Abstract
Background & Aims
T-cell–mediated biliary injury is a feature of primary sclerosing cholangitis (PSC). We studied the roles of CD28- T cells in PSC and their regulation by vitamin D.
Methods
Peripheral and liver-infiltrating mononuclear cells were isolated from blood or fresh liver tissue. We analyzed numbers, phenotypes, functions, and localization patterns of CD28- T cells, along with their ability to activate biliary epithelial cells. We measured levels of tumor necrosis factor (TNF)α in liver tissues from patients with PSC and the effects of exposure to active vitamin D (1,25[OH]2D3) on expression of CD28.
Results
A significantly greater proportion of CD4+ and CD8+ T cells that infiltrated liver tissues of patients with PSC were CD28-, compared with control liver tissue (CD4+: 30.3% vs 2.5%, P < .0001; and CD8+: 68.5% vs 31.9%, P < .05). The mean percentage of CD4+CD28- T cells in liver tissues from patients with PSC was significantly higher than from patients with primary biliary cirrhosis or nonalcoholic steatohepatitis (P < .05). CD28- T cells were activated CD69+CD45RA- C-C chemokine receptor (CCR)7- effector memory and perforin+ granzyme B+ cytotoxic cells, which express CD11a, CX3CR1, C-X3-C motif receptor 6 (CXCR6), and CCR10—consistent with their infiltration of liver and localization around bile ducts. Compared with CD28+ T cells, activated CD28- T cells produced significantly higher levels of interferon γ and TNFα (P < .05), and induced up-regulation of intercellular cell adhesion molecule-1, HLA-DR, and CD40 by primary epithelial cells (3.6-fold, 1.5-fold, and 1.2-fold, respectively). Liver tissue from patients with PSC contained high levels of TNFα; TNFα down-regulated the expression of CD28 by T cells in vitro (P < .01); this effect was prevented by administration of 1,25(OH)2D3 (P < .05).
Conclusions
Inflammatory CD28- T cells accumulate in livers of patients with PSC and localize around bile ducts. The TNFα-rich microenvironment of this tissue promotes inflammation; these effects are reversed by vitamin D in vitro.
Keywords: Interferon, Autoimmunity, Biliary Epithelial Cells, Immune Regulation
Primary sclerosing cholangitis (PSC) is a poorly understood chronic immune-mediated biliary disease lacking effective treatment, as well as validated animal models. Despite features supporting a classic autoimmune etiology standard, immunosuppression is ineffective.1 As a result of progress in genetic studies, several risk loci have been identified for PSC; numerous loci for immunologically relevant proteins (eg, HLA, CD28, interleukin [IL]2RA, and macrophage stimulating 1).2 These findings parallel histologic observations that include a mixed inflammatory cell infiltrate consisting of lymphocytes, plasma cells, neutrophils, natural killer cells, Kupffer cells, and perisinusoidal macrophages.3 The majority of mononuclear cells in the portal infiltrates are T lymphocytes4 that produce high levels of tumor necrosis factor α (TNFα), supporting PSC as a predominantly T-helper 1-mediated disease.5
The cell surface molecule CD28 is a co-stimulatory molecule necessary for T-cell activation, survival, and proliferation,6 and the CD28 locus is a newly recognized risk factor in PSC development. Prior work identified T cells lacking CD28 accumulating in various autoimmune diseases,7,8 and suggested that loss of CD28 occurs at chronic inflammatory sites as a consequence of continuous antigenic stimulation and TNFα exposure.9 CD28- T cells appear to be chronically activated immunopathogenic cells,10 less susceptible to regulation by CD4+CD25+ T regulatory cells (Tregs), thus making them potentially important drivers of persistent chronic inflammation.11 Although immunogenetic profiles underpin the risk of PSC, environmental factors are equally relevant. Vitamin D is an extrinsic factor repeatedly associated with autoimmunity, as well as cholestatic liver diseases.12,13 The local activation of vitamin D by immune cells suppresses the development of proinflammatory effector T cells while increasing the frequency and suppressive function of Tregs.14
We sought to elucidate the mechanisms of biliary injury in PSC using patient-derived samples, to study T-cell infiltration and CD28 expression, alongside intervention with vitamin D. Our data show expansion of CD28- T cells with an activated phenotype in human PSC liver, localization close to bile ducts, release of proinflammatory cytokines, and induction of epithelial cell activation. A TNFα-rich PSC microenvironment was evident and TNFα down-regulated T-cell CD28 expression in vitro, an effect prevented by vitamin D.
Materials and Methods
Human Tissue and Blood
Fresh diseased liver tissue from our transplant program was available, as was nondiseased liver from surgical resections. Whole blood was obtained from healthy volunteers and PSC patients. All samples were collected after local research ethics committee approval and patient consent.
Isolation of peripheral blood and liver-infiltrating mononuclear cells
Peripheral blood mononuclear cells (PBMCs) and liver-infiltrating mononuclear cells (LIMCs) were isolated from peripheral blood and fresh human liver, respectively,15 as described in the Supplementary Materials and Methods section.
Isolation and culture of human primary biliary epithelial cells
Human biliary epithelial cells (BECs) were isolated from liver tissue, and cultured as previously reported16 and further described in the Supplementary Materials and Methods section.
Flow Cytometry
Flow cytometric analysis was performed on blood and liver-infiltrating T cells using a Cyan flow cytometer (Beckman Coulter, Bucks, United Kingdom), and analyzed using FlowJo (version 9; Treestar Inc, Ashland, OR) (see the Supplementary Materials and Methods section and Supplementary Table 1).
Isolation of CD28+/- T-Cell Subsets and Effect of T-Cell–Conditioned Media on BECs
PBMCs from PSC patients were isolated and stained for CD3–fluorescein isothiocyanate, CD4-allophycocyanin, and CD28-phycoerythrin markers to allow isolation of CD3+CD4+/-CD28+/- subsets by fluorescence-activated cell sorting. Isolated cells were activated overnight and their cell-free conditioned media was used to culture primary BECs for 4 days (see the Supplementary Materials and Methods section and Supplementary Table 1).
Detection of Cytokine Expression Ex Vivo
PBMCs and LIMCs from PSC patients were isolated as described earlier, and stimulated in 96-well, round-bottomed tissue culture plates for 6 hours with plate-bound anti-CD3 (OKT3; 5 μg/mL) at 106 cells/well (see the Supplementary Materials and Methods section and Supplementary Table 1).
Long-term In Vitro Culture of CD4+ T Cells
CD4+ T cells were enriched to 94%–98% purity from PSC PBMCs using a negative selection antibody cocktail (StemCell Technologies, Manchester, United Kingdom) and stimulated with aCD3/aCD28 Dynabeads (Life Technologies, Paisley, United Kingdom), IL2 (50 U/mL; Peprotech, London, United Kingdom), in the presence/absence of TNFα (10 ng/mL; Peprotech), with or without 1,25(OH)2D3 (10 nmol/L; ENZO Life Sciences, Exeter, United Kingdom). At 4 days, beads were removed using a magnet and cells were split to 0.5 × 106/mL. Cultures were assessed every 3–4 days and maintained at 0.5–1 × 106/mL. Cytokines and 1,25(OH)2D3 also were re-supplemented at these times, the concentration of IL2 being increased to 100 U/mL after 2 weeks.
