Abstract
Background and purpose
This single institution phase I trial determined the maximum tolerated dose (MTD) of concurrent vorinostat and capecitabine with radiation in non-metastatic pancreatic cancer.
Material and methods
Twenty-one patients received escalating doses of vorinostat (100–400 mg daily) during radiation. Capecitabine was given 1000 mg q12 on the days of radiation. Radiation consisted of 30 Gy in 10 fractions. Vorinostat dose escalation followed the standard 3 + 3 design. No dose escalation beyond 400 mg vorinostat was planned. Diffusion-weighted (DW)-MRI pre- and post-treatment was used to evaluate in vivo tumor cellularity.
Results
The MTD of vorinostat was 400 mg. Dose limiting toxicities occurred in one patient each at dose levels 100 mg, 300 mg, and 400 mg: 2 gastrointestinal toxicities and one thrombocytopenia. The most common adverse events were lymphopenia (76%) and nausea (14%). The apparent diffusion coefficient (ADC) increased in most tumors. Nineteen (90%) patients had stable disease, and two (10%) had progressive disease at time of surgery. Eleven patients underwent surgical exploration with four R0 resections and one R1 resection. Median overall survival was 1.1 years (95% confidence interval 0.78–1.35).
Conclusions
The combination of vorinostat 400 mg daily M–F and capecitabine 1000 mg q12 M–F with radiation (30 Gy in 10 fractions) was well tolerated with encouraging median overall survival.
Keywords: HDAC inhibitor, Magnetic resonance imaging, Neoadjuvant therapy
The only potentially curative therapy for patients with localized pancreatic cancer is complete surgical resection. Even in patients undergoing surgical resection, the median survival is less than 24 months [1]. Although the addition of adjuvant chemotherapy improves survival over surgery alone, the 5 year survival remains 21% [2,3], highlighting the need for better therapies for this disease.
The high rates of margin positivity as well as progression of disease shortly after surgery has led to an interest in neoadjuvant chemoradiation [4]. When combining radiation and systemic therapy, one can either dose escalate radiation or intensify chemotherapy using novel agents while reducing the side effects of radiation by limiting volumes and dose. A phase II trial of gemcitabine 1000 mg/m2 days 1 and 8 of each 21 day cycle and 36 Gy in 2.4 Gy per fraction found that despite the decrease in both dose and volumes of radiation, there was no increase in local–regional failures [5]. This suggests that intensifying chemotherapy using novel agents while reducing radiation dose and volume may be an effective treatment strategy. In a large retrospective study from a prospectively collected database, short-course radiation consisting of 30 Gy in 10 fractions was equivalent in terms of survival and less toxic when compared to standard-course radiation of 50.4 Gy in 28 fractions. The authors concluded that short course radiation maximizes survival, has equivalent local control and minimizes toxicity [6]
Vorinostat is a histone deacetylase (HDAC) inhibitor that is FDA-approved for the treatment of advanced primary cutaneous T cell lymphoma [7,8]. HDACs catalyze the removal of acetyl groups from the lysine residues of proteins, including histones and transcription factors. The exact mechanism by which HDAC inhibition leads to radiosensitization is not known but may in part be due to the prevention of the DNA damage repair process. HDAC inhibitors prevent DNA double-strand break (DSB) repair, as demonstrated by prolonged expression of phosphorylated H2AX (γH2AX), a marker for DNA DSBs, following radiation [9]. [3]. Preclinical studies suggest the importance of having high HDAC inhibitor levels both before and after the tumor cells are irradiated, as the cells attempt to repair the radiation-induced DNA damage [10]. In a phase I study of Vorinostat combined with radiation to the pelvis (30 Gy in 10 fractions over 2 weeks), the maximum-tolerated dose of vorinostat was determined to be 300 mg per day [11].
In pancreatic cancer, fluoropyrimidines are commonly used as radiation sensitizers. Capecitabine, an oral fluoropyrimidine, in combination with radiation therapy, has been shown to have similar efficacy to 5-fluorouracil in the treatment of locally advanced pancreatic cancer [12]. In addition to being a radiosensitizer, vorinostat synergizes with fluoropyrimidines through up-regulation of thymidine phosphorylase [13]. A phase I study of vorinostat and capecitabine in patients with solid tumors demonstrated that the combination was well-tolerated [14]. The recommended phase II dose was determined to be vorinostat 300 mg daily × 2 weeks and capecitabine 1000 mg twice daily × 2 weeks q21 days.
