Abstract
Objective
To investigate the role of advancing age on postoperative complications and revision surgery after fusion for scoliosis.
Methods
A retrospective, cohort study was performed using the Thomson Reuters MarketScan database, examining patients with adult scoliosis who underwent spinal fusion from 2000 to 2009. Primary outcomes included infection, hemorrhage and pulmonary embolism (PE) within 90 days of surgery, and refusion. The effect of increasing age was estimated using the odds ratio (OR) of complications in a multivariate logistic regression analysis, and a Cox proportional hazard model estimated the hazard ratio of refusion.
Results
A total of 8432 patients were included in this study. Overall, the average age was 53.3 years, with 26.90% males and 39% with a Charlson Comorbidity Score of ≥1. Most patients had commercial insurance (66.81%), with 26.03% and 7.16% covered by Medicare and Medicaid, respectively. Increasing age (per 5-year increment) was a significant predictor of hemorrhagic complication (OR, 1.06; confidence interval [CI], 1.01–1.11; P = 0.0196), PE (OR, 1.09; CI, 1.03–1.16; P = 0.0031), infection (OR, 1.04; CI, 1.01–1.07; P = 0.0053), and refusion (hazard ratio, 1.07; CI, 1.02–1.13; P = 0.0103).
Conclusions
In this study, age was associated with increased risk of hemorrhage, PE, infection, and refusion. With the aging population, the role of patient age on postoperative healing and outcomes deserves deeper investigation after repair of adult idiopathic scoliosis.
Keywords: Age, Outcomes, Scoliosis, Spinal fusion
Introduction
Adult scoliosis is a highly prevalent and disabling condition that may develop de novo during adult life or progress from untreated adolescent scoliosis; it is associated with significant health care costs in the United States.1-4 The overall prevalence has been reported to vary widely between 2% and 32%,5-9 and it is predicted to increase as the proportion of elderly individuals steadily increases.2,8-12 In a recent study by Schwab et al.,13 the prevalence of scoliosis was found to be 68% in healthy volunteers older than 60 years. Similarly, several longitudinal studies have reported an increased prevalence of scoliosis with age.1,9,14-18 As the population ages rapidly in the United States, the prevalence of scoliosis will increase, resulting in increased complex surgical intervention for symptomatic spinal disease.19,20 Complications after surgical correction of adult spinal deformity remains a major impediment to operative management. Despite satisfactory outcomes21-23 and functional improvement,24-26 complications and reoperations are significant risks with surgical intervention.22 Relatively high rates of complications have been reported in previous studies evaluating adult patients with spinal deformity.12,27 Reoperation rates have ranged from 10% to 25%,17,28-30 with pseudarthrosis being one of the most common indications.31-33 One major contribution to the high complication rates in the surgical correction of adult spinal deformity is advancing age, with several studies showing a correlation between increasing age and incidence of surgical complications.3,20,23,34-36 Most studies evaluating outcomes after repair of scoliosis have been single-institution studies,28,37-39 with few studies evaluating outcomes at a national level.40 This study analyzes the role of advancing age on postoperative complications (hemorrhage, infection, pulmonary embolus) and revision surgery using a large, national retrospective database analysis.
Methods
Data Source
Data for the study were obtained using Thomson Reuter's MarketScan database, which contains information from Commercial Claims and Encounters, the Medicare Supplemental and Coordination of Benefits, and the Medicaid databases. The database captures patient-level data on clinical use (inpatient and outpatient), pharmaceutical claims, insurance enrollment, and costs, and links these data with detailed patient, provider, and facility information. The MarketScan database is a deidentified database that was deemed to be exempt from review by the institutional review board.
Study Sample
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes were used to identify patients diagnosed with scoliosis (ICD-9-CM 737.30) who underwent spinal fusion (ICD-9 CM: 81.0-81.08) between 2000 and 2009. Based on this study design, all approaches for surgical correction were grouped and do not differentiate based on the type of surgery, which proves to be an unavoidable cofounding factor based on use of an ICD-9 driven data source. All selected patients were required to have 90 days or more of enrollment. Observations with more than 1 spinal fusion used the earliest recorded spinal fusion. Only patients aged 18 years and older at the time of the index hospitalization were included in the analysis. Patients were stratified into 3 groups based on age: group 1, 18–44 years; group 2, 45–64 years; group 3, 65+ years. Comorbidities were tallied and used to calculate a Charlson Comorbidity Index Score41 for each patient.
Postoperative Outcome Variables
Primary outcomes were postoperative complications within 90 days of surgery (hemorrhage or hematoma, infection, and pulmonary embolism [PE]) and the rate of refusion surgery was signified as a subsequent spinal fusion (repeated ICD-9-CM: 81.0-81.08) at a later date from the index spinal fusion surgery. The reasons for repeat fusion were varied and included situations such as adjacent segment disease, revision for poor placement, and implant failure. Complications within 90 days of surgery were assessed at any postoperative hospital admission using ICD-9 diagnosis. All cost estimates were based on patient-reported resource use.