Immunohistochemical Analysis
Dual-color immunohistochemistry was used to localize CD4+ and CD8+ T-cell subsets, and lymphocytes that lacked CD28 expression in human liver tissue (see the Supplementary Materials and Methods section and Supplementary Tables 1 and 2).
RNA Analysis
Total RNA was extracted from normal, PSC, and primary biliary cirrhosis (PBC) liver samples, using the RNeasy mini Kit (Qiagen, Manchester, United Kingdom), and analyzed as described in the Supplementary Materials and Methods section, for expression of TNFα, interferon γ (IFNγ), and IL17A by quantitative polymerase chain reaction analysis.
Cytokine Secretion Assays
The secretion of cytokines and chemokines from PSC LIMCs was investigated using the cytokine array panel A kit (R&D Systems, Abingdon, United Kingdom) (see the Supplementary Materials and Methods section).
Vitamin D Supplementation in PSC Patients
Patients were classified as 25(OH)D sufficient (>50 nmol/L), 25(OH)D insufficient (30–49 nmol/L), or severely 25(OH)D deficient (<30 nmol/L). Routine supplementation is advised for individuals with insufficient vitamin D levels (400 U/day colecalciferol). Those with severe deficiency are treated with high-dose replacement therapy (ergocalciferol capsules 50,000 U/wk for 5 weeks). Serum 25(OH)D levels were correlated with CD28- T-cell frequency in peripheral blood, before and after vitamin D therapy.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA) and SPSS software (SPSS, Inc, Chicago, IL). Data not normally distributed were evaluated using an unpaired Mann–Whitney, Wilcoxon matched-pairs signed rank, and Kruskal–Wallis tests. For correlations of CD28- T-cell frequency and clinical characteristics, Kolmogorov-Smirnov testing and the Spearman rank correlation coefficient were used. P values less than .05 were considered significant.
Results
CD28- T Cells Are More Frequent in Human PSC Liver Tissue
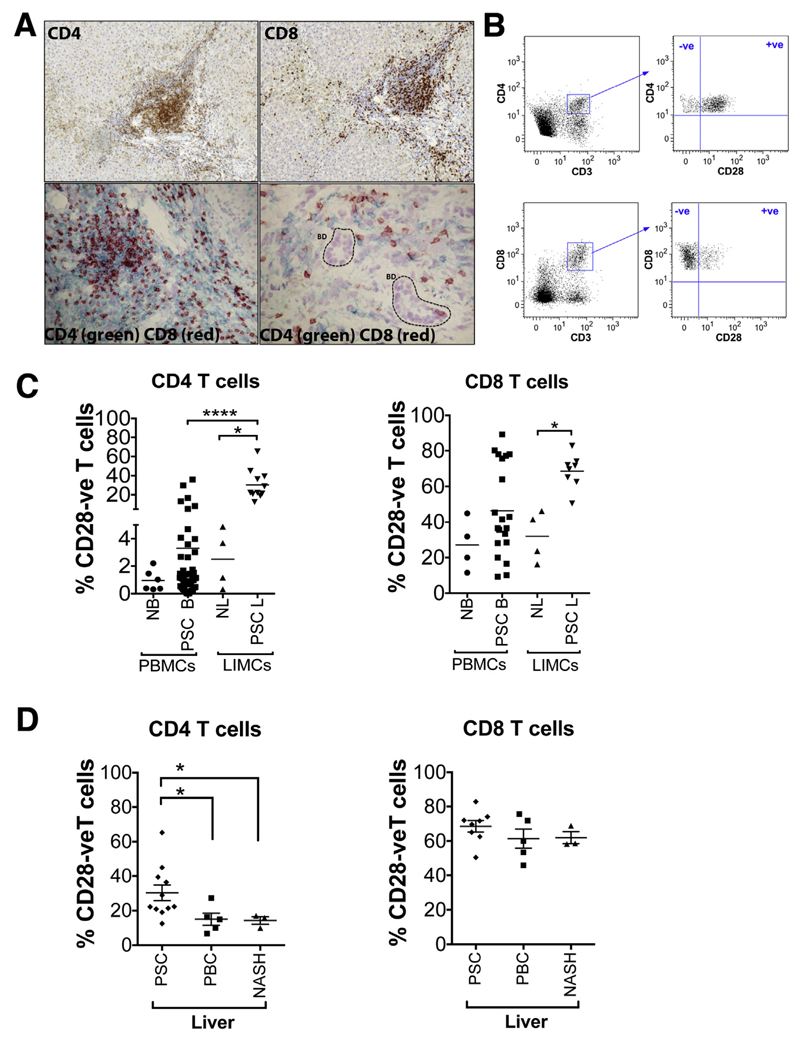
In PSC liver a reversal of the CD4:CD8 ratio (1:1.4) was detected compared with peripheral blood (2.3:1) (data not shown). CD4+ T cells were found localized mainly to the portal tracts, and CD8+ T cells were found localized mainly in the portal areas and in the parenchyma. Both CD4+ and CD8+ T cells were found in proximity to bile ducts (Figure 1A).
Figure 1.
CD28- T cells are more frequent in human PSC liver. (A) Single-color (magnification, 200×) and dual-color immunohistochemistry (CD4 [green] and CD8 [red]; 200× and 400×, respectively) showing localization of CD4+ and CD8+ T cells in human PSC liver tissue. BD, bile duct. (B) Representative flow cytometry dot plots showing the gating strategy defining CD28- T cells. (C) The frequency of CD3+CD4+CD28- and CD3+CD8+CD28- T cells in human PSC blood (n = 50 and n = 20, respectively) and liver (n = 11 and n = 8, respectively) was analyzed by flow cytometry and compared with blood (n = 6 and n = 4, respectively) and liver of healthy controls (n = 4, both). *P < .05, ****P < .0001. The ratio of CD4+CD28- T cells in PSC LIMCs:PBMCs was 9:1 and in normal LIMCs:PBMCs was 3:1. The ratio of CD8+CD28- T cells in PSC LIMCs:PBMCs was 1.4:1 and in normal LIMCs:PBMCs was 1:1. (D) The frequency of CD3+CD4+CD28- and CD3+CD8+CD28- T cells was analyzed in human PSC liver (n = 11 and n = 8, respectively) and compared with PBC (n = 5) and NASH (n = 3) human livers. *P < .05. NB, normal blood; NL, normal liver; PSC B, PSC blood; PSC L, PSC liver.
We studied the proportion of CD4+ and CD8+ T cells that were CD28- (Figure 1B). In the blood of PSC patients, 3.3% of CD4+ T cells were CD28-, whereas in PSC liver tissue this frequency was increased approximately 10-fold to 30.3% (P < .0001). By comparison, the frequencies of CD4+CD28- T cells in normal blood and liver were only 0.96% and 2.5%, respectively. Higher frequencies of CD28- T cells were found in the CD8+ compartment in both healthy controls and PSC patients. In healthy controls the frequencies of CD8+CD28- cells were not significantly different in blood and liver (27.05% and 31.9%, respectively), whereas in PSC, CD8+CD28- cells were increased in the blood (46.35%), and were significantly higher in the PSC liver (68.5%) when compared with uninflamed tissue (31.9%) (P < .05) (Figure 1C). We further studied the frequencies of CD28- T cells in control groups consisting of individuals with PBC and nonalcoholic steatohepatitis (NASH) liver diseases (Figure 1D). The proportion of CD4+ T cells that were CD28- was significantly higher in PSC livers (30.3%) compared with PBC (15.0%; P < .05) and NASH livers (14.2%; P < .05). No significant difference was detected for CD8+CD28- T cells.
