Abstract
The hippocampus receives two major external inputs from the diencephalon, that is, from the supramammillary nucleus (SUM) and nucleus reuniens (RE) of the midline thalamus. These two afferents systems project to separate, nonoverlapping, regions of the hippocampus. Specifically, the SUM distributes to the dentate gyrus (DG) and to CA2 of the dorsal and ventral hippocampus, whereas RE projects to CA1 of the dorsal and ventral hippocampus and to the subiculum. SUM and RE fibers to the hippocampus participate in common as well as in separate functions. Both systems would appear to amplify signals from other sources to their respective hippocampal targets. SUM amplifies signals from the entorhinal cortex (EC) to DG, whereas RE may amplify them from CA3 (and EC) to CA1 of the hippocampus. This “amplification” may serve to promote the transfer, encoding, and possibly storage of information from EC to DG and from CA3 and EC to CA1. Regarding their unique actions on the hippocampus, the SUM is a vital part of an ascending brainstem to hippocampal system generating the theta rhythm of the hippocampus, whereas RE importantly routes information from the medial prefrontal cortex to the hippocampus to thereby mediate functions involving both structures. In summary, although, to date, SUM and RE afferents to the hippocampus have not been extensively explored, the SUM and RE exert a profound influence on the hippocampus in processes of learning and memory.
Keywords: Rhomboid nucleus, Medial septum, Entorhinal cortex, Theta rhythm, Long-term potentiation, Fear memory, Working memory, Trajectory-dependent neurons
1 INTRODUCTION
Whereas much attention has been paid to the description and functional significance of inputs to the hippocampus from the medial septum (MS) and the entorhinal cortex (EC), less consideration has been given to the role of other major afferent systems to the hippocampus (HF). Two prominent, but relatively unexplored, diencephalic inputs to the hippocampus are the supramammillary nucleus (SUM) of the hypothalamus and nucleus reuniens (RE) of the midline thalamus. As will be described herein, an ever increasing body of evidence, however, suggests that the SUM and RE exert a pronounced influence on the hippocampus—which appears largely distinct for each nucleus. Differential actions of SUM and RE on the hippocampus might be expected by the relatively complete segregation of their inputs to HF. Specifically, the SUM distributes to the dentate gyrus (DG) and to the CA2/CA3a region of Ammon’s horn (Haglund et al., 1984; Vertes, 1992), whereas RE projects selectively to CA1 of the dorsal and ventral hippocampus and to the ventral subiculum of HF (Varela et al., 2014; Vertes et al., 2006; Wouterlood et al., 1990). Accordingly, SUM is more positioned to affect early stages of hippocampal processing with projections to DG/CA2, while RE would exert a greater influence on later stages of hippocampal circuitry, perhaps modulating the output of HF with projections to CA1 and to the subiculum.
Presently, we will describe: (1) the direct (and indirect) connections of SUM and RE with the hippocampus (HF), (2) the physiological effects of manipulations of these systems (SUM and RE) on hippocampal activity, and (3) the role of SUM and RE in behavior. Emphasis will be placed on the involvement of SUM in the generation of the hippocampal theta rhythm, and RE as a critical interface between the medial prefrontal cortex (mPFC) and the hippocampus in coordinating functions involving both structures.
2 SUM: ANATOMY
Several reports have shown that the SUM is a major source of afferents to the hippocampus (Amaral and Cowan, 1980; Haglund et al., 1984; Harley et al., 1983; Leranth and Hajszan, 2007; Magloczky et al., 1994; Ohara et al., 2013; Soussi et al., 2010; Vertes, 1992; Vertes and McKenna, 2000; Wyss et al., 1979). For instance, Amaral and Cowan (1980) initially demonstrated that hippocampal injections of horseradish peroxidase in the monkey produced dense retrograde cell labeling in SUM—or equivalent or even greater than that seen in the septum with these injections. SUM fibers distribute selectively to the DG and to CA2/CA3a of the hippocampus and terminate within the upper third of the granule cell layer and adjacent inner molecular layer of DG and within the stratum oriens and pyramidal cell layer of CA2/CA3a. The SUM projection to DG (and CA2) predominantly originates from the lateral two-thirds of SUM and distributes more densely to the inner (supragranular) than to the outer (infraganular) layer of DG (Fig. 1). Medial SUM fibers are mainly restricted to the ventral DG, whereas those of the lateral SUM terminate in the dorsal and ventral DG but most heavily in the dorsal DG (Vertes, 1992). Figure 1 depicts labeled fibers in the DG and in CA2/CA3a of Ammon’s horn of the dorsal hippocampus following a PHA-L injection in lateral SUM (Vertes, 1992).
FIGURE 1.
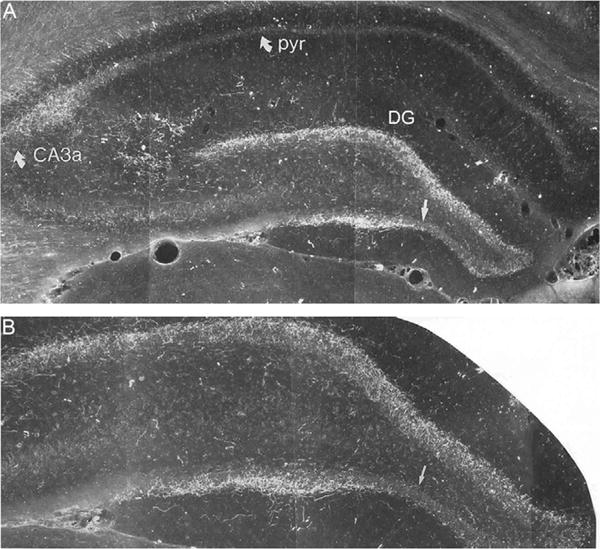
(A) Low-power and (B) high-power darkfield photomicrographs of transverse sections through the dorsal hippocampus showing the patterns of labeling produced by a PHA-L injection in the lateral supramammillary nucleus. Panel (B) taken from panel (A). Note the dense labeling within the granule cell layer of the dentate gyrus (DG), slightly stronger in the dorsal than the ventral blade (straight arrow) of DG, and labeling confined to CA2/CA3a of Ammon’s horn. CA3a, field CA3a of Ammon’s horn; DG, dentate gyrus; pyr, pyramidal cell layer of CA1 of Ammon’s horn.
A number of early studies reported that the SUM-DG projection was largely (or exclusively) excitatory—or glutamatergic. This was based on the demonstration that SUM fibers lacked markers for GABA and predominantly formed asymmetric synapses with DG cells (Kiss et al., 2000; Magloczky et al., 1994; Stanfield and Cowan, 1984). In contrast to this, however, recent reports have described SUM-originating GABAergic terminals in DG—or more specifically have identified a unique subset of SUM fibers projecting to HF that contain release sites for both GABA and glutamate (Boulland et al., 2009; Soussi et al., 2010). For instance, Boulland et al. (2009) demonstrated that SUM fibers terminating in DG contained transporters for both GABA (VGAT) and glutamate (VGLUT2) which were localized to separate vesicles and formed symmetric or asymmetric contacts, respectively, with proximal dendrites/soma of granule cells. Confirming and extending these finding, Soussi et al. (2010) described two distinct pathways from the SUM to DG, with differing transmitter characteristics. One pathway originated from the lateral SUM, distributed most heavily to the dorsal DG, and contained markers for both GABA (GAD65) and glutamate (VGLUT2). The second system originated from the medial SUM, primarily distributed to DG and CA2/CA3a of the ventral hippocampus and only contained VGLUT2+ terminals. Soussi et al. (2010) speculated, for the dual transmitter system, that GABA may act as the main transmitter in the inhibition of target cells, whereas glutamate may serve as a neuromodulator.
