Abstract
Background
We tested interim positron-emission tomography–computed tomography (PET-CT) as a measure of early response to chemotherapy in order to guide treatment for patients with advanced Hodgkin’s lymphoma.
Methods
Patients with newly diagnosed advanced classic Hodgkin’s lymphoma underwent a baseline PET-CT scan, received two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, and then underwent an interim PET-CT scan. Images were centrally reviewed with the use of a 5-point scale for PET findings. Patients with negative PET findings after two cycles were randomly assigned to continue ABVD (ABVD group) or omit bleomycin (AVD group) in cycles 3 through 6. Those with positive PET findings after two cycles received BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone). Radiotherapy was not recommended for patients with negative findings on interim scans. The primary outcome was the difference in the 3-year progression-free survival rate between randomized groups, a noninferiority comparison to exclude a difference of 5 or more percentage points.
Results
A total of 1214 patients were registered; 937 of the 1119 patients (83.7%) who underwent an interim PET-CT scan according to protocol had negative findings. With a median follow-up of 41 months, the 3-year progression-free survival rate and overall survival rate in the ABVD group were 85.7% (95% confidence interval [CI], 82.1 to 88.6) and 97.2% (95% CI, 95.1 to 98.4), respectively; the corresponding rates in the AVD group were 84.4% (95% CI, 80.7 to 87.5) and 97.6% (95% CI, 95.6 to 98.7). The absolute difference in the 3-year progression-free survival rate (ABVD minus AVD) was 1.6 percentage points (95% CI, −3.2 to 5.3). Respiratory adverse events were more severe in the ABVD group than in the AVD group. BEACOPP was given to the 172 patients with positive findings on the interim scan, and 74.4% had negative findings on a third PET-CT scan; the 3-year progression-free survival rate was 67.5% and the overall survival rate 87.8%. A total of 62 patients died during the trial (24 from Hodgkin’s lymphoma), for a 3-year progression-free survival rate of 82.6% and an overall survival rate of 95.8%.
Conclusions
Although the results fall just short of the specified noninferiority margin, the omission of bleomycin from the ABVD regimen after negative findings on interim PET resulted in a lower incidence of pulmonary toxic effects than with continued ABVD but not significantly lower efficacy. (Funded by Cancer Research UK and Others; ClinicalTrials.gov number, NCT00678327.)
The treatment of advanced-stage Hodgkin’s lymphoma with chemotherapy has produced high survival rates. A series of randomized trials has confirmed that doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), first described more than 40 years ago,1 yields cure rates of 70 to 80%, similar to the rates observed with more complex multidrug regimens.2–7 The possible exception is escalated therapy with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), with higher-than-standard doses of etoposide, doxorubicin, and cyclophosphamide.8 This escalated regimen has been shown to yield higher progression-free survival rates than ABVD among previously untreated patients.9,10 Trials in which ABVD and escalated BEACOPP have been directly compared have not shown a significant difference in overall survival, but a meta-analysis of several studies has suggested that the 5-year survival rate may be 5 to 10 percentage points higher with escalated BEACOPP than with ABVD.11
This increment is achieved at the cost of significantly increased short-term and long-term toxic effects. Escalated BEACOPP carries the risk of permanent infertility and prolonged fatigue, and myelodysplasia or acute leukemia develops in a number of patients.12,13 There is also a risk of second solid cancers from the use of radiation therapy. ABVD does not carry these same long-term risks. The long-term toxic effects of treatment for Hodgkin’s lymphoma are important, because the majority of patients have a life expectancy of many years. Although ABVD is generally associated with acceptable adverse-event rates, it carries the risk of serious pulmonary toxic effects as a result of the bleomycin exposure.14 The risk increases with age and with consolidation radiotherapy to the thorax, which is typically used when there are bulky lymph nodes at presentation or when residual masses remain at the end of chemotherapy.
In the context of these observations, we sought to explore the potential for adapting therapy by de-escalating treatment for patients with a good outlook and intensifying it for those at highest risk for treatment failure. Retrospective analyses suggest that positron-emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) after two cycles of ABVD was predictive: patients with low FDG uptake had a 2-year progression-free survival rate of 95%, whereas those with high FDG uptake had a rate of only 13%,15 though more recent estimates are substantially higher. We designed a prospective trial to test a response-adapted approach, performing FDG-PET scans in patients after two cycles of ABVD and modifying treatment according to the results. In patients who had negative PET findings, a randomized comparison tested whether the omission of bleomycin from subsequent cycles had any effect on control of the lymphoma or the toxic effects of therapy. Patients who had positive PET findings underwent intensification with escalated BEACOPP or an accelerated version (BEACOPP-14) involving growth-factor support, both with repeat FDG-PET scanning to evaluate outcomes.
Methods
Eligibility
Previously untreated patients 18 years of age or older with advanced classic Hodgkin’s lymphoma that was confirmed by histologic analysis were eligible if they were fit to receive a full course of combination chemotherapy. Staging comprised clinical assessment; contrast-enhanced computed tomography (CT) of the neck, thorax, abdomen, and pelvis; and bone marrow biopsy. Advanced stage was defined as an Ann Arbor stage of IIB to IV, or stage IIA with adverse features: bulky disease (>33% of the transthoracic diameter or >10 cm elsewhere) or at least three involved sites. Written informed consent was obtained from all patients before trial entry.
