize the invasive cells that have migrated to the upper side of the membrane. Alternatively, cells that have been genetically modified to express fluorescent protein can be imaged and counted by fluorescence microscopy. However, Boyden chamber-based assay does not allow real-time monitoring of cell invasion and the assay needs to be terminated with a limited window. Real Time Cell Analyzer (RTCA) is an alternative technique that allows precise and continuous monitoring of invasion over the course of the assay. RTCA assay uses specially designed culture plates called CIM plates that have gold-coated microelectrodes placed underneath an ECM coated microporous membrane. The membrane is situated at the interface of upper and lower chambers of a two chamber well (Figure 4B). A monolayer of mesothelial or endothelial cells are seeded on top of the matrix to further assess the invasive potential of cancer cells through the cellular barrier. Invasive cells from the MCTS migrate through the matrix/cell/microporous membrane and upon breaching the membrane cause electrical impedance, which is measured by xCELLigence RTCA instrument (Bilandzic et al., 2014). Celigo cytometer also allows real time monitoring of the invasive front, capturing images and calculating area occupied by the leading edge of invading cells (Vinci et al., 2012) (Figure 4C). Invasive index of cells are calculated as the percentage of cells with invasive extensions within the total number of cells (De Wever et al., 2014). Further, sophisticated techniques are available to study migratory patterns of spheroids guided by electric field or gradients of O2 (S Zhao et al., 2013; Mosadegh et al., 2015).
Figure 4. Schematic representing the various spheroid-based invasion assays.
A. In modified-Boyden chamber based invasion assay, a thin layer of spheroid and matrix mixture is coated on the. Within 72 hours, cells begin to disseminate from the spheroid, proteolytically cleave through the matrix and migrate towards the bottom chamber through a matrix coated porous membrane located at the interface of upper and lower chambers. A layer of endothelial cells placed on top of the matrix coated porous membrane allow to determine the invasive potential of the cells through matrix and cellular barriers. B. The schematic represents a CIM plate, which has similar components as Boyden chamber. However, the detection system is different in xCelligence, where invasive potential of tumor cells is determined by measuring electrical impendence imposed by them. C. Spheroid is seeded on a layer of matrix, such as Matrigel, Methylcellulose, and Collagen type I, which is followed by a second layer of matrix. Cells from the embedded spheroid detach from the spheroid, radiating outward, which is measured in real time by Celigo Cytometer.
5.3. Cancer stem cells and their niche
Cancer is thought to arise from cancer stem cells (CSC) or cancer cells with stemness that have the ability to self-renew, and differentiate into different cell types contributing to tumor heterogeneity. Flow cytometric analysis of CD44+/CD24−/low and CD133 CSC involve enzyme-assisted dissociation of tumor into single cell suspension. Unfortunately, sample preparation destroys tumor tissue integrity, which prevents investigation of CSC niche in tumors. Flow cytometric analysis of intact MCTS using COPAS flow cytometers can provide insightful knowledge about the CSC niche. COPAS flow cytometers consist of specially engineered fluidic system that enables measurement of particles ranging in size from 20–1500 μm. Another non-destructive method of studying CSC niche in MCTS involves confocal microscopy. MCTS generated from adherent cells that have been pre-labeled with nucleoside analogue (EdU) are cultured for 7 to 14 days. During this time the nucleoside analogue is diluted in the actively diving cells while being retained in the quiescent cells. The MCTS are fixed, permeabilized, and stained with nuclear stain (TO-PO-3) and visualized by confocal microscopy. Quantification of the cells that retain both EdU and TO-PO-3 reveal percentage of the “label retaining cells” (Robertson et al., 2010).
5.4. Tumor-microenvironment signaling crosstalk
Our current knowledge of the oncogenic signaling pathways and their therapeutic interventions are mostly based on 2D-cultured cells. However, compelling evidences from several studies suggest that the signaling pathways are activated differently in cells within MCTS compared to cells in monolayers. Some recent examples include identifying HER2 homodimerization in MCTS as opposed to HER2 and HER3 heterodimerization in 2D-cultured cells (Pickl et al., 2009), higher HER3 and EGFR activation in tumor spheroids compared to 2D-cultured cells (Pickl et al., 2009), and higher rate of acquired resistance to TRAIL-mediated apoptosis due to decreased expression of death receptors (DR4 and DR5) in breast MCTS (Chandrasekaran et al., 2014). One of the reasons for differential pathway activation between 2D- and 3D-cultured cells is difference in extracellular cues arising from the TME. The ECM components, such as laminin and fibronectin, provide crucial cues that influence cellular functions through activating intracellular signaling pathways. Sitting at the cell-matrix interface, integrins play a critical role in sensing changes in ECM and relaying them intracellularly by activating downstream signaling pathways. For example, binding of integrin α9β-1 to tenascin activates MAPK pathway that promotes survival and proliferation of the medulloblastoma cells (Fiorilli et al., 2008). Integrin αVβ3, overexpressed at the invasive front of malignant melanoma cells and angiogenic blood vessels, increases metastatic potential of melanoma cells (Felding-Habermann et al., 1992; Brooks et al., 1994). The role of focal adhesion kinase (FAK) has been observed in promoting anchorage-independent growth of breast cancer spheroids (Tancioni et al., 2015). Hence, culturing cells in laminin rich ECM (lr-ECM), ECM rich natural hydrogels, and synthetic hydrogels with integrin binding sites may provide the framework needed to study the ECM cues and their role in cancer progression. Spheroids cultured by matrix on-top and matrix-embedded methods enable the study of signal transduction between the ECM and tumor cells, and allow for the evaluation of their effect on the biological properties of tumor cells (Lee et al., 2007; Ritter et al., 2007; Pickl et al., 2009). Similarly cells in the TME play a critical role in cancer progression. Co-culturing MCTS with other cells types, such as immune cells and fibroblasts, can provide insights into host tumor cell interactions (Hauptmann et al., 1993; Esendagli, 2014). Microfluidic devices are suitable for such studies as tumor cells can be co-cultured with multiple cell types in presence of the ECM components.