Using retrograde transsynaptic viral tracers to identify first- and second-order inputs to the dorsal or ventral DG, Ohara et al. (2013) recently showed that the SUM (as well as the MS and EC) was a first-order input to DG and confirmed earlier findings (Soussi et al., 2010; Vertes, 1992) that the medial SUM primarily distributes to the ventral DG and the lateral SUM to the dorsal DG. In line with the notion that the dorsal HF is primarily involved in spatial behaviors and the ventral HF in affective states (Fanselow and Dong, 2010), Ohara et al. (2013) demonstrated that second-order fibers to the ventral DG (largely routed through the medial SUM) primarily originated from “affective” structures such as the ventromedial and dorsal hypothalamus, the preoptic area, and the infralimbic cortex (IL) cortex.
Magloczky et al. (1994) reported that SUM afferents to the hippocampus virtually exclusively target principal cells of the HF. For example, at the light/EM level, they showed that none of the postsynaptic targets of 68-labeled SUM boutons in HF were immunoreactive for inhibitory transmitters. This, however, contrasts with the subsequent findings of Nitsch and Leranth (1996) demonstrating that supramammillary calretinin-positive fibers formed asymmetric synapses with parvalbumin-containing basket cells bordering the granule cell layer and with calbindin-positive cells of the hilus. Nitsch and Leranth (1996) suggested that the conflicting results probably involve the use of different anatomical techniques—especially those used to identify nonprincipal cells of DG.
To conclude, the SUM is a major source of projections to the DG and to the CA2/CA3a region of the hippocampus. Although initial studies indicated that SUM fibers innervating the hippocampus were predominantly excitatory and made synaptic contact almost exclusively with principal cells of HF, subsequent reports: (1) identified a population of lateral SUM cells projecting to DG that contained markers for both GABA and glutamate and (2) showed that a subset of calretinin+fibers of SUM form asymmetric connections with nonprincipal cells of DG.
3 SUM: ELECTROPHYSIOLOGY
In a comprehensive examination of the effects of SUM and the MS on hippocampal activity, Mizumori et al. (1989) demonstrated that prestimulation of either site (SUM or MS) significantly enhanced perforant path (PP)-elicited population spikes at DG. With respect to SUM, multiple lines of evidence led them to propose that the population spike enhancement at DG involved a SUM inhibition of inhibitory interneurons (or basket cells) of DG—and hence a disinhibition and activation of dentate granule cells. Specifically, they demonstrated that (1) SUM stimulation suppressed the activity of about 50% of fast firing (presumed) interneurons of DG, (2) SUM prestimulation paired with PP stimulation (producing potentiation) inhibited the activity of dentate interneurons, and (3) short-term paired pulse depression of PP-elicited population spikes at DG activated dentate interneurons (Mizumori et al., 1989).
In accord with the foregoing, Carre and Harley (1991) showed that glutamate injections into the lateral SUM substantially increased the amplitude of PP-evoked population spikes at DG—which often lasted for 20 min or more. More recently, Nakanishi et al. (2001) demonstrated that SUM stimulation paired with weak tetanic stimulation of the medial PP produced long-lasting potentiation (LTP) of population spikes at DG. It was further shown that LTP was blocked by infusions of the GABA antagonist, picrotoxin, implicating a GABAergic mechanism in this effect (Nakanishi et al., 2001). Conceivably, picrotoxin reversed the inhibitory actions of GABAergic SUM fibers terminating in DG (Boulland et al., 2009; Soussi et al., 2010), thereby activating interneurons, suppressing granule cells, and thus abolishing LTP.
4 SUM: ROLE IN THE THETA RHYTHM
In addition to direct inputs to the hippocampus, SUM distributes to several forebrain structures with connections to the hippocampus, that is, to the RE, the endopiriform nucleus, the medial and lateral septum, and the EC (Vertes, 1992). SUM prominently targets the MS and a subset of cells of the lateral SUM give rise to collateral projections to MS and DG (5–10% of cells) as well as to MS and CA2 (3–5% of cells) (Vertes and McKenna, 2000).
The theta rhythm of the hippocampus is a large amplitude (1–2 mV) nearly sinusoidal oscillation of 5–12 Hz in the behaving rat (Bland, 1986; Buzsáki, 2002; Pignatelli et al., 2012; Vertes and Kocsis, 1997). It is the largest extracellular synchronous signal that can be recorded in the mammalian brain. In their initial report describing theta and its characteristics in the curarized rabbit, Green and Arduini (1954) showed that theta could be readily elicited by natural sensory stimuli as well as by electrical stimulation of the brainstem reticular formation (RF). Shortly thereafter, Petsche, Stumpf, and colleagues (Petsche et al., 1962, 1965) demonstrated the now well-recognized finding that the MS is critical for the generation of theta; that is, the MS contains a population of “pacemaking” cells that drive theta of the HF (Pignatelli et al., 2012; Vertes et al., 2004). Lesions of the MS completely abolish the theta rhythm. While it was originally thought that the brainstem RF directly affected the MS in the elicitation of theta (Petsche et al., 1965), subsequent studies have shown that brainstem actions on the MS are mediated by the SUM, lying between the brainstem RF and MS (Pan and McNaughton, 2004; Vertes et al., 2004).
Specifically, it has been demonstrated that (1) the SUM receives projections from the brainstem RF and in turn strongly targets the MS, (2) SUM contains a population of cells that fire rhythmically synchronous with the theta rhythm (see Fig. 2), (3) electrically or chemically induced activation of SUM drives septal pacemaking cells as well as hippocampal theta, and (4) the reversible suppression of SUM with procaine in anesthetized rats disrupts the spontaneous as well as brainstem RF-elicited rhythmical bursting activity of MS cells as well as hippocampal theta (Bland et al., 1990, 1994, 1995; Kocsis and Vertes, 1994, 1997; Oddie et al., 1994).
FIGURE 2.
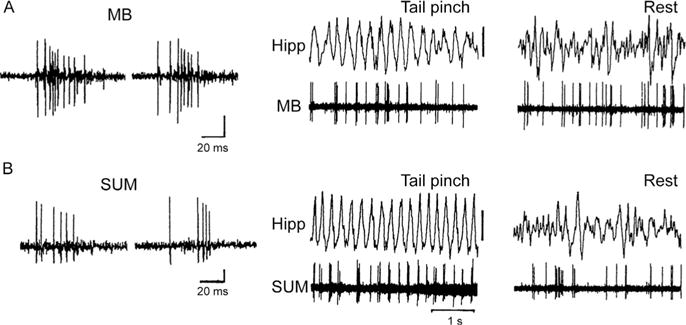
Burst properties of neurons of the mammillary bodies (MB) (A) and the supramammillary nucleus (SUM). (B) during theta (middle traces) and non-theta (right traces) states of the hippocampal EEG in the anesthetized rat. Note that both MB and SUM cells discharge rhythmically in bursts synchronous with the theta rhythm (produced by a tail pinch) but nonrhythmically in the absence of theta (rest). Whereas the theta burst discharge of MB cells is dependent on descending input from the hippocampus to MB via the fornix, the theta rhythmical activity of SUM cells involves the ascending actions of the brainstem reticular formation on SUM (see Vertes et al., 2004 and text).
