Abstract
Uric acid is the final oxidation product of purine metabolism in humans. Xanthine oxidoreductase (XOR) catalyzes oxidative hydroxylation of hypoxanthine to xanthine to uric acid, accompanying the production of reactive oxygen species (ROS). Uric acid usually forms ions and salts known as urates and acid urates in serum. Clinically, overproduction or under-excretion of uric acid results in the elevated level of serum uric acid (SUA), termed hyperuricemia, which has long been established as the major etiologic factor in gout. Accordingly, urate-lowering drugs such as allopurinol, an XOR-inhibitor, are extensively used for the treatment of gout. In recent years, the prevalence of hyperuricemia has significantly increased and more clinical investigations have confirmed that hyperuricemia is an independent risk factor for cardiovascular disease, hypertension, diabetes, and many other diseases. Urate-lowering therapy may also play a critical role in the management of these diseases. However, current XOR-inhibitor drugs such as allopurinol and febuxostat may have significant adverse effects. Therefore, there has been great effort to develop new XOR-inhibitor drugs with less or no toxicity for the long-term treatment or prevention of these hyperuricemia-related diseases. In this review, we discuss the mechanism of uric acid homeostasis and alterations, updated prevalence, therapeutic outcomes, and molecular pathophysiology of hyperuricemia-related diseases. We also summarize current discoveries in the development of new XOR inhibitors.
MeSH Keywords: Cardiovascular Diseases, Gout, Hyperuricemia
Background
Serum uric acid (SUA) concentration is a significant parameter for human health. Alteration of SUA homeostasis has been linked to a number of diseases. For example, an abnormally high SUA level, termed hyperuricemia, is the underlying cause of gout and has been correlated with cardiovascular disease, hypertension, and renal disease. More recent studies have demonstrated that hyperuricemia may directly contribute to the development or progression of these diseases [1–3]. Besides gout, therapies that directly inhibit the production of uric acid may be effective to prevent and/or treat hyperuricemia-related cardiovascular disease and other diseases [4]. Xanthine oxidoreductase (XOR) is a critical enzyme for uric acid production. Since clinical XOR-inhibitor drugs such as allopurinol and febuxostat may have serious adverse effects such as hypersensitivity drug reactions, there is great interest in the development of novel XOR inhibitors for long-term use with less or no adverse effects [5]. This review aims to summarize the critical aspects of uric acid biochemistry, recent advances in molecular pathogenesis, clinical significance of hyperuricemia in cardiovascular disease and other diseases, and new discoveries in XOR inhibitors. This article provides fundamental and updated information about hyperuricemia-related diseases and XOR inhibitors to physicians and scientists who work in this field.