PET Scanning
Patients underwent PET-CT scanning with low-dose unenhanced CT within 28 days before enrollment. PET-CT scans were acquired at 60±10 minutes after the intravenous injection of 350 to 550 MBq of FDG, as reported previously.16 Subsequent PET-CT scanning was performed under the same conditions and on the same scanner as baseline scanning. Scans were centrally reported by a network of national core laboratories in the United Kingdom, Italy, Sweden, Denmark, and Australia.17 Scans were scored by two readers at each core laboratory who were unaware of the patient’s clinical status. Differences were resolved by consensus between two doctors at the same core laboratory, or when agreement could not be reached, by a third doctor at another core laboratory.
Interim PET-CT scanning was performed 9 to 13 days after the preceding dose of chemotherapy. PET findings were scored with the use of a 5-point scale, according to the level of any residual FDG uptake at involved sites on baseline PET.18 A score of 1 (no uptake), 2 (slight uptake but lower than uptake in the normal mediastinal blood pool), or 3 (uptake equal to or slightly above uptake in the blood pool but less than uptake in the liver) was regarded as indicating negative findings, and a score of 4 (uptake moderately higher than uptake in the liver) or 5 (uptake markedly higher than uptake in the liver) was regarded as indicating positive findings.
Trial Design
This was a prospective, randomized, controlled trial to determine whether the omission of bleomycin after negative findings on an interim PET-CT scan could yield a noninferior progression-free survival rate at 3 years, as compared with the rate among patients who continued standard ABVD. It also assessed the progression-free survival rate among patients with positive findings on an interim PET-CT scan, for comparison with historical controls.
After initial staging and a baseline PET-CT scan, all patients received two cycles of standard ABVD chemotherapy, given at full dose and on schedule irrespective of the blood count, if they were well enough to receive treatment. An interim PET-CT scan was performed after the second cycle. The images were transmitted to a national core laboratory for scoring within 72 hours. Patients with a PET score of 1 to 3 were randomly assigned in a 1:1 ratio to continue ABVD or receive the same regimen without bleomycin (AVD) for a further four cycles. Randomization was performed by the Cancer Research UK and University College London Cancer Trials Centre, with stratification according to PET score and center. Patients with a PET score of 4 or 5 received either BEACOPP-14 or escalated BEACOPP (for doses and schedules, see the Supplementary Appendix, available with the full text of this article at NEJM.org), with the regimen chosen in advance by each treatment center. Those receiving BEACOPP-14 had a third PET-CT scan after four cycles, and those receiving escalated BEACOPP were reassessed after three cycles. Patients with negative findings on the third PET-CT scan completed either two further cycles of BEACOPP-14 or one more cycle of escalated BEACOPP. Patients who had positive findings on the third PET-CT scan underwent further salvage treatment in accordance with local protocols.
Patients with negative findings on the interim or third PET-CT scans were not recommended to receive consolidation radiotherapy, although local investigators had discretion to use radiotherapy if they believed it was necessary. Patients with negative findings on the interim PET-CT scan did not undergo repeat PET-CT evaluation at the completion of therapy. Patients underwent clinical evaluation every 3 months in year 1, every 4 months in year 2, every 6 months in year 3, and annually thereafter. A CT scan was obtained at 3 months and at 1 year after the completion of all therapy, but there were no other protocol-mandated CT or PET-CT scans during follow-up.
Trial Oversight
The authors designed the trial, and they vouch for the accuracy and completeness of the data and for the fidelity of this report to the protocol (available at NEJM.org). No commercial support was provided, and no commercial entity had any role in study design, data accrual, data analysis, or manuscript preparation.
Statistical Analysis
This trial was designed as a noninferiority trial in which the principal outcome was the difference between the randomized groups in the rate of progression-free survival at 3 years, measured from the date of registration to the date of first appearance of disease progression, relapse, or death from any cause. On the basis of previous publications, we assumed that 75% of patients would have negative findings on the interim PET-CT scan, with a 3-year progression-free survival rate of 95%. We calculated that 101 events of disease progression, relapse, or death in the randomized group would be required for the trial to have 90% power to exclude a 5-percentage-point difference at 3 years, using a one-sided alpha of 0.025 (target sample size, 950 randomly assigned patients; see the Supplementary Appendix). Secondary outcomes included the progression-free survival rate among patients with positive findings on the interim PET-CT scan who were assigned to BEACOPP; the overall survival rate; and short-term and long-term toxic effects, with assessments including serial evaluations of respiratory function.