5.5. Anti-cancer drug discovery
At the pre-clinical stage of drug discovery, in-vitro models are commonly used for high-throughput screening due to its low cost and rapid turnaround time compared to animal-based studies. However, the drug activity may differ considerably depending on the in-vitro model used for testing. For example, monolayers of breast cancer and HeLa cells are more sensitive to drugs compared to spheroids of these cells (Ma et al., 2012; Abuelba et al., 2015; Lovitt et al., 2015). Although several findings suggest that heterogeneity and physiochemical gradients in tumor spheroids reduce their sensitivity to drugs compared to monolayer of cells, it does not necessarily hold true under all circumstances. A recent study recently demonstrated higher sensitivity of the breast tumor spheroids to trastuzumab compared to 2D cultured cells, as the cells of the spheroid displayed increased activation and dependence to Her2 and Her3 signaling (Pickl et al., 2009). Trastuzumab blocked Her2 and Her3 activation and proliferation of spheroids but not 2D-cultured cells. Another study showed that RAF and MEK inhibitors block the invasion of thyroid carcinoma spheroids (SW1736) but have no effect on migration of SW1736 monolayer cells (Ingeson-carlsson et al., 2015).
Differences in drug distribution and penetration, generation of hypoxia and ROS, enhanced expression of multidrug resistant genes, activation of survival pathways increased cell-cell and cell-matrix adhesions, may explain the differences in drug activity between 3D-cultured and 2D-cultured cells (Vinci et al., 2012). Several studies have shown that increased cell-cell and cell-matrix adhesions may activate downstream signaling pathways leading to changes in gene expression, influencing sensitivity of the cancer cells to drugs. For example, enhanced expression of cell-adhesion molecules, such as lumican, SNED1, DARP32, and miR-146a, increases chemotherapeutic resistance in pancreatic tumor spheroids (Huanwen et al., 2009; Longati et al., 2013). Similarly, interaction of β1-integrin with collagen I, collagen IV, laminin, and fibronectin protects breast cancer and lung cancer cells lines from the cytotoxic effects of various chemotherapeutic drugs (Sethi et al., 1999; Aoudjit et al., 2001). Inhibition of integrin β1 significantly increases the sensitivity of Her2 hyperactivated breast cancer spheroids to trastuzumab and pertuzumab (Weigelt et al., 2010). Laminin-mediated signaling through focal adhesion kinase (FAK) promotes resistance in pancreatic cancer cell spheroids to gemcitabine (Huanwen et al., 2009). Insulin like growth factor-1 receptor upon interacting with fibronectin protects DU145 (prostate cancer cell spheroids) from cytotoxic effects of ceramide and docetaxel (Thomas et al., 2010). Additionally, increased resistance to chemotherapeutic drugs in spheroids may be attributed to activation of hypoxia resistant metabolic pathways, leading to enrichment of CSCs in spheroids (Liao et al., 2014). Generation of ROS in Nox-1 high prostate tumor spheroids drives overexpression of multidrug resistance transporter P-glycoprotein, thereby promoting drug resistance (Wartenberg et al., 2005). Moreover, the effect of tumor-stromal interactions on drug sensitivity of tumor cells can be very complex and context dependent, as demonstrated by a study where colon tumor spheroid co-cultured with stromal cells and treated with various combinations of drugs (Cetuximab, Trastuzumab, Vorinostat and Everolimus, 5-FU/oxaliplatin (FO), 5-FU/irinotecan (FI)) revealed that different microenvironment compositions alter sensitivity of tumor spheroids to drugs (Ingo et al., 2015). This finding underscores the importance of incorporating TME as a critical factor during high-throughput screenings. Poor vascularization and ECM mediated physical interference can slow down drug penetration, distribution, and immune cell infiltration within tumors (Netti et al., 2000). High-resolution 3D images of effector cell (NK cell, Cytotoxic T cell) infiltrating spheroids can be obtained using scanning electron microscopy and transmission electron microscopy (Ma et al., 2012; Klöss et al., 2015). Multi-photon microscopy enables visualization of the penetration of fluorescently labeled drugs and nanoparticles into spheroids (Ma et al., 2012; Zipfel et al., 2003).
6. Concluding remarks
In this review, we discussed about three different 3D models of cancer, while putting emphasis on MCTS as a tool for cancer research. We mainly focused on three key components – techniques for generating spheroids, assays for spheroid characterization, and their applications in oncology research. Although tumor spheroid is one the most widely used in-vitro 3D models, it presents some basic challenges to researchers, such as variability in spheroid size and homogeneity, poor light scattering, and impenetrability of antibodies inside the spheroid posing difficulties in imaging. Size, cell number, and cell density profoundly affect generation of the pathophysiological gradients and biological zones in MCTS, influencing their response to drugs. On one hand compact spheroids present challenges in imaging and drug distribution, on the other hand spheroids made of loosely arranged cells require special care in handling, as they tend to disintegrate easily. Although spheroids present physiochemical gradients and cellular heterogeneity like in-vivo tumors, geometry of drug penetration in spheroids is not exactly similar to in-vivo tumors, largely due to lack of vasculature in spheroids. However, uptake of fluorescent anthracyclines, radio-labeled and superparamagnetic-iron-oxide-nanoparticle-labeled drugs in spheroids still provide insights about tissue penetration and distribution properties of drugs (Bichay et al., 1990). Lastly, automation of scaffold-based spheroids may be impractical as matrix (agarose, Matrigel, cellulose, hydrogel etc.) is temperature sensitive and needs active temperature-control, which might be challenging for automated liquid handling platforms. However, recent advances in imaging and nanotechnology have enabled researchers to overcome some of the technical difficulties associated with spheroids.