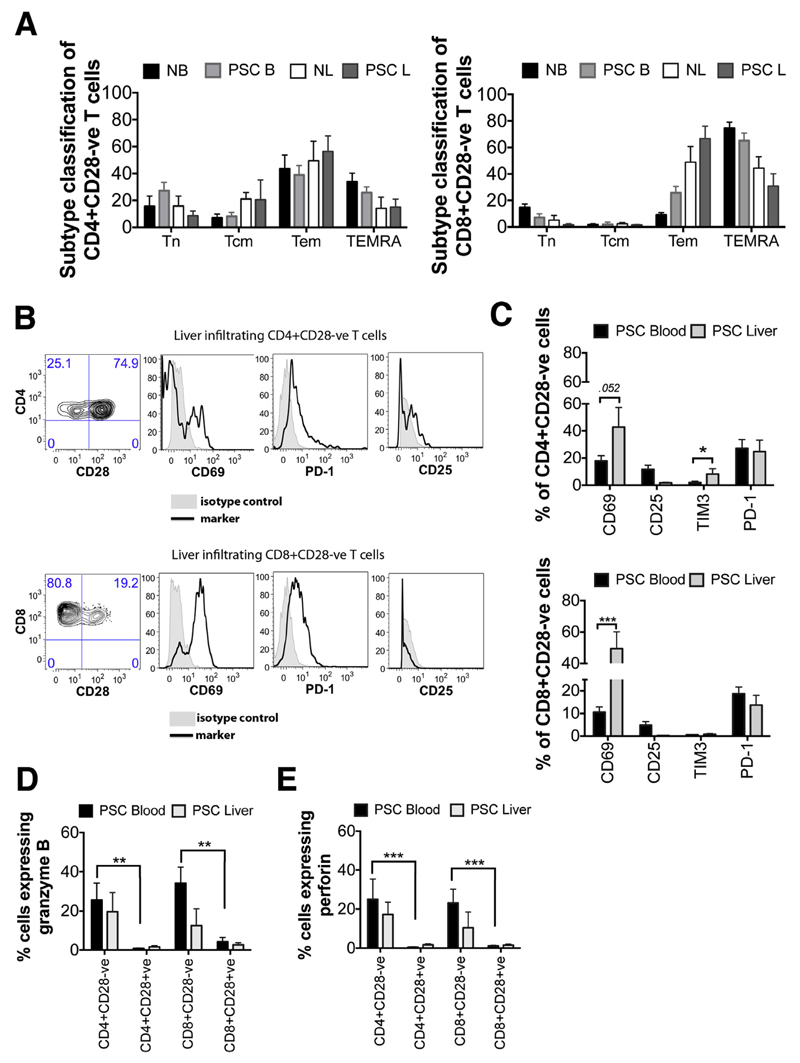
CD28- T Cells Are Activated Memory Cells With Intracellular Stores of Cytotoxic Molecules
CD45RA and CCR7 expression was evaluated to classify CD28- T cells, in PSC and normal control livers, into naive (CD45RA+CCR7+; T naive), central memory (CD45RA-CCR7+; T central memory), effector memory (CD45RA-CCR7-; T effector memory), and terminally differentiated effector memory RA (CD45RA+CCR7-; terminally differentiated effector memory RA) populations (Figure 2A). Our data showed that CD4+CD28- T cells in both blood and liver of PSC patients were mainly effector memory cells. PSC liver-infiltrating CD8+CD28- T cells also were effector memory cells; however, in peripheral circulation they showed a terminally differentiated effector memory RA phenotype (Figure 2A). The expression of activation and exhaustion markers was examined further on CD28- T cells from PSC liver and blood, as shown in the representative contour plots and histograms (Figure 2B). The expression levels between CD28- and CD28+ populations were not significantly different (Supplementary Figure 1A), but differences were seen between CD28- cells isolated from liver vs blood (Figure 2C). A higher proportion of CD4+CD28- T cells from liver expressed the activation marker CD69 (42.8% vs 17.81%; P = .05) (Figure 2C), and more liver-infiltrating CD8+CD28- T cells compared with circulating CD8+CD28- T cells (49.38% vs 10.62%, respectively; P < .0001) (Figure 2C). Expression of the IL2-Rα chain (CD25) was generally low; only 11.7% and 4.8% of peripheral blood CD4+CD28- and CD8+CD28- T cells, respectively, expressed CD25, and expression by liver-infiltrating CD28- cells was less than 2% (Figure 2C). CD8+CD28- T cells from circulation and liver tissue expressed T-cell immunoglobulin domain and mucin domain 3 (TIM3) at very low to undetectable levels (<1%). However, PSC liver-infiltrating CD4+CD28- T cells showed 8.1% TIM3 expression, which was 4 times higher compared with circulating cells (2.0%; P < .05). Circulating CD4+ and CD8+CD28- T cells (27.2% and 18.7%, respectively) expressed programmed cell-death 1, whereas liver-infiltrating cells expressed slightly lower levels (24.6% and 13.7%, respectively) (Figure 2C). Freshly isolated peripheral blood CD4+ and CD8+CD28- T cells contained intracellular stores of granzyme B and perforin, but these cytotoxic molecules were absent from their CD28+ counterparts (P < .01, P < .001) (Figure 2D and E). CD4+ and CD8+CD28- T cells in PSC liver and disease control (DC) (PBC and NASH) groups were phenotypically similar. In the DC group, however, significantly higher CD25 expression was detected (P < .05) (Supplementary Figure 1B).
Figure 2. Phenotypic characterization of CD28- T cells in blood and liver of PSC patients.
(A) The expression of CD45RA and CCR7 on CD4+CD28- and CD8+CD28- T cells was analyzed by flow cytometry for normal blood (NB) PBMCs (n = 4), PSC blood (PSC B) PBMCs (n = 14), normal liver (NL) LIMCs (n = 4), and PSC liver (PSC L) LIMCs (n = 4). Cells were classified into naive (CD45RA+CCR7+; T naive [Tn]), central memory (CD45RA-CCR7+; T central memory [Tcm]), effector memory (CD45RA-CCR7−; T effector memory [Tem]), and terminally differentiated effector memory RA (CD45RA+CCR7-; terminally differentiated effector memory RA [TEMRA]) populations. (B) CD3+CD4+ and CD3+CD8+ cells were selected and CD28- T cells gated as shown in the representative contour plots were analyzed for CD69, programmed cell-death 1 (PD-1), CD25, and TIM3 expression. Representative histograms for the marker (solid line) and its isotype control (shaded area) are shown. (C) Data show the percentage (mean ± SEM) of CD4+CD28- and CD8+CD28- T cells expressing CD69 (n = 23 [blood] and n = 6 [liver]), CD25 (n = 22 [blood] and n = 7 [liver]), TIM3 (n = 14 [blood] and n = 5 [liver]), and PD-1 (n = 14 [blood] and n = 6 [liver]). *P < .05, ***P < .001. (D and E) CD28- and CD28+ T lymphocytes from PSC PBMCs (n = 9) and LIMCs (n = 5) were analyzed by flow cytometry for the presence of intracellular deposits of granzyme B and perforin. **P < .01, ***P < .001.
