Abstract
Background
Observational studies have shown that attentional bias for smoking-related cues is associated with increased craving and relapse. Laboratory experiments have shown that manipulating attentional bias may change craving. Interventions to reduce attentional bias could reduce relapse in smokers seeking to quit. We report a clinical trial of attentional retraining in treatment-seeking smokers.
Methods
This was a double-blind randomised controlled trial that took place in UK smoking cessation clinics. Smokers interested in quitting were randomised to five weekly sessions of attentional retraining (N=60) or placebo training (N=58) using a modified visual probe task from one week prior to quit day. Both groups received 21 mg nicotine patches (from quit day onwards) and behavioural support. Primary outcomes included change in attentional bias reaction times four weeks after quit day on the visual probe task and craving measured weekly using the Mood and Physical Symptoms Scale. Secondary outcomes were changes in withdrawal symptoms, time to first lapse and prolonged abstinence.
Results
No attentional bias towards smoking cues was found in the sample at baseline (mean difference=3 ms, 95%CI=-2, 9). Post-training bias was not significantly lower in the retraining group compared with the placebo group (mean difference=-9 ms, 95%CI=-20, 2). There was no difference between groups in change in craving (p=0.89) and prolonged abstinence at four weeks (risk ratio=1.00, 95%CI=0.70, 1.43).
Conclusions
Taken with one other trial, there appears to be no effect from clinic-based attentional retraining using the visual probe task. Attentional retraining conducted out of clinic may prove more effective.
Clinical trial registration
UK Clinical Trials ISRCTN 54375405.
Keywords: Attentional bias, attentional retraining, cigarette smoking, smoking cessation, craving
1. Introduction
Excessive attention towards drug-related cues is termed attentional bias (Field and Cox, 2008). Theoretical accounts of attentional bias suggest that drug-related cues become salient to users through learning initiated and maintained by repeated pairing to drug reward (Robinson and Berridge, 1993, 2001). Franken (2003, 2007) suggests attentional bias towards drug-related cues influences drug-seeking and increases craving, prompting relapse. Numerous studies report associations between attentional bias and craving intensity for several drug substances (Copersino et al., 2004; Field et al., 2005). Attentional bias has been associated with an increased risk of relapse in smokers (Powell et al., 2010), alcohol users (Cox et al., 2002) and heroin users (Marissen et al., 2006).
Attentional bias is commonly measured with a visual probe task (Bradley et al., 2004; Hogarth et al., 2003). Pairs of words or pictures – one smoking-related and one neutral – are briefly displayed on a computer screen before a probe appears in the location of one of the stimuli that participants must respond to as quickly as possible. Attentional bias is indicated by quicker responses to probes that replace smoking-related stimuli compared to neutral stimuli, indicating that the smoker was attending to the smoking-related stimuli. Other measures of bias include the modified Stroop task, which typically uses word stimuli but can use pictorial stimuli (Cox et al., 2006). Each stimulus is presented in a colour that participants must identify and respond to as quickly as possible. Smokers are slower to name the colour of smoking-related stimuli, indicating that attention is captured by smoking cues (Munafo et al., 2003).
Pre-clinical studies have investigated whether attentional retraining influences attentional bias and craving (Attwood et al., 2008; Field and Eastwood, 2005; Field et al., 2007, 2009a; McHugh et al., 2010; Schoenmakers et al., 2007). In attentional retraining, the probe always appears in the place of either the neutral or drug-related stimuli, thus the user learns to look towards one stimulus type. All these studies have taken place in a laboratory with a single episode of training followed by immediate reassessment of craving in heavy drinkers or smokers not seeking to change their behaviour. Some studies have compared training to attend to a drug-related stimulus with training to avoid them. Differences in attentional bias and craving have been reported (Attwood et al., 2008; Field and Eastwood, 2005). These provide proof of principle that it is possible to manipulate attention and that this may affect craving but leave open whether it is training to attend or training to avoid that is having the effect. Four studies have assessed whether training to avoid a drug-related stimulus reduces attentional bias or craving compared with no training (Field et al., 2007, 2009a; McHugh et al., 2010; Schoenmakers et al., 2007), which is the more clinically relevant comparison. One reported a significant reduction in attentional bias (Schoenmakers et al., 2007) but three found no significant difference (Field et al., 2007, 2009a; McHugh et al., 2010). No studies found that training to avoid reduced craving compared with control. Thus laboratory data suggest it is possible to manipulate attention and this may influence craving in people not looking to quit substance use but the data are not strong.
Clinical studies give more direct evidence that attentional bias can be reduced and that this may affect clinical outcomes. Randomised trials show that attentional retraining is effective for anxiety disorders, reducing both attentional bias and improving symptoms up to four months after treatment (Amir et al., 2009; Schmidt et al., 2009). One uncontrolled trial of attentional retraining in heavy drinkers reported positive results on consumption (Fadardi and Cox, 2009). Another randomised trial with alcohol-dependent patients reported that five training sessions on a modified visual probe task led to reduced attentional bias, earlier discharge from treatment and delayed time to relapse compared with controls (Schoenmakers et al., 2010). Here, we report a randomised trial of multiple sessions of attentional retraining (versus placebo training) on attentional bias, craving, withdrawal severity, and abstinence in people quitting smoking.