The notion has been developed that the SUM converts a steady (nonrhythmical) barrage of activity from the brainstem RF into a rhythmical pattern of discharge which is then relayed to the septum to drive MS pacemaking cells and consequently hippocampal targets in the generation of theta. Accordingly, McNaughton and colleagues (Pan and McNaughton, 2004) have proposed that the SUM encodes the frequency of theta and MS the amplitude of theta. This is supported by the demonstration that suppression of SUM reduces the frequency but not the amplitude of theta, whereas the inactivation of MS (by various methods) significantly decreases the amplitude of theta with little effect on frequency (Kirk and McNaughton, 1993; Lee et al., 1994).
In summary, the SUM appears to have (at least) two distinct effects on the hippocampus: direct actions at the DG and CA2 and indirect ones mediated by the MS. These two effects may be complementary: SUM projections to DG that amplify signals from the EC to DG may do so in conjunction with a SUM triggering of theta to thereby link these two events. Particularly, theta is thought to promote the encoding of information in the hippocampus; that is, signals arriving to the HF from “information-bearing” sources (e.g., EC) concurrently with theta are encoded, whereas information reaching the HF in the absence of theta is not encoded—or not to the same degree as that coinciding with theta (Hasselmo et al., 2002; Vertes, 2005). Accordingly, the SUM may enhance the encoding of information in the HF by both amplifying signals from EC to the hippocampus (DG) and timing them to occur in the presence of theta.
5 SUM: ROLE IN LEARNING AND MEMORY
As might be expected by relatively widespread SUM projections to the limbic forebrain, the SUM has been associated with various functions including stress, anxiety, response to novelty, reward processes, and learning/memory (Aranda et al., 2006, 2008; Choi et al., 2012; Ikemoto, 2005; Ikemoto and Bonci, 2014; Ito et al., 2009; Shahidi et al., 2004a,b). Perhaps owing to the strong SUM projections to the septum and the hippocampus, disruptions of SUM have been shown to severely impair learning/memory over a range of tasks. Although an early report described minor effects on memory with SUM lesions (Pan and McNaughton, 2002), which may have resulted from the rather incomplete destruction of SUM (see Shahidi et al., 2004b), several subsequent studies have demonstrated quite pronounced deficits following lesions/inactivation of the SUM.
In an initial study, Shahidi et al. (2004a) showed that the reversible inactivation of SUM with lidocaine produced marked deficits in consolidation of a passive avoidance task. Specifically, lidocaine, given 5 min following acquisition, but not after 90 or 360 min, disrupted performance on the task. Shahidi et al. (2004b) subsequently demonstrated that suppressing SUM altered performance on spatial reference memory (RM) and working memory (WM) tasks on the water maze; that is, disrupting consolidation on the RM task and both consolidation and retrieval on the WM task. Supporting this, electrolytic lesions (Aranda et al., 2006) or the reversible inactivation of SUM (Aranda et al., 2008) impaired spatial WM on a delayed-matching-to-position task. It was further reported (Aranda et al., 2006) that SUM lesions produced anxiolytic effects on an elevated T-maze. The latter finding is consistent with the demonstration of elevated levels of c-fos expression in SUM to novel environments (Ito et al., 2009; Wirtshafter et al., 1998), and results showing that select populations of SUM cells projecting to HF are affected by immobilization stress (Choi et al., 2012).
Finally, Gutiérrez-Guzmán et al. (2012) recently demonstrated that the selective elimination of serotonergic fibers to SUM impaired performance on a water maze task which was accompanied by a disruption of the hippocampal theta rhythm. The authors had previously shown (Gutiérrez-Guzmán et al., 2011) that the effect of serotonergic (5-HT) denervation of the hippocampus was the opposite to that at SUM; that is, it produced a facilitation of spatial learning, associated with high frequency theta. Based on the established link between theta and memory processing (Buzsáki, 2002; Hasselmo et al., 2002; Vertes, 2005), the authors proposed that manipulations that enhance theta activity, such as blocking 5-HT actions on the septum/hippocampus, promote learning, whereas those that disrupt theta (e.g., removing 5-HT input to SUM) impair learning/memory (Olvera-Cortés et al., 2013).
In summary, the foregoing indicates that the SUM, via direct or indirect actions on the hippocampus, serves a critical role in mnemonic processes as demonstrated by deficits in learning/memory (over a range of tasks) seen with lesions/inactivation of SUM.
6 RE: ANATOMY
The RE lies ventrally on the midline directly above the third ventricle and extends longitudinally virtually throughout the thalamus. RE is the largest of the midline nuclei of the thalamus (Cassel et al., 2013; Vertes et al., 2006, 2015a).
While, as will be discussed, the output of RE is somewhat restricted, mainly targeting “limbic cortices” and the hippocampus, afferents to RE are diverse and widespread, originating from the cortex, hippocampus, basal forebrain, amygdala, hypothalamus, and brainstem (Cassel et al., 2013; Herkenham, 1978; Krout et al., 2002; McKenna and Vertes, 2004; Vertes, 2002). Specifically, RE receives projections from several regions of the (limbic) cortex including the orbitomedial, insular, ectorhinal, perirhinal, and retrosplenial cortices and the hippocampus, and also from various subcortical structures which include the claustrum, lateral septum, bed nucleus of the stria terminalis (BST) and medial, lateral, and magnocellular preoptic nuclei of the basal forebrain; the lateral habenula, paraventricular, and lateral geniculate nuclei of the thalamus; the zona incerta, anterior, ventromedial, lateral, perifornical, posterior, SUM, and dorsal premammillary nuclei of the hypothalamus; and the ventral tegmental area, periaqueductal gray, precommissural nucleus, parabrachial nuclei, laterodorsal tegmental nucleus, and the dorsal and median raphe nuclei of the brainstem (Krout et al., 2002; McKenna and Vertes, 2004; Van der Werf et al., 2002). Although inputs to each of the midline thalamic nuclei have not been examined as thoroughly as those to RE, it appears that afferents to RE are considerably more diverse and widespread than those to other nuclei of the midline thalamus (Groenewegen and Witter, 2004; Van der Werf et al., 2002; Vertes et al., 2015a).
As alluded to previously, RE distributes substantially to limbic cortices and to the hippocampus, with limited projections to subcortical sites. The main cortical targets of RE are the medial and ventral orbital cortices, the IL, prelimbic (PL), and anterior cingulate cortices of the mPFC, the dorsal, and ventral agranular insular cortices, rostral retrosplenial cortex, perirhinal cortex, and the medial and lateral entorhinal cortices (Berendse and Groenewegen, 1991; Cassel et al, 2013; Dolleman-Van der Weel and Witter, 1996; Van der Werf et al., 2002; Varela et al., 2014; Vertes et al., 2006, 2007; Wouterlood, 1991). As recognized early on (Herkenham, 1978) and subsequently confirmed, RE projects massively and in a highly organized manner to the hippocampus (Fig. 3). RE is the predominant, or virtually sole, source of thalamic input to the hippocampus. RE fibers innervating the hippocampus terminate selectively in the stratum lacunosum-moleculare (slm) of CA1 of the dorsal and ventral hippocampus as well as the molecular layer of the subiculum and parasubiculum (Vertes et al., 2006; Wouterlood et al., 1990). This pattern of labeling is depicted in Fig. 3. RE axons form asymmetric (excitatory) contacts predominantly on distal dendrites of pyramidal cells in slm of CA1 and the subiculum (Wouterlood et al., 1990) and to a lesser degree on dendrites in slm of GABAergic neurons located in the strata oriens or radiatum of CA1 (Dolleman-Van der Weel and Witter, 2000). Wouterlood et al. (1990) remarked that “Without exception, the synaptic membrane specializations (of RE terminals in CA1/subiculum) are of the asymmetric type.” There is an essential absence of RE projections to CA2 and CA3 and to the DG of the hippocampus.