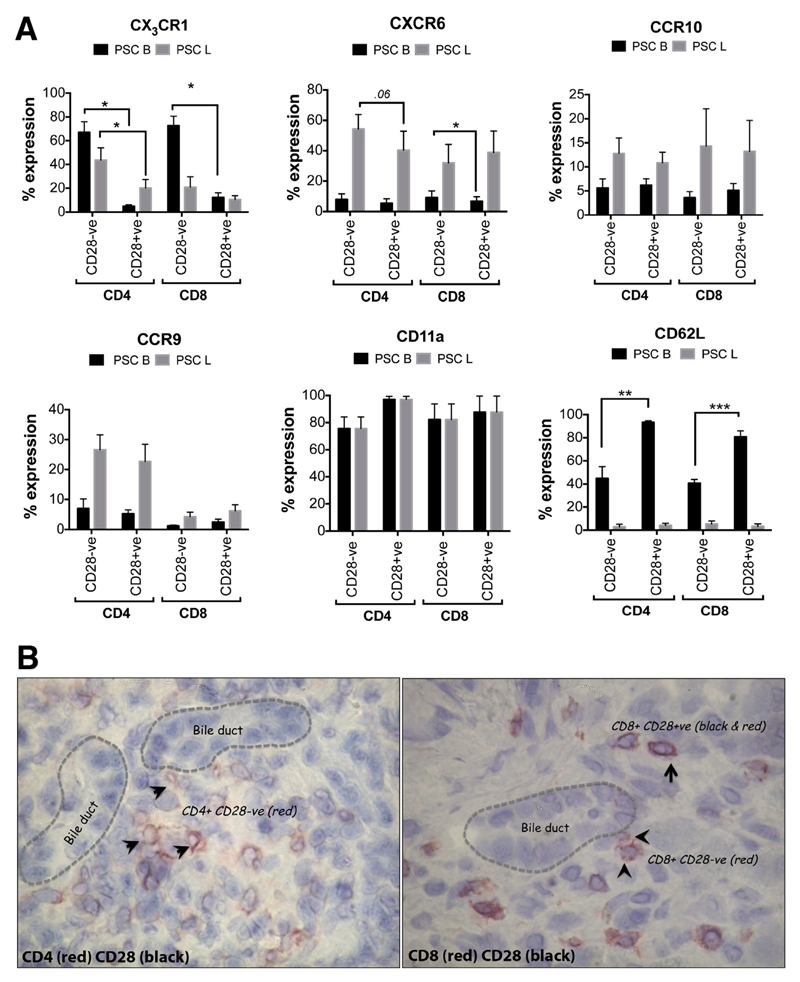
CD28- T Cells Are Equipped With Adhesion Molecules and Chemokine Receptors That Promote Tissue Infiltration and Localization Close to Bile Ducts
We investigated the mechanisms of CD28- T-cell infiltration into liver tissue by studying the expression of adhesion molecules and chemokine receptors previously associated with migration into and retention within the liver. Higher frequencies of CX3CR1+ CD4+ and CD8+CD28- T cells were found in circulation than in liver (66.9% vs 43.5% for CD4+ and 72.5% vs 20.8% for CD8+ in peripheral blood vs liver, respectively). Frequencies of CX3CR1+ CD28+ cells were significantly lower (P < .05) in the blood (Figure 3A). Of all liver-infiltrating CD4+CD28- and CD28+ T cells in the PSC liver, 54.1% and 40.3% expressed CXCR6, respectively, levels much higher than their circulating counterparts. The expression of CXCR6 by CD8+CD28- and CD28+ cells was less than 38%, whereas more than 40% of CD4+ T cells expressed CXCR6. PSC liver-infiltrating CD4+ and CD8+CD28- T cells expressed higher levels of CCR10 compared with their counterparts in the circulation. Moreover, 26.6% and 22.7% of PSC liver-infiltrating CD4+CD28- and CD28+ cells, respectively, expressed CCR9, at levels higher than circulating cells (6.9% and 5.2%, respectively). CCR9 expression was observed in less than 6.5% of total CD8+ T cells in both peripheral circulation and liver of PSC patients. CD11a was expressed at high levels on both CD28- and CD28+ T cells of both lineages in both blood and liver, whereas CD62L (L-selectin) was barely expressed (<6%) by liver-infiltrating CD28- and CD28+ T cells of both lineages, consistent with them being memory/effector T cells. Although CD62L expression was significantly higher in CD4+ and CD8+ T cells in circulation, CD28- T cells from both subsets expressed significantly lower levels (44.8% and 40.6%, respectively) than their CD28+ counterparts (93.3% and 80.7%, respectively) (P < .01, P < .001) (Figure 3A). We further observed that CD28- T cells localize around bile ducts in human PSC liver tissue (Figure 3B).
Figure 3.
CD28- T cells are equipped with adhesion molecules and chemokine receptors that promote tissue infiltration and localization close to the bile ducts. (A) The expression of chemokine receptors CX3CR1 (n = 7 [blood] and n = 6 [liver]), CXCR6 (n = 7 [blood] and n = 6 [liver]), CCR9 (n = 4 [blood] and n = 5 [liver]), and CCR10 (n = 9 [blood] and n = 5 [liver]), and adhesion molecules CD11a (n = 4 [blood] and n = 4 [liver]), and CD62L (n = 7 [blood] and n = 3 [liver]) on CD28- and CD28+ T cells of CD4+ and CD8+ T cells from blood (PSC B) and liver (PSC L) of PSC patients was analyzed using flow cytometry. Data show the percentages of CD28- and CD28+ T cells that express the chemokine receptors. *P < .05, **P < .01, ***P < .001. (B) Representative dual-color immunohistochemistry image showing the localization of CD4+CD28- and CD8+CD28- T cells in human PSC liver tissue (magnification, 400×). Arrowheads point to CD28- ve T cells in red and arrows point to CD28+ T cells in black and red.
The chemokine receptor expression was studied further on PBC patients. PSC liver-infiltrating CD4+CD28- T cells expressed higher levels of CCR9 when compared with PBC (26.6% vs 12.5%) (Supplementary Figure 2B). Similarly, a higher proportion of CD4+CD28+ T cells in PSC liver expressed CCR9 when compared with PBC (22.7% vs 7.1%) (Supplementary Figure 2B). The absolute frequency of CCR9+ T cells in peripheral blood of PSC patients was low, but remained higher compared with PBC patients (CD4+CD28-: 6.9% vs 4.3% and CD4+CD28+: 5.2% vs 3.3%) (Supplementary Figure 2A). In PBC peripheral blood, significantly higher levels of CD8+CD28+ T cells expressed CX3CR1 (50%) when compared with PSC blood (12.1%; P < .05). Higher levels of PBC liver-infiltrating CD8+CD28+/- T cells also expressed CX3CR1 when compared with PSC. A higher proportion of PSC peripheral blood and liver-infiltrating CD4+ and CD8+ CD28- T cells expressed CCR10 compared with PBC (Supplementary Figure 2).