Although organotypic 3D models considerably reduce the time and cost of drug discovery, until we have more sophisticated whole organ culture systems, animal models will still be needed for validating the toxicity and in-vivo activities of drugs. Microfluid-based 3D models of cancer are catching up fast, as they present unique customizable options that can be multiplexed with microscopy. Research groups in Japan recently constructed well organized three dimensional cerebral cortex, pituitary gland and optic cup (eyelids) in vitro (Eiraku et al., 2008, 2011; Suga et al., 2011), thus providing a glimpse of the future possibilities of 3D culture systems. In the near future we will have more advanced cancer models, resulting from the collaboration of tissue engineering and cancer biology, which will allow more intense interrogation of the signaling pathways and their inhibitors. The application of such culture system will not just be limited to studying diseases, but will also revolutionize the field of organ transplantation.
Acknowledgments
We would like to acknowledge Duke Cancer Institute as part of the P30 Cancer Center Support Grant NIH CA014236 (GRD) and Department of Defense grant W81XWH-13-1-0047 (GRD); Dr. Bradley Collins, Dr. Kannan Samy, Dr. Biswajit Mazumder and Pranalee Patel for reviewing the manuscript.
Abbreviations
- 2D
Two-dimensional
- 3D
three-dimensional
- TME
tumor microenvironment
- IBC
inflammatory breast cancer
- MTCS
multicellular tumor spheroids
- ECM
extracellular matrix
- O2
oxygen
- CSC
cancer stem cells
Footnotes
Conflict of interest:
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuelba H, Cotrutz CE, Stoica BA, Stoica L, Olinici D, Petreus T. In vitro evaluation of curcumin effects on breast adenocarcinoma 2D and 3D cell cultures. Romanian Journal of Morphology & Embryology. 2015;56:71–76. [PubMed] [Google Scholar]
- Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A, Lam AK, Sykes EA, Rocheleau JV, Chan WCW. Nature Communications. Vol. 4. Nature Publishing Group; 2013. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri K, Ranjan B, Valérïévitch V, Sinha B. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. PNAS. 2013;110:14843–14848. doi: 10.1073/pnas.1309482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allensworth JL, Evans MK, Aldrich J, Festa RA, Finetti P, Ueno NT, Safi R, Mcdonnell DP, Thiele DJ, Van Laere S, Devi R. ScienceDirect Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Molecular Oncology. 2015;9:1155–1168. doi: 10.1016/j.molonc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allensworth JL, Sauer SJ, Lyerly HK, Morse MA, Devi GR. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF- a -independent mechanism. Breast Cancer Res Treat. 2013;137:359–371. doi: 10.1007/s10549-012-2352-6. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pérez J, Ballesteros P, Cerdán S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. Magma (New York, NY) 2005;18:293–301. doi: 10.1007/s10334-005-0013-z. [DOI] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- Arora AJ, Samal L, Tarpley M, Williams KP, Dewhirst MW, Devi GR, Sauer SJ. Development of a Novel High Content Multiparametric Assay for the Quantitative Analysis of Cell Health in Tumor Emboli in Culture. 2nd Annual Duke Cancer Institute Scientific Retreat.2014. [Google Scholar]
- Artenberg MW, Challenberg MS. Reactive oxygen species-mediated regulation of eNOS and iNOS expression in multicellular prostate tumor spheroids. International Journal of Cancer. 2003;282:274–282. doi: 10.1002/ijc.10928. [DOI] [PubMed] [Google Scholar]
- Baal N, Widmer-Teske R, McKinnon T, Preissner KT, Zygmunt MT. In vitro spheroid model of placental vasculogenesis: does it work? Laboratory investigation; a journal of technical methods and pathology. 2009;89:152–163. doi: 10.1038/labinvest.2008.126. [DOI] [PubMed] [Google Scholar]
- Baker BM, Chen CS. Deconstructing the third dimension - how 3D culture microenvironments alter cellular cues. Journal of Cell Science. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell and tissue research. 2012;347:701–11. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell HS, Whittle IR, Walker M, Leaver HA, Wharton SB. The development of necrosis and apoptosis in glioma: experimental findings using spheroid culture systems *. Neuropathology and Applied Neurobiology. 2001:291–304. doi: 10.1046/j.0305-1846.2001.00319.x. [DOI] [PubMed] [Google Scholar]
- Bertuzzi A, Fasano A, Gandolfi A, Sinisgalli C. Journal of Theoretical Biology. Vol. 262. Elsevier; 2010. Necrotic core in EMT6/ Ro tumour spheroids: Is it caused by an ATP deficit ? pp. 142–150. [DOI] [PubMed] [Google Scholar]
- Bichay T, Adams E, Inch W, Brewer J, BKB HPLC and flow cytometric analyses of uptake of adriamycin and menogaril by monolayers and multicell spheroids. Sel Cancer Ther. 1990;6:153–66. doi: 10.1089/sct.1990.6.153. [DOI] [PubMed] [Google Scholar]
- Bilandzic M, Stenvers KL. Assessment of Ovarian Cancer Spheroid Attachment and Invasion of Mesothelial Cells in Real Time. Journal of Visualized Experiments. 2014;87:e51655. doi: 10.3791/51655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K, Smith H, VanHamel V, Gavaghan D, Kelly C, Fletcher A, Maini P, Callaghan R. Metabolic Alterations During the Growth of Tumour Spheroids. 2014:615–628. doi: 10.1007/s12013-013-9757-7. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Bumpers HL, Janagama DG, Manne U, Basson MD, Katkoori V. Journal of Surgical Research. Vol. 194. Elsevier Inc; 2015. Nanomagnetic levitation three-dimensional cultures of breast and colorectal cancers; pp. 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez Ja, Rodriguez aM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. The Journal of biological chemistry. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S, Marshall JR, Messing JA, Hsu J, King MR. TRAIL-Mediated Apoptosis in Breast Cancer Cells Cultured as 3D Spheroids. 2014:9. doi: 10.1371/journal.pone.0111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafe-jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra J, Bertucci F, Jacquemier J, Xerri L, Dontu G, Stassi G, Xiao Y, Barsky SH, Birnbaum D, Viens P, Wicha MS. Aldehyde Dehydrogenase 1 – Positive Cancer Stem Cells Mediate Metastasis and Poor Clinical Outcome in Inflammatory Breast Cancer. Clinical Cancer Research. 2010;16:45–56. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EN, Gao H, Anfossi S, Mego M, Reddy NG, Debeb B, Giordano A, Tin S, Wu Q, Garza RJ, Cristofanilli M, Mani SA, Croix DA, Ueno NT, Woodward WA, Luthra R, Krishnamurthy S, Reuben JM. Inflammation Mediated Metastasis: Immune Induced Epithelial-To-Mesenchymal Transition in Inflammatory Breast Cancer. PloS one. 2015;10:1–18. doi: 10.1371/journal.pone.0132710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CC, Kim A, Terao S, Gotoh A, Higuchi M. Cell Death and Disease. Vol. 3. Nature Publishing Group; 2012. Consumption of oxygen: a mitochondrial-generated progression signal of advanced cancer; pp. e258–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier AR, Pang X, Zimmerman MI, Zhou H. Molecular Structure of RADA16 - I Designer Self-Assembling Peptide Nano fibers. ACS Nano. 2013:7562–7572. doi: 10.1021/nn401562f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa de Sampaio P, Auslaender D, Krubasik D, Failla AV, Skepper JN, Murphy G, English WR. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed SJ, Le CP, Hassan M, Pon CK, Albold S, Chan KT, Berginski ME, Huang Z, Bear JE, Lane JR, Halls ML, Ferrari D, Nowell CJ, Sloan EK. B2-Adrenoceptor Signaling Regulates Invadopodia Formation To Enhance Tumor Cell Invasion. Breast Cancer Research. 2015;17:145. doi: 10.1186/s13058-015-0655-3. Breast Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science (New York, NY) 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- De Wever O, Hendrix A, De Boeck A, Eertmans F, Westbroek W, Braems G, Bracke ME. Single Cell and Spheroid Collagen Type I Invasion Assay. Methods in Molecular Biology. 2014:1070. doi: 10.1007/978-1-4614-8244-4_2. [DOI] [PubMed] [Google Scholar]
- Delarue M, Montel F, Vignjevic D, Prost J, Joanny J-F, Cappello G. Compressive Stress Inhibits Proliferation in Tumor Spheroids through a Volume Limitation. Biophysical Journal. 2014;107:1821–1828. doi: 10.1016/j.bpj.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrbar M, Schoenmakers R, Christen EH, Fussenegger M, Weber W. Drug-sensing hydrogels for the inducible release of biopharmaceuticals. Nature materials. 2008;7:800–804. doi: 10.1038/nmat2250. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Esch EW, Bahinski A, Huh D. Nature Publishing Group. Vol. 14. Nature Publishing Group; 2015. Organs-on - chips at the frontiers of drug discovery; pp. 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esendagli DRG. Spheroid formation and invasion capacity are differentially influenced by co-cultures of fibroblast and macrophage cells in breast cancer. Molecular biology reports. 2014;41:2885–2892. doi: 10.1007/s11033-014-3144-3. [DOI] [PubMed] [Google Scholar]
- Evans MK. Structural and Functional Analysis of the Caspase-Dependent and -Independent Domains of the X-Linked Inhibitor of Apoptosis Protein in Inflammatory. Breast Cancer Tumor Biology Pathology 2016 [Google Scholar]
- Fedorenko IV, Smalley KSM. The complexity of microenvironment-mediated drug resistance. Genes & cancer. 2015;6:367–368. doi: 10.18632/genesandcancer.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin αv gene expression in human melanoma tumorigenicity. Journal of Clinical Investigation. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorilli P, Partridge D, Staniszewska I, Wang JY, Grabacka M, So K, Marcinkiewicz C, Reiss K, Khalili K, Croul SE. Integrins mediate adhesion of medulloblastoma cells to tenascin and activate pathways associated with survival and proliferation. Lab Investigation. 2008;88:1143–1156. doi: 10.1038/labinvest.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AE, Hoffman RM. In vivo-like growth of human tumors in vitro. PNAS. 1986;83:2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nature Protocols. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harbor Perspectives in Biology. 2011;3:1–21. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani G, Fedi S, Barbucci R. Hybrid magnetic hydrogel: A potential system for controlled drug delivery by means of alternating magnetic fields. Polymers. 2012;4:1157–1169. [Google Scholar]
- Goodwin TJ, Prewett TL, Wolf Da, Spaulding GF. Reduced shear stress: a major component in the ability of mammalian tissues to form three-dimensional assemblies in simulated microgravity. Journal of cellular biochemistry. 1993;51:301–311. doi: 10.1002/jcb.240510309. [DOI] [PubMed] [Google Scholar]
- Gottfried E, Kunz-schughart LA, Ebner S, Mueller-klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Immunobiology. 2016;107:2013–2022. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- Groebe K, Muller-Kleiser W. Distributions of oxygen, nutrient, and metabolic waste concentrations in multicellular spheroids and their dependence on spheroid parameters. Eur Biophys J. 1991;19:169–81. doi: 10.1007/BF00196343. [DOI] [PubMed] [Google Scholar]
- Gupta P, Vermani K, Garg S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discovery Today. 2002;7:569–579. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Zwadlo-klarwasser G, Jansen M, Kirkpatrickt CJ. Macrophages and Multicellular Tumor Spheroids in Co-Culture: A Three -Dimensional Model to Study Tumor-Host Interactions Evidence for Macrophage-Mediated Tumor Cell Proliferation and Migration. American Journal ofPathology. 1993;143:1406–1415. [PMC free article] [PubMed] [Google Scholar]