Results
Patients
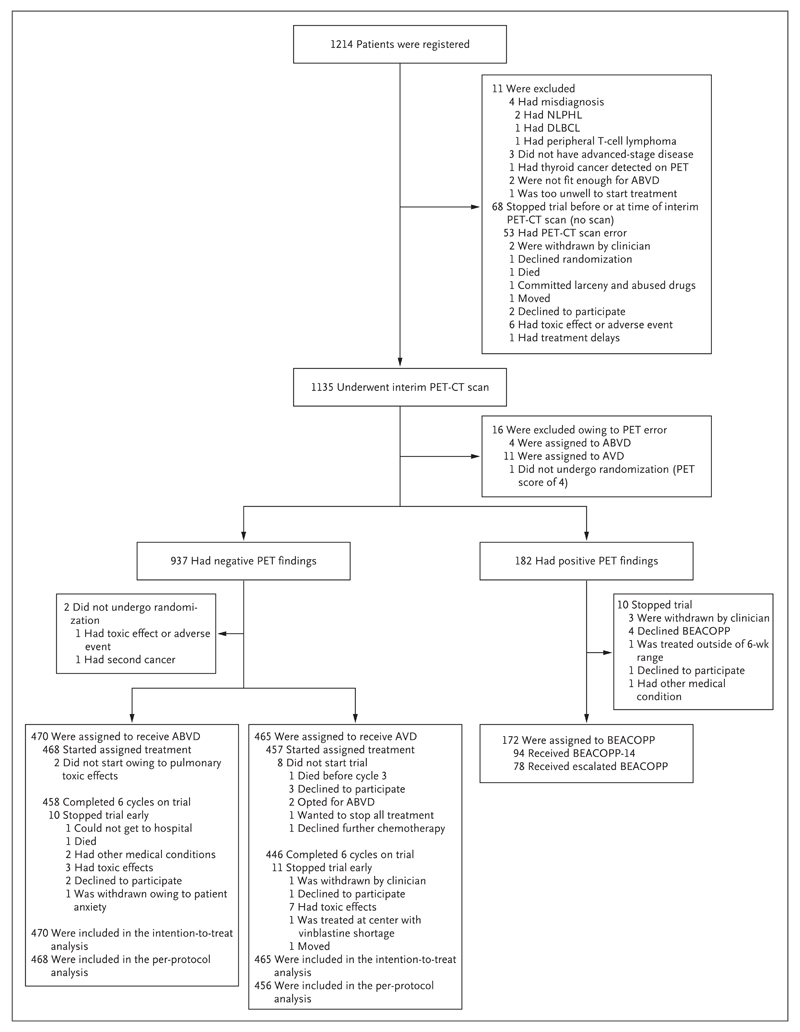
From August 2008 through December 2012, a total of 1203 eligible patients were registered at 138 participating centers in the United Kingdom, Italy, Australia, New Zealand, Norway, Sweden, and Denmark (Fig. 1; a full list of centers and investigators is provided in the Supplementary Appendix). The median age was 33 years (range, 18 to 79), 54.5% of patients were men, and 41.6% had stage II disease, 30.2% stage III disease, and 28.3% stage IV disease. Systemic B symptoms (i.e., weight loss, night sweats, and fever) were present in 61.3% of the patients, and 32.1% had bulky disease.
Figure 1. Registration and Randomization of the Patients and Outcomes of Positron-Emission Tomography–Computed Tomography (PET-CT).
PET findings on the interim PET-CT scan were scored with the use of a 5-point scale, according to the level of any residual uptake of 18F-fluorodeoxyglucose at involved sites on baseline PET and with higher scores indicating greater uptake. A score of 1, 2, or 3 was regarded as indicating negative findings, and a score of 4 or 5 was regarded as indicating positive findings. BEACOPP-14 is an accelerated version of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) that involves growth-factor support. Escalated BEACOPP involves higher-than-standard doses of etoposide, doxorubicin, and cyclophosphamide. ABVD denotes doxorubicin, bleomycin, vinblastine, and dacarbazine, AVD doxorubicin, vinblastine, and dacarbazine, DLBCL diffuse large-B-cell lymphoma, and NLPHL nodular lymphocyte-predominant Hodgkin’s lymphoma.
PET-CT Scans
After two cycles of ABVD, 1119 patients underwent an interim PET-CT scan according to protocol (an additional 16 patients were excluded owing to deviations from the PET-CT protocol). Central review showed a PET score of 1 in 111 patients (9.9%), a score of 2 in 483 (43.2%), a score of 3 in 343 (30.7%), a score of 4 in 144 (12.9%), and a score of 5 in 38 (3.4%). Overall, 937 patients (83.7%) had negative PET findings (a score of 1 through 3). Pretreatment characteristics of the patients with negative findings and those with positive findings are shown in Table 1.
Table 1. Pretreatment Characteristics of Patients Included in the Analyses.*.