Given the in vitro as well as in vivo data on the role of vorinostat as both a radiosensitizer as well as its synergistic activity with fluoropyrimidines, we postulated that it could be combined based on phase I data that showed its safety when combined with radiation alone and with fluoropyrimidines alone. This single institution phase I study (NCT00983268) was conducted to determine the maximum tolerated dose of vorinostat with concurrent capecitabine (1000 mg twice daily) and radiation to a total dose of 30 Gy in 10 fractions. This was followed with two additional weeks of vorinostat alone. Secondary objectives included assessing toxicities, response rate, HDAC activity in peripheral blood mononuclear cells, and changes in the tumor apparent diffusion coefficient (ADC), as measured by diffusion weighted magnetic resonance imaging (DW-MRI) before and during the course of therapy. Given that the desmoplastic reaction seen in pancreatic cancer can make evaluation of the primary tumor challenging, DW-MRI was used to estimate changes in vivo tumor cellularity during the course of therapy [15–18]. Serial changes in the ADC measured pre- and post-treatment have been used successfully to predict treatment response in cancers of the breast [19]. Pilot studies have suggested that pretreatment DW-MRI can be used to predict response to chemoradiation in pancreatic cancer [20]
When investigating the addition of novel agents in cancer treatment, it is useful to be able to measure successful drug activity. Because histone acetylation is a direct downstream consequence of HDAC inhibition, we postulated that the activity of HDAC inhibition could be measured using peripheral blood lymphocytes as surrogate biomarkers for acetylation. Weekly blood samples were collected and analyzed for posttranslational modification of histone H3 lysine residues. We hypothesized that lysine acetylation would increase with therapy and that this could be used as a biomarker of drug efficacy.
Materials and methods
Patient eligibility
Eligible patients had histologically confirmed non-metastatic pancreatic cancer and were treatment naïve. Resectability was determined by a surgical oncologist using the Intergroup definition of borderline resectable pancreatic cancer[21]. Patients had to be ⩾18 years old, be able to provide written informed consent, have an Eastern Cooperative Oncology Group performance status of 0–2, and have adequate hematologic, renal and hepatic function, normal magnesium levels. Ineligibility criteria included prior radiation to any area within the planned radiation field, history of hypersensitivity to fluoropyrimidines or HDACs, Dihydropyrimidine dehydrogenase deficiency, any significant uncontrolled medical conditions, coumadin, pregnancy or breast feeding. The study was conducted in accordance with the principles of the Declaration of Helsinki and in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice.
Dosage and drug administration
Vorinostat was supplied in 100 mg gelatin capsules (Merck & Co.). Subjects were instructed to swallow it whole with food. Patients kept a pill diary and returned the unused drug or empty bottle to confirm compliance with therapy. Vorinostat was administered at the assigned dose level (range 100–400 mg) daily on the days of radiation and then daily M–F for two weeks after completion of radiation. Capecitabine 1000 mg q12 was administered on the days of radiation.
Radiation was given 30 Gy in 10 fractions over 2 weeks using linear accelerators with photon beams of 10 MV or higher energy. There were no planned interruptions ⩾3 days. The prescription point was designated at the intersection of the multiple beams. Gross tumor volume is defined as the primary tumor plus any involved regional nodes which are ⩾1 cm. The clinical target volume was the GTV plus the celiac artery and superior mesenteric artery with a 1 cm expansion radially and 2 cm cranial caudal. The planning target volume was the CTV plus 0.5 cm in all directions. No boost was planned. Radiotherapy utilized 3D conformal CT-based planning. The uniformity requirement was ±5% of the total dose at the prescription point within the planning target volume. To avoid the reported variation in investigator-defined contours [22], all volumes were placed by a single GI radiation oncologist (BC) following review with a GI radiologist.
Patients deemed unresectable at post-treatment imaging were given the option of continuing on study at systemic doses of vorinostat 300 mg daily × 2 weeks and capecitabine 1000 mg q12 × 2 weeks q21 days or coming off study.
Dose escalation design
This study utilized a standard 3 + 3 dose escalation design with vorinostat being escalated. The study schema is illustrated in Fig. 1. Capecitabine and radiation doses were constant at all dose levels. The doses of vorinostat studied were 100 mg, 200 mg, 300 mg, and 400 mg. There were no plans to dose escalate above 400 mg. If the 400 mg dose was tolerated, that would be considered the MTD and the recommended phase II dose. Intra-patient dose escalation was not allowed.
Fig. 1.