Uric Acid Production and Removal
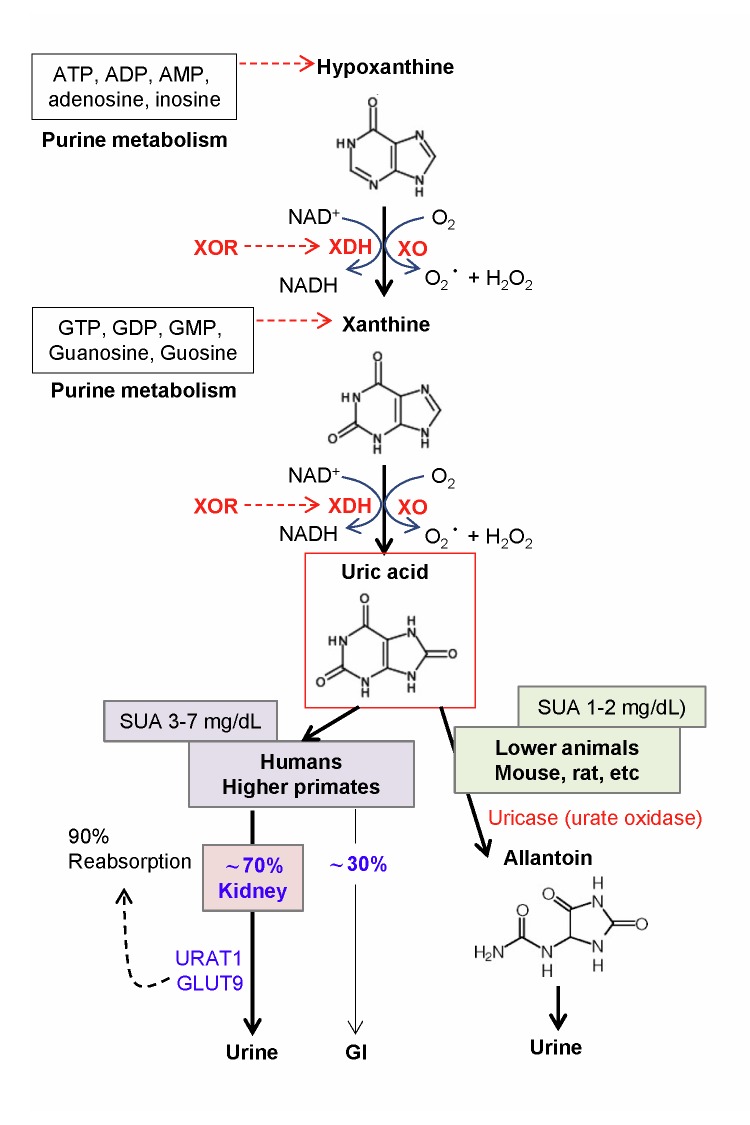
Uric acid (2,6,8-trioxypurine) is a heterocyclic purine derivative with a molecular weight of 158 Da. Uric acid was first found in bladder stones in 1776 by the Swedish pharmacist Scheele [6]. It is a weak acid and forms a singly-charged hydrogen or acid urate ion at the biological pH of 7.4. The water solubility of uric acid is relatively low and is sensitive to temperature. The saturation point of uric acid is 392 μmol/L (6.6 mg/dL) at 37°C and pH 7.4 [7]. In humans and higher primates, uric acid is the final oxidation (breakdown) product of purine (adenine and guanine) metabolism (Figure 1). About 70% of uric acid is removed from the urine and 30% is removed from the gastrointestinal route [8]. In the kidney, at least 90% of the filtered uric acid is reabsorbed by specific anion transporters, including URAT1 and GLUT9 [8]. URAT1 reabsorbs urate and secretes anionic organic compounds such as lactate, ketone bodies, and xenobiotics through a counter-transport process. However, in other lower animals such as rats and mice, the enzyme uricase (urate oxidase) further oxidizes uric acid to allantoin, which is 100 times more water-soluble than uric acid and has more efficient excretion from the urine [9]. Humans and higher primates lack a functional uricase gene.
Figure 1.
Uric acid production and removal in humans and mice. Xanthine oxidoreductase (XOR) catalyzes the oxidation of hypoxanthine to xanthine to uric acid, accompanied with the generation of reactive oxygen species, superoxide anion (O2−), and hydrogen peroxide (H2O2). Uric acid is the final oxidation product of purine (adenine and guanine) metabolism in humans and higher primates, and is removed from renal and gastrointestinal routes. In lower animals such as rats and mice, the enzyme uricase (urate oxidase) further oxidizes uric acid to allantoin for more efficient removal from the urine. Humans and higher primates lack a functional uricase gene.
Purines are important biomolecules, including ATP, GTP, cyclic AMP, NADH, and coenzyme A. Purines are building blocks of DNA and RNA. In general, purines are found in a high concentration in meat and meat products, including sweetbreads, anchovies, sardines, liver, beef kidneys, brains, herring, mackerel, and scallops [10]. Beer (from the yeast) and gravy also contain a high amount of purines.
