Abstract
OBJECTIVE
This study examined association between depressive and menstrual symptoms in adolescent girls in a three-year longitudinal study. It was hypothesized that menstrual symptoms would increase in early adolescence and decrease in later adolescence; girls with greater depressive symptoms would report greater menstrual symptoms; and effects would persist after adjusting for general somatic complaints.
METHODS
A community sample of girls (N = 262) enrolled in an observational study by age cohort (11, 13, 15, 17 years) completed three annual visits. Girls completed the Menstrual Symptom Questionnaire and the Children’s Depression Inventory at each time point, along with the Youth Self Report to assess general somatic complaints.
RESULTS
Menstrual symptoms increased significantly across adolescence (linear age B=10.2, SE=3.7, p=.006), and began to plateau in later adolescence (quadratic age B=−0.27, SE=0.12, p=0.020). Depressive symptoms at study entry were significantly associated with menstrual symptoms (B=0.44, SE=0.08, p<.001). When general somatic complaints were included in the models, the effect of depressive symptoms on menstrual symptoms remained significant for the sum score (B=0.23, SE=0.09, p=0.015) and the menstrual somatic symptoms subscale (B=0.14, SE=0.04, p=0.001). After adjusting for somatic complaints, initial report of depressive symptoms predicted change in menstrual symptoms only for girls with the lowest menstrual symptoms sum score (B=0.39, SE=0.17, p=0.025). Initial report of somatic complaints predicted change in menstrual symptoms (B=0.37, SE=0.16, p=0.020).
CONCLUSION
Girls with higher depressive symptoms and higher somatic complaints are at greater risk for experiencing menstrual symptoms and increasing symptoms across adolescence, with a heightened vulnerability for girls with lower baseline menstrual symptoms.
Keywords: Menstrual symptoms, depression, adolescence, female, menstruation, somatic
The prevalence of dysmenorrhea (pain accompanying menses) and related menstrual symptoms (nausea, vomiting, backaches, headaches and related somatic complaints) is highest during adolescence, with estimates ranging from 20% up to 90% depending on method of assessment (1). Moreover, dysmenorrhea and related menstrual symptoms are the leading cause of school and work absences among female adolescents, with 14% to 52% reporting absenteeism related to menstrual symptoms (1-3). Menstrual symptoms during adolescence and even into adulthood can be normal and are often associated with normal reproductive physiology. However, entry into adolescence is also a period of increased vulnerability to depression (4) and somaticizing (5). As a result, it is currently not clear whether dysmenorrhea and related menstrual symptoms present a new context for youth who are already prone to depressive symptoms and somaticizing, or whether menstrual symptoms are distinct from somaticizing more broadly (6). Given the established association between depressive symptoms and disorder with episodic and recurrent pain, (7, 8) understanding how depressive symptoms influence adolescent girls’ experiences of menstrual pain and somatic complaints may have important epidemiological and clinical implications. Specifically, if menstrual and depressive symptoms are associated, course of treatment for menstrual symptoms may need to vary dependent on the level of depressive symptoms experienced by a given adolescent, either because depressive symptoms often exacerbate the experience of pain, or due to comorbidity in depressive and somatic symptoms. Further, understanding the general trajectory for menstrual symptoms may aid in identifying when increasing symptoms are normative, and when they are indicative of an underlying problem.
Changing Menstrual Symptoms across Reproductive Maturity
Menstrual symptoms are thought to change over the reproductive years. In the months after menarche, menstrual bleeding may or may not be associated with ovulation. Ovulatory menstrual cycles are thought to be associated with more menstrual symptoms or dysmenorrhea (3, 9). In other words, women who are not experiencing ovulatory cycles (for whatever reason) may not have problematic menstrual symptoms. As reviewed by Rosenfield (10), it is thought that cycles generally become ovulatory within a year after menarche for most adolescents, but there is considerable individual variability (11-13). About half of the menstrual cycles are anovulatory two years post-menarche (14, 15) and by the fifth postmenarcheal year about 25% are anovulatory (15-17). Therefore, younger adolescents, in the early months after menarche may not have menstrual symptoms, but as they develop further, their symptoms may progress. Therefore, prior evidence would suggest that menstrual symptoms would increase in middle adolescence and then level off in later adolescence. Although several studies have evaluated menstrual symptoms in adolescents, few studies have evaluated this in a longitudinal manner to understand the course of menstrual symptoms in the early months to years after menarche.
Menstrual Symptoms and Somatic Complaints in Adolescence
While dysmenorrhea and menstrual symptoms are expected to increase in adolescence, a well-documented increase in general somatic complaints also occurs at that time, with significantly higher symptoms reported by adolescent girls than boys (18). This appears to be the case even when menstrual symptoms are excluded (19). Importantly, there have been some inconsistencies in the approach taken to distinguish general somatic and psychosomatic complaints from menstrual symptoms. Some studies exclude any symptoms for women that could be related to menses (e.g., 20, 21), other studies code somatic complaints in a way that eliminates symptoms that occur less than weekly (e.g., 22), and still others do not distinguish menstrual symptoms from somatic symptoms at all (e.g., 23). Thus, it is not clear to what extent menstrual symptoms are distinct from somatization more broadly or whether increases in menstrual symptoms may be at least partially attributable to somatizing.
Depressive and Menstrual Symptoms
Recent studies investigating psychological and behavioral risk factors of menstrual symptoms in young adult and premenopausal women have implicated depression as an important risk factor to consider when evaluating how females experience menstrual symptoms (24, 25). As it stands, much of the menstrual health literature has focused on the etiology and epidemiology of premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD), a condition marked by severe depressive symptoms, irritability, and tension only during the premenstrual phase (26, 27); notably, few studies assessing PMS or PMDD examine adolescent girls. As PMDD does not reflect the norm of adolescent experiences, other studies of more normative populations are needed. In recent years, several community-based studies have emerged focusing on depression as a risk factor for increased frequency and severity of menstrual symptoms (25, 28-31). Recent studies on young adult and premenopausal women have also shown that higher perceived stress, anxious/depressive style and anxiety sensitivity are associated with increased reports of menstrual distress and symptoms (31, 32). Finally, in one of the few studies to examine psychosocial factors related to menstrual symptoms in adolescent girls, Dorn and colleagues (28) found that greater depressive symptoms were associated greater menstrual symptoms (both pain and affective/somatic complaints) in a cross-sectional study of girls age 11 to 17. Together, these findings suggest that the experience of menstrual pain and symptoms may differ for females with greater depressive symptoms or a depressive disorder.