CD28- T Cells Release Proinflammatory Cytokines and Induce Activation of BECs
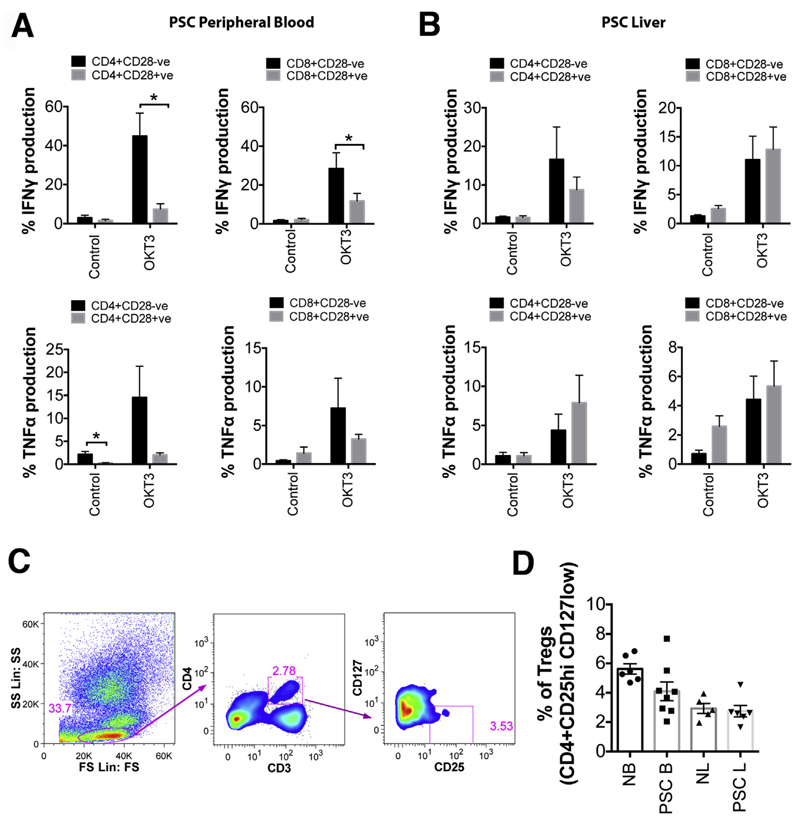
To further examine the role of CD28- T cells in PSC pathology, we studied their ability to produce proinflammatory cytokines immediately after isolation. More than 40% of peripheral blood CD4+CD28- T cells produced IFNγ after 6 hours of stimulation with anti-CD3, which was significantly higher compared with their CD28+ counterparts (P < .05). Peripheral blood CD8+CD28- T cells produced significantly more IFNγ (28%) when compared with CD8+CD28+ T cells (P < .05) (Figure 4A). Both CD4+ and CD8+CD28- T cells secreted more TNFα (14.5% and 7.2%, respectively) than CD28+ T cells (Figure 4A). PSC liver-infiltrating CD28- T cells from both CD4+ and CD8+ subsets produced IFNγ (16.6% and 11.0%, respectively), but levels were not significantly different from their CD28+ counterparts (Figure 4B). Liver-infiltrating cells also produced TNFα but at a lower level than IFNγ. In contrast to blood, liver-infiltrating CD28+CD4+ and CD8+ T cells produced more TNFα when compared with CD28- T cells (Figure 4B).
Figure 4.
CD28- T cells release proinflammatory cytokines and their supernatants are able to activate human primary BECs in vitro. Data from (A) 6 PSC peripheral blood samples and (B) 6 PSC liver samples showing TNFα and IFNγ production by CD4+CD28+/- and CD8+CD28+/-. *P < .05. (C) Representative flow cytometry plots showing the gating strategy for defining T-regulatory cells. (D) Data show the percentage of CD3+CD4+ T cells that are CD25hi CD127low, in blood and liver of normal and PSC patients (normal blood [NB], n = 6; PSC blood [PSC B], n = 8; normal liver [NL], n = 5; PSC liver [PSC L], n = 6). Each shape represents the value from each individual, and the line represents the mean value ± SEM.
Studies have reported that Tregs can inhibit cytokine production by CD28- T cells.11 In PSC liver tissue we detected low frequencies of CD3+CD4+CD25hiCD127low Tregs at levels similar to those seen in normal tissue (Figure 4C and D).
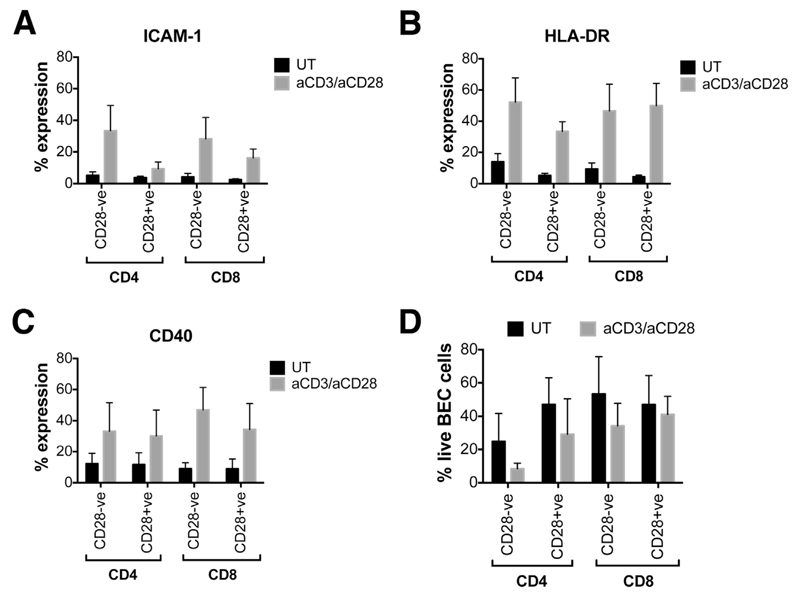
Because CD28- and CD28+ T cells showed different proinflammatory cytokine profiles, we were interested to observe the effect of their secretomes on BEC activation and survival. Conditioned media from activated CD4+CD28- and CD8+CD28- T cells induced the expression of intercellular cell adhesion molecule-1 (ICAM1), HLA-DR, and CD40 (Figure 5A-C) on primary BECs. No significant differences, however, were detected on the effects between CD28- and CD28+ T cells. Neither CD28- nor CD28+ cells induced leukocyte function-associated antigen 3 expression (data not shown). Only 8% of BECs were viable after 4 days of culture with activated CD4+CD28- T-cell–conditioned media, whereas 29%–41% of BECs were alive when cultured with the media of all other subsets (Figure 5D).
Figure 5.
Supernatants from CD28- T cells are able to activate human primary BECs in vitro. (A–C) Data show the indirect effects of untreated (UT) and aCD3-/aCD28-treated, cell-sorted CD28- and CD28+ T cells on the percentage expression of ICAM1, HLA-DR, and CD40 (n = 5) on BECs. (D) Data show the percentage of live BECs after 4 days of coculture with the T-cell–conditioned media (n = 3).