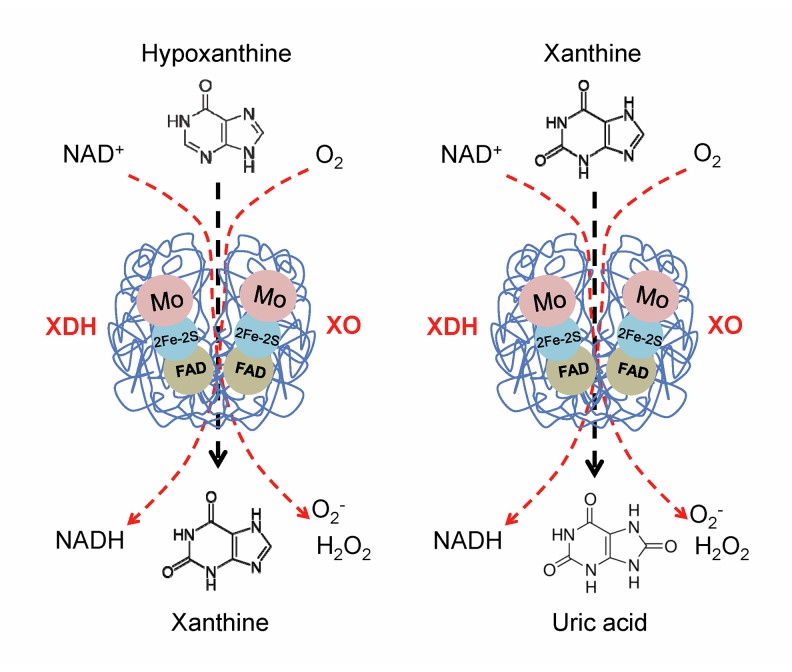
XOR is a critical, rate-limiting enzyme in purine metabolism. It catalyzes the last 2 steps of purine catabolism, the oxidation of hypoxanthine to xanthine and the oxidation of xanthine to uric acid, by utilizing either NAD+ or O2 [11]. As a result of these reactions, 2 reactive oxygen species (ROS), superoxide anion (O2−) and hydrogen peroxide (H2O2), are produced. XOR is therefore a critical source of uric acid and ROS. XOR has 2 forms: xanthine dehydrogenase (XDH) and xanthine oxidase (XO). XDH prefers NAD+ as the substrate and XO prefers O2 [11]. Most XOR in the liver exists in its XDH form, but it can be converted to XO form by reversible sulfhydryl oxidation or by irreversible proteolytic modification [11,12]. XOR is also present in the intestines, mammary gland, cardiac and skeletal muscle, corneal epithelium, and endothelial cells of vascular vessels [13–15]. XOR is a large homodimer enzyme with a molecular mass of 290 kDa [16]. Each subunit (145 kDa) contains 1 molybdenum (Mo) cofactor, 2 ferredoxin iron-sulfur clusters (2Fe-2S), and 1 adenine dinucleotide (FAD) cofactor (Figure 2). During the oxidative hydroxylation of substrates, the Mo center receives 2-electron reduction from Mo (VI) to Mo (IV), and passes electrons via [2Fe–2S] clusters to the FAD cofactor [11,17].
Figure 2.
Structure of xanthine oxidoreductase (XOR). XOR is a large homodimer enzyme; each subunit contains 1 molybdenum (Mo) cofactor, 2 ferredoxin iron-sulfur clusters (2Fe-2S), and 1 adenine dinucleotide (FAD) cofactor. XOR controls the sequential oxidative hydroxylation of hypoxanthine to xanthine then to uric acid, and generates 2 reactive oxygen species (ROS), superoxide anion (O2−) and hydrogen peroxide (H2O2). XOR has 2 forms: xanthine dehydrogenase (XDH) and xanthine oxidase (XO). XDH prefers NAD+ as the substrate and XO prefers O2.