2. Methods
2.1. Design
This double-blind placebo controlled randomised trial took place in National Health Service (NHS) stop smoking clinics, a nationwide network of clinical support for smokers operating to standard protocols. Weekly withdrawal-orientated behavioural support was given immediately prior to and after quit day and 21mg 24 hour nicotine patches were provided for 8-12 weeks. Participants received five sessions of attentional retraining or a dummy “placebo” training procedure. The design and methods are described in detail elsewhere (Begh et al., 2013).
2.2. Recruitment
Participating general practices and stop smoking services wrote to their patients offering trial participation as a way of achieving abstinence. The trial team screened participants and booked them into a clinic.
2.3. Participants
Eligible participants were 18 years or over, smoked at least 10 cigarettes per day and had normal or corrected-to-normal vision. We excluded people already on smoking cessation medication and who had such severe medical or psychiatric problems to make participation impossible. Almost all people with stable medical and psychiatric problems were included. Detailed inclusion/exclusion criteria are reported in Begh et al. (2013).
2.4. Materials
Eighteen picture pairs of smoking-related and neutral pictures were used across attentional bias assessment and training tasks. These pictures have been used in previous research (McClernon et al., 2007, 2008). In the assessment version of the visual probe task and pictorial Stroop task, 12 picture pairs were used. In both the retraining and placebo visual probe task, 12 picture pairs were used, consisting of six pictures that featured in the assessment version of the task and six pictures that did not. Four neutral picture pairs that had not been used in the assessment or training versions of the task were used for practice trials before each task.
2.5. Study procedures
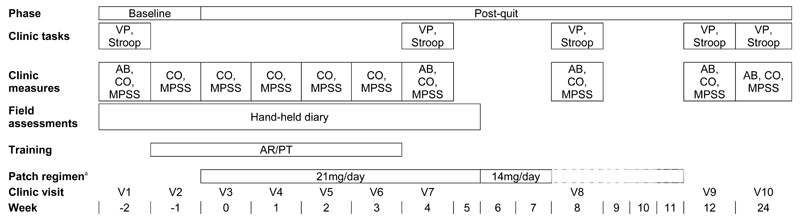
Figure 1 displays the timeline of the study procedures and treatment plan. The trial statistician produced the sequence that allocated participants 1:1 to either attentional retraining or placebo training, using a computer-generated simple randomisation scheme ordered in random permuted blocks of four. An independent programmer entered the sequence on to a dedicated online trial database, which was accessed by study staff in clinics. Participants were instructed that they would be randomly allocated to a group with or without training but were not informed of their resulting allocation. Participants and study staff were thus blinded to group allocation, as were the statisticians.
Figure 1.
Timeline of procedures and clinic visits. V=visit; VP=visual probe task; AR=attentional retraining; PT=placebo training; AB=attentional bias; CO=carbon monoxide; MPSS=Mood and Physical Symptoms Scale; mg=milligrams; aDashed lines indicate that patch regimen ranged from 8-12 weeks
There were ten clinic visits. At baseline, eligibility was checked, consent given and demographic and smoking information collected, and a quit day set for two weeks’ time. At each visit, behavioural support was provided. All participants received £15 compensation for participating in study procedures at each follow-up session.
2.6. Assessments
At each clinic visit, cigarette consumption was assessed and exhaled carbon monoxide (CO) was measured. Craving and withdrawal were measured weekly with the combined nine-item Mood and Physical Symptoms Scale-Craving (MPSS-C) and Mood and Physical Symptoms Scale-Mood (MPSS-M), which scored withdrawal symptoms, urge intensity and frequency from 1 (‘not at all’) to 7 (‘extremely’). Craving intensity was measured by summing the scores for items on strength and frequency of urges. Craving and withdrawal scores are presented as means. Questionnaire assessments were completed by telephone if necessary. Participants carried an electronic diary for eight weeks and recorded lapses occurring after quit day.
Attentional bias was assessed using the visual probe and pictorial Stroop task at the baseline visit, and at four weeks, eight weeks, three months, and six months after quit day. The visual probe assessment comprised 192 trials presented in two blocks, with each picture pair presented for 500 ms. Eight practice trials with neutral picture pairs were presented before the first assessment block. Presentation of the picture pairs and probes were counterbalanced, i.e. each permutation of picture pair and probe type was presented within each block. Thus, each type of probe appeared in the location of the smoking-related and neutral picture with equal frequency. Each block of trials were presented in a new random order for each participant, using EPrime version 2 (Psychology Software Tools Inc., Pittsburgh PA). The assessment took 16 minutes.
An index of attentional bias was calculated by subtracting the median reaction times (RTs) for smoking-related pictures from the median RTs for neutral pictures. Positive scores reflected an attentional bias towards smoking cues. We discarded incorrect responses and those that timed out after 2000 ms. These scoring procedures are consistent with previous studies (Attwood et al., 2008; Field and Eastwood, 2005; Field et al., 2007, 2009a; McHugh et al., 2010; Schoenmakers et al., 2007, 2010).