- Hickey RJ, Malkas ÆLH, Sandoval ÆJA. Three-dimensional neuroblastoma cell culture: proteomic analysis between monolayer and multicellular tumor spheroids. 2008:1229–1234. doi: 10.1007/s00383-008-2245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokazu K, Hiroshi H, Kazuhito T, Masahiro H, Sei-ichi N. Indolequinone-rhodol conjugate as a fluorescent probe for hypoxic cells: enzymatic activation and fluorescence properties. Med Chem Commun. 2010;1:50–53. [Google Scholar]
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: An underestimated tool is catching up again. Journal of Biotechnology. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Holliday DL, Brouilette KT, Markert A, Gordon LA, Jones JL. Research article Novel multicellular organotypic models of normal and malignant breast: tools for dissecting the role of the microenvironment in breast cancer progression. Breast Cancer Research. 2009;11:1–11. doi: 10.1186/bcr2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ding Y, Sun XS, Nguyen TA. Peptide Hydrogelation and Cell Encapsulation for 3D Culture of MCF-7. Breast Cancer Cells. 2013:8. doi: 10.1371/journal.pone.0059482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Herrera AI, Luo Z, Prakash O, Sun XS. Structural Transformation and Physical Properties of a Hydrogel-Forming Peptide Studied by NMR, Transmission Electron Microscopy, and Dynamic Rheometer. Biophysical Journal. 2012:103. doi: 10.1016/j.bpj.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huanwen W, Zhiyong L, Xiaohua S, Xinyu R, Kai W, Tonghua L. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Molecular cancer. 2009;8:125. doi: 10.1186/1476-4598-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Versatility, Modulation, and Signaling in Cell Adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingeson-carlsson C, Martinez-monleon A, Nilsson M. Experimental Cell Research. Vol. 338. Elsevier; 2015. Differential effects of MAPK pathway inhibitors on migration and invasiveness of BRAF V600E mutant thyroid cancer cells in 2D and 3D culture; pp. 127–135. [DOI] [PubMed] [Google Scholar]
- Ingo O, Ilmberger C, Magosch S, Joka M, Jauch K, Mayer B. Journal of Biotechnology. Vol. 205. Elsevier B.V; 2015. Impact of the spheroid model complexity on drug response; pp. 14–23. [DOI] [PubMed] [Google Scholar]
- Ivanov DP, Parker TL, Walker Da, Alexander C, Ashford MB, Gellert PR, Garnett MC. Multiplexing spheroid volume, resazurin and acid phosphatase viability assays for high-throughput screening of tumour spheroids and stem cell neurospheres. PLoS ONE. 2014;9:1–14. doi: 10.1371/journal.pone.0103817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganathan H, Gage J, Leonard F, Srinivasan S, Souza GR, Dave B, Godin B. Three-Dimensional In Vitro Co-Culture Model of Breast Tumor using Magnetic Levitation. Scientific Reports. 2014;4:6468. doi: 10.1038/srep06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen A, Young J, Nielsen JE, Joensen UN, Toft BG, Rajpert-De Meyts E, Loveland KL. Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. British journal of cancer. 2014;110:2604–14. doi: 10.1038/bjc.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce Ja, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Jenabi JM, Zhang J, Keshelava N. E-Cadherin Cell-Cell Adhesion in Ewing Tumor Cells Mediates Suppression of Anoikis through Activation of the ErbB4 Tyrosine Kinase. Oncogene. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakasheva TA, Waldron TJ, Eruslanov E, Kim S, Lee J, Brien SO, Hicks PD, Basu D, Singhal S, Malavasi F, Rustgi AK. CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer. Cancer Research. 2015;75:4074–4086. doi: 10.1158/0008-5472.CAN-14-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehua X, Feng W, Xiaohong P, Renpu L, Jing M, Fanpeng K, Bo T. Tumor, High selectivity imaging of nitroreductase using a near-infrared fluorescence probe in hypoxic. Chemical Communications. 2013;49:2554–2556. doi: 10.1039/c3cc38980d. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and Bioengineering. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- Khaitan D, Chandna S, Arya M, Dwarakanath B. Differential mechanisms of radiosensitization by 2-deoxy-D-glucose in the monolayers and multicellular spheroids of a human glioma cell line. Cancer Biology and Therapy. 2006;5:1142–51. doi: 10.4161/cbt.5.9.2986. [DOI] [PubMed] [Google Scholar]
- Klöss S, Chambron N, Gardlowski T, Weil S, Koch J, Klöss S. Cetuximab reconstitutes pro-inflammatory cytokine secretions and tumor-infiltrating capabilities of sMICA-inhibited NK cells in HNSCC tumor spheroids. Frontiers in immunology. 2015;6:1–18. doi: 10.3389/fimmu.2015.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. Journal of biomolecular screening: the official journal of the Society for Biomolecular Screening. 2004;9:273–285. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]
- Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. International Journal of Experimental Pathology. 1998;79:1–23. doi: 10.1046/j.1365-2613.1998.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev D, Bochev I, Ivanova-todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S, Kyurkchiev D, Ivanova-todorova E. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World Journal of Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor ER, Scheel C, Irving J, Sorensen PHB. Anchorage-independent multicellular spheroids as an in vitro model of growth signaling in Ewing tumors. Oncogene. 2002;21:307–318. doi: 10.1038/sj.onc.1205053. [DOI] [PubMed] [Google Scholar]
- Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nature Protocols. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman HL, Dashner EJ, Lucey M, Vermeulen P, Dirix L, Van Laere S, van Golen KL. Modeling and characterization of inflammatory breast cancer emboli grown in vitro. International journal of cancer Journal international du cancer. 2013;132:2283–94. doi: 10.1002/ijc.27928. [DOI] [PubMed] [Google Scholar]