Hyperuricemia
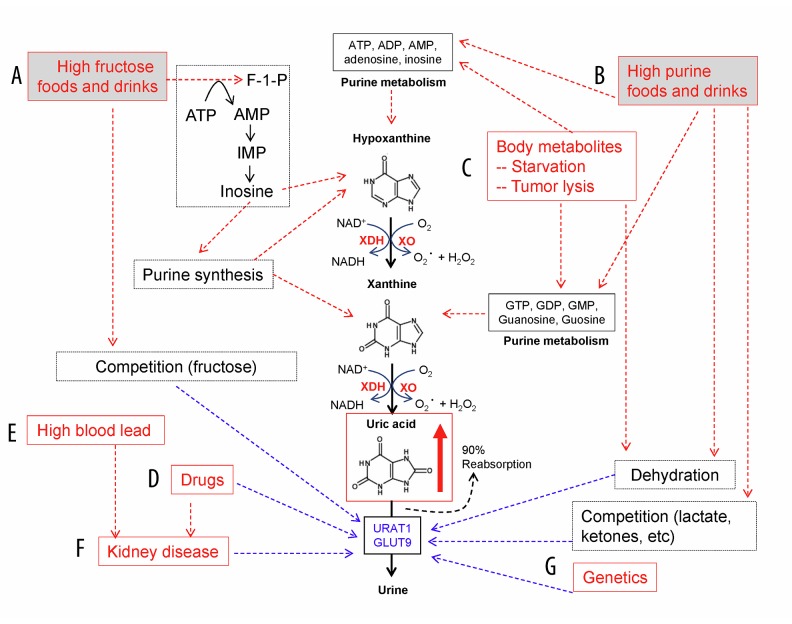
In humans, normal SUA levels are 2.6–5.7 mg/dL (155–339 μmol/L) for premenopausal women and 3.5–7.0 mg/dL (208–416 μmol/L) for men and postmenopausal women [9,10,18]. In most other mammals, such as rats and mice, normal blood levels of uric acid are 1–2 mg/dL because uric acid is further oxidized to allantoin by uricase [9]. The loss of uricase in humans and higher primates occurred about 15 million years ago [19], resulting in a relatively higher SUA level than that in lower animals. This evolutionary event may provide survival advantages for humans, such as increasing antioxidant capacity [20] and regulating sodium retention and blood pressure [21]. High SUA may be also related to alertness, intelligence, and longevity [22], as well as innate immune functions [23]. However, in the modern era such advantages of high SUA may turn into risk factors for many human diseases. Modern humans have dramatically increased the consumption of purine-rich foods, alcohol, and soft drinks sweetened with fructose, which can cause overproduction of uric acid and increase levels of SUA, termed hyperuricemia. On the other hand, hyperuricemia could also result from the under-excretion of uric acid from the kidney due to renal dysfunction and/or influence by certain metabolites and medications [24] (Figure 3). Currently, the estimated prevalence of hyperuricemia in the US general population is 21% [18], and it ranges from 13% to 25% in China [25]. A large number of studies have reported that hyperuricemia plays a pathophysiological role in many human diseases, including gout, cardiovascular disease, diabetes, and kidney disease.
Figure 3.
Major causes of hyperuricemia. (A) High dietary intake of high-fructose foods and drinks, which increase the production of inosine and purines. Fructose competes with uric acid for the secretion in the kidney. (B) High dietary intake of rich purine foods and drinks. More purines are metabolized to produce more uric acid. High intake of alcohol (ethanol) may increase lactic acid and ketones and cause dehydration, while decreasing uric acid removal in the kidney. (C) Change of body metabolisms. Starvation may enhance the body metabolism of its own (purine-rich) tissues for energy. Chemotherapy may cause tumor lysis, increasing purine degradation and uric acid production (tumor lysis syndrome). (D) Medications. Some drugs, such as anti-uricosurics and diuretics, may decrease the excretion of uric acid from the kidney. Drugs can also cause renal dysfunction, which decreases the excretion of uric acid from the kidney. (E) Elevated blood lead levels may cause renal dysfunction and decrease uric acid excretion. (F) Many different types of kidney diseases may affect uric acid secretion. (G) Genetic factors. The gene SLC2A9 encodes a protein that helps to transport uric acid in the kidney. Several single-nucleotide polymorphisms of SLC2A9 affect the secretion of uric acid in the kidney
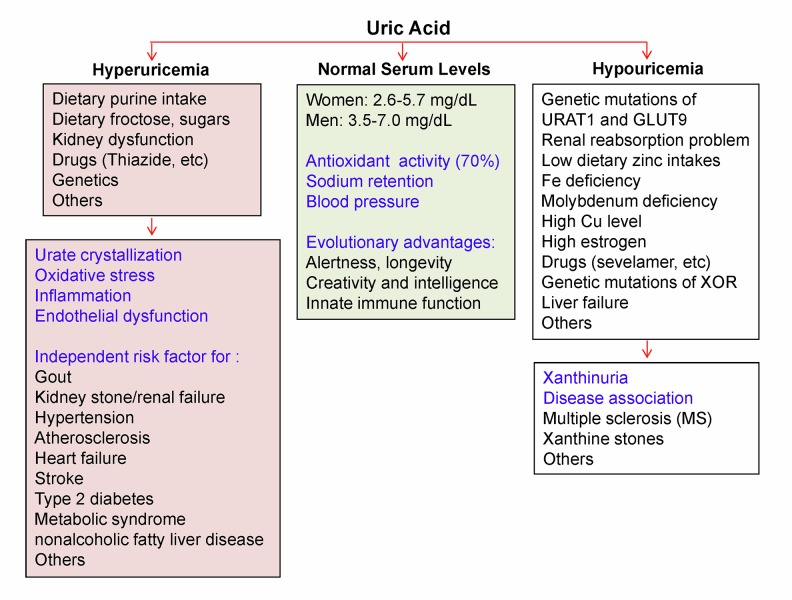
The homeostasis of SUA is mainly governed by hepatic production and renal removal. Acquired deficiency of XOR due to allopurinol therapy or liver disease can decrease uric acid production [26]. Several rare inherited disorders of XOR deficiency cause an abnormally low level of SUA and a high serum level of xanthine, termed hypouricemia and xanthinuria, respectively [27]. Hereditary genetic mutations of the urate transporter URAT1 (RHUC1) and glucose transporter 9 (GLUT9; RHUC2) have been reported to cause hypouricemia due to deficiency of reabsorption of kidney-filtered uric acid [28,29]. It may also be caused by uric acid oxidation due to treatment with uricase, or decreased renal tubular reabsorption due to acquired disorders [30] (Figure 4). The present review focuses on hyperuricemia and its related diseases.