| Characteristic | Patients with Negative PET Findings | Patients with Positive PET Findings Who Received BEACOPP (N = 172) | All Eligible Patients (N = 1203) | |
|---|---|---|---|---|
| ABVD (N = 470) | AVD (N = 465) | |||
| Age at registration — yr | ||||
| Median | 32 | 33 | 32 | 33 |
| Range | 18–79 | 18–76 | 18–70 | 18–79 |
| Age — no. (%) | ||||
| 18–24 yr | 121 (25.7) | 117 (25.2) | 47 (27.3) | 299 (24.9) |
| 25–44 yr | 231 (49.1) | 223 (48.0) | 75 (43.6) | 576 (47.9) |
| 45–59 yr | 80 (17.0) | 81 (17.4) | 32 (18.6) | 213 (17.7) |
| ≥60 yr | 38 (8.1) | 44 (9.5) | 18 (10.5) | 115 (9.6) |
| Male sex — no. (%) | 261 (55.5) | 252 (54.2) | 92 (53.5) | 656 (54.5) |
| ECOG performance status — no. (%)† | ||||
| 0 | 340 (72.3) | 354 (76.1) | 123 (71.5) | 889 (73.9) |
| 1 | 113 (24.0) | 96 (20.6) | 40 (23.3) | 271 (22.5) |
| 2 | 11 (2.3) | 9 (1.9) | 6 (3.5) | 28 (2.3) |
| 3 | 6 (1.3) | 6 (1.3) | 2 (1.2) | 14 (1.2) |
| Missing | 0 | 0 | 1 (0.6) | 1 (0.1) |
| Ann Arbor stage — no. (%) | ||||
| II | 195 (41.5) | 197 (42.4) | 73 (42.4) | 500 (41.6) |
| III | 157 (33.4) | 140 (30.1) | 34 (19.8) | 363 (30.2) |
| IV | 118 (25.1) | 128 (27.5) | 65 (37.8) | 340 (28.3) |
| B symptoms — no. (%)‡ | 287 (61.1) | 277 (59.6) | 121 (70.3) | 738 (61.3) |
| Bulky disease — no. (%) | 133 (28.3) | 150 (32.3) | 79 (45.9) | 386 (32.1) |
| International prognostic score — no. (%)§ | ||||
| 0 or 1 | 170 (36.2) | 172 (37.0) | 34 (19.8) | 404 (33.6) |
| 2 or 3 | 219 (46.6) | 224 (48.2) | 84 (48.8) | 579 (48.1) |
| ≥4 | 75 (16.0) | 67 (14.4) | 52 (30.2) | 209 (17.4) |
| Missing | 6 (1.3) | 2 (0.4) | 2 (1.2) | 11 (0.9) |
Positron-emission tomographic (PET) findings on the interim PET–computed tomographic (PET-CT) scan were scored with the use of a 5-point scale, according to the level of any residual uptake of 18F-fluorodeoxyglucose at involved sites on baseline PET and with higher scores indicating greater uptake. A score of 1, 2, or 3 was regarded as indicating negative findings, and a score of 4 or 5 was regarded as indicating positive findings. ABVD denotes doxorubicin, bleomycin, vinblastine, and dacarbazine, AVD doxorubicin, vinblastine, and dacarbazine, and BEACOPP bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone.
Values for the Eastern Cooperative Oncology Group (ECOG) performance status range from 0 to 5, with higher scores indicating greater disability.
Systemic B symptoms include weight loss, night sweats, and fever.
The international prognostic score ranges from 0 to 7, with higher scores indicating increased risk.
Randomization of Patients with Negative PET Findings
All but 2 of the 937 patients with negative findings on the interim PET-CT scan underwent randomization, with 470 assigned to the ABVD group and 465 assigned to the AVD group. Only 10 patients did not receive the assigned therapy: 8 in the AVD group (2 elected to continue ABVD, 5 withdrew for other reasons, and 1 died before starting cycle 3) and 2 in the ABVD group (both were treated with AVD owing to pulmonary toxic effects) (Fig. 1). The number of patients completing a total six cycles of treatment was 458 in the ABVD group (97.9%) and 446 in the AVD group (97.6%). In the ABVD group, only 4.3% of the total planned bleomycin doses were omitted and 3.2% were given at less than 90% of the planned dose. One patient (in the ABVD group) died during treatment, 10 withdrew owing to toxic effects, and 10 withdrew for other reasons. Consolidation radiotherapy was administered to 12 patients (2.6%) in the ABVD group and 20 (4.3%) in the AVD group.
Outcomes in the Randomized Group with Negative PET Findings
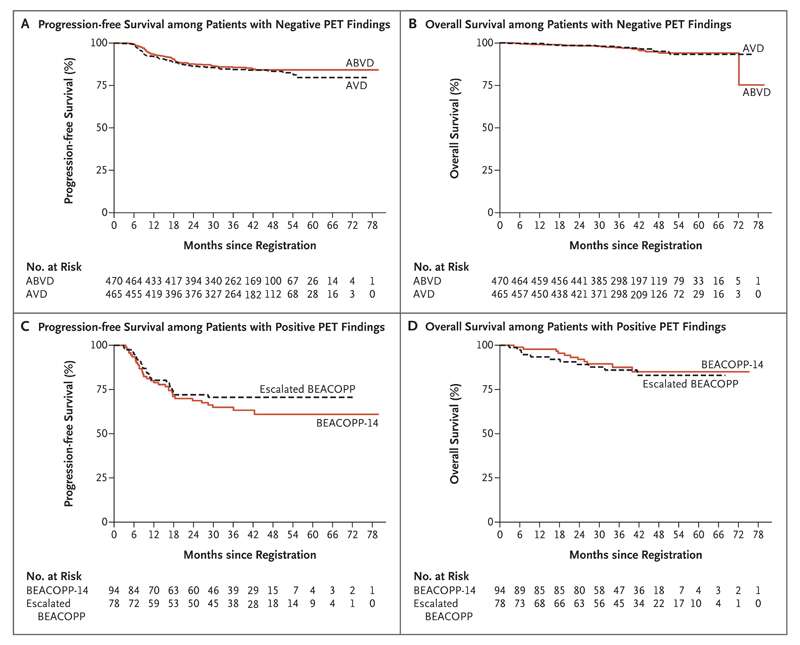