Statistical Analysis
This retrospective cohort study reviewed the association of age with select outcomes among adult patients with scoliosis undergoing spinal fusion. A multivariate Cox proportional hazard model was selected to study refusion. Complications for infection and hemorrhagic and PE within 90 days of the operation were evaluated with multivariate logistic models. The rate of refusion was analyzed using the multivariate Cox proportional hazard regression models. Year of operation, a patient's Charlson Score, insurance type, gender, and age as a continuous variable were included in each model. Analyses report respective hazard ratios or odds ratios (ORs) with 95% confidence intervals (CIs). Statistical testing and associated P values are from a χ2 distribution. All analyses were conducted with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Patient Cohort
A total of 8453 patients met the inclusion criteria for this study. Selected patients were 18 years and older with diagnosis of scoliosis and underwent spinal fusion as a treatment procedure. 22.39% of patients had a Charlson Comorbidity Score of 1, with 9.90% and 6.70% having a score of 2 and 3, respectively. Most of the patients (66.81%) were privately insured, with 26.03% and 7.16% covered by Medicare and Medicaid, respectively. Of the total number of patients, 25.66% were aged 18–44 years, 47.89% were 45–64 years, and 26.45% were 65+ years. Overall, the mean age ± standard deviation was 53.3 ± 18.04 years (group 1, 27.2 ± 9.17 years; group 2, 56.5 ± 5.34 years; group 3, 73.0 ± 5.47 years). Among patients in group 1, 1.43% had a Charlson Score of 3 or more, compared with 6.24% and 12.65% in groups 2 and 3, respectively. Complete demographic characteristics of the cohorts are listed in Table 1.
Table 1. Demographics by Age Group.
| Total | Age Group | P Value | |||
|---|---|---|---|---|---|
| 18–44 years | 45–64 years | 65+ years | |||
| Total, number (col%) | 8432 (100) | 2164 (100) | 4038 (100) | 2230 (100) | < 0.0001 |
| Age, mean (standard deviation) | 53.3 (18.04) | 27.2 (9.17) | 56.5 (5.34) | 73.0 (5.47) | < 0.0001 |
| Commercial insurance | 5633 (66.81) | 1773 (81.93) | 3860 (95.59) | 00 (00.0) | |
| Medicaid | 604 (7.16) | 391 (18.07) | 142 (3.52) | 71 (3.18) | |
| Medicare | 2195 (26.03) | 36 (0.89) | 2159 (96.82) | < 0.0001 | |
| Gender | |||||
| Male | 2268 (26.90) | 649 (29.99) | 986 (24.42) | 633 (28.39) | |
| Female | 6164 (73.10) | 1515 (70.01) | 3052 (75.58) | 1597 (71.61) | < 0.0001 |
| Charlson Score, number (col%) | |||||
| 0 | 5144 (61.01) | 1735 (80.18) | 2423 (60.00) | 986 (44.22) | < 0.0001 |
| 1 | 1888 (22.39) | 324 (14.97) | 952 (23.58) | 612 (27.44) | < 0.0001 |
| 2 | 835 (9.90) | 74 (3.42) | 411 (10.18) | 350 (15.70) | < 0.0001 |
| ≥3 | 565 (6.70) | 31 (1.43) | 252 (6.24) | 282 (12.65) | < 0.0001 |
| Follow-up days, mean (standard deviation) | 669.6 (664.31) | 671.1 (653.9) | 644.3 (636.72) | 713.9 (718.96) | 0.0004 |
col%, Column percentage.
Postoperative Outcome Variables
Overall, the most common complication within 90 days was infection, with a rate of 11.27% (group 1, 9.24%; group 2, 11.29%; group 3, 13.18%) (Table 2). The next most common complication was postoperative pneumonia, with a rate of 10.48% (group 1, 9.38%; group 2, 8.84%; group 3, 14.53%). The rate of hemorrhagic complication was 3.90% (group 1, 2.59%; group 2, 4.41%; group 3, 4.53%). The rate of refusion in this study was 2.87% (group 1, 2.03%; group 2, 3.52%; group 3, 2.51%, with the average ± standard deviation number of years to refusion being 1.8 ± 1.78 years, 1.7±1.7 years, and 1.9±1.94 years, respectively). The overall mortality within 90 days was 0.32% (group 1, 0.14%; group 2, 0.27%; group 3, 0.58%).
Table 2. Outcomes by Age.
| Total | Age Group | P Values | |||
|---|---|---|---|---|---|
| 18–44 years | 45–64 years | >65 years | |||
| Total patients, number (col%) | 8432 (100) | 2164 (100) | 4038 (100) | 2230 (100) | 0.0004 |
| Hemorrhage within 90 days, number (col%) | 335 (3.9) | 56 (2.59) | 178 (4.41) | 101 (4.53) | 0.0006 |
| Infections within 90 days, number (col%) | 950 (11.27) | 200 (9.24) | 456 (11.29) | 294 (13.18) | 0.0002 |
| Pulmonary embolism within 90 days, number (col%) | 262 (3.1) | 42 (1.94) | 117 (2.90) | 103 (4.62) | < 0.0001 |
| Pneumonia within 90 days, number (col%) | 884 (10.48) | 203 (9.38) | 357 (8.84) | 324 (14.53) | < 0.0001 |
| Death in 90 days, number (col%) | 27 (0.32) | 3 (0.14) | 11 (0.27) | 13 (0.58) | 0.0254 |
| Refusion number (col%) | 242 (2.8) | 44 (2.03) | 142 (3.52) | 56 (2.51) | 0.0019 |
| Years to refusion or censor, mean (standard deviation) | 1.8 (1.79) | 1.8 (1.78) | 1.7 (1.70) | 1.9 (1.0.94) | 0.0001 |
col%, Column percentage.