Figure 4.
Human serum uric acid (SUA) homeostasis and abnormal changes. Normal SUA levels in humans are higher than in mice because humans lack a functional uricase gene. This evolutionary event may provide survival advantages for humans. However, in the modern era such advantages of high SUA may become risk factors for many human diseases. Several factors could cause overproduction of uric acid or under-excretion of uric acid from the kidney, leading to an abnormally high level of SUA, termed hyperuricemia. Hyperuricemia plays a pathophysiological role in many human diseases. On the other hand, several factors could cause an abnormally low level of SUA, termed hypouricemia, which may also be associated with many diseases.
Clinical Studies of Hyperuricemia-Related Diseases
Hyperuricemia has long been established as the major etiologic factor in gout. When SUA levels go beyond its saturation point, monosodium urate begins to crystallize and deposit into peripheral joints and surrounding tissues to cause gout or gouty arthritis. The worldwide prevalence of gout ranges from 0.1% to 10% [31]. Prevalence of gout in most countries in North America and Western Europe ranges from 1% to 4% [32]. The pooled prevalence of hyperuricemia and gout in mainland China from 2000 to 2014 was 13.3% and 1.1%, respectively [25]. The prevalence of hyperuricemia and gout in Taiwanese aborigines is 41.4% and 11.7%, respectively [33]; the direct causal mechanisms of such high prevalence in this ethnic group are unknown.
The association of hyperuricemia with cardiovascular disease was described 2 centuries ago [1]. A large member of studies reported that SUA levels correlate with cardiovascular diseases, including ischemic heart disease and heart failure, as well as hypertension and stroke. For a long time, however, the observed association between SUA elevations and cardiovascular disease was considered to be “epiphenomenal” and not causal; it remains controversial whether SUA has a causal relationship with these diseases [34]. In the last few years, several large clinical studies have confirmed that hyperuricemia is a significant and independent risk factor for hypertension and ischemic heart disease and heart failure, after an extensive adjustment for almost all of the possible confounding conditions [2,35,36]. The impact of uric acid on these diseases seems in a dose-dependent manner. For example, the overall risk of cardiovascular disease mortality increased 15% for each increase of 1 mg/dl of uric acid [4]. Hyperuricemia is also a causal factor for renal disease, metabolic syndrome, insulin resistance, type 2 diabetes, and nonalcoholic fatty liver disease, with a linear dose-response relationship [37,38]. High levels of uric acid may directly impair the cardiovascular system and/or through XOR-induced oxidative stress [4]. Urate-lowering drugs such as allopurinol play an important role in the prevention and treatment of cardiovascular diseases. Indeed, this hypothesis is strongly supported by recent clinical studies [4,39,40]. Thus, uric acid is a central player, not an innocent bystander, in cardiovascular disease and many other diseases [41].