At a median of 41.2 months (range, 2.0 to 79.7) of follow-up after randomization, 142 events of disease progression, relapse, or death had occurred; events did not differ significantly between the patients who continued ABVD and those who received AVD (hazard ratio with AVD, 1.13; 95% confidence interval [CI], 0.81 to 1.57; P = 0.48) (Table 2 and Fig. 2). The 3-year progression-free survival rate was 85.7% (95% CI, 82.1 to 88.6) in the ABVD group and 84.4% (95% CI, 80.7 to 87.5) in the AVD group, with a difference (ABVD minus AVD) at 3 years of 1.6 percentage points (95% CI, −3.2 to 5.3). The two groups also showed similar 3-year overall survival rates: 97.2% (95% CI, 95.1 to 98.4) with ABVD and 97.6% (95% CI, 95.6 to 98.7) with AVD. A per-protocol analysis that excluded the 10 patients who did not receive the randomly assigned treatment had similar results (Table 2). Subgroup analysis of the randomly assigned patients showed no significant difference in progression-free survival between ABVD and AVD according to baseline characteristics such as age, sex, disease stage, international prognostic score, or the presence or absence of bulky disease or according to PET score (Fig. S3 in the Supplementary Appendix), although the hazard ratio favored ABVD for patients without B symptoms (hazard ratio with AVD, 1.76; 95% CI, 1.04 to 2.97).
Table 2. Outcomes of Therapy.
| Outcome | ABVD (N = 470) | AVD (N = 465) | BEACOPP (N = 172) | All Eligible Patients (N = 1203)* |
|---|---|---|---|---|
| Alive without disease progression — no. of patients | 402 | 391 | 117 | 999 |
| Alive after disease progression — no. of patients | 49 | 57 | 33 | 142 |
| Died — no. of patients | 19 | 17 | 22 | 62 |
| From Hodgkin’s lymphoma | 4 | 8 | 10 | 24† |
| Owing to initial therapy | 4 | 0 | 4 | 8 |
| Owing to salvage therapy | 4 | 1 | 5 | 10 |
| From second cancer | 4‡ | 6§ | 0 | 11† |
| From cardiac event | 1 | 1 | 1 | 4† |
| From cause unrelated to Hodgkin’s lymphoma or treatment | 2 | 1 | 2 | 5 |
| 3-Yr progression-free survival (95% CI) — %¶|| | 85.7 (82.1–88.6) | 84.4 (80.7–87.5) | 67.5 (59.7–74.2) | 82.6 (80.2–84.7) |
| 3-Yr overall survival (95% CI) — %** | 97.2 (95.1–98.4) | 97.6 (95.6–98.7) | 87.8 (81.5–92.1) | 95.8 (94.4–96.8) |
| Second cancer — no. of patients | 13 | 11 | 3 | 29 |
| Ann Arbor stage III or IV and age ≤60 yr | ||||
| 3-Yr progression-free survival (95% CI) — % | 82.1 (76.5–86.5) | 82.1 (76.3–86.4) | 63.9 (52.9–72.9) | 79.8 (76.3–82.9) |
| 3-Yr overall survival (95% CI) — % | 95.9 (92.2–97.9) | 97.8 (94.8–99.1) | 87.8 (78.9–93.0) | 94.6 (92.5–96.2) |
This includes patients who dropped out before randomization, declined BEACOPP, or who were ineligible for the randomized comparison owing to a nonprotocol PET-CT scan after two cycles of ABVD.
An additional four deaths occurred in patients who withdrew from the trial before randomization: two from Hodgkin’s lymphoma (one from early progression and one after withdrawal from the trial owing to toxic effects from ABVD), one from cardiac causes after the interim PET-CT scan but before further protocol therapy, and one from a second cancer after the completion of treatment off protocol owing to other medical problems.
One patient each died from esophageal cancer, T-cell acute lymphoblastic leukemia, lung cancer, and mesothelioma.
One patient each died from colon cancer, neuroendocrine cancer, acute myeloid leukemia, ovarian carcinoma, T-cell lymphoma, and Kaposi’s sarcoma.
The absolute difference (ABVD minus AVD) in the intention-to-treat analysis was 1.6 percentage points (95% confidence interval [CI], −3.2 to 5.3). The difference was calculated by applying the hazard ratio to the 3-year estimate in the AVD group, the group with more events of disease progression, relapse, or death. With adjustment for stratification factors (PET score and center), the absolute difference was 1.0 percentage points (95% CI, −4.3 to 5.0). In the per-protocol analysis, the absolute difference was 1.3 percentage points (95% CI, –3.7 to 5.1).
The hazard ratio (AVD vs. ABVD) in the intention-to-treat analysis was 1.13 (95% CI, 0.81 to 1.57; two-tailed P=0.48 for the null hypothesis of a hazard ratio of 1.00; one-tailed P=0.11 for the null hypothesis of a hazard ratio of ≥1.39 [i.e., a difference of 5 percentage points from 80.7% to 85.7%] vs. the alternative hazard ratio of <1.39). In the per-protocol analysis, the hazard ratio was 1.10 (95% CI, 0.79 to 1.53; P=0.58).