FIGURE 3.
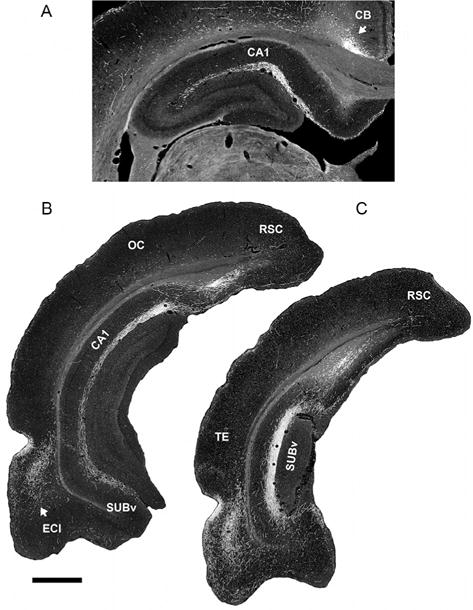
Low-magnification darkfield photomicrographs showing patterns of labeling in the dorsal (A) and ventral hippocampus (B) and (C) produced by an injection of PHA-L in nucleus reuniens of the midline thalamus. Note the dense concentration of labeled fibers restricted to the stratum lacunosum-moleculare of CA1 of the dorsal (A) and ventral (B) hippocampus and the molecular layer of the ventral subiculum (C). CA1, field CA1 of Ammon’s horn; CB, cingulum bundle; ECl, lateral entorhinal cortex; OC, occipital cortex; RSC, retrosplenial cortex; SUBv, ventral subiculum; TE, temporal cortex. Scale bar for (A) = 600 μm and for (B) and (C) = 1000 μm.
While, as indicated, RE distributes fairly widely throughout “limbic cortices,” projections to the mPFC, especially to IL and PL, are pronounced (Cassel et al, 2013; Vertes et al., 2006, 2015b). This, in part, has led to the examination of RE cells with possible collateral projections to the mPFC and to the hippocampus. In an initial report, using retrograde fluorescent techniques, Hoover and Vertes (2012) demonstrated that approximately 3–9% of RE cells projected, via collaterals, to the HF and mPFC. Figure 4 shows representative samples of retrogradely double-labeled cells in RE following injections of Fluorogold in the mPFC and Fluororuby in the ventral hippocampus (Hoover and Vertes, 2012). Although such cells (collateralizing) were rather dispersed throughout RE, they were most numerous in the lateral one-third of RE, just medial to the lateral wings of RE. It was further shown that, while intermingled, RE neurons projecting to one structure or to the other (non-branching) were preferentially localized to distinct subregions of RE; that is, cells projecting to the mPFC were concentrated in the lateral wings of RE (or perireuniens nucleus), while those distributing to the hippocampus were most abundant in the rostral pole of RE. Finally, an approximately 10-fold greater number of RE cells projected to the ventral than to the dorsal hippocampus (Hoover and Vertes, 2012).
FIGURE 4.
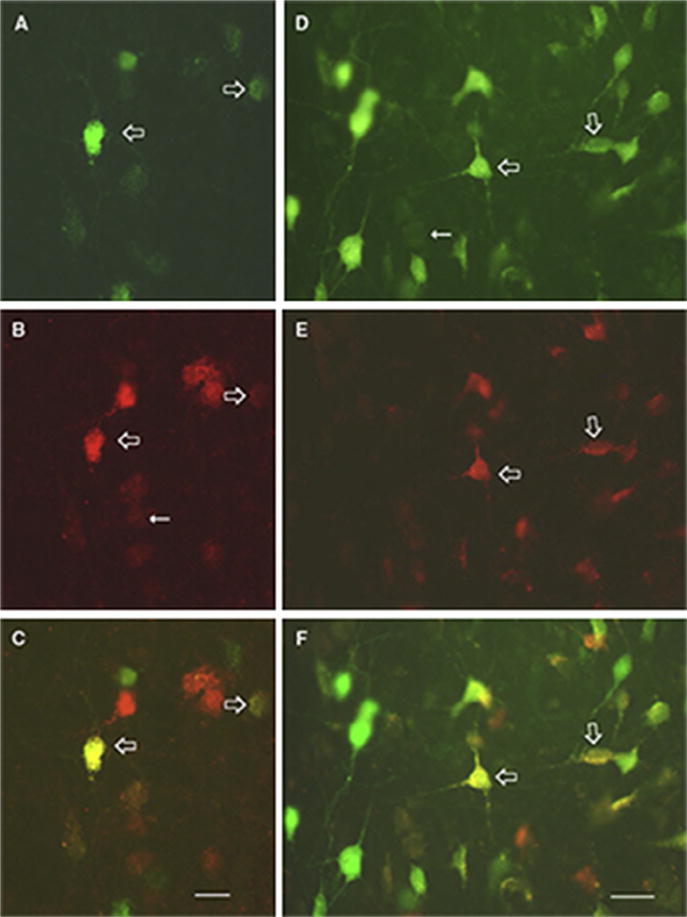
Photomicrographs depicting single- and double-labeled cells in the nucleus reuniens following Fluorogold (FG) injections in the medial prefrontal cortex and Fluororuby (FR) injections in the ventral hippocampus for two rats (A–C) and (D–F). (A) and (D) show FG-labeled cells (green (gray in the print version)), (B) and (E) show FR-labeled neurons (red (dark gray in the print version)), and (C) and (F) (open arrows in all panels) show double-labeled cells (yellow (white in the print version)) for each case. Scale bars = 20 μm.
In accord with the foregoing, Varela et al. (2014) reported that on average 8% of RE cells, spanning the rostrocaudal axis, gave rise to collateral projections to the hippocampus and to the ventral mPFC. Interestingly, they further showed that only about 1% of hippocampal neurons (of the subiculum) projected dually (via collaterals) to RE and to the mPFC. It was suggested that RE cells with branching projections to the hippocampus and to the mPFC may play a critical role in systems consolidation of memory or in the synchronization of the theta rhythm during exploratory behaviors (Varela et al., 2014).
While it is well recognized that the hippocampus strongly targets the mPFC (Carr and Sesack, 1996; Ferino et al., 1987; Hoover and Vertes, 2007; Jay and Witter, 1991), interestingly there are no return projections from the mPFC to the hippocampus (Laroche et al., 2000; Vertes, 2004). The demonstration, however, of dense projections from the mPFC to RE, and, in turn, from RE to the hippocampus (Vertes, 2002; Vertes et al., 2006) suggests that RE may be a principal route for the transfer of information from the mPFC to the hippocampus—thus completing an important functional loop between these structures. Supporting this, at the ultrastructural level, mPFC fibers distributing to the RE have been shown to form asymmetric (excitatory) contacts on proximal dendrites of RE cells projecting to the hippocampus (Fig. 5; Vertes et al., 2007). As will be discussed, there is developing view that reuniens is most directly involved in functions that depend on the interactions of the mPFC and the hippocampus. Accordingly, the loop from the mPFC to the hippocampus through RE (mPFC>RE>HF) appears critical in mediating the actions of the mPFC on the hippocampus for a range of behaviors (see below).
FIGURE 5.