Multivariate Analysis
Using a multivariate logistic regression analysis, our study showed that within 90 days of surgery, increasing age (per 5-year increment) was a significant predictor of hemorrhagic complication (OR, 1.06; CI, 1.01–1.11; P = 0.0196), PE (OR, 1.09; CI, 1.03–1.16; P = 0.0031) and infection (OR, 1.04; CI, 1.01–1.07; P = 0.0053) (Table 3). Analysis of refusion using the Cox proportional hazard model showed a 7% increased risk of having refusion surgery for every 5-year age increment (hazard ratio, 1.07; CI, 1.02–1.13; P = 0.0103). At 10 years after primary fusion, Kaplan-Meier survival estimates for refusion were 89.7%, 82.7%, and 87.9% for age groups 1, 2, and 3, respectively (Figure 1).
Table 3. Logistic Regression: Effect of Age (Per 5-Year Increments) on Complication Rate and Refusion Within 90 Days of Surgery.
| Bivariate | Multivariate | |||
|---|---|---|---|---|
| Estimate (95% Confidence Interval) | P Value | Estimate (95% Confidence Interval) | P Value | |
| Hemorrhagic complication | 1.05 (1.02–1.09) | 0.0021 | 1.06 (1.01–1.11) | 0.0196 |
| Infectious complication | 1.04 (1.02–1.06) | < 0.0001 | 1.04 (1.01–1.07) | 0.0053 |
| Pulmonary embolism | 1.11 (1.07–1.16) | < 0.0001 | 1.09 (1.03–1.16) | 0.0031 |
| Refusion or reoperation | – | – | 1.07 (1.02–1.13) | 0.0103 |
Figure 1.
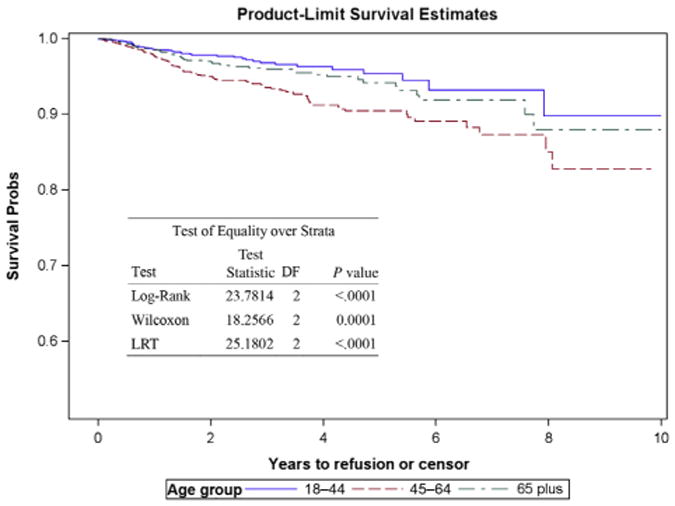
Kaplan-Meier survival estimates for refusion stratified by age groups: 18–44 years; 45–64 years; 65+ years.
Discussion
Adult spinal deformity has a significant and measurable impact on health-related quality of life, affecting several domains, including pain, function, self-image, and mental health.8,9,15,24 There is increasing evidence suggesting the effectiveness of surgical treatment for adult spinal deformity; however, the relatively high reported rate of potential surgical complications has facilitated a trend toward nonoperative management, notwithstanding that longitudinal data on the effectiveness of conservative care are lacking.1,3,15,16
Complications and reoperations after surgical repair for adult scoliosis remain an important concern. There remains a paucity of data evaluating and quantifying the impact of age on outcomes after primary fusion for adult scoliosis. Despite the increased risk of complications and mortality, elderly patients can benefit from significantly greater improvement in disability and reduced pain after scoliosis surgery.36 Scheer et al.42 showed that elderly patients (≥65 years) who underwent 3-column posterior subtraction osteomies for correction of spinal deformity were more likely to reach a minimum clinically important difference compared with younger patients (≤45 years) in terms of 1- and 2-year Physical Component Scores and Scoliosis Research Society pain and function scores. This again emphasizes the beneficial role of surgery in elderly patients, despite the incidence of complications.