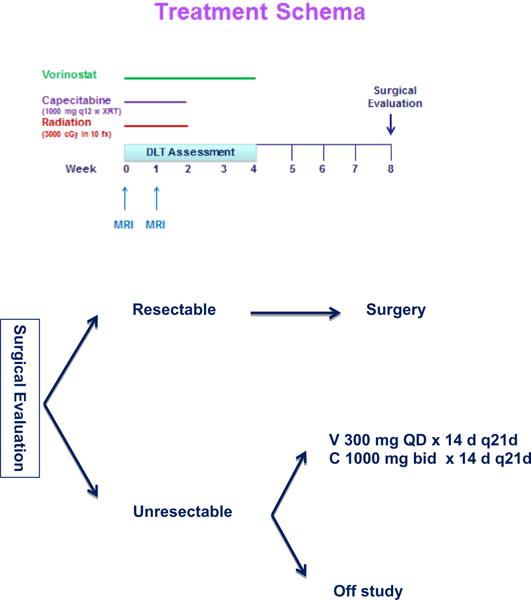
Study schema. Subjects received capecitabine, vorinostat and radiation (30 Gy in 10 fractions) followed by two additional weeks of vorinostat. Subjects deemed unresectable were given the option of continuing capecitabine and vorinostat or coming off study and receiving standard of care treatment with their oncologist.
Dose-limiting toxicities (DLTs) were defined as any grade 3 or higher non-hematologic adverse event with the exception of alopecia, fatigue, and anorexia, grade 3 or higher nausea and/or vomiting that persists ⩾48 h despite optimal medical management, grade 4 neutropenia lasting for ⩾7 days in duration, grade ⩾3 febrile neutropenia; any grade 4 thrombocytopenia; any grade 5 hematologic toxicity, any study-related toxicity that required a hold of chemotherapy and/or radiation of >14 days, or inability to deliver >75% of planned chemotherapy and/or radiation therapy in the two-week period. DLTs for MTD determination were assessed during chemoradiation and for two weeks following completion of study drug.
Pretreatment and follow-up studies
Pretreatment physical examination, medical history, vital signs, performance status evaluation, complete blood count (CBC) with differential and platelets, comprehensive metabolic panel, CA-19-9 and pregnancy test (for women with child bearing potential) were required within two weeks of starting treatment. Electrocardiogram (EKG) and tumor evaluation with CT scan or MRI were required within 30 days of start of therapy.
During chemoradiation therapy up through the time of surgery, each patient was seen weekly by an attending physician for toxicity assessment which included weekly CBC with differential and platelets, comprehensive metabolic panel, and history and physical examination.
Peripheral blood mononuclear cells (PBMCs) were collected prior to start of therapy, and then weekly while taking study drug, and on the day of surgery. If the patient did not have surgery, the final PBMC was collected 6–8 weeks after the last dose of radiation.
DW-MRI was obtained prior to the start of therapy and one week ±2 days after start of chemoradiation. Although initially a mandatory component of the study, it was later made optional when several patients were unable to enroll in the study due to contraindications to MRI.
Patients undergoing surgical resection were followed every 3 months for progression and/or survival. Adjuvant chemotherapy was left to the discretion of the treating medical oncologist. Patients who did not undergo surgical resection and elected to continue on capecitabine and vorinostat were seen every three weeks with CBC, comprehensive metabolic panel and CA-19–9. Disease evaluation was obtained at the beginning of each 3-week cycle. If the patient opted to discontinue the study drugs they were followed for progression and/or survival every 3 months.
HDAC activity assay
Cells were sonicated in RIPA buffer + PIC. Cell lysate was run on SDS–PAGE at 200V for 45–60 min and then transferred onto membrane. Membrane was blocked overnight at 4 °C in 5% milk plus 0.1% PBS-T. Membrane was then rinsed with PBS-T. Pan H3 (1:500 in 1% milk plus PBS-T, Millipore 05–928) was added to one membrane and incubated for two hours at room temperature. K9K14 antibody (1:1000 in 1% milk plus PBS-T, Millipore 06-599) was added to the other membrane and incubated for 2 h at room temperature. Membranes were washed for 5 min × 3 with PBS-T. RB800 antibody (1:3000 in 1% milk plus PBS-T, Odyssey, 926-32211) was added for one hour at room temperature. Membranes were washed for 5 min × 3 with PBS-T and developed on Odyssey.