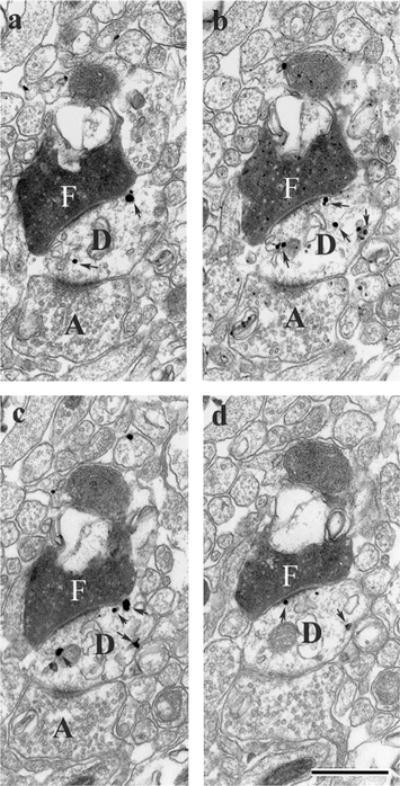
Series of electron micrographic sections through the nucleus reuniens of the midline thalamus (a–d) showing asymmetric contacts of a single PHA-L-labeled (F) fibers from the medial prefrontal cortex onto a labeled dendritic shaft, identified by the presence of numerous silver intensified gold deposits (arrows in D) of a RE cell retrogradely from a Fluorogold injection in the ventral hippocampus. Note also the presence of asymmetric contacts of an unlabeled fiber (A) on the same labeled dendrite segment (D). Scale bar = 1 μm.
7 RE: ELECTROPHYSIOLOGY
While several recent reports have described the functional properties of RE, surprisingly few studies have examined the electrophysiological characteristics of RE neurons or the effects of their activation (or suppression) on target structures. Nonetheless, consistent with marked RE projections to the HF and mPFC, reuniens has been shown to exert strong excitatory actions at CA1 of the hippocampus and at the mPFC (Bertram and Zhang 1999; Dolleman-Van der Weel et al., 1997; Viana Di Prisco and Vertes, 2006). Dolleman-Van der Weel et al. (1997) demonstrated that RE stimulation produced large negative-going field potentials (sink) at slm of CA1 as well as paired pulse facilitation at CA1. Bertram and Zhang (1999) subsequently compared the effects of RE and CA3 stimulation on population responses (field EPSPs and spikes) at CA1 and reported that RE actions at CA1 were equivalent to, and in some cases considerably greater than, those of CA3 at CA1. They concluded that the RE projection to the hippocampus “allows for the direct and powerful excitation of the CA1 region. This thalamohippocampal connection bypasses the trisynaptic/commissural pathway that has been thought to be the exclusive excitatory drive to CA1” (Bertram and Zhang, 1999). Viana Di Prisco and Vertes (2006) confirmed the excitatory effects of RE on the hippocampus and further showed that RE stimulation produced large monosynaptically elicited evoked responses dorsoventrally throughout the mPFC, with most pronounced actions (latency and amplitude) at the ventral mPFC—or at PL and IL.
In recent studies, Lisman and colleagues (Duan et al., in press; Lisman et al., 2010; Zhang et al., 2012a,b) provided evidence that RE is a critical component of a complex circuitry which may contribute to schizophrenia—or a circuitry that is central to the glutamate/NMDA receptor (NMDAR) hypofunction model of schizophrenia (Coyle, 1996, 2006; Javitt and Zukin, 1991). More specifically, several symptoms of schizophrenia, notably abnormal delta frequency oscillations in the cortex, are produced by systemic or intrathalamic infusions of NMDA antagonists (Buzsáki, 1991; Zhang et al., 2012a). In this regard, Zhang et al. (2012a) showed that the systemic administration of the NMDAR antagonist, ketamine, increased the firing rate of neurons in RE and in CA1 of the hippocampus of awake rats, together with an associated increase in the power of delta oscillations in both structures. The increases in neuronal firing and delta power in RE and HF were also produced by intra-RE infusions of ketamine and were blocked by injections of muscimol into RE. The foregoing suggests a vital role for RE in ketamine-induced (or NMDA antagonist-induced) hyperactivity and delta oscillations in HF—two hallmarks of schizophrenia (Boutros et al., 2008; Fehr et al., 2001; Lodge and Grace, 2007, 2011). In a recent report, using optogenetic techniques, Duan et al. (in press) showed that driving RE fibers at their termination in the dorsal hippocampus at delta frequencies significantly impaired performance on a WM task. As was convincingly demonstrated, activating RE fibers at delta frequencies (light-on condition) severely altered performance, whereas in the absence of such activation (light-off condition) performance was normal.
As was described, RE fibers distributing to CA1 of the hippocampus terminate in slm of CA1 (Vertes et al., 2006, 2015b; Wouterlood et al., 1990). It is also the case (Cappaert et al., 2015; Desmond et al., 1994) that projections from layer III of the medial and lateral EC to CA1 terminate in the slm—or on distal apical dendrites of CA1 cells. The convergence of RE and EC inputs to slm suggests that the slm may be an important site for the interactions of these two systems in the modulation/control of CA1 activity. In this regard, Desmond et al. (1994) drew key similarities between these two sets of afferents to slm of CA1 stating that “The n. reuniens and entorhinal cortical synapses in CA1 share a number of features, e.g., the small size of their axons, the spherical synaptic vesicles filling the presynaptic element, and the asymmetric synaptic contracts.”
Whereas the precise nature of interactions of RE and EC inputs to CA1 is presently unknown, possible insight is gained by examining the interactive effects of CA3 and entorhinal (PP) afferents to CA1 (Takahashi and Magee, 2009). For instance, Takahashi and Magee (2009) described the important findings that the coactivation of Schaffer collateral (SC) and PP input to stratum radiatum and to slm of CA1, respectively, produced supralinear depolarizing responses (EPSPs) at distal dendrites of CA1 cells—or plateau potentials. In effect, they stated, “the distal dendrites of CA1 pyramidal neurons supralinearly summate moderate levels of SC and PP input through the generation of a large prolonged plateau potential.” The plateau potentials were dependent on the timing of afferent input to back propagating action potentials in the distal dendrites (slm) of CA1 cells—with the consequent recruitment of voltage-gated calcium channels and NMDARs (Larkum et al., 1999; Takahashi and Magee, 2009). The dendritic plateau potentials, in turn, gave rise to a large after-depolarization of CA1 neurons, a shift from single spiking to a bursting pattern of discharge of these cells, and importantly to the long-term potentiation (LTP) of the PP input to slm.
Among other things, the foregoing demonstrates that inputs to distal apical dendrites of CA1 cells from CA3 and the EC summate to modify the firing pattern of CA1 cells (favoring bursts) as well as the efficacy of the PP-CA1 synapse, supporting LTP. As pointed out by Takahashi and Magee (2009), the combined effects signal to downstream regions that “there exists a certain level of coactivity among specific entorhinal and CA3 ensembles.”
In a similar manner, it seems likely that reuniens and layer III entorhinal inputs to slm may interact in basically the same way (and employ similar mechanisms) as shown for SC and PP inputs to distal dendrites of CA1 cells. Specifically, the coincident activation of RE and EC inputs to CA1 could enhance the effectiveness of entorhinal afferents to CA1, possibly shifting CA1 cells into a burst mode with associated long-term changes (LTP) at the entorhinal–CA1 synapse.
8 RE: ROLE IN LEARNING AND MEMORY
A number of recent reports have described the effects of lesions/inactivation of RE on behavior (Cholvin et al., 2013; Davoodi et al., 2009, 2011; Dolleman-Van der Weel et al., 2009; Eleore et al., 2011; Hallock et al., 2013; Hembrook and Mair, 2011; Hembrook et al., 2012; Ito et al., in press; Loureiro et al., 2012; Mitchell et al., 2014; Prasad et al., 2013; Saalmann, 2014; Xu and Südhof, 2013). While a consensus has not been reached, it appears that RE (or RE and the dorsally adjacent rhomboid nucleus (RH)) is critically involved in behaviors that depend on interactions between the hippocampus and the mPFC.