While the link between depressive symptoms and menstrual symptoms is not well-understood, two potential frameworks could provide insight into why the two are related. First, research has established an association between depressive symptoms and somatization (19). This association has been consistently found in adolescence (22, 23, 33), a period of time marked by increasing depressive symptoms (34). To the extent that menstrual symptoms are comorbid with broader somatic complaints, depressive symptoms would be expected to be associated with somaticizing around menstruation as well. Second, depressive symptoms are known to exacerbate or co-occur with various forms of chronic and episodic pain (e.g., recurrent headaches, abdominal pain, and musculoskeletal pain; 8). Although a comprehensive review of the depression and pain literature is beyond the scope of this article, several key findings from relevant studies are highlighted. In a four-year longitudinal study of youth (9 to 16 years) at risk for psychopathology, Egger and colleagues (7) found that girls with major depressive disorder (MDD) were 13 times more likely to report musculoskeletal pains and 4 times more likely to report headaches compared to girls without depression. Interestingly, the associations between complaints of pain and affective disorders (anxiety, depression) were significantly stronger in girls than boys.
As indicated previously, relatively little is known about the association between depressive symptoms and menstrual symptoms, particularly in pubertal-aged adolescent girls. Moreover, to date no studies have examined these relations longitudinally. Understanding how depressive symptoms influence the experience of the menstrual cycle in newly menarcheal girls may hold important implications for promoting health and wellbeing in adolescent girls. As such, the goal of this study was three-fold. First, the trajectory of menstrual symptoms across age was estimated, with the expectation that symptoms would increase in early and middle adolescence, followed by a plateau later in adolescence. Second, the association between depressive and menstrual symptoms was examined, with the expectation that depressive symptoms at study entry would be predictive of an increase in menstrual symptoms across adolescence. Finally, relations between depressive and menstrual symptoms after adjusting for somatic complaints were examined, using a three-year longitudinal study of adolescent girls.
Methods
Participants
Adolescent girls (N = 262) were recruited from an urban teen health center and the surrounding community to participate in a longitudinal study on smoking, mood, bone and reproductive health (35). Girls were enrolled in the study by age cohort (11, 13, 15, and 17 years) based on the cross-sequential design (36). Exclusionary criteria included (1) non-English speaking, (2) pregnancy/breastfeeding within the past 6 months, (3) primary amenorrhea (>16 years) or secondary amenorrhea (<6 cycles/ year not due to hormonal contraception), (4) body mass index less than the first percentile or body weight greater than 300 pounds, (5) medication/medical disorder influencing bone health, and (6) psychological disabilities impairing comprehension or compliance in the study. While pregnancy or recent birth was part of the exclusion criteria, girls who became pregnant during the course of the study remained in the study under a modified protocol. Across the three years, 24 girls (9%) reported having given birth. In addition to the above criteria for the parent study, only girls who were menarcheal during the course of the study and reported on menstrual symptoms during at least one visit (n = 247, 94.3%) were included in the analyses. Girls who were premenarcheal at initial report of do not contribute menstrual symptoms data at baseline, but were included for all time points after first menses. Hormone contraceptives were not an exclusion criterion of the parent study as it was desired to have the sample more representative of a wider range of community adolescents. However, girls were excluded for time points where they reported using depot medroxyprogesterone acetate [DMPA (n = 36)] because an expected side effect is amenorrhea; thus girls without menses would have difficulty reporting associated menstrual symptoms. As a result, 137 girls (55%) had MSQ data available at all three study time points, 65 girls (26%) had data at two and 45 girls (31%) had data at one time point. The sample size by year is presented in Table 1. The study received approval from the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Table 1.
Descriptive statistics by age cohort
| Total (N = 247) |
Age 11 (n = 40) |
Age 13 (n = 51) |
Age 15 (n = 87) |
Age 17 (n = 69) |
P-value | |
|---|---|---|---|---|---|---|
| Initial Report | ||||||
| Age (years) | 15.1 (2.1) | 11.6 (0.3) | 13.6 (0.3) | 15.6 (0.3) | 17.6 (0.3) | <0.001 |
| Body Mass Index | 24.3 (6.2) | 21.6 (5.5) | 22.8 (4.3) | 25.3 (6.5) | 25.8 (6.7) | <0.001 |
| Gynecological Age | 2.7 (2.3) | −0.5 (0.9) | 1.2 (1.2) | 3.2 (1.3) | 5.0 (1.6) | <0.001 |
| Oral Contraceptive Use | 19% (47) | 0% (0) | 6% (3) | 18% (16) | 41% (28) | <0.001 |
| Length of Menses (days) | 5.4 (1.9) | 5.5 (18) | 5.6 (2.1) | 5.5 (1.3) | 5.1 (2.4) | 0.52 |
| Depressive Symptoms | 46.6 (10.9) | 45.1 (9.3) | 42.4 (9.7) | 47.9 (10.8) | 49.1 (12.0) | <0.001 |
| Somatic Complaints | 56.6 (6.3) | 55.9 (5.9) | 56.0 (6.3) | 57.4 (6.7) | 56.4 (6.0) | 0.49 |
| Menstrual Symptoms Sum Score | 51.4 (17.0) | 38.1 (10.2) | 45.0 (14.4) | 57.0 (17.8) | 56.8 (15.3) | <0.001 |
| Abdominal Pain | 21.4 (8.3) | 15.5 (5.6) | 19.2 (7.2) | 23.9 (8.5) | 23.3 (7.9) | <0.001 |
| Back Pain | 5.9 (3.3) | 3.9 (1.9) | 4.9 (2.6) | 6.7 (3.6) | 7.0 (3.3) | <0.001 |
| Somatic Symptoms | 22.0 (7.8) | 16.7 (4.8) | 19.0 (6.6) | 24.2 (8.1) | 24.4 (7.5) | <0.001 |
| Year 1 Variables | N= 204 | 11 | 39 | 87 | 67 | |
| Depressive Symptoms | 47.3 (11.2) | 47.7 (9.0) | 42.4 (10.3) | 47.9 (10.8) | 49.4 (12.0) | 0.02 |
| Somatic Complaints | 56.9 (6.5) | 58.0 (7.5) | 55.9 (6.5) | 57.4 (6.7) | 56.6 (6.1) | 0.59 |
| Menstrual Symptoms Sum Score | 53.6 (17.2) | 37.1 (7.8) | 45.0 (15.2) | 57.0 (17.8) | 57.0 (15.4) | <0.001 |
| Year 2 Variables | N= 185 | 22 | 49 | 65 | 49 | |
| Depressive Symptoms | 44.5 (10.7) | 43.7 (8.6) | 41.8 (9.5) | 46.1 (11.1) | 45.5 (11.9) | 0.17 |
| Somatic Complaints | 55.2 (6.6) | 54.9 (5.7) | 55.1 (7.7) | 56.6 (7.0) | 53.4 (4.5) | 0.08 |
| Menstrual Symptoms Sum Score | 50.5 (16.5) | 39.3 (12.4) | 44.7 (15.6) | 55.3 (16.7) | 55.1 (14.7) | <0.001 |
| Year 3 Variables | N= 197 | 37 | 48 | 65 | 47 | |
| Depressive Symptoms | 41.6 (8.5) | 40.9 (10.0) | 39.8 (5.6) | 43.4 (9.2) | 41.8 (8.6) | 0.20 |
| Somatic Complaints | 53.2 (4.3) | 52.5 (3.5) | 53.2 (4.0) | 54.0 (5.2) | 52.7 (4.0) | 0.32 |
| Menstrual Symptoms Sum Score | 49.8 (16.2) | 40.7 (11.9) | 45.4 (13.5) | 54.0 (16.7) | 55.6 (16.9) | <0.001 |
Note. Values presented are unadjusted percentages [%( n)] or means (standard deviations) for each age cohort. Initial report includes values at Year 1 or year with first reported value. P-values are from ANOVA for evaluating differences between age cohort means and from the chi-square test for comparing differences between age cohort proportions of oral contraceptive use.