In this large retrospective study of 8432 patients, we demonstrated that increasing age was associated with postoperative complications including hemorrhage, infection, PE, and refusion. Here, we have emphasized the importance of age predicting hemorrhage development, allowing clinicians to better anticipate such morbidity in older persons. Our results correlate with several studies43-47 that have reported age-associated increase in incidence of complications after spinal surgeries. A retrospective study by Smith et al.36 evaluating outcomes in 206 adult patients with scoliosis showed a 4- and 5-fold increase in the number of minor complications (eg, airway edema, mild pleural effusion, cerebro-spinal fluid leak, venous thrombosis, superficial infection) and major complications (eg, deep wound infection, excessive blood loss, hemothorax, PE myocardial infarction, nerve root injury), respectively, in the oldest age cohort (65–85 years) compared with the youngest (25–44 years). Another study by Carreon et al.35 illustrated that the prevalence of major complications (wound infection, pneumonia, renal failure, neurologic deficit, myocardial infarction) increased with age in 101 patients aged 65 years and older undergoing spinal surgery. These studies suggest that age can potentially increase the baseline risk for surgical complication and should be included in the preoperative risk assessment when counseling elderly patients.
Infection and hemorrhagic complications are common concerns in major spinal procedures. In our study, increasing age was associated with increased risk of infection. This increased risk could be associated with our higher comorbidity index in older patients. This correlates with reported increase in surgical complications (pulmonary, bleeding/hematoma, renal, cardiac, infectious, neurologic, and thromboembolic) in association with medical comorbidity.40 Elderly patients are more likely to present with more comorbidities, which increases their baseline risk of postoperative complications. The extent of surgical complexity, surgical approach, and reoperations can also influence the incidence of adverse outcomes.48 Older patients often present with advanced and more challenging spinal diseases combined with decreased bone integrity, degenerative changes, kyphotic deformities, and increased rigidity of spinal deformity.23,49 These cumulative spinal diseases can all increase operating room time, blood loss, and hospital length of stay. Furthermore, there can be bleeding risk related to medications common to the aging population that interfere with the coagulation cascade as well as bleeding events related to age-related tissue integrity changes. Consequently, there is a potential increase in the baseline risk of surgical complications after primary fusion for scoliosis.
Differences in baseline risk factors such as gender, hypertension, or smoking have previously been shown to influence the rate of hemorrhagic complications.50-52 Our study demonstrated that age is a significant contributor to hemorrhagic complications. Patients should therefore be evaluated for risk of hemorrhage with standard risk factors such as hypertension, bleeding disorders, blood vessel abnormalities, and use of anticoagulants to minimize the confounding effects of these variables on surgical complications. Based on our findings, age should be included in the pre-operative risk assessments in patients with scoliosis considering surgery. Duke University Medical Center has recently adopted a Perioperative Optimization of Senior Health Program with the aim of improving outcomes in elderly patients (>65 years) undergoing lumbar spinal fusion. In this program, a geriatrician-led team in addition to the neurosurgical staff evaluate patients daily throughout their hospital stay to manage medical comorbidities, treat pain, prevent delirium, and coordinate rehabilitation. Our preliminary results show a significant reduction in the incidence of postoperative complications (urinary tract infection and pneumonia), hospital length of stay, and 30-day readmission rates. Such a highly customized intervention has the potential to be beneficial to elderly patients undergoing spinal surgery, and the present study supports the necessity of developing such novel approaches to surgical intervention in the elderly.
In balancing the risk of hemorrhage, clinicians must consider the risk of PE, a complication that is often reported after spinal surgeries. The incidence of PE in adult patients undergoing spinal surgery is confounded by various attributable factors such as age, obesity, gender, and tobacco consumption, which increase the baseline risk after spinal surgeries.53-57 Controversy continues regarding the use of systemic anticoagulant in the prevention of PE, given the risk of bleeding in the postoperative phase.56-59 Despite the controversy, patients should be evaluated on an individual basis, with inclusion of attributable risk factors for PE in the decision algorithm for preventive measures after major spinal surgery. Providers should continue to encourage early ambulation and the use of sequential compression devices to prevent postoperative deep venous thrombosis/PE.
Reoperation (or refusion) rates have been downtrending over the past several decades as surgical techniques become more advanced and minimally invasive, despite the confounding factor that revision rates undoubtedly increase over time. Some variability exists in reported rates of reoperation, partly as a result of surgical approaches and the broad spectrum of associative postoperative complications. Luhmann et al.29 documented a 3.9% reoperation rate with an average follow-up of 5.7 years and Richards et al.39 reported a rate of 12.37% within the first 5 years of surgery. Our study showed a lower reoperation rate of 2.8%, with an average of 1.8 ± 1.79 years to reoperation. The broad spectrum of causes for reoperation can account for the variability in reported rates. Infection, pseudarthrosis, pain, and symptomatic implants are among the leading causes of reoperation after primary fusion for adult scoliosis.29,33,39 Our results showed a nonlinear relationship between age and revision, with the highest revision rate in the middle age group. We believe this can be attributed to predicted years left in a person's life incentivizing reoperation because there would be a hypothetical greater number of years remaining to benefit from such revision. Our study does not support age as the only factor contributing to risk of revision. Notwithstanding the nonlinear relationship, given the increased odds of surgical complications with increasing age,3,35,60,61 and medical comorbidities,40 elderly patients are at greatest risk for complication-driven reoperations. This has negative implications on patients' quality of life and health care resource use. The cost associated with reoperation was illustrated by McCarthy et al.62 in a study of 484 patients undergoing spinal surgery for adult spinal deformity. These investigators reported an increase of more than 70% in the average cost as a result of readmission for spine-related operations. This potentially creates an added financial burden on health care institutions, providers, and patients. With the constant advancement in technology used in spinal surgeries and the minimal invasiveness of the procedures, the occurrence of fewer postoperative complications will drive down the need for reoperations in some patients.