DW-MRI
DW-MRI was obtained prior to the start of therapy and one week ±2 days after start of chemoradiation. MRI examinations were performed on a Philips 3T Achieva MR scanner with a 16-channel torso coil (Philips Healthcare, Best, The Netherlands). DW-MRIs were acquired with a single-shot spin echo (SE) echo planar imaging (EPI) sequence in three orthogonal diffusion encoding directions (x, y, and z). For 5 patients, TR = “shortest” (range = 4693–7357 ms), TE = “shortest” (46.7 ms), Δ= 22.6 ms, δ= 9.7 ms, and 8 signal acquisitions were acquired. For 8 patients, TR = 1650 ms, TE = 57 ms, Δ= 28.1 ms, δ = 18.2 ms, and 4 signal acquisitions were acquired. ADC maps were calculated from the b = 50 and 500 s/mm2 images. The ADC maps were calculated from the diffusion weighted data (b = 0 and 500 s/mm2). Regions of interest (ROI) were drawn by a region growing algorithm (RGA) on T1-weighted anatomical images with multiple slices combined to create a volume of interest (VOI) which was then mapped to the ADC space to calculate the mean ADC for each volume.
Statistical methods
Due to the nature of the study, descriptive statistics were used to summarize the demographics, adverse events, and response. A Kaplan–Meier curve was used to estimate survival.
Results
Study population
Twenty-one patients were accrued in one of four dose cohorts between November 2009 and December 2012. The study population included 1 resectable patient, 12 borderline resectable patients, and 8 unresectable patients. Ages ranged from 44 to 82, with a median age of 67.
Safety
All 21 patients were included in the safety evaluation. Table 1 lists all of the adverse events assessed to be at least possibly related to study therapy. The most common study related ⩾3 grade adverse events were lymphopenia (76%) and nausea (14%).
Table 1.
Treatment related adverse events of any grade. Summary of the incidence of highest grade toxicity deemed possibly related to study therapy.
| Grade 1 (%) |
Grade 2 (%) |
Grade 3 (%) |
Grade 4 (%) |
|
|---|---|---|---|---|
| Gastrointestinal | ||||
| Nausea | 6 (29) | 8 (39) | 3 (14) | 0 (0) |
| Vomiting | 8 (39) | 4 (19) | 1 (5) | 0 (0) |
| Diarrhea | 8 (39) | 1 (5) | 1 (5) | 0 (0) |
| Constipation | 3 (14) | 2 (10) | 0 (0) | 0 (0) |
| Anorexia | 4 (19) | 3 (14) | 0 (0) | 0 (0) |
| Dysgeusia | 2 (10) | 3 (14) | 0 (0) | 0 (0) |
| Mucositis | 3 (14) | 1 (5) | 0 (0) | 0 (0) |
| Heartburn/dyspepsia | 1 (5) | 3 (14) | 0 (0) | 0 (0) |
| Xerostomia | 3 (14) | 0 (0) | 0 (0) | 0 (0) |
| Flatulence | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 3 (14) | 1 (5) | 0 (0) |
| ALT | 4 (19) | 1 (5) | 0 (0) | 0 (0) |
| AST | 4 (19) | 0 (0) | 0 (0) | 0 (0) |
| Alkaline phosphatase | 2 (10) | 0 (0) | 0 (0) | 0 (0) |
| Hyperbilirubinemia | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Constitutional | ||||
| Fatigue | 7 (33) | 9 (43) | 1 (5) | 0 (0) |
| Weight loss | 3 (14) | 1 (5) | 0 (0) | 0 (0) |
| Dizziness | 1 (5) | 1 (5) | 0 (0) | 0 (0) |
| Hematologic | ||||
| Lymphopenia | 1 (5) | 3 (14) | 16 (76) | 0 (0) |
| Leukopenia | 3 (14) | 0 (0) | 1 (5) | 0 (0) |
| Anemia | 5 (24) | 1 (5) | 0 (0) | 0 (0) |
| Thrombocytopenia | 8 (39) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Metabolic/laboratory | ||||
| Hyponatremia | 7 (33) | 0 (0) | 0 (0) | 0 (0) |
| Hyperglycemia | 1 (5) | 9 (43) | 1 (5) | 0 (0) |
| Hypokalemia | 3 (14) | 0 (0) | 1 (5) | 1 (5) |
| Hypomagnesemia | 2 (10) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Neurologic | ||||
| Memory | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Impairment/forgetfulness | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Headache Tremor |
2 (10) | 0 (0) | 0 (0) | 0 (0) |
| Dermatologic | ||||
| Pruritus | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Dry skin | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
One DLT occurred at dose levels 1 (100 mg), 3 (300 mg) and 4 (400 mg). These dose levels were expanded with an additional three patients in each cohort and no further DLTs were observed. Two DLTs were gastrointestinal (nausea and vomiting, diarrhea, dehydration) and one was thrombocytopenia.