Procedure
Parents provided consent for their daughter’s participation and girls provided assent. Following initial screening for eligibility, participants completed three annual study visits at an urban children’s hospital. As the parent study included measurement of hormones, attempts were made for study visits to be conducted during day 5 to 9 of their menstrual cycle. Due to irregularity of cycles, if a girl had reached day 45 without a period the visit was scheduled (median day in cycle for girls who reported on menstrual symptoms ranged from 10 to 11 days for the annual visits). After parental consent and adolescent assent, girls completed a battery of questionnaires including a measure of general menstrual symptoms and depressive symptoms; parents were not present at this time. Additionally, anthropometric measures were collected during a physical examination conducted by a physician or nurse practitioner. Enrollment for the first visit took place from December 2003 to October 2007.
Measures
Depressive symptoms
Depressive symptoms were measured at each annual visit via the Children’s Depression Inventory (CDI; 37), a 27-item self-report measure. Participants were asked to report on depressive symptoms during the last two weeks. Girls were scheduled to complete study visits (including the CDI) during days 10 to 11 (median) of their menstrual cycle (day 1 represents the first day of girls’ last menstrual period). Therefore, reports of depressive symptoms occurred during the pre- and perimenstrual phase and later in the follicular phases of the cycle (i.e., the estimated 3-4 days prior to starting her most recent menstrual period, her menstrual period itself, and the estimated 5-8 days after her menstrual period ended). The CDI demonstrated high reliability across the study (Cronbach’s α = 0.87 to .90). T scores were used in the analyses (mean = 50; SD = 10), with scores ≥ 65 considered clinically significant (37). Depressive symptoms reported at the first annual visit, representing the baseline measurement, were used in the analyses.
Somatic complaints
General somatic complaints were assessed via self-report using the somatic complaints subscale of the Youth Self Report (38). This subscale consists of 10 items with responses on a three-point Likert scale ranging from not true (0) to very true/often true (2). Youth are asked to respond based on their experiences in the past 6 months. Items are distinct from somatization around menstrual symptoms (e.g., “I feel dizzy or lightheaded” and “I feel overtired without good reason”). Higher scores indicate more somaticizing. Somatic complaints reported at the first annual visit, representing the baseline measurement, were used in the analyses.
Menstrual symptoms
The Menstrual Symptom Questionnaire (MSQ; 39), a 24 item self-report measure, was used to assess pre- and peri-menstrual symptoms. This includes menstrual pain (e.g. ‘I have cramps that begin on the first day of my period’) as well as affective and somatic symptoms (e.g., ‘I feel depressed for several days before my period’ and ‘My breasts feel tender and sore a few days before my period’). Girls rated each item on a Likert scale from 1 (never) to 5 (always); girls rate symptoms in general (i.e., “Chose the response which best describes you”). In order to minimize construct overlap between depressive symptoms and menstrual symptoms, individual items from the MSQ that correlated with items on the CDI at r ≥ .20 were excluded. Ultimately, 5 items reflecting affective complaints (i.e., I feel irritable, easily agitated, and am impatient a few days before my period; I feel depressed for several days before my period; For several days before my period, I feel exhausted, lethargic, or tired; I feel weak and dizzy during my period; I feel tense and nervous before my period) were removed. Higher MSQ scores represent increased symptoms. Based on a recent study examining the factor structure of the MSQ in adolescent females (39), a total sum score (18 items, possible scores ranged from 18 to 90; α = .90 across study) as well as a three factor structure [abdominal pain (9 items, possible scores ranged from 9 to 45; α = .86 across study), somatic complaints (6 items, possible scores ranged from 6 to 30; α = .69 to .64), and back pain (3 items, possible scores ranged from 3 to 15; α =.84 to .85)] were used in this study. Higher scores represent greater total number and frequency of symptoms. Menstrual symptoms reported at all three annual visits were included in the analyses.
Demographics and covariates
Other participant characteristics were measured and examined as potential covariates. Race/ethnicity was determined by parental report. Socioeconomic status (SES), as measured by the Hollingshead Four Factor Index of Social Status (40), was estimated by parent report of occupation and education with scores ranging from 14 to 66 (MSES = 37.4, SD = 13.6). Height and weight, which were obtained in triplicate by trained nursing personnel, were used to calculate body mass index (kg/m2). Pubertal maturation was determined by physical examination by trained clinicians using visualization, breast palpation and inspection of pubic hair as categorized by Tanner criteria (41). Gynecological age and pubertal timing were based on age-at-menarche (in years and months) as obtained through a clinician interview with the adolescent. Pubertal timing groups (early, on-time, and late) were created based on the sample distribution of age at menarche within white and black racial groups in this study. Using common cutoffs for timing groups (42), girls who were 1 SD or more below the mean (within their racial group) were coded as early timing, and those who were 1 SD or more above the mean were coded as late timing; remaining girls were coded as on-time. Finally, girls reported on average duration of menstruation and current use of hormonal contraceptives (use within last two weeks) during the clinician interview, with oral contraceptive pills, depot medroxyprogesterone acetate, transdermal patch, and intravaginal ring most commonly reported.