The hazard ratio (AVD vs. ABVD) in the intention-to-treat analysis was 0.90 (95% CI, 0.47 to 1.74; P=0.76).
Figure 2. Progression-free and Overall Survival.
Panel A shows progression-free survival among patients with negative PET findings after two cycles of ABVD who underwent randomization, Panel B overall survival among patients with negative PET findings who underwent randomization, Panel C progression-free survival among patients with positive PET findings, and Panel D overall survival among patients with positive PET findings.
Analysis of possible predictors of treatment failure after negative findings on the interim PET-CT scan indicated that the initial Ann Arbor stage was associated with the risk of disease progression, with a 3-year progression-free survival rate of 90.0% (95% CI, 86.4 to 92.6) among patients with stage II disease, versus 83.1% (95% CI, 78.2 to 87.0) among those with stage III disease and 79.6% (95% CI, 73.8 to 84.2) among those with stage IV disease (P<0.001). A similar but less strong effect was seen for prognostic score, and older patients had a higher rate of events than younger patients. There was no significant association with bulky disease, B symptoms, or PET score (Tables S1 and S2 in the Supplementary Appendix), nor did this change when data from the small number of patients who were treated with consolidation radiotherapy were censored. The toxic effects of continued ABVD treatment were greater than those of AVD, with more grade 3 or 4 respiratory events (Table 3). In a longitudinal analysis, the absolute difference between the ABVD group and AVD group in the change in the diffusing capacity of the lung for carbon monoxide (DLco) from baseline to the completion of therapy was −7.4 percentage points (95% CI, 5.1 to 9.7; P<0.001); this effect persisted at 1 year, with an absolute difference between groups of −4.6 percentage points (95% CI, 1.6 to 7.5; P = 0.003).
Table 3. Grade 3 or 4 Adverse Events among Patients with Negative PET Findings Who Started Their Assigned Treatment.*.
| Event | ABVD, Cycles 1 and 2 (N =1203) | ABVD, Cycles 3–6 (N = 468) | AVD, Cycles 3–6 (N = 457) | BEACOPP-14 (N = 94) | Escalated BEACOPP (N = 78) |
|---|---|---|---|---|---|
| number (percent) | |||||
| Any blood or bone marrow event | 711 (59) | 280 (60) | 273 (60) | 68 (72) | 58 (74) |
| Neutropenia | 694 (58) | 275 (59) | 269 (59) | 59 (63) | 52 (67) |
| Thrombocytopenia† | 16 (1) | 6 (1) | 15 (3) | 18 (19) | 33 (42) |
| Any cardiac event | 9 (1) | 6 (1) | 2 (<0.5) | 1 (1) | 0 |
| Any constitutional symptom | 36 (3) | 18 (4) | 13 (3) | 11 (12) | 11 (14) |
| Fatigue† | 14 (1) | 14 (3) | 5 (1) | 8 (9) | 3 (4) |
| Fever | 16 (1) | 4 (1) | 7 (2) | 2 (2) | 9 (12) |
| Any infection | 76 (6) | 68 (15) | 47 (10) | 35 (37) | 33 (42) |
| Febrile neutropenia† | 24 (2) | 22 (5) | 10 (2) | 10 (11) | 20 (26) |
| Any neurologic event | 20 (2) | 23 (5) | 14 (3) | 9 (10) | 3 (4) |
| Any pulmonary or upper respiratory event† | 8 (1) | 15 (3) | 3 (1) | 4 (4) | 4 (5) |
| Dyspnea† | 5 (<0.5) | 9 (2) | 1 (<0.5) | 2 (2) | 2 (3) |
| Pneumonitis | 0 | 5 (1) | 1 (<0.5) | 0 | 2 (3) |
| Any vascular event | 18 (1) | 23 (5) | 12 (3) | 8 (9) | 2 (3) |
| Thrombosis or embolism related to vascular access | 4 (<0.5) | 4 (1) | 1 (<0.5) | 0 | 0 |
| Thrombosis, thrombus, or embolism | 14 (1) | 20 (4) | 11 (2) | 8 (9) | 2 (3) |
| Any clinical adverse eventठ| 188 (16) | 143 (31) | 96 (21) | 52 (55) | 47 (60) |
| Any grade 3 or 4 adverse event | 771 (64) | 322 (69) | 299 (65) | 75 (80) | 65 (83) |
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3. BEACOPP-14 is an accelerated version of BEACOPP that involves growth-factor support. Escalated BEACOPP involves higher-than-standard doses of etoposide, doxorubicin, and cyclophosphamide.
P<0.05 for the comparison of ABVD with AVD during cycles 3 through 6.
Blood or bone marrow events and laboratory events were excluded.
P<0.005 for the comparison of ABVD with AVD during cycles 3 through 6.