For instance, Hembrook and Mair (2011) initially showed that RE/RH lesions significantly altered performance on a delayed nonmatch to sample radial arm maze task which is sensitive to damage to the HF or to the mPFC (Mair et al., 1998; McDonald and White, 1993; Porter and Mair, 1997), but were without effect on a visuospatial reaction time task sensitive to alterations of the striatum or the motor cortex. Hembrook et al. (2012) subsequently examined the effects of reversible inactivation of RE/RH: (1) on a delayed nonmatch to position task in an operant chamber sensitive to lesions of the hippocampus or the mPFC and (2) on a variable choice radial maze delayed nonmatching task responsive to hippocampal but not to mPFC lesions (Porter et al., 2000). RE/RH inactivation significantly disrupted performance on the delayed nonmatch to position task but not on the radial maze task. The authors concluded that “RE and RH affect measures of spatial WM that depend on interactions between the hippocampus and the mPFC, but not measures that depend on the hippocampus alone” (Hembrook et al., 2012).
Using a different set of tasks, Cassel and colleagues similarly concluded that RE/RH selectively participate in functions requiring the cooperative actions of the hippocampus and the mPFC (Cassel et al., 2013; Cholvin et al., 2013; Loureiro et al., 2012). Specifically, lesions of RE/RH had no effect on either the acquisition or short-term retention (5-day postacquisition) of a water maze task but disrupted long-term retention (25 days) on the task (Loureiro et al., 2012). As was pointed out, recent memory (5 days) involves the hippocampus, whereas remote memory (25 days) enlists both the hippocampus and the mPFC (Broadbent et al., 2006; Clark et al., 2005; Lopez et al., 2012; Teixeira et al., 2006). In a follow-up report, Cholvin et al. (2013) compared the effects of selective inactivation of the hippocampus, the mPFC, or the RE/RH on a standard water maze task and on a “double-H” water maze task that places demands on both the hippocampus (place identification) and the mPFC (strategy shifting) for successful completion. Only hippocampal inactivation impaired performance on the standard water maze task, whereas inactivation of the hippocampus, mPFC, or the RE/RH disrupted performance, and to a similar degree, on the double-H water maze task. According to the authors, the hippocampus serves a recognized role in spatial memory, the mPFC in set shifting, and RE/RH may act “as the coordinator of this processing” (Cholvin et al., 2013).
Finally, Hallock et al. (2013) compared the effects of RE/RH suppression on two versions of a conditional discrimination T-maze task: one involving a WM component and the other not. RE/RH inactivation severely disrupted performance on the WM, but not on the conditional discrimination, version of the task, leading the authors to conclude that RE/RH is a necessary component of WM performance which is “thought to depend on the hippocampal–prefrontal circuit.”
As described, the hippocampus projects significantly to the mPFC, but there are no return from the mPFC to the hippocampus (Hoover and Vertes, 2007; Laroche et al., 2000; Vertes, 2004). In the absence of direct actions of the mPFC on the HF, reuniens appears to serve as a critical functional link between these structures. Accordingly, deficits in tasks which reportedly involve interactions between the mPFC and hippocampus may mainly result from interrupting the flow of information from the mPFC to the hippocampus with RE lesions. In this regard, two recent reports have demonstrated that RE is vital for routing information from the mPFC to the HF in memory-associated functions (Ito et al., in press; Xu and Südhof, 2013).
Using an impressive combination of tracing, viral vector, optogenetic, and electrophysiological techniques, Xu and Südhof (2013) described an mPFC–RE–hippocampal circuit responsible for fear memory specificity and generalization. More precisely, in a series of experiments, they demonstrated how altering the balance of activity within this circuit affects responses to fearful stimuli—along a specificity–generalization continuum. The authors had previously shown that disruption of the mPFC led to an overgeneralization of fear memory (Xu et al., 2012) and proceeded to examine the circuitry responsible for this effect (Xu and Südhof, 2013). Using viral tracing techniques, they confirmed previous demonstrations that the mPFC (mainly IL and PL) distributes massively to RE, and that the medial PFC also sends strong projections to other subcortical sites, prominently, the mediodorsal nucleus (MD) of thalamus and the striatum (Vertes, 2002, 2004). Focusing then on these three sites (RE, MD, striatum), they showed that the disruption of mPFC projections to RE, but not to the other two structures, produced an overgeneralization of contextual fear memory—comparable to that seen with alterations of the mPFC. Following this, they demonstrated that suppressing (with toxins) or enhancing the output of reuniens produced either an overgeneralization or a reduction of contextual fear memory (respectively) which was associated with decreases or increases in levels of c-fos expression in CA1 of the hippocampus. Finally, they demonstrated that two differing patterns of RE stimulation had opposite effects on fear memory; that is, phasic stimulation (15 pulses/30 Hz) produced an overgeneralization of fear memory, whereas tonic stimulation (4 Hz) reduced fear generalization. The phasic stimulation may have disrupted RE–hippocampal communication, much like toxins applied to RE (see above), thus similarly resulting in an overgeneralization of contextual fear memory. In summary, the combined findings indicate a critical role for RE in the processing of fear memory/memory in the HF—or as the authors concluded that reuniens “determines the specificity and generalization of memory attributes to a particular context by processing information from the mPFC en route to the hippocampus” (Xu and Südhof, 2013).
In a similar vein, Ito et al. (in press) recently demonstrated that information transferred from the mPFC to hippocampus via RE is essential for goal-directed spatial coding. Specifically, it had previously been shown that place cells in CA1 of the hippocampus not only code for location (place) but acquire the capacity for signaling future paths to remembered goals (Catanese et al., 2014; Frank et al., 2000; Pfeiffer and Foster, 2013; Wood et al., 2000). For instance, CA1 cells were found to fire differentially in the central stem of a continuous T-maze (i.e., before the choice point) dependent on whether a rat makes a left or right turn in the maze (Wood et al., 2000). In essence, the discharge of these CA1 cells predicted intended movement toward a goal—or displayed “trajectory-dependent” firing characteristics. Ito et al. (in press) addressed the process whereby CA1 cells acquired this property. They initially showed that CA1 contained a large percentage of “trajectory-dependent” neurons, which contrasted with few such cells in CA3—which was attributed to the fact that RE projects to CA1 but not to CA3 of the hippocampus (Cassel et al., 2013; Vertes et al., 2006, 2015b, Wouterlood et al., 1990). They then identified significant populations of trajectory-dependent neurons in both the RE and the medial PFC (see also Baeg et al., 2003; Fujisawa et al., 2008), and proceeded to show that the irreversible (with ibotenic acid lesions) or the reversible (with optogenetic techniques) inactivation of RE reduced the trajectory-dependent firing of CA1 cells. Finally, by comparing the activity of mPFC, RE, and CA1 neurons during correct and incorrect choices on the T-maze, Ito et al. (in press) demonstrated that firing predicted the successful choice for correct trials (prospective coding), but not for the incorrect trials—wherein activity instead reflected the previous choice on the maze (retrospective coding). Based on their findings, the authors concluded that “projections from mPFC, via the RE, are crucial for representation of the future path during goal-directed behavior and point to the thalamus as a key node in networks for long-range communication between cortical regions involved in navigation” (Ito et al., in press).