Statistical Analyses
Analyses were performed using SAS v.9.2. Descriptive information and cohort differences in variables were analyzed using analysis of variance for continuous measures and chi-square test for categorical measures. All continuous variables were approximately normally-distributed. Associations between baseline depressive symptoms and longitudinal measures of menstrual symptoms were analyzed using random effects models (Proc Mixed in SAS v.9.2) with random intercepts, random slopes, and time-varying covariates. Models were estimated using an autoregressive covariance structure and restricted maximum likelihood estimation, which accommodates missing data, allows for the use of all data points available to estimate a model, and minimizes bias from missing data. (43) The following variables were tested as potential confounders of the association between depressive symptoms and menstrual symptoms: chronological age, race/ethnicity (white vs. other), SES, BMI, physical activity, gynecological age, pubertal timing (early vs. other), current contraceptive use (2 week duration), average duration of menstruation, and nulliparity. After controlling for chronological age, BMI, gynecological age, average duration of menstruation, and current contraceptive use were associated with depressive symptoms and/or menstrual symptoms at p < .20 and were included as covariates in the full longitudinal model. Current contraceptive use (i.e., past 2 weeks) and average duration of menstruation were included in the models as time varying covariates; baseline BMI and gynecological age were included as time-invariant covariates. Models were estimated separately for MSQ total sum scores and subscales (abdominal pain, somatic complaints, and back pain). Residual plots were used to check model assumptions and possible influential points. To examine whether effects of depressive symptoms on menstrual symptoms persisted after adjusting for general somatic complaints, models were estimated that controlled for baseline somatic complaints.
Results
Descriptive Statistics and Age Cohort Differences
Descriptive statistics are presented in Table 1. On average, girls were aged 15.1 years (SD = 2.1), and the majority were white (61 %) or African American (34%) with some mixed race/other (5%). Race was dichotomized as white versus non-white for analyses. Most participants were in later the stages of puberty at Year 1 (e.g., Tanner breast stage I and II < 2%, III = 9 %, IV = 15%, and V = 74%), and 83.5% were post-menarcheal (mean gynecological age = 2.7 years, SD = 2.3). Age differences were observed for many of the health and behavior measures (Table 1). As expected, older girls demonstrated greater physical and pubertal maturation, and were more likely to endorse current use of hormonal contraceptives.
Longitudinal Models of Menstrual Symptoms
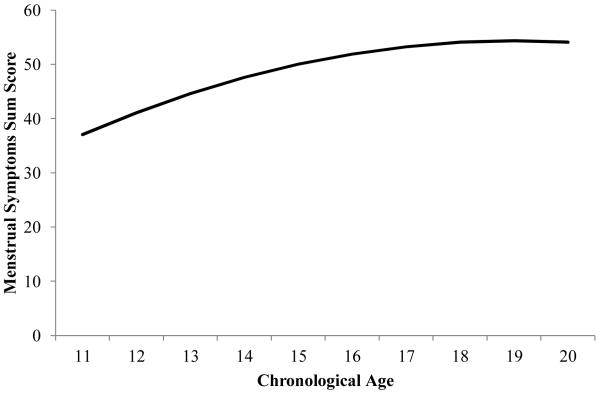
Overall, menstrual symptoms increase with age (B = 10.2, SE = 3.7, p = 0.006) with a deceleration in the increase as girls approach the end of adolescence (B = −0.27, SE = 0.12, p = 0.020; see Figure 1). When covariates were included, baseline depressive symptoms significantly predicted menstrual symptoms (see Table 2) such that higher depressive symptoms were associated with higher menstrual symptoms. Consistent with this finding, baseline depressive symptoms also significantly and positively predicted abdominal pain, somatic complaints, and back complaints subscales (see Table 2). Of note, baseline menstrual symptoms, depressive symptoms, and somatic complaints were all intercorrelated (r = 0.33-0.49).
Figure 1.
Trajectory of Menstrual Symptoms across Age.
Table 2.
Effect of Initial Report of Depressive Symptoms on MSQ Sum and Subscale Scores, accounting for covariates.
| MSQ Sum Scale Score | Abdominal Pain | Somatic Menstrual Symptoms |
Back Pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | B | S.E. | P | B | S. E. | P | B | S. E. | P | B | S.E. | P |
| Intercept | −25.3 | 29.9 | 0.40 | −8.8 | 15.1 | 0.56 | −9.1 | 14.7 | 0.54 | −11.88 | 6.50 | 0.068 |
| Age (Slope) | 6.4 | 3.7 | 0.080 | 2.62 | 1.86 | 0.16 | 2.12 | 1.81 | 0.24 | 1.82 | 0.80 | 0.023 |
| Age*Age (Quadratic) |
−0.18 | 0.11 | 0.11 | −0.08 | 0.06 | 0.16 | −0.06 | 0.06 | 0.29 | −0.05 | 0.03 | 0.039 |
| Body Mass Index | −0.40 | 0.16 | 0.011 | −0.16 | 0.08 | 0.042 | −0.16 | 0.07 | 0.032 | −0.06 | 0.03 | 0.049 |
| Gynecological Age | 2.53 | 0.51 | <.001 | 1.19 | 0.26 | <.001 | 1.11 | 0.24 | <.001 | 0.27 | 0.10 | 0.009 |
| Oral Contraceptives |
−0.83 | 1.27 | 0.51 | 0.09 | 0.64 | 0.89 | −0.66 | 0.64 | 0.30 | −0.01 | 0.28 | 0.97 |
| Length of menstruation |
0.92 | 0.38 | 0.010 | 0.39 | 0.19 | 0.042 | 0.52 | 0.19 | <.001 | 0.07 | 0.08 | 0.42 |
| Initial Report CDI | 0.44 | 0.08 | <.001 | 0.17 | 0.04 | <.001 | 0.23 | 0.04 | <.001 | 0.05 | 0.02 | 0.034 |
Menstrual Symptoms and Somatic Complaints
To examine whether depressive symptoms continued to be associated with menstrual symptoms after adjusting for the effect of somatic complaints, baseline somatic complaints was included as an additional covariate (see Table 3). Results indicate that when somatic complaints are accounted for, the effect of depressive symptoms remains significant for the MSQ sum score and the somatic symptoms subscale. However, the size of the effect of depressive symptoms on menstrual symptoms was smaller (B = .44 versus B = .23) when somatic symptoms were included.