- Leonard F, Godin B. 3D In Vitro Model for Breast Cancer Research Using Magnetic Levitation and Bioprinting Method. Methods in Molecular Biology. 2016:239–251. doi: 10.1007/978-1-4939-3444-7_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL. Cell. Vol. 139. Elsevier Ltd; 2009. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling; pp. 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang G, Zhang X, Li B, Chen Y, Lu T, Lu TJ, Xu F. Magnetic Hydrogels and Their Potential Biomedical Applications. Advanced Functional Materials. 2013;23:660–672. [Google Scholar]
- Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9:1–13. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnology Journal. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang C, Gao Q, Liu X, Tong Z. Magnetic hydrogels with supracolloidal structures prepared by suspension polymerization stabilized by Fe2O3 nanoparticles. Acta Biomaterialia. 2010;6:275–281. doi: 10.1016/j.actbio.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, Janes SM. Magnetic Resonance Imaging of Mesenchymal Stem Cells Homing to Pulmonary Metastases Using Biocompatible Magnetic Nanoparticles. Cancer research. 2009;69:8862–8868. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longati P, Jia X, Eimer J, Wagman A, Witt M-R, Rehnmark S, Verbeke C, Toftgård R, Löhr M, Heuchel RL. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC cancer. 2013;13:95. doi: 10.1186/1471-2407-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo C, Frongia C, Jorand R, Fehrenbach J, Weiss P, Maandhui A, Gay G, Ducommun B, Lobjois V. Live cell division dynamics monitoring in 3D large spheroid tumor models using light sheet microscopy. Cell Division. 2011;6:1–8. doi: 10.1186/1747-1028-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovitt CJ, Shelper TB, Avery VM. Evaluation of chemotherapeutics in a three-dimensional breast cancer model. Journal of Cancer Research and Clinical Oncology. 2015;141:951–9. doi: 10.1007/s00432-015-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. Journal of Chemical Biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HL, Jiang Q, Han S, Wu Y, Tomshine JC, Wang D, Gan Y, Zou G, Liang XJ. Multicellular tumor spheroids as an in vivo-like tumor model for three-dimensional imaging of chemotherapeutic and nano material cellular penetration. Molecular Imaging. 2012;11:487–498. [PubMed] [Google Scholar]
- Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. Journal of controlled release: official journal of the Controlled Release Society. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes AC, Baran ET, Lisboa P, Reis RL, Azevedo HS. Microfluidic Fabrication of Self-Assembled Peptide-Polysaccharide Microcapsules as 3D Environments for Cell Culture. Biomacromolecules. 2012;13:4039–4048. doi: 10.1021/bm301332z. [DOI] [PubMed] [Google Scholar]
- Mironi-Harpaz I, Berdichevski A, Seliktar D. Fabrication of PEGylated Fibrinogen: A Versatile Injectable Hydrogel Biomaterial. 2014 doi: 10.1007/978-1-4939-1047-2_6. [DOI] [PubMed] [Google Scholar]
- Monjaret F, Fernandes M, Duchemin-pelletier E, Argento A, Degot S, Young J. Fully Automated One-Step Production of Functional 3D Tumor Spheroids for High-Content Screening. Journal of Laboratory Automation. 2015 doi: 10.1177/2211068215607058. [DOI] [PubMed] [Google Scholar]
- Mosadegh B, Lockett MR, Minn KT, Simon Ka, Gilbert K, Hillier S, Newsome D, Li H, Hall AB, Boucher DM, Eustace BK, Whitesides GM. A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials. 2015;52:262–271. doi: 10.1016/j.biomaterials.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Muhitch JW, O’Connor KC, Blake Da, Lacks DJ, Rosenzweig N, Spaulding GF. Characterization of aggregation and protein expression of bovine corneal endothelial cells as microcarrier cultures in a rotating-wall vessel. Cytotechnology. 2000;32:253–263. doi: 10.1023/A:1008117410827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdan S. Electro-responsive drug delivery from hydrogels. Journal of Controlled Release. 2003;92:1–17. doi: 10.1016/s0168-3659(03)00303-1. [DOI] [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. 2000;60:2497–2503. [PubMed] [Google Scholar]
- Nguyen DM, Sam K, Tsimelzon A, Li X, Wong H, Mohsin S, Clark GM, Hilsenbeck SG, Elledge RM, Allred DC, Connell PO, Chang JC. Human Cancer Biology Molecular Heterogeneity of Inflammatory Breast Cancer: A Hyperproliferative Phenotype. Clinical Cancer Research. 2006;12:5047–5055. doi: 10.1158/1078-0432.CCR-05-2248. [DOI] [PubMed] [Google Scholar]
- Nguyen K, West J. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- Nickerson CA, Goodwin TJ, Ott CM, Buchanan KL, Uicker WC, Emami K, Leblanc CL, Ramamurthy R, Mark S, Vanderburg CR, Hammond T, Pierson DL, Terlonge J, Blanc CLLE. Three-Dimensional Tissue Assemblies: Novel Models for the Study of Salmonella enterica Serovar Typhimurium Pathogenesis Three-Dimensional Tissue Assemblies: Novel Models for the Study of Salmonella enterica Serovar Typhimurium Pathogenesis. 2001;69:7106–7120. doi: 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem G, Sercan O, Guven U, Uslu R, Uysal A, Goksel G, Ayla S, Bilir A. Cancer stem cell differentiation: TGF β 1 and versican may trigger molecules for the organization of tumor spheroids. Oncology reports. 2014;32:641–649. doi: 10.3892/or.2014.3252. [DOI] [PubMed] [Google Scholar]
- Olsen TR, Mattix B, Casco M, Herbst A, Williams, Colby Tarasidis A, Evans G, Jenkins L, McMahan CL, Simionescu D, Visconti RP, Alexis F. Processing cellular spheroids for histological examination. Journal of histotechnology. 2014;37:138–142. [Google Scholar]