The pictorial Stroop task provided an additional measure of cognitive bias to assess the generalisation of training. As in the visual probe task assessment, 192 trials were presented in four blocks. Each block consisted of smoking-related or neutral pictures only. Eight practice trials were offered. Each trial began with a fixation cross presented in the centre of the computer screen followed by a picture outlined in red, blue, yellow, or green. Participants were required to indicate the colour of the border as quickly as possible by pressing the corresponding colour-labelled keys on the keyboard. The picture remained on the screen until a response was given or timed out after 2000 ms. Each block of trials was presented in a new random order for each participant. A Stroop bias score for each participant was calculated by subtracting median RTs to probes that replaced neutral pictures from median RTs to probes that replaced smoking-related pictures. Positive scores indicated a bias towards smoking cues.
2.7. Intervention
A modified visual probe task was used to deliver both the attentional retraining and placebo training. Both lasted 16 minutes. The placebo training was identical to the assessment condition and the active retraining differed only in that the probe appeared behind the neutral stimulus on all occasions, thus training attention away from smoking cues. Training occurred on five weekly occasions, starting one week prior to quit day.
2.8. Primary and secondary outcome measures
This was a trial to establish proof of concept prior to a phase III trial in which smoking abstinence would be the primary outcome. The two primary outcomes were change in attentional bias as measured by the visual probe task and intensity of craving at four weeks post-quit. Craving severity is positively associated with risk of relapse in smokers attempting to quit (Zhou et al., 2009).
The secondary outcomes were smoking abstinence, time to first lapse, and change in severity of tobacco withdrawal symptoms. Smoking abstinence was assessed using the Russell standard (West et al., 2005), which allowed a grace period of two weeks and imputes participants who are lost to follow-up as smokers. Time to first lapse was taken from the electronic diary, supplemented by retrospective report in the clinic assessment. A lapse was assumed if a person had a CO reading that implied smoking (CO≥10). Change in withdrawal was assessed with the MPSS-M.
2.9. Sample size
A previous laboratory study (Attwood et al., 2008) found that a change in attentional bias of 26 ms was associated with a change in craving and therefore if our intervention changed attentional bias by this amount there was a good prospect that this would lead to an observable reduction in craving. Using a repeated measures design, assuming a correlation of -0.13 between baseline and follow-up attentional bias measures taken from an unpublished trial, would require a sample size of 42 people in each arm to detect this 26 ms effect with a 5% type I error rate and 80% power.
Pharmacotherapy reduces average craving by between 0.5 and 1 point on a 5-point Mood and Physical Symptoms Scale (MPSS; Aveyard et al., 2008; West et al., 1999), so a similar effect size from attentional retraining would be worth detecting. Allowing for the repeated measures design, and assuming a correlation of 0.41 between baseline and follow-up craving measures taken from a previous trial (Aveyard et al., 2008), 53 participants in each arm would be required to detect this change in craving with 80% power.
2.10. Analysis
The effect of attentional retraining on change in attentional bias in both visual probe and Stroop assessments was analysed in a one-way analysis of covariance (ANCOVA), with a between-subjects factor of group (retraining versus placebo) and adjustment for baseline attentional bias scores. We then tested whether the effect of retraining was modified by whether or not participants achieved abstinence using a multiplicative interaction term. We also explored whether the strength of attentional bias exhibited at baseline (low/high) modified retraining effects on attentional bias. Bias scores less than zero were classified as low attentional bias and zero or above as high bias. The analysis was carried out on all data collected initially and then with imputation for missing RT and questionnaire data but gave nearly identical results and is not reported.
We modelled change in craving and withdrawal symptoms using mixed-effects regression models with an autoregressive variance-covariance structure to account for repeated measures. The regression model incorporated a random effect for participant which allowed each participant to have their own baseline level of change in craving/withdrawal. The variables were time, time squared and group (retraining/placebo). Following standard procedures for the MPSS (West and Hajek, 2004), we adjusted for baseline MPSS-M (withdrawal) scores but not baseline MPSS-C (craving) scores. To test the effect of treatment on change in craving or withdrawal we included interaction terms between the linear and quadratic time trends and group. We then included abstinence status (abstainers/non-abstainers) and strength of attentional bias exhibited at baseline (low/high) and appropriate interaction terms to explore whether these factors modified retraining effects on craving or withdrawal. For withdrawal analyses, we followed recommendations of the Society for Research on Nicotine and Tobacco work group (Shiffman et al., 2004) and performed analyses initially in abstainers only and then by intention-to treat (ITT).
We calculated risk ratios (RRs) and corresponding 95% CIs to examine the impact of training on abstinence. Proportional hazards modelling was used for time to first lapse. Analyses were performed using Stata 12.0 (StataCorp, 2011, College Station, TX: StataCorp LP).