Outcomes in the Group with Positive PET Findings
Of the 182 patients with positive findings on an interim PET-CT scan according to protocol, 94 received BEACOPP-14, 78 received escalated BEACOPP, 6 were withdrawn from the trial to undergo different salvage treatments, and 4 elected to continue ABVD. The results of a third PET-CT scan were available for 160 patients, of whom 119 (74.4%) had negative findings. Of these patients, 20 went on to receive consolidation radiotherapy. In the group with positive PET findings overall, there have been 22 deaths and 55 events of disease progression, relapse, or death as follow-up continues (Table 2). Further chemotherapy was given for consolidation after salvage therapy in 24 patients (additional cycles of BEACOPP in 11, other salvage regimens in 6, and high-dose therapy in 7), 1 patient received brentuximab vedotin, and 43 received radiotherapy. The 3-year progression-free survival rate for the group as a whole was 67.5% (95% CI, 59.7 to 74.2), and the overall survival rate was 87.8% (95% CI, 81.5 to 92.1) (Fig. 2). The nonrandomized comparison of BEACOPP-14 and escalated BEACOPP did not show a significant difference in outcomes between regimens, and toxic effects were broadly similar, except for higher rates of thrombocytopenia and febrile neutropenia with escalated BEACOPP (Table 3).
Discussion
The principal aim of this trial was to determine whether an interim FDG-PET scan could be used to guide the de-escalation of therapy for patients with a high probability of cure after ABVD therapy and escalation for those at higher risk for treatment failure. The intention was to reserve more intensive treatment for patients whose poor prognosis justified the added risk. The overall results of this approach appear favorable as compared with those of our previous studies that involved full-course ABVD and more consolidation radiotherapy.5,6 This trial population as a whole had a progression-free survival rate of 82.6% (83.7% among patients <60 years of age), and only 78 of the 1203 patients (6.5%) received radiotherapy, as compared with a progression-free survival rate of 75% and 80% in the two preceding trials, in which consolidation radiotherapy was given in 38% and 53% of patients, respectively. In all these studies, patients with stage II disease but systemic symptoms or other adverse features were included, because standard care is usually with a full course of chemotherapy, but it is evident that the results in this group are generally better than in patients with stage III and IV disease.
The primary outcome measure was the progression-free survival rate among patients who were randomly assigned to continue or stop bleomycin after negative findings on an interim PET-CT scan; the aim was to exclude a difference of 5 percentage points at 3 years. The observed upper boundary of the 95% confidence interval (5.3 percentage points) was just over this margin, and this is probably due to the lower-than-expected 3-year progression-free survival rate of 85.7% in the ABVD group, which would require more events to exclude a 5-percentage-point difference.
Subgroup analysis that was designed to identify those at higher risk for treatment failure also showed similar progression-free survival rates in the two groups, except for the paradoxical finding that there was an apparent advantage to continuing ABVD in those without B symptoms. This is not easily explained on biologic grounds. Adherence to the protocol-specified therapy was good, with excellent delivery of the first two cycles of ABVD and more than 90% of bleomycin doses given as planned for those in the ABVD group. An important feature of the protocol of the present trial was the advice to proceed with full-dose chemotherapy irrespective of the blood count whenever possible, on the basis of previous studies that showed the feasibility of this approach.19,20 Our findings may be compared with those of the recent German Hodgkin Study Group (GHSG) HD13 trial involving patients with early-stage Hodgkin’s lymphoma, which used only two cycles of ABVD before involved-field radiotherapy and showed that the 5-year rate of freedom from treatment failure was 3.9 percentage points higher with ABVD than with AVD.21
We conclude from these findings that the inclusion of bleomycin in the first two cycles may still make a positive contribution to the control of disease, but its omission after negative findings on an interim PET-CT scan carries a minimal risk of treatment failure, estimated in our trial to be 1.6%, and there was no significant difference in survival between the randomized groups. This approach does, however, reduce toxic effects; in particular, we found that the omission of bleomycin lowered the incidence of fatigue and respiratory events and led to better preservation of DLco.
We found that the rate of recurrence among patients with negative findings on an interim PET-CT scan was higher than the rate in retrospective series. This has been a common finding in other large prospective trials that took the same approach. Despite very careful quality control and rigorous prospective central review of all interim scans, the 3-year progression-free survival rate among patients with negative findings on an interim PET-CT scan in this trial was 84.9%, as compared with 95% in one large retrospective series.15 A U.S. Intergroup trial showed a 1-year progression-free survival rate of 85%,22 and the Italian HD0607 trial showed a 4-year failure-free survival rate of 85%,23 confirming that the negative predictive value of interim PET imaging is less than that previously reported. The consistency of this finding across different groups suggests that this is a reflection of the technical validity of the test rather than errors of interpretation. Analysis of baseline prognostic factors suggested that patients with a higher Ann Arbor stage or international prognostic score had a higher likelihood of recurrence after negative findings on an interim PET-CT scan. Neither bulky disease nor the PET score (1 to 3) had a significant effect on the risk of recurrence.