Vorinostat 400 mg daily was determined to be the maximum tolerated dose with the study regimen of twice daily capecitabine (1000 mg) on the days of radiation.
Response
Table 2 lists the outcomes of the study subjects. Response was assessed by RECIST criteria [23]. Nineteen (90%) patients had stable disease, and two (10%) had progressive disease at the time of tumor re-evaluation. Ten of the twelve original borderline resectable patients underwent surgical exploration. Of these 10 patients, four underwent resection with three having R0 resections and one had an R1 resection. Of the six remaining patients, four were noted to be locally unresectable at surgery and an additional two were deemed unresectable secondary to the finding of metastatic disease at the time of surgery. Four of the original twelve (33%) borderline resectable patients ultimately underwent an R0 resection. None of the patients deemed initially to be unresectable patients were converted to resectable status.
Table 2.
Patient outcomes.
| Pt # (age, M/F) | Dose level | DLT | Pre tx status | Response | Surgery (Y/N) | Subsequent therapy |
|---|---|---|---|---|---|---|
| 1 (82, M) | 1 | N | Borderline | SD | X 2 | Cap/vori × 6 |
| 2 (73, F) | 1 | Y | Borderline | SD | N | N |
| 3 (61, F) | 1 | N | Borderline | SD | Y (yT3N1) | N |
| 4 (73, F) | 1 | N | Borderline | SD | Y (yT3N1 + RP) | Gemcitabine |
| 5 (75, M) | 1 | N | Borderline | SD | Y, unresectable | N (Pt choice) |
| 6 (61, M) | 1 | N | Borderline | SD | N | FOLFIRINOX |
| 7 (68, M) | 2 | N | Resectable | SD | Y (yT3N0) | Gemcitabine |
| 8 (63, M) | 2 | N | Unresectable | SD | N | N (Pt choice) |
| 9 (81, F) | 2 | N | Unresectable | PD | N | N |
| 10 (71, M) | 3 | N | Unresectable | SD | N | Cap/vori × 6 |
| 11 (76, M) | 3 | N | Unresectable | SD | N | Gemcitabine |
| 12 (74, F) | 3 | Y | Borderline | SD | Y (yT2N1) | Gemcitabine |
| 13 (55, M) | 3 | N | Unresectable | SD | N | 2nd opinion |
| 14 (44, F) | 3 | N | Borderline | SD | Y Liver met bx | FOLFIRINOX |
| 15 (63, M) | 3 | N | Unresectable | SD | N | Cap/vori × 2 |
| 16 (49, F) | 4 | N | Borderline | PD | Y Liver met bx | Gemcitabine |
| 17 (60, F) | 4 | Y | Unresectable | SD | N | Gemcitabine |
| 18 (51, F) | 4 | N | Unresectable | SD | N | Cap/vori × 3 |
| 19 (67, M) | 4 | N | Borderline | SD | Y (yT3N1) | Gemcitabine |
| 20 (74, M) | 4 | N | Borderline | SD | Y, unresectable | FOLFOX |
| 21 (54, M) | 4 | N | Borderline | SD | Y, unresectable | FOLFIRINOX |
Abbreviations: SD, stable disease; PD, progressive disease; Cap, capecitabine; vori, vorinostat; RP, retroperitoneal margin. Pre-treatment status denotes determination of resectability. Subjects 3, 7, 12, and 19 underwent an R0 resection. Subject 4 underwent an R1 resection with a positive retroperitoneal margin. Subjects 14 and 16 underwent exploratory surgery and were found to have liver metastasis; therefore, resection was not performed. Subjects 5, 20, and 21 underwent exploratory surgery but were noted to have unresectable disease at the time of surgery. Subject 1 underwent exploratory surgery and was noted to have left renal vein involvement; therefore, resection was not performed. After subsequent treatment with six cycles of systemic capecitabine and vorinostat, he was deemed potentially resectable. Unfortunately, his second surgery was delayed secondary to cholangitis. At the time of his second surgery, he was noted to have peritoneal metastases, and surgery was terminated.
One subject underwent exploratory surgery after chemoradiation therapy and was noted to have left renal vein involvement and resection was not pursued given the extent of disease. He opted to go onto continuation therapy with capecitabine and vorinostat. Interestingly, after six cycles of capecitabine and vorinostat, he was deemed potentially resectable. Unfortunately, he developed cholangitis, which delayed surgery and at the time of his second exploratory surgery, he was noted to have peritoneal metastases and surgery was abandoned.