3. Results
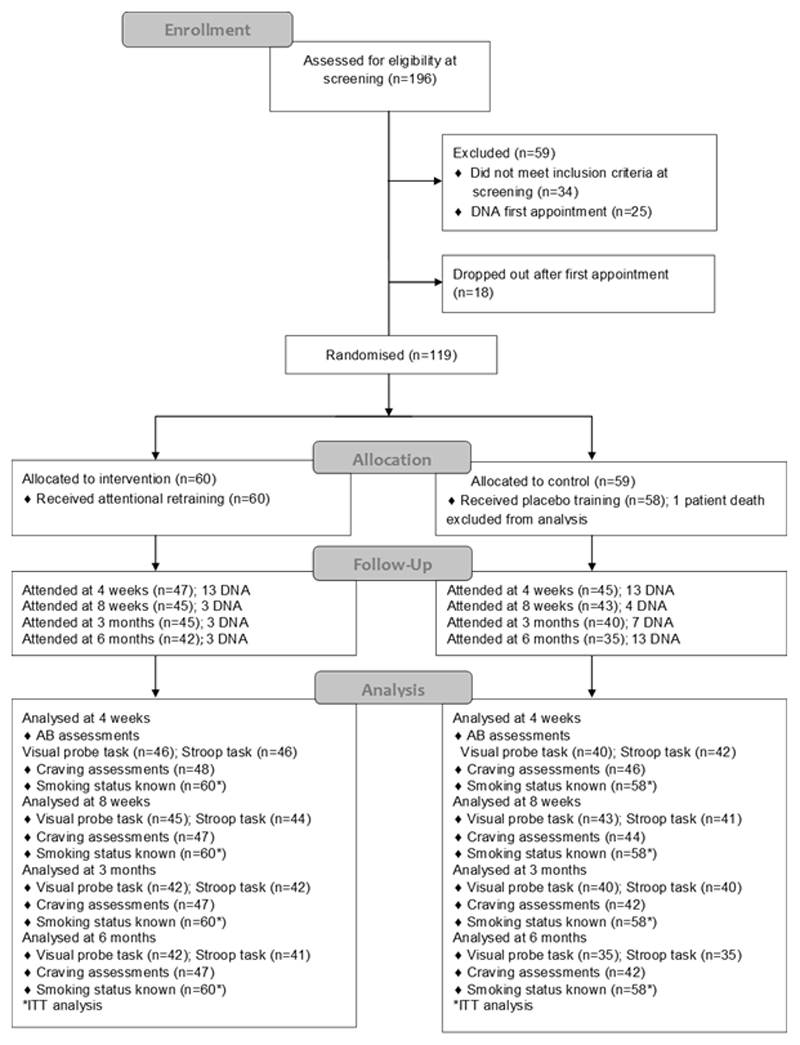
Recruitment took place between April 2011 and October 2012. Of the 196 participants screened, 119 were randomised (Figure 2). We excluded data from one participant who died shortly after enrolment.
Figure 2.
Flow chart of participants
Participants were on average 45 years old, smoked approximately 20 cigarettes a day and were moderately dependent with an FTND score of 5.5 (Table 1). There were no group differences in baseline characteristics (p>0.07). The median number of clinic visits attended by all participants was 9 out of 10 visits, with no difference between groups (p=0.18). The median number of training sessions attended was 4.5 out of 5 and was similar across both groups (p=0.80). Nicotine patch use did not differ between groups (p=0.84).
Table 1.
Participant baseline characteristics
| All (n=118) |
Attentional retraining (n=60) |
Placebo training (n=58) |
|
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Age in years mean (SD) | 44.8 (12.7) | 46.5 (12.7) | 43.0 (12.7) |
| Gender ratio (M:F) | 49:69 | 26:34 | 23:35 |
| Cigarettes smoked per day | 20.8 (9.2) | 21.8 (9.9) | 19.8 (8.5) |
| Age started smoking (years) | 16.5 (4.4) | 16.6 (4.0) | 16.4 (4.7) |
| FTNDa | 5.5 (2.3) | 5.3 (2.4) | 5.7 (2.1) |
| Visual probe task bias | 3.2 (29.8) | 1.0 (24.3) | 5.5 (34.5) |
| Pictorial Stroop task bias | 4.9 (70.0) | 9.9 (62.9) | -0.3 (59.1) |
| MPSS-Cb | 5.3 (1.1) | 5.4 (1.0) | 5.3 (1.2) |
| MPSS-Mc | 2.9 (1.2) | 3.1 (1.2) | 2.7 (1.2) |
FTND, Fagerstrom Test for Nicotine Dependence, range 0-10 where higher scores indicate higher level of dependence
Mood and Physical Symptoms Scale-Craving (combined) mean score for urge strength and intensity, each rated 1-7 where higher values indicate higher level of cigarette craving
Mood and Physical Symptoms Scale-Mood (combined) mean score for seven withdrawal symptoms, each rated 1-7 where higher values indicate higher level of withdrawal.
Of the 118 participants, 73% provided follow-up visual probe data at four weeks post quit; 75% at eight weeks; 69% at three months and 64% at six months. Pictorial Stroop task data were provided by 75% of participants at four weeks, 72% at eight weeks, 69% at three months and 64% at six months. Trials with errors were removed; error rates on each task were less than 3%.