TNFα Induces the Differentiation of CD28- T Cells and Vitamin D Overcomes This Effect
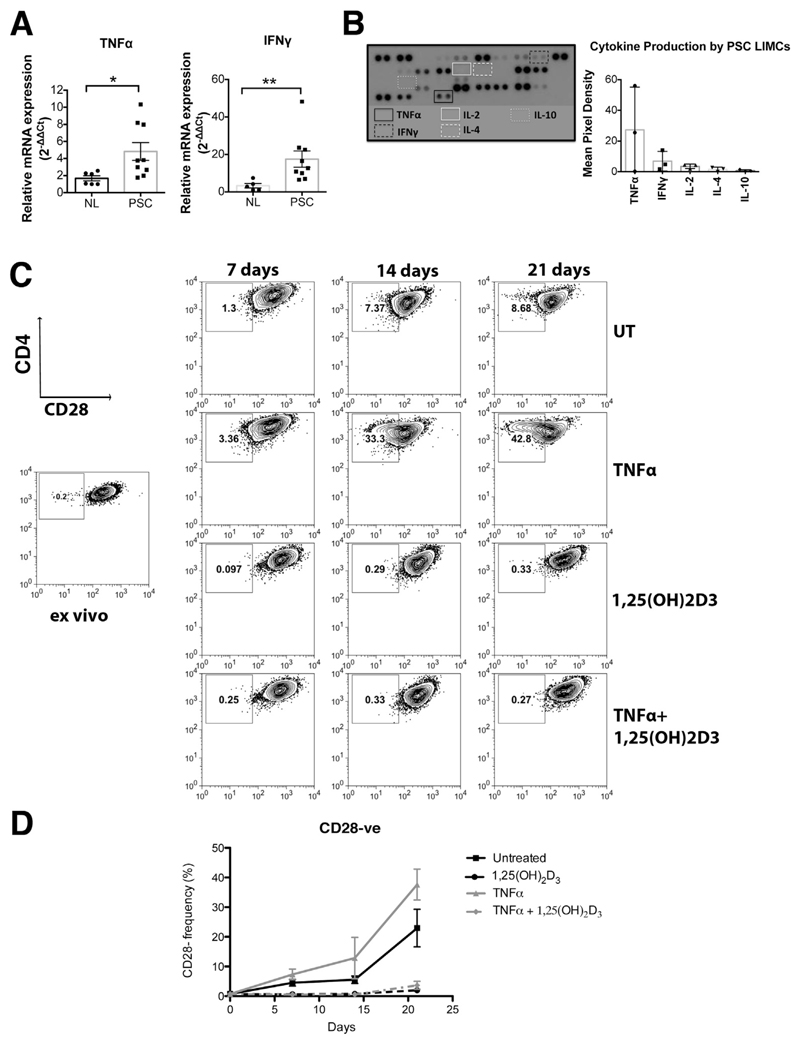
We detected significantly increased levels of TNFα and IFNγ messenger RNA in the PSC liver microenvironment compared with normal tissue (4.8 vs 1.7, P < .05; and 17.5 vs 3.2, P < .01, respectively) (Figure 6A) and increased levels of IL17A messenger RNA relative to PBC (Supplementary Figure 3A). PSC liver-infiltrating mononuclear cells were able to release TNFα and IFNγ after 24 hours of culture even without stimulation. IL2, IL4, and IL10 were low to undetectable (Figure 6B). PSC LIMCs also were producers of IL17, IL23, IL6, IL8, CCL2, CCL3, and CCL4 (Supplementary Figure 3B).
Figure 6.
TNFα enhances the emergence of CD28- T cells and 1,25(OH)2D3 overcomes this effect. (A) TNFα and IFNγ messenger RNA (mRNA) expression in 6 normal liver (NL) and 9 PSC liver tissues was measured by quantitative polymerase chain reaction. Scatter dot plots show relative mRNA levels in diseased livers with respect to 1 NL tissue (mean ± SEM). *P < .05, **P < .01. (B) Cytokines and chemokines released from 3 PSC LIMCs as analyzed with the Human Cytokine Array kit (R&D Systems, Abingdon, United Kingdom). Expression levels are reported as mean pixel density in arbitrary units. (C) CD4+ T cells from blood of PSC patients were stimulated with aCD3/aCD28 beads and cultured for 21 days in the presence or absence of TNFα, with or without 1,25(OH)2D3. The frequency of CD28- cells was measured at 0, 7, 14, and 21 days by flow cytometry as shown in the representative contour plots. (D) Data from 7 donors.
We cultured PSC CD4+ T cells with recombinant (r)TNFα in vitro and found a significant increase in CD4+CD28- T cells after 7–21 days. After 21 days, 22.9% of CD4+ T cells spontaneously lost CD28 expression whereas this expression increased to 37.6% in the presence of exogenous rTNFα. Given the immunomodulatory effects of vitamin D we tested further whether the active form of vitamin D 1,25(OH)2D3 had an effect on CD28 expression in the presence and/or absence of TNFα. Our data showed that 1,25(OH)2D3 markedly suppressed the development of CD28- T cells (2%; P < .05) in vitro, even in cultures in which exogenous rTNFα was present (3.7%; P < .05) (Figure 6C and D).
Frequencies of CD4+CD28- T Cells in PSC Patients Supplemented With Vitamin D
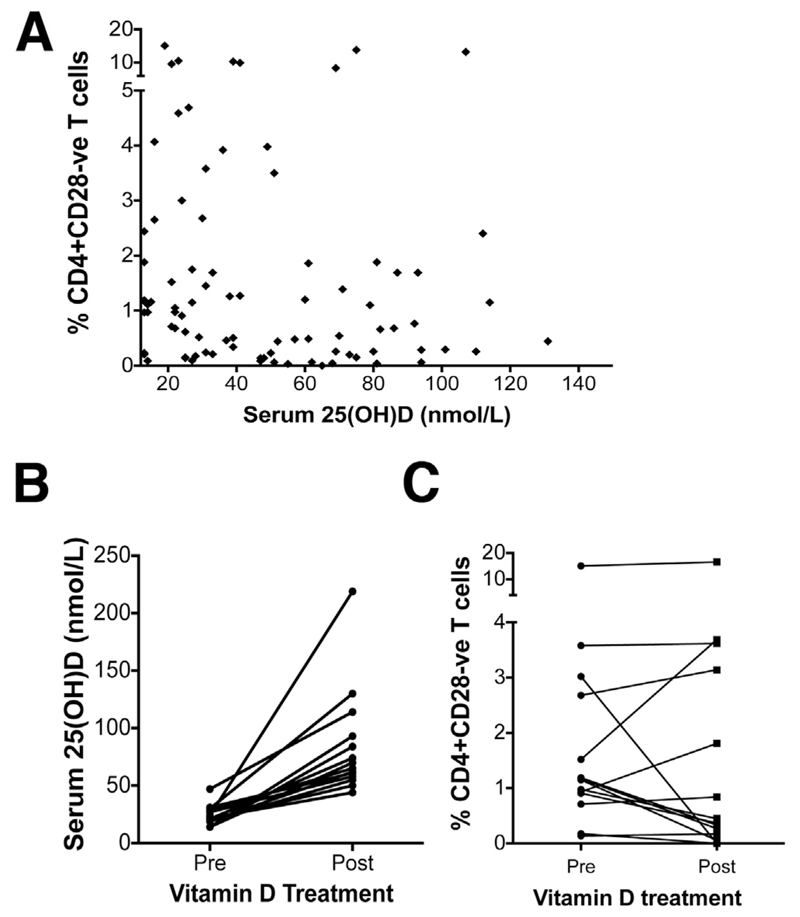
To examine whether vitamin D status influences CD28- T-cell frequencies in PSC patients we measured their peripheral blood CD28- T-cell frequencies and serum vitamin D (25[OH]D) concentration. A trend toward a negative correlation with serum vitamin D was seen (nonparametric Spearman rank correlation test: P = .1699; and Spearman ρ(r): −0.1451) (Figure 7A). No significant associations were found between peripheral CD28- T-cell frequency and patient age, ethnicity, severity/activity of liver disease, or inflammatory bowel disease status (data not shown). In 14 PSC patients with severe vitamin D deficiency who returned for a routine clinical review, we repeated CD4+CD28- T-cell frequency and vitamin D measurements. Our data showed that supplementation of vitamin D increased levels sufficiently (Figure 7B) and in 7 of these patients the CD4+CD28- T-cell frequency was reduced by 5.8-fold (Figure 7C).