Although not a primary endpoint, the median overall survival for these patients was 1.1 years (95% confidence interval 0.78–1.35) which compares favorably with other phase I studies of chemoradiation in this patient population.
Histone modification
Weekly blood samples were collected and analyzed for post-translational modification of histone H3 lysine residues. Fig. 2 depicts the results in the first subject, who demonstrated the expected increase in acetylation. After five days of radiation and vorinostat, increased histone acetylation was detected. When vorinostat was stopped for six days (lane 6), this level decreased. Increases in acetylation were seen in six (28.6%) of the subjects, suggesting that vorinostat functioned as expected. For patients where this did not occur, the samples were not processed immediately. Given the small sample size as well as the lack of change in tumor size, changes in histone acetylation in PBMCs could not be correlated with changes in tumor size.
Fig. 2.
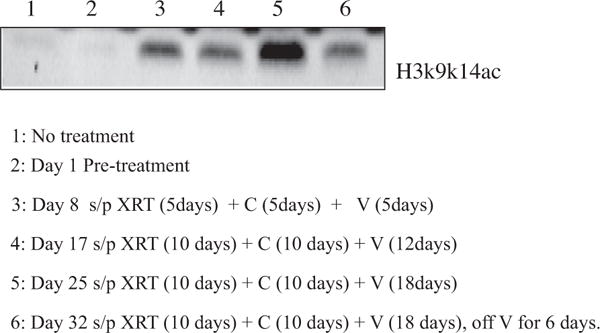
Vorinostat results in increased lysine acetylation of H3 in PBMCs: Abbreviations: XRT, Radiation; C, Capecitabine; V, Vorinostat.
DW-MRI
Thirteen subjects underwent pre- and post-treatment DW-MRI scans. Fig. 3 demonstrates the volume used to calculate the mean ADC and illustrates an increase in ADC after one week of chemoradiation. Eighty-five percent of the subjects had the expected increase in ADC, with 54% showing an increase of at least 10%. Given the small sample size as well as the lack of change in tumor volumes, we were unable to correlate ADC changes with the observed changes in tumor size at the completion of chemoradiation (Table 3).
Fig. 3.
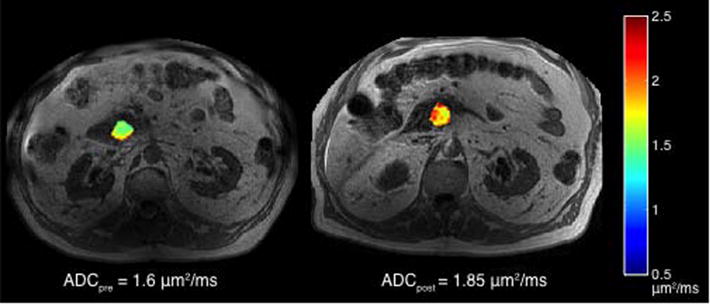
DW-MRI pre and post start of therapy shows increase in ADC: pre-treatment DW-MRI was obtained prior to therapy. Post therapy DW-MRI was obtained after one week of chemoradiation. Images were obtained on a 3T MRI.
Table 3.
DW-MRI changes in ADC pre and post start of therapy.
| Pt # | Tumor ADC
|
Tumor size
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean tumor ADC (μm2/ms) (Pre) |
St dev tumor ADC (Pre) |
Mean tumor ADC (μm2/ms) (Post) |
St dev tumor ADC (Post) |
% change in mean ADC (%) |
Size (cm) (Pre) |
Size (cm) (Post) |
% change in size (%) |
|
| 1 | 1.601 | 0.209 | 1.845 | 0.17 | 15 | 2.2 | 1.6 | −27 |
| 3 | 1.437 | 0.253 | 1.501 | 0.33 | 4 | 2.1 | 2 | − 5 |
| 4 | 1.798 | 0.402 | 1.973 | 0.304 | 10 | 2.9 | 3 | 3 |
| 5 | 0.911 | 0.28 | 1.569 | 0.175 | 72 | 2.2 | 2.5 | 14 |
| 6 | 1.372 | 0.24 | 2.138 | 0.305 | 56 | 2.5 | 2.7 | 8 |
| 7 | 1.203 | 0.225 | 1.398 | 0.219 | 16 | 3.5 | 2.7 | −23 |
| 8 | 1.497 | 0.312 | 1.506 | 0.306 | 1 | 6 | 5.1 | −15 |
| 9 | 1.72 | 0.353 | 2.101 | 0.357 | 22 | 4.6 | 5.4 | 17 |
| 10 | 1.407 | 0.264 | 1.096 | 0.308 | −22 | 3.7 | 3.7 | 0 |
| 11 | 1.948 | 0.4 | 1.976 | 0.394 | 1 | 3.6 | 3.9 | 8 |
| 12 | 1.374 | 0.2 | 1.478 | 0.273 | 8 | 2 | 2 | 0 |
| 13 | 0.898 | 0.143 | 1.096 | 0.223 | 22 | 3.5 | 3.4 | −3 |
| 18 | 1.269 | 0.221 | 1.197 | 0.217 | −6 | 5.6 | 4 | −29 |
DW-MRI was obtained prior to the start of therapy and after one week of therapy in thirteen subjects. Tumor size was assessed by pancreas protocol CT scan prior to the start of therapy and approximately 8 weeks later.