3.1. Attentional bias assessment
A one-sample t-test revealed no significant attentional bias towards smoking cues at baseline on the visual probe task (t[115]=1.16, p=0.25, mean difference=3.21; Table S1) or Stroop task (t[117]=0.87, p=0.39, mean difference=4.87; Table S2). A paired t-test indicated that there was no significant change in reaction times towards neutral pictures from pre-training to post-training in the retraining group (t[43]=0.52, p=0.61, mean difference=10.31). There was no effect of retraining on change in attentional bias on either task measure. At four weeks post-quit, attentional bias was slightly but not significantly (p=0.11) lower in the retraining group by 9 ms (95% CI=-20, 2) on the visual probe task. There was no evidence that the effect of retraining was modified by abstinence status (p=0.29; Figure S1) or strength of attentional bias (p=0.26; Figure S2) for the multiplicative interaction terms.
Stroop data indicated that bias scores at four weeks post-quit were marginally higher in the retraining group by 5 ms (95% CI=-15, 26) but not significantly (p=0.62). Retraining was not modified by abstinence status, (p=0.64) or strength of attentional bias (p=0.82) for the multiplicative interaction terms. There were no retraining effects on change in attentional bias at any follow-up session (Table 2).
Table 2.
Effects of retraining on attentional bias over time
| B | p value | 95% CI | |
|---|---|---|---|
| Visual probe task bias RT | |||
| Baseline | -4 | 0.42 | -15, 6 |
| 4 weeks | -9 | 0.11 | -20, 2 |
| 8 weeks | 7 | 0.12 | -3, 18 |
| 3 months | -2 | 0.78 | -13, 10 |
| 6 months | -1 | 0.81 | -14, 11 |
| Pictorial Stroop task bias RT | |||
| Baseline | 10 | 0.36 | -12, 32 |
| 4 weeks | 5 | 0.62 | -15, 26 |
| 8 weeks | 13 | 0.30 | -12, 37 |
| 3 months | -11 | 0.42 | -37, 16 |
| 6 months | -6 | 0.64 | -33, 20 |
B – Beta regression coefficient; RT - reaction time (milliseconds)
3.2. Craving assessment
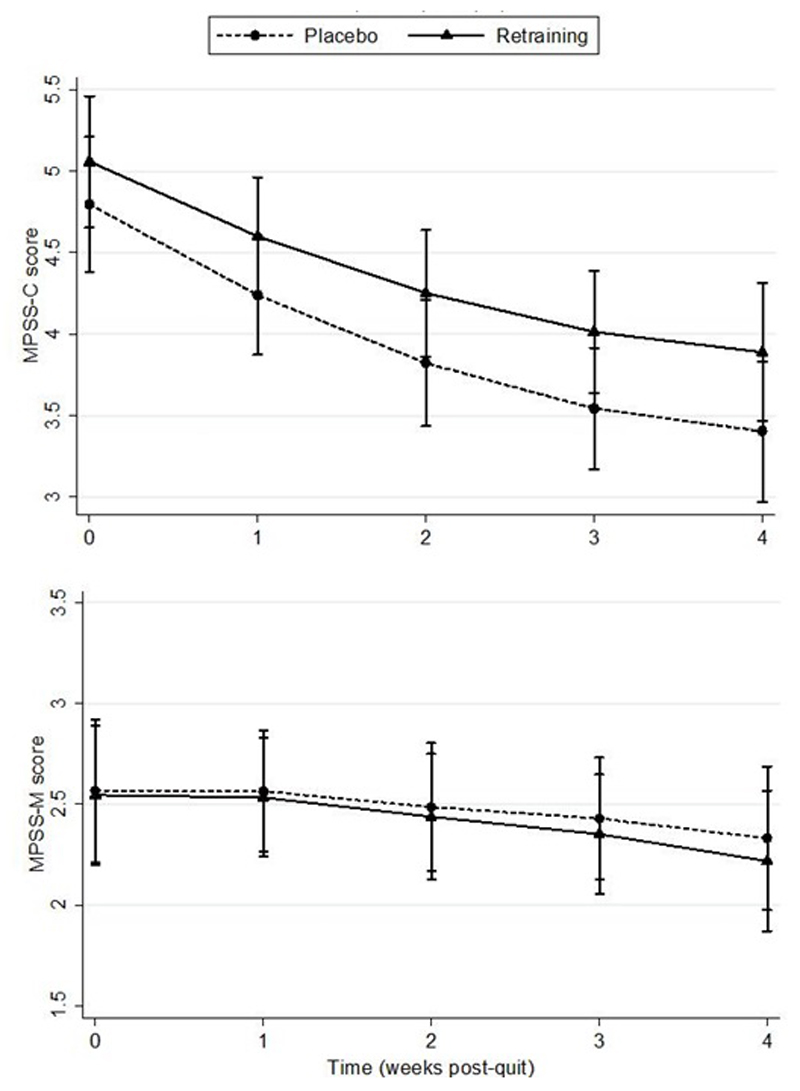
Multi-level modelling analyses revealed that change in craving from quit day to four weeks was similar in the retraining group and the placebo group, with no evidence of a significant difference (p=0.89). At four weeks post-quit, craving was approximately half a point higher in the retraining group than the placebo group (Figure 3a). There was no evidence that abstinence status modified the effect of retraining on craving (p=0.84). Craving was higher in non-abstainers in the retraining group compared with the placebo group and similar in both groups for abstainers (Figure S3). Similarly there was no evidence that the strength of attentional bias exhibited at baseline modified retraining effects on craving (p=0.97; Figure S4).