Mortality associated with spinal surgery remains a devastating adverse outcome, with several confounding variables that can affect the overall incidence. Despite the rarity of this phenomenon, the risk factors for mortality are an essential component of presurgical evaluation to optimize patient care. Our study showed a trend toward increased incidence of mortality with increasing age. Our findings are similar to a larger study of 108,419 patients by Smith et al.48 evaluating the rates and causes of mortality associated with spine surgery. These investigators showed that the mortality among patients aged 21 years or older was almost double the rate in patients younger than 21 years (2.0 vs. 1.3 deaths per 1000 patients). When stratified by age groups, the same study illustrated a dramatic age-associated increase in mortality: 0.9 and 34.3 deaths per 1000 patients, aged 20–39 and ≥90 years, respectively. However, other variables such as the existence of medical comorbidities can increase the mortality rate after spinal surgery. A recent study of 87,162 cases of deformity (scoliosis: n = 50,553) from the Scoliosis Research Society by Shaffrey et al.63 demonstrated an overall mortality of 1.5 per 1000 cases, with respiratory (36.6%), cardiovascular events (24.4%), and sepsis (9.2%) being the 3 most common causes. Based on these studies, the risk of mortality should be assessed preoperatively using variables such as patient age and the existence of medical comorbidities. Notwithstanding the relatively low incidence of mortality in spine surgery, continuous efforts to improve patient safety and optimize clinical outcomes should be encouraged.
This study has several limitations that must be acknowledged. First, the use of ICD-9 codes inherently depends on accurate coding, which leads to intrinsic poor reliability. However, this coding system is commonly used to classify diagnoses and every effort is made to ensure accuracy, making the coding construct an appropriate (although not perfect) approach to analysis. Beyond this, clinical factors such as disease severity and health care quality of life are not in the dataset, nor does this study account for the types of surgical approaches, as mentioned previously. Radiographic factors including spinopelvic modifiers (sagittal vertical axis, pelvic tilt, pelvic incidence/lumbar lordosis mismatch) were not available. In addition, our study could not capture variables such as smoking history and obesity, which are known factors that increase baseline risk of PE. Furthermore, operative effect could not be determined because metrics such as Scoliosis Research Society and quality of life scores are not included in the database. Also, because patients were selected using diagnosis and procedure codes, miscoding may be present, and because readmission data were used to assess complications, the analysis may miss the perioperative hematomas and superficial infections of such surgeries. We attempted to reduce this inaccuracy by using ICD-9 and Current Procedural Terminology codes, allowing for greater data accuracy. This study was retrospective and nonrandomized. Despite these limitations, we believe that our study provides a valuable addition to the literature investigating and quantifying the impact of advancing age on outcomes after primary spinal fusion for adult scoliosis.
Conclusions
As health care costs continue to increase, increasing attention is focused on reducing surgical complications in patients undergoing spine surgery for scoliosis. In this retrospective cohort analysis, age was associated with increased risk of hemorrhagic events, PE, infection, and reoperation. With the aging population, the role of patient age on postoperative healing and outcomes after repair of adult scoliosis deserves deeper investigation. Future studies should analyze the differences in mortality in older persons based on type of surgery to optimize the approach to surgically repairing adult scoliosis in the aging population. Overall, we find that physicians should work with elderly patients to balance the anticipated symptomatic relief from surgery with the age-associated increased risk of surgical complications in the decision algorithm when considering surgical intervention for scoliosis.