Discussion
Pancreatic cancer is a deadly disease with few people having surgically resectable disease at the time of diagnosis. The only up front resectable patient in the study completed the protocol therapy and underwent an R0 resection. None of the eight patients with locally advanced unresectable disease at diagnosis were converted to resectable. The study accrued 12 borderline resectable pancreatic cancer patients, and of these 12, ten subsequently went on to surgical exploration.
One of the reasons pancreatic cancer is such a difficult cancer to treat is the dense desmoplastic stroma, which can interfere with delivery of chemotherapy to the tumor itself. This desmoplastic stroma may also be the reason why early changes in ADC seen on DW-MRI did not correlate with the CT assessment of the tumor post treatment. However, further analysis was hampered by the lack of Response Evaluation Criteria in Solid Tumors (RECIST) [24] responses seen from the therapy. There are several limitations to our study including the lack of voxel-by-voxel correlation between repeated scans. As studies have shown that the pancreas is prone to respiratory motion ([25,26], correction for respiratory motion using 4D acquisition would be worthwhile in future studies of serial imaging for testing response to radiation.
While there were no partial responses seen, it should be noted that 19 (90%) of the subjects had stable disease on therapy. The two patients that progressed included a patient who was initially borderline resectable who had 29% shrinkage in the primary tumor but developed a new right lobe liver lesion on re-imaging after chemoradiation. The other patient who progressed had unresectable disease at diagnosis and had significant growth of her primary tumor on re-imaging.
As observed in other phase I studies of vorinostat [27,28], this study showed that histone acetylation is rapidly induced and then returns to baseline. Samples that had not been immediately processed did not show the expected increase in acetylation. As pancreatic tumors rarely show radiologic response, it is not possible to determine whether patients with higher degrees of acetylation correlate with increased response.
In conclusion, this phase I study demonstrated that vorinostat 400 mg daily and capecitabine 1000 mg q12 on the days of radiation (30 Gy in 10 fractions) were well tolerated. Of note, although only four subjects opted for continuation of systemic doses of capecitabine and vorinostat after they were determined to be unresectable, two of these subjects received six cycles of vorinostat and capecitabine, including one who stopped for an attempt at surgical resection. Given the small number of patients, it does suggest that this combination has activity and may be worth exploring in phase II trials in pancreatic cancer. Newer regimens for pancreatic cancer have more efficacy than single agent fluoropyrimidine [29,30] and therefore further analysis of vorinostat might best be performed using these newer multi-agent regimens as the backbone.
Acknowledgments
Role of funding
The funding sources had no role in the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication, and did not have access to study data. The corresponding author had full access to all of the data and the final responsibility to submit for publication. The authors are solely responsible for the study design as well as the collection, analysis and interpretation of data; writing of the manuscript; and in the decision to submit the manuscript for publication.
Financial support
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) from general research support provided by Merck & Co., Inc. Study was also supported by the Vanderbilt-Ingram Cancer Center Support Grant P30CA68485, NCI U01CA142565, UL1TR000445. RGA was funded in part by the AUR GE Radiology Research Academic Fellowship.
Footnotes
Conflicts of interest
Emily Chan, M.D., Ph.D.: Advisory boards for Merrimack, Amgen, Bayer, Taiho, Castle Biosciences, Genentech, Lilly, Imclone.
Lori R. Arlinghaus, Ph.D.: None.
Dana Cardin, M.D.: Advisory board for Merrimack.
Laura Goff, M.D.: Research funding: Astellas Pharma, Millenium, Roche, Amgen, Pfizer, Onyx.