Figure 3.
a. Mood and Physical Symptoms Score-Craving (MPSS-C; 95% CI) for an average patient by group from quit day to 4 weeks, b. Mood and Physical Symptoms Score-Mood (MPSS-M; 95% CI) for an average patient by group from quit day to 4 weeks
3.3. Withdrawal assessment
In analyses of abstainers only, no significant difference was found for change in withdrawal from quit day to four weeks between the retraining group and placebo group (p=0.97; Figure 3b). Re-running the model with all participants included did not change the findings (p=0.81). There was no evidence that abstinence status (p=0.74; Figure S5) or strength of attentional bias at baseline (p=0.82; Figure S6) modified the effect of retraining on withdrawal symptoms.
3.4. Prolonged abstinence
There was no significant difference between groups in the proportion of smokers achieving prolonged abstinence at any time point (Table 3).
Table 3.
CO-verified abstinence by group
| Attentional retraining (n=60) |
Placebo training (n=58) |
||
|---|---|---|---|
| Time after randomisation | % (n) | % (n) | Risk ratio (95% CI) |
| 4 weeks | 50.0 (30) | 50.0 (29) | 1.00 (0.70, 1.43) |
| 8 weeks | 38.3 (23) | 31.0 (18) | 1.24 (0.75, 2.04) |
| 3 months | 31.7 (19) | 22.4 (13) | 1.41 (0.77, 2.59) |
| 6 months | 16.7 (10) | 15.5 (9) | 1.07 (0.47, 2.45) |
3.5. Time to first lapse
The median time to lapse was 8 days in the retraining group (95% CI=4, 16) and 7 days in the control group (95% CI=4, 20). Cox regression analyses revealed no significant difference between groups (HR=0.81, p=0.35, 95% CI=0.52, 1.26).
4. Discussion
There was no evidence of attentional bias prior to treatment in dependent smokers seeking help to stop smoking. Five sessions of attentional retraining starting prior to a quit attempt and continuing for the first 4 weeks had no effect on attentional bias, craving, withdrawal symptoms, time to first lapse or abstinence compared with placebo training when both were offered with standard smoking cessation support. There was no evidence of effect seen only in those who managed to remain abstinent or in those who exhibited higher attentional bias at baseline.
Some but not all experimental studies of attentional retraining for smokers have shown that they modify attentional bias (Attwood et al., 2008; Field et al., 2009a; McHugh et al., 2010). These laboratory studies have used more training trials than we used in a single session. Our study offered more training trials in total than in these experiments, albeit spread across five weeks and some occurring after the quit date, when many quit attempts had already failed and hence participants’ motivation to attend may have been reduced. It could therefore be that we need to provide more training sessions prior to quitting, although the inconsistency of the laboratory data and failure to observe an effect in those who maintained abstinence does not argue strongly for that. Overall, five sessions of retraining did not change attention towards neutral cues, indicating that this procedure did not train attention in the intended direction.
It is important to note that a significant attentional bias towards smoking cues was not evident in these dependent smokers at baseline. Most studies have reported attentional bias in smokers not motivated to quit (Bradley et al., 2003, 2004) and some in smokers motivated to quit (Cane et al., 2009), but not all studies have done so (McHugh et al., 2010). In all these studies, some individuals showed attentional bias while others did not. We found no evidence of a difference in effect when we split the sample into those who exhibited attentional bias at baseline and those who did not, suggesting that absence of attentional bias may not explain the lack of retraining effects observed in this study. However, the study was not powered to detect differences in attentional bias and clinical outcomes in this subgroup and it may be that the effects of retraining are greater in those with greater pre-treatment bias. Training those who do not exhibit attentional bias to focus their attention even more strongly away from smoking cues may still be effective. Two retraining studies in people with anxiety disorder found response times to social threat stimuli were comparable to neutral stimuli pre-training indicating no evidence of attentional bias at baseline (Amir et al., 2008; Amir et al., 2009). However, training produced bias away from anxiety-provoking stimuli and reduced anxiety compared with controls. We found no evidence that the retraining procedure produced a bias against smoking stimuli and no effect on craving.
There was also no evidence that retraining on the visual probe task affected responses to the Stroop task. Similarly, no other study has demonstrated that the effects of attentional retraining generalise to other attentional tasks (Field et al., 2007, 2009a; Schoenmakers et al., 2007). Correlations between attentional bias tasks are generally poor and may reflect the fact that different tasks measure different aspects of attentional processing (Wiers and Stacy, 2006). As no initial effect of retraining was found on the visual probe task, we can assume that the intervention did not change any cognitive biases towards smoking cues. A further consideration is that reaction time tasks have poor internal reliability (Ataya et al., 2012) and low test-retest reliability (Marks et al., 2014; Schmuckle, 2005; Spiegelhalder et al., 2011). Even if the procedure could retrain attention, the unreliability of the visual probe task as a measure of attention makes interpreting the outcomes difficult. Direct measures such as eye-tracking, may be more ecologically reliable indices of attention (Field and Cox, 2008). Although the modified visual probe task was not an effective attentional retraining procedure in this study, modifying attentional bias measured by other tasks may yield different findings.