Acknowledgments
The National Institutes of Health KM1 CA 156687 grant supplied funding for the collection, management, analysis, and interpretation of the data. The National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117 supported the Duke University Clinical and Translational Science Awards team. S.P. Lad has consulted for and received grant support from Medtronic Inc., Boston Scientific, and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center, which has received research funding from National Institutes of Health KM1 CA 156687, Medtronic Inc., Boston Scientific, and St. Jude Medical. S.P. Lad had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations and Acronyms
- CI
Confidence interval
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- OR
Odds ratio
- PE
Pulmonary embolism
Footnotes
Conflict of interest statement: The remaining authors report no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Birknes JK, White AP, Albert TJ, Shaffrey CI, Harrop JS. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63:94–103. doi: 10.1227/01.NEU.0000325485.49323.B2. [DOI] [PubMed] [Google Scholar]
- 2.Bridwell KH. Where to stop the fusion distally in adult scoliosis: L4, L5, or the sacrum? Instr Course Lect. 1996;45:101–107. [PubMed] [Google Scholar]
- 3.Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976) 2007;32:2238–2244. doi: 10.1097/BRS.0b013e31814cf24a. [DOI] [PubMed] [Google Scholar]
- 4.Mok JM, Cloyd JM, Bradford DS, Hu SS, Deviren V, Smith JA, et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976) 2009;34:832–839. doi: 10.1097/BRS.0b013e31819f2080. [DOI] [PubMed] [Google Scholar]
- 5.Carter OD, Haynes SG. Prevalence rates for scoliosis in US adults: results from the first National Health and Nutrition Examination Survey. Int J Epidemiol. 1987;16:537–544. doi: 10.1093/ije/16.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Francis RS. Scoliosis screening of 3,000 college-aged women. The Utah Study–phase 2. Phys Ther. 1988;68:1513–1516. [PubMed] [Google Scholar]
- 7.Kostuik JP, Bentivoglio J. The incidence of low back pain in adult scoliosis. Acta Orthop Belg. 1981;47:548–559. [PubMed] [Google Scholar]
- 8.Perennou D, Marcelli C, Herisson C, Simon L. Adult lumbar scoliosis. Epidemiologic aspects in a low-back pain population. Spine (Phila Pa 1976) 1994;19:123–128. doi: 10.1097/00007632-199401001-00001. [DOI] [PubMed] [Google Scholar]
- 9.Robin GC, Span Y, Steinberg R, Makin M, Menczel J. Scoliosis in the elderly: a follow-up study. Spine (Phila Pa 1976) 1982;7:355–359. doi: 10.1097/00007632-198207000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sansur CA, Smith JS, Coe JD, Glassman SD, Berven SH, Polly DW, Jr, et al. Scoliosis research society morbidity and mortality of adult scoliosis surgery. Spine (Phila Pa 1976) 2011;36:E593–597. doi: 10.1097/BRS.0b013e3182059bfd. [DOI] [PubMed] [Google Scholar]
- 11.Trobisch P, Suess O, Schwab F. Idiopathic scoliosis. Dtsch Arztebl Int. 2010;107:875–883. doi: 10.3238/arztebl.2010.0875. quiz 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadla S, Maltenfort MG, Ratliff JK, Harrop JS. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus. 2010;28:E3. doi: 10.3171/2009.12.FOCUS09254. [DOI] [PubMed] [Google Scholar]
- 13.Schwab F, Dubey A, Gamez L, El Fegoun AB, Hwang K, Pagala M, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005;30:1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 14.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024–2029. doi: 10.1097/01.brs.0000179086.30449.96. [DOI] [PubMed] [Google Scholar]
- 15.Glassman SD, Carreon LY, Shaffrey CI, Polly DW, Ondra SL, Berven SH, et al. The costs and benefits of nonoperative management for adult scoliosis. Spine (Phila Pa 1976) 2010;35:578–582. doi: 10.1097/BRS.0b013e3181b0f2f8. [DOI] [PubMed] [Google Scholar]
- 16.Glassman SD, Schwab FJ, Bridwell KH, Ondra SL, Berven S, Lenke LG. The selection of operative versus nonoperative treatment in patients with adult scoliosis. Spine (Phila Pa 1976) 2007;32:93–97. doi: 10.1097/01.brs.0000251022.18847.77. [DOI] [PubMed] [Google Scholar]
- 17.Pichelmann MA, Lenke LG, Bridwell KH, Good CR, O'Leary PT, Sides BA. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976) 2010;35:219–226. doi: 10.1097/BRS.0b013e3181c91180. [DOI] [PubMed] [Google Scholar]
- 18.Yeo JM, Vertinsky AT, Chew JB, Heran MK, Shewchuk J, Malfair D, et al. Imaging in adult scoliosis: preoperative assessment and postoperative complications. Semin Musculoskelet Radiol. 2011;15:143–150. doi: 10.1055/s-0031-1275597. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Loeser JD, Bigos SJ, Ciol MA. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis, and procedure. J Bone Joint Surg Am. 1992;74:536–543. [PubMed] [Google Scholar]
- 20.Deyo RA, Ciol MA, Cherkin DC, Loeser JD, Bigos SJ. Lumbar spinal fusion. A cohort study of complications, reoperations, and resource use in the Medicare population. Spine (Phila Pa 1976) 1993;18:1463–1470. [PubMed] [Google Scholar]