Table 3.
Effects of Initial Report of Depressive Symptoms and Somatic Complaints on Menstrual Symptoms
| MSQ Sum Score | Abdominal Pain | Somatic Symptoms | Back pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | B | SE | P | B | SE | P | B | SE | P | B | SE | P |
| Intercept | −57.27 | 30.16 | 0.058 | −22.67 | 15.30 | 0.14 | −21.78 | 14.76 | 0.14 | −16.06 | 6.56 | 0.015 |
| Age (Slope) | 6.46 | 3.63 | 0.075 | 2.65 | 1.84 | 0.15 | 2.10 | 1.79 | 0.24 | 1.80 | 0.80 | 0.024 |
| Age*Age(Quadratic) | −0.18 | 0.11 | 0.10 | −0.08 | 0.06 | 0.16 | −0.06 | 0.06 | 0.29 | −0.05 | 0.03 | 0.042 |
| BMI | −0.42 | 0.15 | 0.006 | −0.17 | 0.08 | 0.024 | −0.16 | 0.07 | 0.016 | −0.06 | 0.03 | 0.036 |
| Gynecological Age | 2.56 | 0.50 | <0.001 | 1.20 | 0.25 | <0.001 | 1.13 | 0.23 | <0.001 | 0.28 | 0.10 | 0.007 |
| Oral Contraceptives | −0.65 | 1.26 | 0.61 | 0.16 | 0.64 | 0.80 | −0.59 | 0.63 | 0.35 | 0.01 | 0.28 | 0.96 |
| Length of menstruation |
0.94 | 0.37 | 0.012 | 0.40 | 0.19 | 0.037 | 0.53 | 0.19 | 0.005 | 0.07 | 0.08 | 0.42 |
| Baseline Somatic Complaints |
0.72 | 0.15 | <0.001 | 0.32 | 0.08 | <0.001 | 0.30 | 0.07 | <0.001 | 0.10 | 0.03 | 0.001 |
| Baseline CDI | 0.23 | 0.09 | 0.015 | 0.08 | 0.05 | 0.099 | 0.14 | 0.04 | <0.001 | 0.02 | 0.02 | 0.29 |
Predicting Change in Menstrual Symptoms
To examine whether depressive symptoms were associated with change in menstrual symptoms, models predicting subsequent menstrual symptoms (i.e., from data derived at T2 and T3) controlling for symptoms at baseline (i.e., T1) were estimated. Those models also accounted for baseline BMI, gynecological age, oral contraceptives, and average length of menstruation. While overall there was no effect of depressive symptoms on change in menstrual symptoms (B = 0.149, SE = 0.086, p = 0.083), when stratified by tertiles of initial report of menstrual symptoms sum score, there was a significant effect of depressive symptoms on change in MSQ symptoms among girls in the lowest third for the baseline MSQ symptoms sum score (B = 0.398, SE = 0.169, p = 0.025). Said differently, depressive symptoms at baseline were not generally associated with whether there were longitudinal increases or decreases in menstrual symptoms; however, for girls with lower levels of menstrual symptoms at baseline, higher depressive symptoms at baseline was associated with a significant increase in menstrual symptoms at subsequent time points. This association remained significant for girls in the lowest tertile of baseline MSQ symptoms when somatic complaints was included in the model (B=0.394, SE = 0.183, p=0.036). Baseline somatic complaints independently predicted increases in menstrual symptoms sum score and abdominal pain and somatic symptoms subscale scores (Table 4).
Table 4.
Effects of Initial Report of Depressive Symptoms and Somatic Complaints on Change in Menstrual Symptoms
| MSQ Sum Score | Abdominal Pain | Somatic Symptoms | Back pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | B | SE | P | B | SE | P | B | SE | P | B | SE | P |
| Intercept | 20.71 | 45.82 | 0.65 | −1.04 | 22.90 | 0.96 | 8.52 | 23.19 | 0.71 | −0.46 | 10.11 | 0.96 |
| Age (Slope) | −4.45 | 5.42 | 0.41 | −0.44 | 2.71 | 0.87 | −2.16 | 2.76 | 0.43 | −0.29 | 1.20 | 0.81 |
| Age*Age (Quadratic) | 0.15 | 0.17 | 0.36 | 0.02 | 0.08 | 0.81 | 0.07 | 0.08 | 0.39 | 0.01 | 0.04 | 0.73 |
| Body Mass Index | −0.17 | 0.15 | 0.26 | −0.13 | 0.07 | 0.071 | −0.03 | 0.07 | 0.64 | −0.02 | 0.03 | 0.63 |
| Gynecological Age | 0.74 | 0.64 | 0.26 | 0.29 | 0.32 | 0.36 | 0.59 | 0.31 | 0.062 | 0.11 | 0.13 | 0.42 |
| Oral Contraceptives | −1.90 | 1.52 | 0.21 | −0.29 | 0.76 | 0.71 | −0.94 | 0.79 | 0.24 | −0.57 | 0.35 | 0.10 |
| Length of menstruation | 1.15 | 0.58 | 0.048 | 0.06 | 0.29 | 0.83 | 0.91 | 0.29 | 0.002 | 0.18 | 0.13 | 0.17 |
| Initial Report of Menstrual Symptoms |
0.66 | 0.06 | <.001 | 0.61 | 0.06 | <.001 | 0.57 | 0.06 | <.001 | 0.57 | 0.06 | <.001 |
| Initial Report of Somatic Complaints |
0.37 | 0.16 | 0.020 | 0.19 | 0.08 | 0.016 | 0.18 | 0.07 | 0.015 | 0.05 | 0.03 | 0.14 |
| Initial Report of CDI | 0.06 | 0.09 | 0.50 | 0.04 | 0.05 | 0.36 | 0.01 | 0.05 | 0.77 | 0.02 | 0.02 | 0.28 |
Discussion
The goal of this study was to examine the association between depressive symptoms and menstrual symptoms (both pain and associated somatic complaints) in pubertal-aged adolescent girls across a three-year longitudinal study. Examining this association during a developmental period marked by increasing risk for depression and rapid physical and biological changes (i.e., menarche), this study extended the depression and pain literature (7, 8, 44, 45) to better understand how depressive symptoms influence for adolescent girls’ experience of menstrual symptoms. Additionally, this study contributes to the growing literature examining the developmental progression of normative menstrual symptoms in pubertal adolescent girls and the longitudinal association between depressive symptoms and menstrual symptoms (e.g., 46).