Other experimental data on the effects of retraining suggests that increasing attentional bias increases craving in smokers (Attwood et al., 2008), but training to reduce attentional bias does not reduce it (Attwood et al., 2008; Field et al., 2009a; McHugh et al., 2010). The results of this study are therefore consistent with other experimental work, but also show that attentional retraining administered in a clinic does not reduce craving in smokers seeking to quit and with other data suggesting that there is only a weak association between attentional bias and craving for cigarettes overall (Field et al., 2009b).
This study also demonstrated that this retraining procedure had no effect on the clinically relevant outcomes of time to first lapse and abstinence. Previous experimental studies have shown no changes in smoking behaviour (Attwood et al., 2008) but, as participants had no motivation to quit in these studies, this was hard to interpret. Since the time of writing, another study found that three clinic sessions of attentional retraining had no effect on craving and smoking behaviour in smokers enrolled in a smoking cessation programme (Lopes et al., 2014). This contrasts with a single randomised trial of attentional retraining for alcohol dependence, which showed some changes in drinking behaviour (Schoenmakers et al., 2010). The neural pathways may differ between dependencies, so findings in one addiction may not generalise to others.
There are several strengths of the study. Previous experimental studies have used volunteers with no interest in quitting smoking and conducted short-term assessments in the laboratory. This study is amongst the first to provide a smoking retraining intervention in a clinical context. The double blind design and rigorous analysis gives confidence that retraining smokers on a modified visual probe task in a clinical setting is not effective and that this procedure did not retrain attention.
We administered baseline assessments of attentional bias while participants were still smoking. Some investigators have reported that attentional bias is more marked when regular smokers have been deprived of nicotine (Field et al., 2004; Freeman et al., 2012), although the weight of evidence suggests that there is no difference between satiated and deprived smokers (Mogg and Bradley, 2002; Munafo et al., 2003; Wertz et al., 2001).
Recent studies suggest the effects of retraining may depend on context. One study found that heavy drinkers who performed two sessions of retraining to avoid alcohol-related stimuli in their own homes reduced the frequency of drinking compared with controls (McGeary et al., 2014). A trial of daily retraining via a personal digital assistant (PDA) over one-week reduced attentional bias and craving in a group trained to avoid smoking-related stimuli (Kerst and Waters, 2014). The common factor in these studies is that the retraining occurred in participants’ natural environments and theory suggests this is where it may be more effective (Christiansen et al., 2014).
In conclusion, this clinical trial is amongst the first to examine the efficacy of multiple sessions of attentional retraining in smokers attempting cessation. We found that retraining on a modified visual probe task in a clinical setting had no effect on attentional bias, craving, withdrawal symptoms and relapse in smokers and therefore provides no evidence of clinical benefit. As such, these procedures should not be used in clinical practice in their current form.
Supplementary Material
Role of funding source
The study was funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship (DRF-2009-02-15) awarded to Rachna Begh. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR DRF programme or the Department of Health. The UK Centre for Tobacco and Alcohol Studies (UKCTAS), a UKCRC Public Health Research Centre of Excellence, is gratefully acknowledged.
Footnotes
Contributors
Begh, Aveyard, Munafo, Shiffman, Ferguson, Sutton and Holder designed the study. Begh, Aveyard, Holder, Nichols and Mohammed analysed the data. All authors contributed to the draft of the manuscript and approved the final version.
Conflict of interest
Aveyard has done research and consultancy for manufacturers of smoking cessation medication. Ferguson has consulted for GlaxoSmithKline Consumer Healthcare on matters relating to smoking cessation and has received researcher-initiated project grant funding from Pfizer (through the GRAND initiative).