- 21.Ali RM, Boachie-Adjei O, Rawlins BA. Functional and radiographic outcomes after surgery for adult scoliosis using third-generation instrumentation techniques. Spine (Phila Pa 1976) 2003;28:1163–1169. doi: 10.1097/01.BRS.0000067267.04011.91. discussion: 1169-1170. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro GS, Taira G, Boachie-Adjei O. Results of surgical treatment of adult idiopathic scoliosis with low back pain and spinal stenosis: a study of long-term clinical radiographic outcomes. Spine (Phila Pa 1976) 2003;28:358–363. doi: 10.1097/01.BRS.0000048502.62793.0C. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S, Delecrin J, Passuti N. Surgical treatment of idiopathic scoliosis in adults: an age-related analysis of outcome. Spine (Phila Pa 1976) 2002;27:1742–1748. doi: 10.1097/00007632-200208150-00011. [DOI] [PubMed] [Google Scholar]
- 24.Albert TJ, Purtill J, Mesa J, McIntosh T, Balderston RA. Health outcome assessment before and after adult deformity surgery. A prospective study. Spine (Phila Pa 1976) 1995;20:2002–2004. doi: 10.1097/00007632-199509150-00009. discussion: 2005. [DOI] [PubMed] [Google Scholar]
- 25.Dickson JH, Mirkovic S, Noble PC, Nalty T, Erwin WD. Results of operative treatment of idiopathic scoliosis in adults. J Bone Joint Surg Am. 1995;77:513–523. doi: 10.2106/00004623-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Grubb SA, Lipscomb HJ, Suh PB. Results of surgical treatment of painful adult scoliosis. Spine (Phila Pa 1976) 1994;19:1619–1627. doi: 10.1097/00007632-199407001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Charosky S, Guigui P, Blamoutier A, Roussouly P, Chopin D Study Group on Scoliosis. Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients. Spine (Phila Pa 1976) 2012;37:693–700. doi: 10.1097/BRS.0b013e31822ff5c1. [DOI] [PubMed] [Google Scholar]
- 28.Cook S, Asher M, Lai SM, Shobe J. Reoperation after primary posterior instrumentation and fusion for idiopathic scoliosis. Toward defining late operative site pain of unknown cause. Spine (Phila Pa 1976) 2000;25:463–468. doi: 10.1097/00007632-200002150-00012. [DOI] [PubMed] [Google Scholar]
- 29.Luhmann SJ, Lenke LG, Bridwell KH, Schootman M. Revision surgery after primary spine fusion for idiopathic scoliosis. Spine (Phila Pa 1976) 2009;34:2191–2197. doi: 10.1097/BRS.0b013e3181b3515a. [DOI] [PubMed] [Google Scholar]
- 30.Scheer JK, Tang JA, Smith JS, Klineberg E, Hart RA, Mundis GM, Jr, et al. Reoperation rates and impact on outcome in a large, prospective, multicenter, adult spinal deformity database: clinical article. J Neurosurg Spine. 2013;19:464–470. doi: 10.3171/2013.7.SPINE12901. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976) 2006;31:2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Bridwell KH, Lenke LG, Rinella AS, Edwards C., 2nd Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine (Phila Pa 1976) 2005;30:468–474. doi: 10.1097/01.brs.0000153392.74639.ea. [DOI] [PubMed] [Google Scholar]
- 33.Ramo BA, Richards BS. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis: five-year update on a previously published cohort. Spine (Phila Pa 1976) 2012;37:1211–1217. doi: 10.1097/BRS.0b013e31824b6b05. [DOI] [PubMed] [Google Scholar]
- 34.Benz RJ, Ibrahim ZG, Afshar P, Garfin SR. Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res. 2001;384:116–121. doi: 10.1097/00003086-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Carreon LY, Puno RM, Dimar JR, 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85-A:2089–2092. doi: 10.2106/00004623-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Smith JS, Shaffrey CI, Glassman SD, Berven SH, Schwab FJ, Hamill CL, et al. Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine (Phila Pa 1976) 2011;36:817–824. doi: 10.1097/BRS.0b013e3181e21783. [DOI] [PubMed] [Google Scholar]
- 37.Bago J, Ramirez M, Pellise F, Villanueva C. Survivorship analysis of Cotrel-Dubousset instrumentation in idiopathic scoliosis. Eur Spine J. 2003;12:435–439. doi: 10.1007/s00586-001-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barr SJ, Schuette AM, Emans JB. Lumbar pedicle screws versus hooks. Results in double major curves in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1997;22:1369–1379. doi: 10.1097/00007632-199706150-00016. [DOI] [PubMed] [Google Scholar]
- 39.Richards BS, Hasley BP, Casey VF. Repeat surgical interventions following “definitive” instrumentation and fusion for idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31:3018–3026. doi: 10.1097/01.brs.0000249553.22138.58. [DOI] [PubMed] [Google Scholar]
- 40.Patil CG, Santarelli J, Lad SP, Ho C, Tian W, Boakye M. Inpatient complications, mortality, and discharge disposition after surgical correction of idiopathic scoliosis: a national perspective. Spine J. 2008;8:904–910. doi: 10.1016/j.spinee.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 42.Scheer JK, Lafage V, Smith JS, Deviren V, Hostin R, McCarthy IM, et al. Impact of age on the likelihood of reaching a minimum clinically important difference in 374 three-column spinal osteotomies: clinical article. J Neurosurg Spine. 2014;20:306–312. doi: 10.3171/2013.12.SPINE13680. [DOI] [PubMed] [Google Scholar]