Figure 7.
CD4+CD28- T cells in PSC patients supplemented with vitamin D. (A) Serum 25(OH)D levels of 92 PSC patients were correlated with the frequency of CD4+CD28- T cells in circulation. Each dot represents the value from each individual. (B) PSC patients who had insufficient serum vitamin D levels were supplemented with vitamin D as part of their medical treatment. The levels of serum 25(OH) vitamin D before and after supplementation are shown. (C) Data show the percentage of CD4+CD28- T cells after several weeks when serum vitamin D levels reached sufficiency. Data were analyzed using the Wilcoxon matched-pairs signed rank test (P = .669).
Discussion
We provide evidence that a significant proportion of PSC liver-infiltrating T cells lack CD28, an important immunoregulatory protein implicated in PSC pathogenesis by genetic association studies. We showed that CD28- T cells are recently activated memory/effector cells, equipped with a combination of adhesion molecules and chemokine receptors that allow infiltration into liver tissue and specific localization around bile ducts. Upon re-activation, CD28- memory/effector T cells release high levels of TNFα and IFNγ proinflammatory cytokines, which can activate neighboring BECs to express adhesion and costimulatory molecules. The important role of local TNFα is suggested by the finding of high intrahepatic levels of TNFα in PSC and its ability to induce CD28 loss in cultured T cells. The ability of vitamin D to prevent this effect suggests a novel therapeutic action of this vitamin in PSC. However, more extensive studies are required to evaluate this effect, particularly in larger cohorts of PSC patients, with vitamin D supplementation remaining a speculative approach to intervention at this time.
We show that CD28- T cells express high levels of CD69 and no CD45RA, suggesting an active effector/memory phenotype. Both CD4+ and CD8+CD28- T cells expressed programmed cell-death 1, a marker associated with both activated and exhausted T cells.17,18 PSC liver-infiltrating CD4+CD28- T cells expressed low levels of TIM3, which generally is considered a marker of T-cell exhaustion, suggesting that CD28- cells in PSC liver are activated effector cells.19 TIM3 levels were higher on CD28- than CD28+ cells, further suggesting that CD28- cells have undergone many cycles of activation. The higher CD25 expression on CD28- cells of the DC group agrees with our data showing the presence of more CD28- cells in PSC because CD25 expression is dependent on CD28-mediated signals. Both CD4+ and CD8+ CD28- cells readily produced proinflammatory cytokines upon activation and contained perforin and granzyme B.
Our data suggest mechanisms to explain the accumulation of CD28- T cells in human PSC liver tissue by migration from peripheral blood. We found that CD28- T cells are equipped with CD11a and chemokine receptors CX3CR1, CXCR6, and CCR10, which allow their infiltration into tissue and migration toward BECs, which strongly express the chemokine ligands CX3CL1, CXCL16, and CCL28.20–22 This recruitment may be amplified in a paracrine fashion because CD28- T cells could release TNFα and IFNγ proinflammatory cytokines, which increase adhesion molecule expression and chemokine secretion from BECs. The ability of CD28- T cells to up-regulate ICAM1, HLA-DR, and CD40 on BECs could promote BECs as T-cell targets in PSC. Increased ICAM1 expression on bile ducts is a characteristic feature of liver diseases in which bile ducts are the major targets of immune-mediated destruction, such as PSC and PBC.23 Increased binding of effector cells to cholangiocyte ICAM1 not only promotes accumulation but also allows recognition of major histocompatibility complex antigens and the activation of cytolytic mechanisms that induce cholangiocyte apoptosis.24
Previous studies showed the ability of Tregs to inhibit cytokine production by CD28- T cells but not their proliferation.11 In PSC liver tissue we detected low frequencies of CD4+CD25hiCD127low Tregs at levels similar to those seen in normal tissue, and lower than the frequencies previously reported for other autoimmune and chronic inflammatory conditions.25 A reduction in the frequency of peripheral blood Tregs and in Foxp3+ T cells in PSC liver, with an apparent impaired suppressive capacity, recently was reported.26 Reduced numbers of Tregs in PSC liver therefore may lead to uncontrolled cytokine production by CD28- T cells. Other investigators have shown that TNFα can modulate the expression of CD28 at a transcriptional level.9 Our data showed high levels of TNFα in human PSC liver, suggesting that the local cytokine microenvironment may promote the differentiation and accumulation of CD28- T cells. We also showed that activated liver-infiltrating CD28+ T cells produce high levels of TNFα, which may act in an autocrine manner to down-regulate their own CD28 expression. CD28 signaling is required for IL2 production, which in turn is needed for induction of Tregs. Therefore, in PSC liver, in which 30.3% of CD4+ T cells are CD28- T cells, IL2 production likely is defective and this might be a contributing factor for the reduced frequency of Tregs in PSC liver tissue. Because both CD28- and CD28+ T cells localize close to bile ducts and produce TNFα, we suggest that such local cytokine production can induce the emergence of CD28- T cells and the amplification of the inflammatory response as indicated by their ability to activate BECs. TNFα also can activate BECs to express receptors such as tumor necrosis factor receptor superfamily members involved in apoptosis and cholangiocyte death,6 further implicating TNFα in the pathogenesis of biliary destruction in PSC. Correspondingly, in our coculture system, we observed that peripheral blood CD4+CD28-, which produced the highest levels of TNFα, promoted the greatest death of BECs. CD28- T cells also are cytotoxic cells, expressing perforin and granzyme B, and other investigators have shown the synergistic effect of them in cholangiocyte injury.27
We also show that 1,25(OH)2D3 can increase the absolute expression of CD28 on in vitro stimulated cells and prevent emergence of the CD28- population during long-term culture, even in the presence of exogenous rTNFα. This finding is consistent with recent data showing that vitamin D could increase the median fluorescence of CD28 in CD4+ T cells from healthy controls and multiple sclerosis patients during in vitro stimulation.28 We observed that in a subset of PSC patients with profound deficiency in vitamin D, the frequency of CD28- T cells in the circulation was reduced after supplementation and correction of hypovitaminosis. However, in as many patients an increase or stability of CD28- T-cell frequency was observed. No significant clinical differences were detected between the cohorts experiencing a reduction in CD28- frequency vs those in whom CD28- frequency remained stable/increased (Supplementary Table 3). We suggest that, in vivo, a more complex set of immunoregulatory interactions is likely to participate and other factors in the disease background of the patients also might affect results. Moreover, we speculate that in patients in whom supplementation of vitamin D had no obvious effect, it actually may have prevented the further expansion of CD28- proinflammatory cells. We therefore highlight a potential therapeutic use of vitamin D in PSC that merits further in-depth evaluation.