References
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The Effect of a Single-Session Attention Modification Program on Response to a Public-Speaking Challenge in Socially Anxious Individuals. J Abnorm Psychol. 2008;117:860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention Modification Program in Individuals With Generalized Anxiety Disorder. J Abnorm Psychol. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Bums M, Chen X. Attention Training in Individuals With Generalized Social Phobia: A Randomized Controlled Trial. J Consult Clin Psych. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafo MR. Internal reliability of measures of substance-related cognitive bias. Drug Alcohol Depen. 2012;121:148–151. doi: 10.1016/j.drugalcdep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Attwood AS, O’Sullivan H, Leonards U, Mackintosh B, Munafo MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103:1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. Brit Med J. 2008;336:1223–+. doi: 10.1136/bmj.39545.852616.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begh R, Munafo MR, Shiffman S, Ferguson SG, Nichols L, Mohammed MA, Holder RL, Sutton S, Aveyard P. Attentional bias retraining in cigarette smokers attempting smoking cessation (ARTS): Study protocol for a double blind randomised controlled trial. BMC Public Health. 2013;13:1176. doi: 10.1186/1471-2458-13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Field M, Mogg K, De Houwer J. Attentional and evaluative biases for smoking cues in nicotine dependence: component processes of biases in visual orienting. Behav Pharmacol. 2004;15:29–36. doi: 10.1097/00008877-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: Vigilance for cigarette-related cues in smokers. Psychol Addict Behav. 2003;17:66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Cane JE, Sharma D, Albery IP. The addiction Stroop task: examining the fast and slow effects of smoking and marijuana-related cues. J Psychopharmacol. 2009;23:510–519. doi: 10.1177/0269881108091253. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Schoenmakers TM, Field M. Less than meets the eye: Reappraising the clinical relevance of attentional bias in addiction. [accessed on 10/12/14];Addict Behav. doi: 10.1016/j.addbeh.2014.10.005. [DOI] [PubMed]
- Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JCY, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiat Res. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-stroop test: Theoretical considerations and procedural recommendations. Psychol Bull. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depen. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Fadardi JS, Cox WM. Reversing the sequence: Reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depen. 2009;101:137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M. Experimental manipulation of attentional biases in heavy drinkers: do the effects generalise? Psychopharmacol. 2007;192:593–608. doi: 10.1007/s00213-007-0760-9. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Tyler E, Schoenmakers T. Attentional bias modification in tobacco smokers. Nicotine Tob Res. 2009;11:812–822. doi: 10.1093/ntr/ntp067. [DOI] [PubMed] [Google Scholar]
- Field M, Eastwood B. Experimental manipulation of attentional bias increases the motivation to drink alcohol. Psychopharmacol. 2005;183:350–357. doi: 10.1007/s00213-005-0202-5. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Eye movements to smoking-related cues: effects of nicotine deprivation. Psychopharmacol. 2004;173:116–123. doi: 10.1007/s00213-003-1689-2. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Craving and cognitive biases for alcohol cues in social drinkers. Alcohol Alcoholism. 2005;40:504–510. doi: 10.1093/alcalc/agh213. [DOI] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IHA. A Meta-Analytic Investigation of the Relationship Between Attentional Bias and Subjective Craving in Substance Abuse. Psychol Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depen. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Franken I. Craving, cue-reactivity, attentional bias and relapse in addiction. Eur Neuropsychopharm. 2007;17:S214. [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuro-Psychoph. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Freeman T, Morgan C, Beesley T, Curran HV. Drug cue induced overshadowing: selective disruption of natural reward processing by cigarette cues amongst abstinent but not satiated smokers (vol 42, pg 161, 2012) Psychol Med. 2012;42:1341. doi: 10.1017/S0033291711001139. [DOI] [PubMed] [Google Scholar]
- Hogarth LC, Mogg K, Bradley BP, Duka T, Dickinson A. Attentional orienting towards smoking-related stimuli. Behav Pharmacol. 2003;14:153–160. doi: 10.1097/00008877-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Kerst WF, Waters AJ. Attentional retraining administered in the field reduces smokers’ attentional bias and craving. Health Psychol. 2014;33:1232–1240. doi: 10.1037/a0035708. [DOI] [PubMed] [Google Scholar]
- Lopes FM, Pires AV, Bizarro L. Attentional bias modification in smokers trying to quit: A longitudinal study about the effects of number of sessions. J Subst Abuse Treat. 2014;47:50–57. doi: 10.1016/j.jsat.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101:1306–1312. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- Marks KR, Pike E, Stoops WW, Rush CR. Test-retest reliability of eye tracking during the visual probe task in cocaine-using adults. Drug Alcohol Depen. 2014;145:235–237. doi: 10.1016/j.drugalcdep.2014.09.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacol. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Meadows SP, Amir N, Gibb BE. Computer-delivered, home-based, attentional retraining reduces drinking behavior in heavy drinkers. Psychol Addict Behav. 2014;28:559–562. doi: 10.1037/a0036086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Hearon BA, Calkins AW, Otto MW. Attentional Bias and Craving in Smokers: The Impact of a Single Attentional Training Session. Nicotine Tob Res. 2010;12:1261–1264. doi: 10.1093/ntr/ntq171. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. J Psychopharmacol. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Munafo M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, ex-smokers and never-smokers on the modified Stroop task. J Psychopharmacol. 2003;17:310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacol. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The Neural Basis of Drug Craving - An Incentive-Sensitization Theory of Addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. J Abnorm Psychol. 2009;118:5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. Eur J Pers. 2005;19:595–605. [Google Scholar]
- Schoenmakers T, Wiers RW, Jones BT, Bruce G, Jansen ATM. Attentional re-training decreases attentional bias in heavy drinkers without generalization. Addiction. 2007;102:399–405. doi: 10.1111/j.1360-0443.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IFM, Goertz AG, Van Kerkhof DHAT, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depen. 2010;109:30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6:599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Jahne A, Kyle SD, Beil M, Doll C, Feige B, Riemann D. Is Smoking-related Attentional Bias a Useful Marker for Treatment Effects? Behav Med. 2011;37:26–34. doi: 10.1080/08964289.2010.543195. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav. 2001;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- West R, Courts S, Beharry S, May S, Hajek P. Acute effect of glucose tablets on desire to smoke. Psychopharmacol. 1999;147:319–321. doi: 10.1007/s002130051174. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacol. 2004;177:195–199. doi: 10.1007/s00213-004-1923-6. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Handbook of implicit cognition and addiction. Sage. 2006 doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XL, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34:365–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.