- Onodera Y, Nam J, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/ RAP1 and O-GlcNAc pathways. 2014:124. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-king CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461–468. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- Price A, Edwards CM, Evans MK, Devi G. Elucidating a role for the translation initiation factor, eIF4G1, in resistance to therapy in inflammatory breast cancer (IBC) Cancer Research. 2015;75:1007. [Google Scholar]
- Quail D, Joyce J. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasey JS, Grunbaum Z, Krohn K, Nelson N, Chin L, Mar N, Nelson N, Chin LAY. Comparison of Binding of [3H] Misonidazole and [14C] Misonidazole in Multicell Spheroids. Radiation Research. 1985;101:473–479. [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett aB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature biotechnology. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Perez-torres M, Rinehart C, Guix M, Dugger T, Engelman J, Arteaga CL. Cancer Therapy: Preclinical Human Breast Cancer Cells Selected for Resistance to rastuzumab In vivo Overexpress Epidermal Growth Factor Receptor and ErbB Ligands and Remain Dependent on the ErbB Receptor Network. Clinical Cancer Research. 2007;13:4909–4920. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Robertson FM, Ogasawara MA, Ye Z, Chu K, Pickei R, Debeb BG, Woodward WA, Hittelman WN, Cristofanilli M, Barsky SH. Imaging and Analysis of 3D Tumor Spheroids Enriched for a Cancer Stem Cell Phenotype. Journal of Biomolecular Screening. 2010;15:820–829. doi: 10.1177/1087057110376541. [DOI] [PubMed] [Google Scholar]
- Roux L, Schellingerhout D, Volgin A, Maxwell D, Ishihara K, Gelovani J. Optimizing Imaging of 3D Multicellular Tumor Spheroids with Fluorescent Reporter Proteins using Confocal Microscopy. Microsc Microanal. 2008;14:734–736. [PubMed] [Google Scholar]
- Schwartz Ma, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annual review of cell and developmental biology. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Senkowski W, Zhang X, Olofsson MH, Isacson R, Hoglund U, Gustafsson M, Nygren P, Linder S, Larsson R, Fryknas M. Three-Dimensional Cell Culture-Based Screening Identifies the Anthelmintic Drug Nitazoxanide as a Candidate for Treatment of Colorectal Cancer Three-Dimensional Cell Culture-Based Screening Identi fi es the Anthelmintic Drug Nitazoxanide as a Candidate fo. Molecular Cancer Therapeutics. 2015;14:1504–16. doi: 10.1158/1535-7163.MCT-14-0792. [DOI] [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon aC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, Haslett C. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature medicine. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Singh M, Close DA, Mukundan S. Production of Uniform 3D Microtumors in Hydrogel Microwell Arrays for Measurement of Viability, Morphology, and Signaling Pathway Activation. ASSAY and Drug Development Technologies. 2015:570–583. doi: 10.1089/adt.2015.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O, Mitlo T, Hesley J, Luke S, Owens W, Cromwell EF. High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. Assay Drug Dev Technol. 2015;13:402–414. doi: 10.1089/adt.2015.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smans K, Royaux I, Chypre M, Swinnen JV, Danie VW. Cancer Cells Differentially Activate and Thrive on De Novo Lipid Synthesis Pathways in a Low-Lipid Environment. PLoS ONE. 2014;9:13–19. doi: 10.1371/journal.pone.0106913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HA, Kang Y. The Metastasis-Promoting Roles of Tumor-Associated Immune Cells. 2014 doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu M-M, Bankson JA, Gelovani JG, Killian TC, Arap W, Pasqualini R. Three-dimensional tissue culture based on magnetic cell levitation. Nature Nanotechnology. 2010;5:291–6. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylli SS, Kaye AH, Lock P. Invadopodia: At the cutting edge of tumour invasion. Journal of Clinical Neuroscience. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- Sulkowska M, Wincewicz A, Sulkowski S, Koda M, Kanczuga KL. Relations of TGF-beta1 with HIF-1 alpha, GLUT-1 and longer survival of colorectal cancer patients. Pathology. 2009;41:254–60. doi: 10.1080/00313020802579318. [DOI] [PubMed] [Google Scholar]
- Sun L, Li G, Chen X, Chen Y, Jin C, Ji L, Chao H. Azo-Based Iridium(III) Complexes as Multicolor Phosphorescent Probes to Detect Hypoxia in 3D Multicellular Tumor Spheroids. Scientific Reports. 2015;5:14837. doi: 10.1038/srep14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hori Y, Yamamoto T, Urashima T, Ohara Y, Tanaka H. 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells. 2015:1–7. doi: 10.1042/BSR20150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamulevicius P, Streffer C. Metabolic imaging in tumours by means of bioluminescence. British journal of cancer. 2000:1102–1112. doi: 10.1038/bjc.1995.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancioni I, Miller NLG, Uryu S, Lawson C, Jean C, Chen XL, Kleinschmidt EG, Schlaepfer DD. FAK activity protects nucleostemin in facilitating breast cancer spheroid and tumor growth FAK activity protects nucleostemin in facilitating breast cancer spheroid and tumor growth. 2015 doi: 10.1186/s13058-015-0551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. Journal of cell science. 2010;123:4201–4213. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- Thomas F, Holly JMP, Persad R, Bahl A, Perks CM. Fibronectin confers survival against chemotherapeutic agents but not against radiotherapy in DU145 prostate cancer cells: Involvement of the insulin like growth factor-1 receptor. Prostate. 2010;70:856–865. doi: 10.1002/pros.21119. [DOI] [PubMed] [Google Scholar]