On the basis of these findings, it does not appear that the results are worse in the group of patients who would normally have received consolidation radiotherapy on the basis of bulky disease or residual masses, and the overall results suggest that the approach of omitting radiotherapy after negative findings on an interim PET-CT is appropriate. It is possible that the negative predictive value might be improved by performing the scan after one cycle rather than two,24 but this requires prospective evaluation, as does the use of biologic stratification with the use of immunohistochemical or gene-expression analysis, both of which have been proposed to be predictive of treatment failure.25,26
The escalation of therapy for patients with positive findings on an interim PET-CT scan was effective in roughly two thirds of cases, with a 3-year progression-free survival rate of 67.5%. This rate was substantially higher than that observed in retrospective series in which patients continued ABVD15 and is similar to the rates in other series in which patients received BEACOPP after positive findings on an interim PET scan, such as the U.S. Intergroup trial and the Italian HD0607 trial.22,23 None of these was a randomized comparison, so the precise effect of escalation remains uncertain, despite the historical controls. Even after escalation, there was a suggestion that patients with an interim PET score of 5 are at a higher risk for relapse than those with lower scores, with 20 treatment failures among the 38 patients. This suggests that alternative approaches such as early myeloablative therapy or combinations with antibody–drug conjugates should be tested in this group, although the number of patients involved is small. BEACOPP-14 and escalated BEACOPP appeared similar in efficacy in this nonrandomized comparison, which is consistent with data from the GHSG HD15 trial, in which a formal comparison suggested substantial similarity.27
In conclusion, disease control of advanced Hodgkin’s lymphoma after interim-PET–adapted therapy showed overall outcomes at least as good as in our previous studies. Longer follow-up will be required to establish whether reduction in the use of bleomycin and consolidation radiotherapy may affect long-term morbidity and mortality.
Supplementary Material
Acknowledgments
Supported by Cancer Research UK (reference CRUK/07/033), Leukaemia and Blood Cancer New Zealand, and Cancer Australia.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–9. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478–84. doi: 10.1056/NEJM199211193272102. [DOI] [PubMed] [Google Scholar]
- 3.Viviani S, Bonadonna G, Santoro A, et al. Alternating versus hybrid MOPP and ABVD combinations in advanced Hodgkin’s disease: ten-year results. J Clin Oncol. 1996;14:1421–30. doi: 10.1200/JCO.1996.14.5.1421. [DOI] [PubMed] [Google Scholar]
- 4.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–14. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PWM, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–18. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]
- 6.Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the Stanford V regimen and ABVD in the treatment of advanced Hodgkin’s lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol. 2009;27:5390–6. doi: 10.1200/JCO.2009.23.3239. [DOI] [PubMed] [Google Scholar]
- 7.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–91. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–54. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 9.Mounier N, Brice P, Bologna S, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25:1622–8. doi: 10.1093/annonc/mdu189. [DOI] [PubMed] [Google Scholar]
- 10.Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–12. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 11.Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:943–52. doi: 10.1016/S1470-2045(13)70341-3. [DOI] [PubMed] [Google Scholar]
- 12.Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31:231–9. doi: 10.1200/JCO.2012.44.3721. [DOI] [PubMed] [Google Scholar]
- 13.Eichenauer DA, Thielen I, Haverkamp H, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood. 2014;123:1658–64. doi: 10.1182/blood-2013-07-512657. [DOI] [PubMed] [Google Scholar]
- 14.Martin WG, Ristow KM, Habermann TM, Colgan JP, Witzig TE, Ansell SM. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23:7614–20. doi: 10.1200/JCO.2005.02.7243. [DOI] [PubMed] [Google Scholar]
- 15.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 16.Barrington SF, Mackewn JE, Schleyer P, et al. Establishment of a UK-wide network to facilitate the acquisition of quality assured FDG-PET data for clinical trials in lymphoma. Ann Oncol. 2011;22:739–45. doi: 10.1093/annonc/mdq428. [DOI] [PubMed] [Google Scholar]
- 17.Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–33. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 18.Barrington SF, Mikhaeel NG, Kosta-koglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boleti E, Mead GM. ABVD for Hodgkin’s lymphoma: full-dose chemotherapy without dose reductions or growth factors. Ann Oncol. 2007;18:376–80. doi: 10.1093/annonc/mdl397. [DOI] [PubMed] [Google Scholar]
- 20.Evens AM, Cilley J, Ortiz T, et al. G-CSF is not necessary to maintain over 99% dose-intensity with ABVD in the treatment of Hodgkin lymphoma: low toxicity and excellent outcomes in a 10-year analysis. Br J Haematol. 2007;137:545–52. doi: 10.1111/j.1365-2141.2007.06598.x. [DOI] [PubMed] [Google Scholar]
- 21.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–27. doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 22.Press OW, Li H, Schöder H, et al. US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxy-glucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016 Apr 11; doi: 10.1200/JCO.2015.63.1119. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallamini A, Rossi A, Patti C, et al. Interim PET-adapted chemotherapy in advanced Hodgkin lymphoma (HL): results of the second interim analysis of the Italian GITIL/FIL HD0607 Trial. Hematol Oncol. 2015;33s:163. abstract. [Google Scholar]
- 24.Hutchings M, Kostakoglu L, Zaucha JM, et al. In vivo treatment sensitivity testing with positron emission tomography/computed tomography after one cycle of chemotherapy for Hodgkin lymphoma. J Clin Oncol. 2014;32:2705–11. doi: 10.1200/JCO.2013.53.2838. [DOI] [PubMed] [Google Scholar]
- 25.Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol. 2013;31:692–700. doi: 10.1200/JCO.2012.43.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–9. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.