Menstrual Symptoms across Adolescence
Most studies of adult and adolescent females examine a narrow range of menstrual symptoms (e.g., cramps or abdominal pain) or disorder (e.g., dysmenorrhea or PMDD); in contrast, the present study examined a spectrum of symptoms experienced by adolescent girls including abdominal pain, somatic complaints and back pain. Consistent with prior research (47), menstrual symptoms were increasing in severity across adolescence. As stated earlier, such an increase may also have a physiologic rationale related to ovulation. Specifically, menstrual cycles for at least the first year or two postmenarche may be anovulatory; once cycles become ovulatory dysmenorrhea may increase as the production of prostaglandins increases. We hypothesized that menstrual symptoms would decrease later in adolescence; while that hypothesis was not confirmed, we did observe a deceleration of menstrual symptoms, indicating a curvilinear relationship between age and menstrual symptoms. Perhaps a decline would be observed later than the age of our sample such as in early adulthood (i.e., beyond age 20).
Depressive Symptoms and Menstrual Symptoms
Findings from this study indicate that depressive symptoms are positively associated with menstrual symptoms. Further, for girls who experienced lower levels of menstrual pain and somatic complaints at baseline, higher depressive symptoms was associated with a significant increase in subsequent menstrual symptoms. This effect was found, albeit to a lesser extent, even after adjusting for general somatic symptoms. Our findings are consistent with research investigating the association between depression and pain (e.g., headaches, abdominal pain, musculoskeletal pain; 7, 8, 45) and more recent cross-sectional studies on menstrual symptoms (25, 28-32). Although it is possible that girls with greater depressive symptoms are objectively experiencing more menstrual symptoms, a person's perception of pain, outside of extreme physical trauma, often has little to do with the actual sensory receptors and more to do with attribution of the stimulus. In turn, girls with greater depressive symptoms may be more acutely aware of menstrual changes and associated symptoms due to increased self-focus and greater preoccupation with bodily sensations and less able to effectively cope with and manage new physical symptoms (e.g, cramps, breast tenderness and general malaise; 32, 48).
The link between depressive symptoms and menstrual symptoms was attenuated when general somatic complaints were included; this adds further credence to the possibility that high menstrual symptoms may not exclusively have a physiological origin. Interestingly, general somatic complaints predicted both amounts of menstrual symptoms and change in menstrual symptoms across time, suggesting that general somaticizing, and potentially comorbid depressive symptoms, may be operating to increase girls’ experiences of menstrual symptoms. Girls’ reports of menstrual symptoms may also be influenced by cultural and social expectations of the menstrual experience communicated to girls by their mothers, media or other influential sources of information (48). In order to better elucidate pathways of risk for menstrual symptoms, future studies should investigate mechanisms related to global pain perception and coping as well as attitudes and expectation of the menstrual experience.
Limitations
Despite novel findings from this study, conclusions based upon this research are limited by several methodological issues. Because the measure of menstrual symptoms (MSQ) assessed a spectrum of symptoms and lacked a specified timeframe (e.g., one menstrual cycle), it is unclear whether stability of menstrual symptoms across the study reflects a developmentally stable trait or is an artifact of the measure. Furthermore, it is unclear whether the stability in the association between menstrual symptoms and depressive symptoms is simply a reflection of stable measures (i.e., MSQ and CDI scores did not change on average). Future studies are needed to replicate findings using the MSQ with clear time frames and may benefit from following girls for greater than three years to capture change in menstrual and depressive symptoms.
Although the use of a longitudinal design offered the advantage of examining the stability of the relationship between depressive symptoms and menstrual symptoms, the directionality of this association should be interpreted with caution. Based on the depression and pain literature, (7, 45) girls with depressive symptoms may be more likely to perceive or experience menstrual symptoms differently than girls without depressive symptoms. However, it is also plausible that the anticipation and experience of severe menstrual pain could influence depressive symptoms. Hence, studies examining the temporal association of depressive symptoms and menstrual symptoms are needed and may benefit from the inclusion of a clinical sample (i.e., adolescents referred for dysmenorrhea or MDD).
A final limitation is that the mechanism of these associations and trajectories of symptoms was not explored. Although the mechanism could include psychosocial factors, neuroendocrine mechanisms may also play a role. Most often estrogen and progesterone has been implicated (although not always tested) in depressive symptomatology in adult women. (49-51) A recent study showed that hormone contraceptive users (where hormone concentrations are altered) had lower depressive symptoms and were less likely to report past suicide attempts than those on no- or low efficacy contraceptive methods.(52) Additionally earlier reports show that estradiol was lower in adult women diagnosed with major depressive disorder compared to controls (53) and that if rapid changes in estrogen occur particularly in the late luteal phase, postpartum or the perimenopausal periods, depressive episodes may ensue in those women who may be more vulnerable to depression (54, 55). We are unaware of studies examining such factors in adolescent girls in a non-clinical sample
Conclusions and Implications
Although some menstrual symptoms represent a normative experience of reproductive age, dysmenorrhea and related menstrual symptoms remains the leading cause of absenteeism at school and work among female adolescents (1, 2, 47). In turn, a better understanding of factors related to increased risk for menstrual symptoms among adolescents holds great importance. This study demonstrated that girls with higher depressive symptoms consistently report higher menstrual symptoms. Additionally, menstrual symptoms and somatic complaints appear to be somewhat distinct, albeit related, constructs. These findings provide important information for the diagnosis and treatment of menstrual symptoms in adolescent girls. For example, conducting brief depression and somatic complaint screenings with adolescent girls presenting for menstrual symptom complaints may prove to be an effective way to identify girls at risk for missing school or work, and in turn, intervene in girls with depression who may not otherwise be seen by a provider.