- Thomas ZI, Gibson W, Sexton JZ, Aird KM, Ingram SM, Aldrich A, Lyerly HK, Devi GR, Williams KP. British Journal of Cancer. Vol. 104. Nature Publishing Group; 2011. Targeting GLI1 expression in human inflammatory breast cancer cells enhances apoptosis and attenuates migration; pp. 1575–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt MW, Anseth KS. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnology Bioengineering. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Advanced Drug Delivery Reviews. 2011;63:1257–1266. doi: 10.1016/j.addr.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Torisawa Y, Takagi A, Nashimoto Y, Yasukawa T, Shiku H, Matsue T. A multicellular spheroid array to realize spheroid formation, culture, and viability assay on a chip. Biomaterials. 2007;28:559–566. doi: 10.1016/j.biomaterials.2006.08.054. [DOI] [PubMed] [Google Scholar]
- Tseng H, Gage JA, Shen T, Haisler WL, Neeley SK, Shiao S, Chen J, Desai PK, Liao A, Hebel C, Raphael RM, Becker JL, Souza GR. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Scientific Reports. 2015;5:13987. doi: 10.1038/srep13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Y-C, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uroukov IS, Patton D. Optimizing environmental scanning electron microscopy of spheroidal reaggregated neuronal cultures. Microsc Res Tech. 2008;71:792–801. doi: 10.1002/jemt.20621. [DOI] [PubMed] [Google Scholar]
- Vermeulen PB, Van Golen KL, Dirix LY. Angiogenesis, lymphangiogenesis, growth pattern, and tumor emboli in inflammatory breast cancer: A review of the current knowledge. Cancer. 2010;116:2748–2754. doi: 10.1002/cncr.25169. [DOI] [PubMed] [Google Scholar]
- Verveer PJ, Swoger J, Pampaloni F, Greger K, Marcello M, Stelzer EHK. High-resolution three-dimensional imaging of large specimens with light sheet – based microscopy. Nature. 2007;4:311–313. doi: 10.1038/nmeth1017. [DOI] [PubMed] [Google Scholar]
- Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles Sa. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenta S, Doetsch J, Klieser WM, Schughart LAK. Metabolic Imaging in Multicellular Spheroids of Oncogene-transfected Fibroblasts. Journal of Histochemistry & Cytochemistry. 2000;48:509–522. doi: 10.1177/002215540004800409. [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Hoffmann E, Schwindt H, Gru F, Petros J, Arnold JRS, Sauer H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS letters. 2005;579:4541–4549. doi: 10.1016/j.febslet.2005.06.078. [DOI] [PubMed] [Google Scholar]
- Waschow M, Letzsch S, Boettcher K, Kelm J. High-content analysis of biomarker intensity and distribution in 3D microtissues. Nature Methods. 2012:9. [Google Scholar]
- Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Seminars in cancer biology. 2008;18:311–21. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Research and Treatment. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiswald L-B, Guinebretière J-M, Richon S, Bellet D, Saubaméa B, Dangles-Marie V. In situ protein expression in tumour spheres: development of an immunostaining protocol for confocal microscopy. BMC cancer. 2010;10:106. doi: 10.1186/1471-2407-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Liao Q, Hu Y, You L, Zhou L, Zhao Y. A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Brazilian journal of medical and biological research. 2013;46:634–42. doi: 10.1590/1414-431X20132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley BR, Li X, Zhang N, Wen X. Magnetic-directed patterning of cell spheroids. Journal of Biomedical Materials Research Part A. 2014;102:1537–1547. doi: 10.1002/jbm.a.34797. [DOI] [PubMed] [Google Scholar]
- Williams KP, Allensworth JL, Ingram SM, Smith GR, Aldrich AJ, Sexton JZ, Devi GR. Cancer Letters. Vol. 337. Elsevier Ireland Ltd; 2013. Quantitative high-throughput efficacy profiling of approved oncology drugs in inflammatory breast cancer models of acquired drug resistance and re-sensitization; pp. 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B, Mohan Raj BK, Robinson EE, Foty RA, Corbett SA. Threedimensional culture regulates Raf-1 expression to modulate fibronectin matrix assembly. Molecular biology of the cell. 2006;17:3386–3396. doi: 10.1091/mbc.E05-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. The Journal of Cell Biology. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y, Wang Y. Growth factors and epithelial-stromal interactions in prostate cancer development. International Review Cytology. 2000;199:65–116. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- Yulyana Y, Ho IAW, Sia KC, Newman JP, Toh XY, Endaya BB, Chan JKY, Gnecchi M, Huynh H, Chung AYF, Lim KH, Leong HS, Iyer NG, Hui KM, Lam PYP. Paracrine Factors of Human Fetal MSCs Inhibit Liver Cancer Growth Through Reduced Activation of IGF-1R/ PI3K/ Akt Signaling. Molecular Therapy. 2015;23:746–756. doi: 10.1038/mt.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Roger DK, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Xiaohua L, Xinghui G, Yangyang Z, Wen S, Huimin M. Nitroreductase Detection and Hypoxic Tumor Cell Imaging by a Designed Sensitive and Selective Fluorescent Probe, 7-[(5-Nitrofuran-2-yl)methoxy]-3H-phenoxazin-3-one. Anal Chem. 2013;85:3926–2932. doi: 10.1021/ac400750r. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gao R, Devreotes PN, Mogilner A, Zhao M. 3D Arrays for High Throughput Assays of Cell Migration and Electrotaxis. Cell Biology International. 2013;37:995–1002. doi: 10.1002/cbin.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C, Zhao RC. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Lukemia. 2009;23:925–933. doi: 10.1038/leu.2008.384. [DOI] [PubMed] [Google Scholar]
- Zimmermann JA, Mcdevitt TC. Cytotherapy. Vol. 16. Elsevier Inc; 2014. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion; pp. 331–345. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nature biotechnology. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]