Our findings are important in the context of a disease that lacks therapy and has poorly refined animal models in which to test new therapies. We recognize that the increased frequency of CD28- T cells is a feature of chronic inflammatory and autoimmune disease. Notably, our data show a significant difference in the frequency of CD4+CD28- T cells in PSC liver, compared with PBC and NASH, with PSC liver-infiltrating T cells also showing high CCR9 expression, further suggesting the specificity of these cells for PSC. It is important, however, to highlight the inherent difficulty of comparing and contrasting liver diseases that are fundamentally distinct because of the different patterns of liver injury and response to injury over time. Although CD28- T cells were detected in all 3 chronic liver diseases studied, we believe specificity lies in the antigen specificity of these cells. Genetic associations at the CD28 locus have been identified in several autoimmune diseases (eg, PSC, celiac disease, type 1 diabetes, rheumatoid arthritis, alopecia areata, and autoimmune thyroid disease), but notably not in well-powered assessments in inflammatory bowel disease or PBC. The basis of the profound pleiotropy observed for risk genes in immune-mediated conditions is poorly understood, and our findings provide the contextual data for a PSC-centered evaluation of CD28 risk variants.
The frequent, and often profound, deficiency of vitamin D associated with liver disease may make the mechanism particularly applicable to cholestatic liver disease; indeed, increased numbers of CD4+CD28- T cells have been reported in association with bile duct damage in PBC.29 Our vitamin D data are provocative and interesting, albeit presently still are a hypothesis-generating concept at this time.
In summary, in PSC patients we show the significance of CD28- cells, which release proinflammatory cytokines and cytotoxic mediators and express the necessary receptors to allow their localization close to the bile ducts and induce their activation. We provide evidence that TNFα, which is enriched in the PSC liver microenvironment, can enhance the accumulation of CD28- T cells and show that this effect can be overcome with vitamin D.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.04.003.
Funding
Supported in part by the National Institute for Health Research, Wellcome Trust (RCHX1704), and Primary Sclerosing Cholangitis Partners Seeking a Cure; and by the German Research Foundation (BR182/1-1 to T.B.).
The views expressed are those of the authors(s) and not necessarily those of the National Health System, the National Institute for Health Research, or the Department of Health.
Abbreviations used in this paper
- BEC
biliary epithelial cell
- CCR
C-C chemokine receptor
- CXCR
C-X3-C motif receptor
- DC
disease control
- ICAM1
intercellular cell adhesion molecule-1
- IFNγ
interferon γ
- IL
interleukin
- LIMC
liver infiltrating mononuclear cell
- NASH
nonalcoholic steatohepatitis
- PBC
primary biliary cirrhosis
- PBMC
peripheral blood mononuclear cell
- PSC
primary sclerosing cholangitis
- rTNFα
recombinant tumor necrosis factor α
- TIM3
T-cell immunoglobulin domain and mucin domain 3
- TNFα
tumor necrosis factor α
- Treg
T regulatory cell
Footnotes
Author names in bold designate shared co-first authors.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet. 2013;382:1587–1599. doi: 10.1016/S0140-6736(13)60096-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto E, Lindor KD, Homburger HA, et al. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049–1055. doi: 10.1016/s0025-6196(12)60897-0. [DOI] [PubMed] [Google Scholar]
- 4.Ponsioen CY, Kuiper H, Ten Kate FJ, et al. Immunohistochemical analysis of inflammation in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1999;11:769–774. doi: 10.1097/00042737-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bo X, Broome U, Remberger M, et al. Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut. 2001;49:131–141. doi: 10.1136/gut.49.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noel PJ, Boise LH, Thompson CB. Regulation of T cell activation by CD28 and CTLA4. Adv Exp Med Biol. 1996;406:209–217. doi: 10.1007/978-1-4899-0274-0_22. [DOI] [PubMed] [Google Scholar]
- 7.Fasth AE, Cao D, van Vollenhoven R, et al. CD28nullCD4+ T cells–characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand J Immunol. 2004;60:199–208. doi: 10.1111/j.0300-9475.2004.01464.x. [DOI] [PubMed] [Google Scholar]
- 8.Markovic-Plese S, Cortese I, Wandinger KP, et al. CD4+CD28- costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–1194. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryl E, Vallejo AN, Matteson EL, et al. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 11.Thewissen M, Somers V, Hellings N, et al. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol. 2007;179:6514–6523. doi: 10.4049/jimmunol.179.10.6514. [DOI] [PubMed] [Google Scholar]
- 12.L Ng KV, Nguyen LT. The role of vitamin D in primary biliary cirrhosis: possible genetic and cell signaling mechanisms. Gastroenterol Res Pract. 2013;2013:602321. doi: 10.1155/2013/602321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowry EM. Vitamin D: evidence for its role as a prognostic factor in multiple sclerosis. J Neurol Sci. 2011;311:19–22. doi: 10.1016/j.jns.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liaskou E, Zimmermann HW, Li KK, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys EH, Williams KT, Adams DH, et al. Primary and malignant cholangiocytes undergo CD40 mediated Fas dependent apoptosis, but are insensitive to direct activation with exogenous Fas ligand. PLoS One. 2010;5:e14037. doi: 10.1371/journal.pone.0014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei F, Zhong S, Ma Z, et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A. 2013;110:E2480–E2489. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 19.Sakhdari A, Mujib S, Vali B, et al. Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection. PLoS One. 2012;7:e40146. doi: 10.1371/journal.pone.0040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heydtmann M, Lalor PF, Eksteen JA, et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 21.Isse K, Harada K, Zen Y, et al. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 22.Eksteen B, Miles A, Curbishley SM, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- 23.Volpes R, van den Oord JJ, Desmet VJ. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology. 1990;12:59–65. doi: 10.1002/hep.1840120110. [DOI] [PubMed] [Google Scholar]
- 24.Ayres RC, Neuberger JM, Shaw J, et al. Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut. 1993;34:1245–1249. doi: 10.1136/gut.34.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oo YH, Weston CJ, Lalor PF, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 26.Sebode M, Peiseler M, Franke B, et al. Reduced FOXP3+ regulatory T cells in patients with primary sclerosing cholangitis are associated with IL-2RA gene polymorphisms. J Hepatol. 2014;60:1010–1016. doi: 10.1016/j.jhep.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Shivakumar P, Mourya R, Bezerra JA. Perforin and granzymes work in synergy to mediate cholangiocyte injury in experimental biliary atresia. J Hepatol. 2014;60:370–376. doi: 10.1016/j.jhep.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kickler K, Ni Choileain S, Williams A, et al. Calcitriol modulates the CD46 pathway in T cells. PLoS One. 2012;7:e48486. doi: 10.1371/journal.pone.0048486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isse K, Harada K, Sato Y, et al. Characterization of biliary intra-epithelial lymphocytes at different anatomical levels of intrahepatic bile ducts under normal and pathological conditions: numbers of CD4+CD28- intra-epithelial lymphocytes are increased in primary biliary cirrhosis. Pathol Int. 2006;56:17–24. doi: 10.1111/j.1440-1827.2006.01913.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.