Acknowledgments
Source of Funding: This research was supported in part by Grant Number R01 DA 16402, National Institute of Drug Abuse, NIH, PI: Lorah D. Dorn, PhD and by USPHS Grant # TR000077-04 from the National Center for Research Resources, NIH and by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), under Grant # T32HP10027.
ACRONYMS
- BMI
Body Mass Index
- CDI
Children’s Depression Inventory
- MDD
Major Depressive Disorder
- MSQ
Menstrual Symptoms Questionnaire
- PMDD
Premenstrual Dysphoric Disorder
- SD
Standard Deviation
- SE
Standard Error
- SES
Socioeconomic Status
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest.
References
- 1.French L. Dysmenorrhea in Adolescents: Diagnosis and Treatment. Pediatric Drugs. 2008;10(1):1–7. doi: 10.2165/00148581-200810010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Davis AR, Westhoff CL. Primary Dysmenorrhea in Adolescent Girls and Treatment with Oral Contraceptives. Journal of Pediatric and Adolescent Gynecology. 2001;14(1):3–8. doi: 10.1016/s1083-3188(00)00076-0. doi: http://dx.doi.org/10.1016/S1083-3188(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 3.Klein JR, Litt IF. Epidemiology of Adolescent Dysmenorrhea. Pediatrics. 1981;68(5):661–4. [PubMed] [Google Scholar]
- 4.Ge X, Natsuaki MN, Conger RD. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Dev Psychol. 2006;18:253–73. doi: 10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- 5.Aro H, Taipale V. Impact of timing of puberty on psychosomatic symptoms among fourteen- to sixteen-year old Finnish girls. Child Dev. 1987;58(1):261–8. doi: 10.2307/1130306. [DOI] [PubMed] [Google Scholar]
- 6.Egger HL, Costello EJ, Erkanli A, Angold A. Somatic Complaints and Psychopathology in Children and Adolescents: Stomach Aches, Musculoskeletal Pains, and Headaches. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):9. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Egger HL, Costello EJ, Erkanli A, Angold A. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains and headaches. J Am Acad Child Adolesc Psychiatry. 1999;38:852–60. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and Functional Disability in Chronic Pediatric Pain. The Clinical Journal of Pain. 2001;17(4):341–9. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Alvin PE, Litt IF. Current status of the etiology and management of dysmenorrhea in adolescence. Pediatrics. 1982;70(4):516–25. [PubMed] [Google Scholar]
- 10.Rosenfield RL. Adolescent Anovulation: Maturational Mechanisms and Implications. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-1770. Epub 2013/08/06. PubMed PMID: 23913942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. Epub 1970/01/01. PubMed PMID: 5419031. [PubMed] [Google Scholar]
- 12.Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118(5):2245–50. doi: 10.1542/peds.2006-2481. Epub 2006/11/03. PubMed PMID: 17079600. [DOI] [PubMed] [Google Scholar]
- 13.Legro R, Lin HM, Demers LM, Lloyd T. Rapid Maturation of the Reporductive Axis during Perimenarche Independent of Body Composition. The Journal of Clinical Endocrinology & Metabolism. 2000;85(3):1021–5. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- 14.Vollman RF. The menstrual cycle. Major problems in obstetrics and gynecology. 1977;7:1–193. Epub 1977/01/01. PubMed PMID: 836520. [PubMed] [Google Scholar]
- 15.Metcalf MG, Skidmore DS, Lowry GF, Mackenzie JA. Incidence of ovulation in the years after the menarche. J Endocrinol. 1983;97(2):213–9. doi: 10.1677/joe.0.0970213. Epub 1983/05/01. PubMed PMID: 6854190. [DOI] [PubMed] [Google Scholar]
- 16.Apter D, Vihko R. Premenarcheal endocrine changes in relation to age at menarche. Clin Endocrinol (Oxf) 1985;22(6):753–60. doi: 10.1111/j.1365-2265.1985.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 17.Apter D, Vihko R. Hormonal patterns of the first menstrual cycles. In: Venturoli C, Flamigni C, Givens JR, editors. Adolescence in Females. Yearbook Medical; Chicago: 1985. pp. 215–38. [Google Scholar]
- 18.Rauste-von Wright M, von Wright J. Longitudinal study of psychosomatic symptoms in healthy 11-18 year old girls and boys. Journal of Psychosomatic Research. 1981;25(number6):525–34. doi: 10.1016/0022-3999(81)90106-9. [DOI] [PubMed] [Google Scholar]
- 19.Barsky AJ, Peekna HM, Borus JF. Somatic Symptom Reporting in Women and Men. J Gen Intern Med. 2001;16:10. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke KPRK. Symptoms in the community: Prevalence, classification, and psychiatric comorbidity. Archives of Internal Medicine. 1993;153(21):2474–80. doi: 10.1001/archinte.1993.00410210102011. [DOI] [PubMed] [Google Scholar]
- 21.Neitzert CS, Davis C, Kennedy SH. Personality factors related to the prevalence of somatic symptoms and medical complaints in a healthy student population. Br J Med Psychol. 1997;70(1):93–101. doi: 10.1111/j.2044-8341.1997.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 22.Bohman H, Jonsson U, Paaren A, Knorring L, Olsson G, Knorring A. Prognostic significance of functional somatic symptoms in adolescence: A 15 year community-based follow-up study of adolescents with depression compared with healthy peers. BMC Psychiatry. 2012;12(90) doi: 10.1186/1471-244X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly JB, Wherry JN, Wiesner DC, Reed DH, Rule JC, Jolly JM. Mediating role of anxiety in self-reported somatic complaints of depressed adolescents. J Abnorm Child Psychol. 1994;22(6):691–702. doi: 10.1007/BF02171996. [DOI] [PubMed] [Google Scholar]
- 24.Pinkerton JV, Guico-Pabia CJ, Taylor HS. Menstrual cycle-related exacerbation of disease. American Journal of Obstetrics and Gynecology. 2010;202(3):221–31. doi: 10.1016/j.ajog.2009.07.061. doi: http://dx.doi.org/10.1016/j.ajog.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartlage SA, Brandenburg DL, Kravitz HM. Premenstrual Exacerbation of Depressive Disorders In a Community-Based Sample in the United States. Psychosom Med. 2004;66(5):698–706. doi: 10.1097/01.psy.0000138131.92408.b9. [DOI] [PubMed] [Google Scholar]