- 43.Memtsoudis SG, Vougioukas VI, Ma Y, Gaber-Baylis LK, Girardi FP. Perioperative morbidity and mortality after anterior, posterior, and anterior/posterior spine fusion surgery. Spine (Phila Pa 1976) 2011;36:1867–1877. doi: 10.1097/BRS.0b013e3181c7decc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pumberger M, Chiu YL, Ma Y, Girardi FP, Vougioukas V, Memtsoudis SG. Perioperative mortality after lumbar spinal fusion surgery: an analysis of epidemiology and risk factors. Eur Spine J. 2012;21:1633–1639. doi: 10.1007/s00586-012-2298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoenfeld AJ, Carey PA, Cleveland AW, 3rd, Bader JO, Bono CM. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J. 2013;13:1171–1179. doi: 10.1016/j.spinee.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 46.Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ., Jr Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93:1577–1582. doi: 10.2106/JBJS.J.01048. [DOI] [PubMed] [Google Scholar]
- 47.Street JT, Lenehan BJ, DiPaola CP, Boyd MD, Kwon BK, Paquette SJ, et al. Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J. 2012;12:22–34. doi: 10.1016/j.spinee.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Smith JS, Saulle D, Chen CJ, Lenke LG, Polly DW, Jr, Kasliwal MK, et al. Rates and causes of mortality associated with spine surgery based on 108,419 procedures: a review of the Scoliosis Research Society Morbidity and Mortality Database. Spine (Phila Pa 1976) 2012;37:1975–1982. doi: 10.1097/BRS.0b013e318257fada. [DOI] [PubMed] [Google Scholar]
- 49.Winter RB, Lonstein JE, Denis F. Pain patterns in adult scoliosis. Orthop Clin North Am. 1988;19:339–345. [PubMed] [Google Scholar]
- 50.Ialenti MN, Lonner BS, Verma K, Dean L, Valdevit A, Errico T. Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33:372–376. doi: 10.1097/BPO.0b013e3182870325. [DOI] [PubMed] [Google Scholar]
- 51.Marks M, Petcharaporn M, Betz RR, Clements D, Lenke L, Newton PO. Outcomes of surgical treatment in male versus female adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 2007;32:544–549. doi: 10.1097/01.brs.0000256908.51822.6e. [DOI] [PubMed] [Google Scholar]
- 52.Sucato DJ, Hedequist D, Karol LA. Operative correction of adolescent idiopathic scoliosis in male patients. A radiographic and functional outcome comparison with female patients. J Bone Joint Surg Am. 2004;86-A:2005–2014. doi: 10.2106/00004623-200409000-00020. [DOI] [PubMed] [Google Scholar]
- 53.Brambilla S, Ruosi C, La Maida GA, Caserta S. Prevention of venous thromboembolism in spinal surgery. Eur Spine J. 2004;13:1–8. doi: 10.1007/s00586-003-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dearborn JT, Hu SS, Tribus CB, Bradford DS. Thromboembolic complications after major thoracolumbar spine surgery. Spine (Phila Pa 1976) 1999;24:1471–1476. doi: 10.1097/00007632-199907150-00013. [DOI] [PubMed] [Google Scholar]
- 55.Ferree BA, Wright AM. Deep venous thrombosis following posterior lumbar spinal surgery. Spine (Phila Pa 1976) 1993;18:1079–1082. doi: 10.1097/00007632-199306150-00019. [DOI] [PubMed] [Google Scholar]
- 56.Platzer P, Thalhammer G, Jaindl M, Obradovic A, Benesch T, Vecsei V, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77:755–760. doi: 10.1080/17453670610012944. [DOI] [PubMed] [Google Scholar]
- 57.Rokito SE, Schwartz MC, Neuwirth MG. Deep vein thrombosis after major reconstructive spinal surgery. Spine (Phila Pa 1976) 1996;21:853–858. doi: 10.1097/00007632-199604010-00016. discussion: 859. [DOI] [PubMed] [Google Scholar]
- 58.Cain JE, Jr, Major MR, Lauerman WC, West JL, Wood KB, Fueredi GA. The morbidity of heparin therapy after development of pulmonary embolus in patients undergoing thoracolumbar or lumbar spinal fusion. Spine (Phila Pa 1976) 1995;20:1600–1603. doi: 10.1097/00007632-199507150-00008. [DOI] [PubMed] [Google Scholar]
- 59.Hull R, Hirsh J, Jay R, Carter C, England C, Gent M, et al. Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med. 1982;307:1676–1681. doi: 10.1056/NEJM198212303072704. [DOI] [PubMed] [Google Scholar]
- 60.Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976) 2008;33:2179–2184. doi: 10.1097/BRS.0b013e31817c0428. [DOI] [PubMed] [Google Scholar]
- 61.Swank S, Lonstein JE, Moe JH, Winter RB, Bradford DS. Surgical treatment of adult scoliosis. A review of two hundred and twenty-two cases. J Bone Joint Surg Am. 1981;63:268–287. [PubMed] [Google Scholar]
- 62.McCarthy IM, Hostin RA, Ames CP, Kim HJ, Smith JP, Boachie-Adjei O, et al. Total hospital costs of surgical treatment for adult spinal deformity: an extended follow-up study. Spine J. 2014;14:2326–2333. doi: 10.1016/j.spinee.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 63.Shaffrey E, Smith JS, Lenke LG, Polly DW, Jr, Chen CJ, Coe JD, et al. Defining rates and causes of mortality associated with spine surgery: comparison of 2 data collection approaches through the Scoliosis Research Society. Spine (Phila Pa 1976) 2014;39:579–586. doi: 10.1097/BRS.0000000000000201. [DOI] [PubMed] [Google Scholar]