9 CONCLUSION
As described herein, the hippocampus receives two major extrinsic inputs from the diencephalon: from the supramammillary nucleus and the nucleus reuniens of the midline thalamus. Interestingly, these two afferents systems project to separate or nonoverlapping regions of the hippocampus. Specifically, the SUM distributes to the DG and to CA2 of the hippocampus, whereas RE projects selectively to the slm of CA1 of the dorsal and ventral hippocampus and to the molecular layer of the subiculum. With the exception of a restricted SUM input to CA3a, both systems essentially avoid CA3.
The pattern of distribution of SUM and RE fibers to the HF suggests shared as well as separate effects on hippocampal activity. Regarding their common effects, both systems appear to amplify signals from other sources to their respective targets in the HF; that is, SUM amplifies signals from the EC to DG, whereas RE may amplify them from CA3 and EC to CA1 of the hippocampus. This process may promote the transfer, encoding, and possibly storage of information from EC to DG (SUM) or from CA3/EC to CA1 (RE). Regarding the unique actions of the two systems, the SUM is an integral part of an ascending brainstem–diencephalo–septohippocampal system controlling the hippocampal theta rhythm, whereas RE serves a critical role in the transfer of information from the mPFC to the hippocampus, mediating behaviors involving both structures.
In summary, although SUM and RE inputs to the hippocampus have been less explored than other major afferents to the hippocampus, the SUM and RE exert a pronounced influence on the activity of the hippocampus. Lesions/inactivation of either system severely disrupts hippocampal-dependent processes of learning and memory, particularly allocentric spatial working memory.
Acknowledgments
This work was supported by NIMH Grant MH099590 to R.P.V.
References
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Aranda L, Santín LJ, Begega A, Aguirre JA, Arias JL. Supramammillary and adjacent nuclei lesions impair spatial working memory and induce anxiolitic-like behavior. Behav Brain Res. 2006;167:156–164. doi: 10.1016/j.bbr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Aranda L, Begega A, Sánchez-López J, Aguirre JA, Arias JL, Santín LJ. Temporary inactivation of the supramammillary area impairs spatial working memory and spatial reference memory retrieval. Physiol Behav. 2008;94:322–330. doi: 10.1016/j.physbeh.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland BH, Colom LV, Ford RD. Responses of septal θ-on and θ-off cells to activation of the dorsomedial-posterior hypothalamic region. Brain Res Bull. 1990;24:71–79. doi: 10.1016/0361-9230(90)90289-c. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD, Colom LV, Vertes RP. Extrinsic modulation of medial septal cell discharges by the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:649–660. doi: 10.1002/hipo.450040604. [DOI] [PubMed] [Google Scholar]
- Bland BH, Konopacki J, Kirk IJ, Oddie SD, Dickson CT. Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethane-anesthetized rat. J Neurophysiol. 1995;74:322–333. doi: 10.1152/jn.1995.74.1.322. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Jenstad M, Boekel AJ, Wouterlood FG, Edwards RH, Storm-Mathisen J, Chaudhry FA. Vesicular glutamate and GABA transporters sort to distinct sets of vesicles in a population of presynaptic terminals. Cereb Cortex. 2009;19:241–248. doi: 10.1093/cercor/bhn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learn Mem. 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience. 1991;41:351–364. doi: 10.1016/0306-4522(91)90332-i. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cappaert NLM, Van Strien NM, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. fourth. Academic Press; San Diego: 2015. pp. 511–574. [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Carre GP, Harley CW. Population spike facilitation in the dentate gyrus following glutamate to the lateral supramammillary nucleus. Brain Res. 1991;568:307–310. doi: 10.1016/0006-8993(91)91415-w. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese J, Viggiano A, Cerasti E, Zugaro MB, Wiener SI. Retrospectively and prospectively modulated hippocampal place responses are differentially distributed along a common path in a continuous T-maze. J Neurosci. 2014;34:13163–13169. doi: 10.1523/JNEUROSCI.0819-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WK, Wirtshafter D, Park HJ, Lee MS, Her S, Shim I. The characteristics of supramammillary cells projecting to the hippocampus in stress response in the rat. Korean J Physiol Pharmacol. 2012;16:17–24. doi: 10.4196/kjpp.2012.16.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Hav Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Naghdi N, Akbari E. Effect of reversible inactivation of the reuniens nucleus on spatial learning and memory in rats using Morris water maze task. Behav Brain Res. 2009;198:130–135. doi: 10.1016/j.bbr.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Davoodi FG, Motamedi F, Akbari E, Ghanbarian E, Jila B. Effect of reversible inactivation of reuniens nucleus on memory processing in passive avoidance task. Behav Brain Res. 2011;221:1–6. doi: 10.1016/j.bbr.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Scott CA, Jane JA, Jr, Levy WB. Ultrastructural identification of entorhinal cortical synapses in CA1 stratum lacunosum-moleculare of the rat. Hippocampus. 1994;4:594–600. doi: 10.1002/hipo.450040509. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol. 1996;364:637–650. doi: 10.1002/(SICI)1096-9861(19960122)364:4<637::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Witter MP. Nucleus reuniens thalami innervates gamma aminobutyric acid positive cells in hippocampal field CA1 of the rat. Neurosci Lett. 2000;278:145–148. doi: 10.1016/s0304-3940(99)00935-0. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of water-maze learning. Brain Struct Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Duan AR, Varela C, Zhang Y, Shen Y, Xiong L, Wilson MA, Lisman JE. Delta frequency optogenetic stimulation of a thalamic nucleus reuniens is sufficient to produce working memory deficits; relevance to schizophrenia. Biol Psychiatry. doi: 10.1016/j.biopsych.2015.01.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleore L, López-Ramos JC, Guerra-Narbona R, Delgado-García JM. Role of reuniens nucleus projections to the medial prefrontal cortex and to the hippocampal pyramidal CA1 area in associative learning. PLoS One. 2011;6:e23538. doi: 10.1371/journal.pone.0023538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, Elbert T. Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biol Psychiatry. 2001;50:108–116. doi: 10.1016/s0006-3223(01)01122-2. [DOI] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The Rat Nervous System. third. Academic Press; San Diego: 2004. pp. 408–441. [Google Scholar]
- Gutiérrez-Guzmán BE, Hernández-Pérez JJ, González-Burgos I, Feria-Velásco A, Medina R, Guevara MÁ, López-Vázquez MÁ, Olvera-Cortés ME. Hippocampal serotonin depletion facilitates place learning concurrent with an increase in CA1 high frequency theta activity expression in the rat. Eur J Pharmacol. 2011;652:73–81. doi: 10.1016/j.ejphar.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Guzmán BE, Hernández-Pérez JJ, López-Vázquez MÁ, Fregozo CS, Guevara MÁ, Olvera-Cortés ME. Serotonin depletion of supramammillary/posterior hypothalamus nuclei produces place learning deficiencies and alters the concomitant hippocampal theta activity in rats. Eur J Pharmacol. 2012;682:99–109. doi: 10.1016/j.ejphar.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Haglund L, Swanson LW, Kohler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:171–185. doi: 10.1002/cne.902290204. [DOI] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Lacaille JC, Galway M. Hypothalamic afferents to the dorsal dentate gyrus contain acetylcholinesterase. Brain Res. 1983;270:335–339. doi: 10.1016/0006-8993(83)90609-1. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978;177:589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct. 2012;217:191–209. doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology. 2005;30:1088–1095. doi: 10.1038/sj.npp.1300660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76:329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Shirao T, Doya K, Sekino Y. Three-dimensional distribution of Fos-positive neurons in the supramammillary nucleus of the rat exposed to novel environment. Neurosci Res. 2009;64:397–402. doi: 10.1016/j.neures.