Jordan D. Berlin, M.D.: None.
Alexander Parikh, M.D.: None.
Richard G. Abramson, M.D.: ICON Medical Imaging, consultant.
Thomas E. Yankeelov, Ph.D.: None.
Scott Hiebert, Ph.D.: None.
Nipun Merchant, M.D.: None.
Srividya Bhaskara, Ph.D.: None.
A. Bapsi Chakravarthy, MD: Bayer/Onyx.
References
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–49. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 4.Lowy AM. Neoadjuvant therapy for pancreatic cancer. J Gastrointest Surg. 2008;12:1600–8. doi: 10.1007/s11605-008-0482-2. [DOI] [PubMed] [Google Scholar]
- 5.Small W, Berlin J, Freedman GM, Lawrence T, Talamonti MS, Mulcahy MF, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008;26:942–7. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 6.Breslin TM, Hess KR, Harbison DB, Jean ME, Cleary KR, Dackiw AP, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–32. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 7.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–52. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 8.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–9. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5:1967–74. doi: 10.1158/1535-7163.MCT-06-0022. [DOI] [PubMed] [Google Scholar]
- 10.Chinnaiyan P, Cerna D, Burgan WE, Beam K, Williams ES, Camphausen K, et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin Cancer Res. 2008;14:5410–5. doi: 10.1158/1078-0432.CCR-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ree AH, Dueland S, Folkvord S, Hole KH, Seierstad T, Johansen M, et al. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 2010;11:459–64. doi: 10.1016/S1470-2045(10)70058-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim YJ, Lee WJ, Woo SM, Kim TH, Han SS, Kim BH, et al. Comparison of capecitabine and 5-fluorouracil in chemoradiotherapy for locally advanced pancreatic cancer. Radiat Oncol. 2013;8:160. doi: 10.1186/1748-717X-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Gennaro E, Piro G, Chianese MI, Franco R, Di Cintio A, Moccia T, et al. Vorinostat synergises with capecitabine through upregulation of thymidine phosphorylase. Br J Cancer. 2010;103:1680–91. doi: 10.1038/sj.bjc.6605969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang P, Oza A, Townsley C, Siu L, Pond G, Sarveswaran P, et al. A phase I study of vorinostat (VOR) in combination with capecitabine (CAP) in patients (pts) with advanced solid tumors. :3576. 18_suppl ed. [Google Scholar]
- 15.Anderson AW, Xie J, Pizzonia J, Bronen RA, Spencer DD, Gore JC. Effects of cell volume fraction changes on apparent diffusion in human cells. Magn Reson Imaging. 2000;18:689–95. doi: 10.1016/s0730-725x(00)00147-8. [DOI] [PubMed] [Google Scholar]
- 16.Charles-Edwards EM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248:894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 18.Dudeck O, Zeile M, Pink D, Pech M, Tunn PU, Reichardt P, et al. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2008;27:1109–13. doi: 10.1002/jmri.21358. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Abramson RG, Arlinghaus LR, Kang H, Chakravarthy AB, Abramson VG, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50:195–204. doi: 10.1097/RLI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuneo KC, Chenevert TL, Ben-Josef E, Feng MU, Greenson JK, Hussain HK, et al. A pilot study of diffusion-weighted MRI in patients undergoing neoadjuvant chemoradiation for pancreatic cancer. Transl Oncol. 2014;7:644–9. doi: 10.1016/j.tranon.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–95. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fokas E, Clifford C, Spezi E, Joseph G, Branagan J, Hurt C, et al. Comparison of investigator-delineated gross tumor volumes and quality assurance in pancreatic cancer: Analysis of the pretrial benchmark case for the SCALOP trial. Radiother Oncol. 2015;117:432–7. doi: 10.1016/j.radonc.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.11) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Klaassen R, Bennink RJ, van Tienhoven G, Bijlsma MF, Besselink MG, van Berge Henegouwen MI, et al. Feasibility and repeatability of PET with the hypoxia tracer [(18)F]HX4 in oesophageal and pancreatic cancer. Radiother Oncol. 2015;116:94–9. doi: 10.1016/j.radonc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Jones BL, Schefter T, Miften M. Adaptive motion mapping in pancreatic SBRT patients using Fourier transforms. Radiother Oncol. 2015;115:217–22. doi: 10.1016/j.radonc.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–31. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor OA, Heaney ML, Schwartz L, Richardson S, Willim R, MacGregor-Cortelli B, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–73. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 29.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 30.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]