- 26.Braverman PK. Premenstrual Syndrome and Premenstrual Dysphoric Disorder. Journal of Pediatric and Adolescent Gynecology. 2007;20(1):3–12. doi: 10.1016/j.jpag.2006.10.007. doi: http://dx.doi.org/10.1016/j.jpag.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Yonkers KA, O'Brien PMS, Eriksson E. Premenstrual syndrome. The Lancet. 2008;371(9619):1200–10. doi: 10.1016/S0140-6736(08)60527-9. doi: http://dx.doi.org/10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorn LD, Negriff S, Huang B, Pabst S, Hillman J, Braverman P, Susman EJ. Menstrual symptoms in adolescent girls: Association with smoking, depressive symptoms, and anxiety. Journal of Adolescent Health. 2009;44(3):237–43. doi: 10.1016/j.jadohealth.2008.07.018. Epub 2008 Oct 29. PubMed Central PMCID: PMCPMC2667342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto K, Okazaki A, Sakamoto Y, Funatsu M. The Relationship between Premenstrual Symptoms, Menstrual Pain, Irregular Menstrual Cycles, and Psychosocial Stress among Japanese College Students. Journal of PHYSIOLOGICAL ANTHROPOLOGY. 2009;28(3):129–36. doi: 10.2114/jpa2.28.129. [DOI] [PubMed] [Google Scholar]
- 30.Angst J, Sellaro R, Stolar M, Merikangas KR, Endicott J. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatrica Scandinavica. 2001;104(2):110–6. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- 31.Gollenberg AL, Hediger ML, Mumford SL, Whitcomb BW, Hovey KM, Wactawski-Wende J, Schisterman EF. Perceived stress and severity of perimenstrual symptoms: the BioCycle Study. J Womens Health (Larchmt) 2010;19(5):959–67. doi: 10.1089/jwh.2009.1717. Epub 2010/04/14. PubMed PMID: 20384452; PubMed Central PMCID: PMCPMC2875955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigmon ST, Dorhofer DM, Rohan KJ, Boulard NE. The impact of anxiety sensitivity, bodily expectations, and cultural beliefs on menstrual symptom reporting: a test of the menstrual reactivity hypothesis. Journal of Anxiety Disorders. 2000;14(6):615–33. doi: 10.1016/s0887-6185(00)00054-2. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry. 1997;36(5):661–8. doi: 10.1097/00004583-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–17. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- 35.Dorn LD, Susman EJ, Pabst S, Huang B, Kalkwarf H, Grimes S. Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Arch Pediatr Adolesc Med. 2008;162(12):1181–8. doi: 10.1001/archpedi.162.12.1181. PubMed Central PMCID: PMCPMC2660672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychol Methods. 2000;5(1):44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M, Barth RP, Lloyd EC, Green RL, James S, Leslie LK, Landsverk J, Kovacs M. Children's Depression Inventory. The Children's Depression Inventory (CDI) Predictors of placement moves among children with and without emotional and behavioral disorders. Psychopharmacology Bulletin. 2007;1985;1521(1)(4):46–55. PubMed PMID: HaPI-305065 Number of References: 2. References: [Google Scholar]
- 38.Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. University of Vermont; Burlington, VT: 1991. [Google Scholar]
- 39.Negriff S, Dorn LD, Hillman JB, Huang B. The measurement of menstrual symptoms: Factor structure of the Menstrual Symptom Questionnaire in adolescent girls. J Health Psychol. 2009;14(7):899–908. doi: 10.1177/1359105309340995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollingshead AB. A Four-Factor Classification of Social Status. Yale University CT Press; New Haven, CT: 1975. [Google Scholar]
- 41.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge X, Brody GH, Conger RD, Simons RL. Pubertal maturation and African American children’s internalizing and externalizing symptoms. Journal of Youth & Adolescence. 2006;35(4):528–37. doi: 10.1007/s10964-006-9046-5. PubMed PMID: 21604117. [DOI] [Google Scholar]
- 43.Hofer SM, Hoffman L. Statistical analysis with incomplete data: A developmental perspective. In: Little TD, Bovaird JA, Card NA, editors. Modeling contextual effects in longitudinal studies. 2007. pp. 13–32. [Google Scholar]
- 44.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central Processing of Noxious Somatic Stimuli in Patients With Irritable Bowel Syndrome Compared With Healthy Controls. The Clinical Journal of Pain. 2010;26(2):104–9. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker LS, Smith CA, Garber J, Claar RL. Appraisal and Coping with Daily Stressors by Pediatric Patients with Chronic Abdominal Pain. J Pediatr Psychol. 2007;32(2):206–16. doi: 10.1093/jpepsy/jsj124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiesner J, Poulin F. Developmental Associations Between Adolescent Change in Depressive Symptoms and Menstrual-Cycle-Phase-Specific Negative Affect During Early Adulthood. J Youth Adolesc. 2012;41(10):1325–38. doi: 10.1007/s10964-011-9722-y. [DOI] [PubMed] [Google Scholar]
- 47.Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68(5):661–4. Epub 1981/11/01. PubMed PMID: 7312467. [PubMed] [Google Scholar]
- 48.Brooks-Gunn J, Ruble DN. The menstrual attitude questionnaire. Psychosom Med. 1980;42(5):503–12. doi: 10.1097/00006842-198009000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biological Psychiatry. 1998;44(9):798–811. doi: 10.1016/s0006-3223(98)00169-3. doi: http://dx.doi.org/10.1016/S0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS. Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of Applied Physiology. 2001;91(6):2785–801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential Behavioral Effects of Gonadal Steroids in Women with and in Those without Premenstrual Syndrome. New England Journal of Medicine. 1998;338(4):209–16. doi: 10.1056/NEJM199801223380401. PubMed PMID: 9435325. [DOI] [PubMed] [Google Scholar]
- 52.Keyes KM, Cheslack-Postava K, Westhoff C, Heim CM, Haloossim M, Walsh K, Koenen K. Association of hormonal contraceptive use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178(9):1378–88. doi: 10.1093/aje/kwt188. PubMed PMID: 24043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young EA, Midgley RA, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Archives of General Psychiatry. 2000;57(12):1157–62. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
- 54.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: The harvard study of moods and cycles. Archives of General Psychiatry. 2006;63(4):385–90. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 55.Payne JL. The role of estrogen in mood disorders in women. Int Rev Psychiatry. 2003;15(3):280–90. doi: 10.1080/0954026031000136893. PubMed PMID: 15276966. [DOI] [PubMed] [Google Scholar]