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, Moser M-B. A prefrontal-thalamo-hippocampal circuit for goal-directed coding. Nature. doi: 10.1038/nature14396. in press. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, McNaughton N. Mapping the differential effects of procaine on frequency and amplitude of reticularly elicited hippocampal rhythmical slow activity. Hippocampus. 1993;3:517–526. doi: 10.1002/hipo.450030411. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csáki A, Bokor H, Shanabrough M, Leranth C. The supramammillo-hippocampal and supramammillo-septal glutamatergic/aspartatergic projections in the rat: a combined [3H]D-aspartate autoradiographic and immunohistochemical study. Neuroscience. 2000;97:657–669. doi: 10.1016/s0306-4522(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–7052. doi: 10.1523/JNEUROSCI.14-11-07040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Phase relations of rhythmic neuronal firing in the supramammillary nucleus and mammillary body to the hippocampal theta activity in urethane anesthetized rats. Hippocampus. 1997;7:204–214. doi: 10.1002/(SICI)1098-1063(1997)7:2<204::AID-HIPO7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsáki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog Brain Res. 2007;163:63–84. doi: 10.1016/S0079-6123(07)63004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Herbeaux K, Cosquer B, Engeln M, Muller C, Lazarus C, Kelche C, Bontempi B, Cassel JC, Pereira de Vasconcelos AP. Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus. 2012;22:827–841. doi: 10.1002/hipo.20943. [DOI] [PubMed] [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloczky Z, Acsady L, Freund TF. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus. 1994;4:322–334. doi: 10.1002/hipo.450040316. [DOI] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MC. Lesions of the frontal cortex, hippocampus, and intralaminar thalamic nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. 2014;34:15340–15346. doi: 10.1523/JNEUROSCI.3289-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, McNaughton BL, Barnes CA. A comparison of supramammillary and medial septal influences on hippocampal field potentials and single-unit activity. J Neurophysiol. 1989;61:15–31. doi: 10.1152/jn.1989.61.1.15. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Saito H, Abe K. The supramammillary nucleus contributes to associative EPSP-spike potentiation in the rat dentate gyrus in vivo. Eur J Neurosci. 2001;13:793–800. doi: 10.1046/j.1460-9568.2001.01446.x. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Leranth C. GABAergic neurons in the rat dentate gyrus are innervated by subcortical calretinin-containing afferents. J Comp Neurol. 1996;364:425–438. doi: 10.1002/(SICI)1096-9861(19960115)364:3<425::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Oddie SD, Bland BH, Colom LV, Vertes RP. The midline posterior hypothalamic region comprises a critical part of the ascending brainstem hippocampal synchronizing pathway. Hippocampus. 1994;4:454–473. doi: 10.1002/hipo.450040408. [DOI] [PubMed] [Google Scholar]
- Ohara S, Sato S, Tsutsui K, Witter MP, Iijima T. Organization of multisynaptic inputs to the dorsal and ventral dentate gyrus: retrograde trans-synaptic tracing with rabies virus vector in the rat. PLoS One. 2013;8:e78928. doi: 10.1371/journal.pone.0078928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera-Cortés ME, Gutiérrez-Guzmán BE, López-Loeza E, Hernández-Pérez JJ, López-Vázquez MA. Serotonergic modulation of hippocampal theta activity in relation to hippocampal information processing. Exp Brain Res. 2013;230:407–426. doi: 10.1007/s00221-013-3679-x. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci. 2002;16:1797–1809. doi: 10.1046/j.1460-9568.2002.02267.x. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog Neurobiol. 2004;74:127–166. doi: 10.1016/j.pneurobio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Petsche H, Gogolak G, Stumpf C. Significance of rabbit’s septum as a relay station between midbrain and hippocampus. I. Control of hippocampus arousal activity by septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Petsche H, Gogolak G, Van Zwieten PA. Rhythmicity of septal cell discharges at various levels of reticular excitation. Electroencephalogr Clin Neurophysiol. 1965;19:25–33. doi: 10.1016/0013-4694(65)90004-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Beyeler A, Leinekugel X. Neural circuits underlying the generation of theta oscillations. J Physiol Paris. 2012;106:81–92. doi: 10.1016/j.jphysparis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Porter MC, Mair RG. The effects of frontal cortical lesions on remembering depend on the procedural demands of tasks performed in the radial arm maze. Behav Brain Res. 1997;87:115–125. doi: 10.1016/s0166-4328(96)02272-3. [DOI] [PubMed] [Google Scholar]
- Porter MC, Burk JA, Mair RG. A comparison of the effects of hippocampal or prefrontal cortical lesions on three versions of delayed non-matching-to-sample based on positional or spatial cues. Behav Brain Res. 2000;109:69–81. doi: 10.1016/s0166-4328(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Prasad JA, Macgregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Saalmann YB. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst Neurosci. 2014;8:83. doi: 10.3389/fnsys.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S, Motamedi F, Bakeshloo SA, Taleghani BK. The effect of reversible inactivation of the supramammillary nucleus on passive avoidance learning in rats. Behav Brain Res. 2004a;152:81–87. doi: 10.1016/j.bbr.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Shahidi S, Motamedi F, Naghdi N. Effect of reversible inactivation of the supramammillary nucleus on spatial learning and memory in rats. Brain Res. 2004b;1026:267–274. doi: 10.1016/j.brainres.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Soussi R, Zhang N, Tahtakran S, Houser CR, Esclapez M. Heterogeneity of the supramammillary-hippocampal pathways: evidence for a unique GABAergic neurotransmitter phenotype and regional differences. Eur J Neurosci. 2010;5:771–785. doi: 10.1111/j.1460-9568.2010.07329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BB, Cowan WM. An EM autoradiographic study of the hypothalamo-hippocampal projection. Brain Res. 1984;309:299–307. doi: 10.1016/0006-8993(84)90596-1. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. PHA-L analysis of projections from the supramammillary nucleus in the rat. J Comp Neurol. 1992;326:595–622. doi: 10.1002/cne.903260408. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, McKenna JT. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse. 2000;38:281–293. doi: 10.1002/1098-2396(20001201)38:3<281::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Groenewegen HJ, Witter MP. Thalamus. In: Paxinos G, editor. The Rat Nervous System. fourth. Academic Press; San Diego: 2015a. pp. 335–390. [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015b doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Di Prisco G, Vertes RP. Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Stratford TR, Shim I. Placement in a novel environment induces fos-like immunoreactivity in supramammillary cells projecting to the hippocampus and midbrain. Brain Res. 1998;789:331–334. doi: 10.1016/s0006-8993(97)01555-2. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG. Innervation of entorhinal principal cells by neurons of the nucleus reuniens thalami. Anterograde PHA-L tracing combined with retrograde fluorescent tracing and intracellular injection with lucifer yellow in the rat. Eur J Neurosci. 1991;3:641–647. doi: 10.1111/j.1460-9568.1991.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. Evidence for an input to the molecular layer and the stratum granulosum of the dentate gyrus from the supramammillary region of the hypothalamus. Anat Embryol. 1979;156:165–176. doi: 10.1007/BF00300012. [DOI] [PubMed] [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Südhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes d oscillations on the hippocampus. J Neurophysiol. 2012a;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Buonanno A, Vertes RP, Hoover WB, Lisman JE. NR2C in the thalamic reticular nucleus; effects of the NR2C knockout. PLoS One. 2012b;7:e41908. doi: 10.1371/journal.pone.0041908. [DOI] [PMC free article] [PubMed] [Google Scholar]