Molecular Mechanisms of Hyperuricemia-Related Diseases
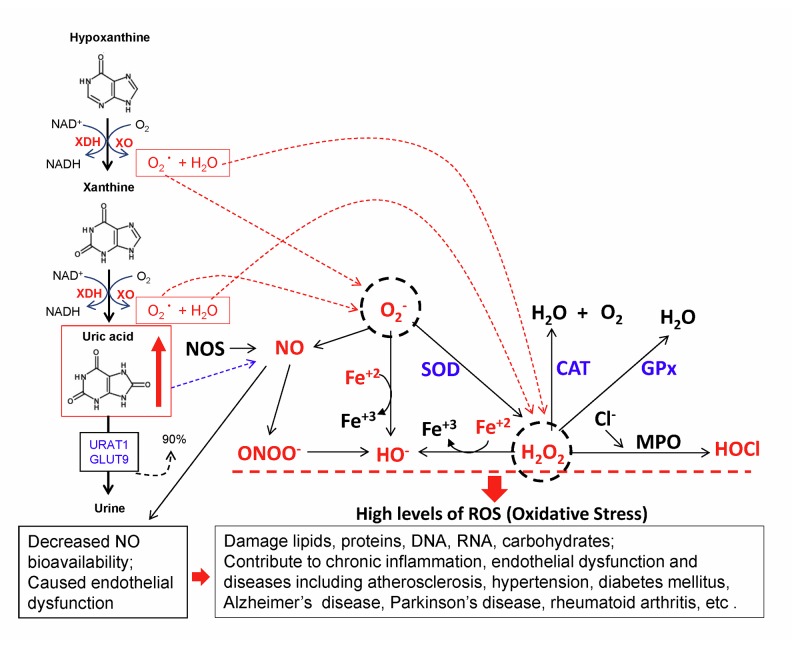
Gout is a kind of arthritis caused by a buildup of uric acid crystals in the joints as a result of hyperuricemia. Furthermore, it has been hypothesized that hyperuricemia contributes to the progression of cardiovascular disease through oxidative stress, systemic inflammation, and endothelial dysfunction [4]. As mentioned above, XOR produces O2− and H2O2 when it catalyzes the oxidation of hypoxanthine to xanthine to uric acid (Figure 5). O2− readily reacts with NO, reducing NO bioavailability, which is a main cause of endothelial dysfunction. In fact, the reaction between O2− and NO is 3 times faster than the O2− dismutation by superoxide dismutase (SOD) [41]. O2− and H2O2 not only directly cause oxidative damage to cells, but also can be converted to peroxynitrate (ONOO−), hydroxyl anion (OH−), and hypochorous acid (HOCl), which are more toxic to the cells, by damaging proteins, lipids, carbohydrates, DNA, RNA, subcellular organelles, and cell systems [42,43]. For example, ONOO− has a cytotoxic potential about 1000 times higher than that of H2O2 [44]. Increased XOR activity and oxidative stress have been observed in cardiovascular disease in humans [45] and experimental animals [46]. Interestingly, high levels of XOR activity has been identified in human endothelial cells from the microvasculature of several tissues, and XOR is detected in the cytoplasm of endothelial cells and on the outer surface of the plasma membrane [47]. Interestingly, circulating XOR can bind to the surface of endothelial cells by glycosaminoglycans (GAGs) and induce oxidative stress and endothelial dysfunction [48]. Indeed, hyperuricemia-related endothelial dysfunction has been observed in both rats [49] and humans, and inhibition of XOR has been shown to improve endothelial functions [50].
Figure 5.
Hyperuricemia and oxidative stress. Increased XOR activity enhances production of uric acid and reactive oxygen species, O2− and H2O2, which could be converted to more toxic ROS, peroxynitrate (ONOO−), hydroxyl anion (OH−), and hypochorous acid (HOCl), and damage proteins, lipids, carbohydrates, DNA, RNA, subcellular organelles and cell systems. O2− readily reacts with NO, reducing NO bioavailability, which is a main cause of endothelial dysfunction. Thus, hyperuricemia-induced oxidative stress, chronic inflammation, and endothelial dysfunction can contribute to the development and progression of many human diseases.
The Oxidant-Antioxidant Paradox Mechanisms of Uric Acid
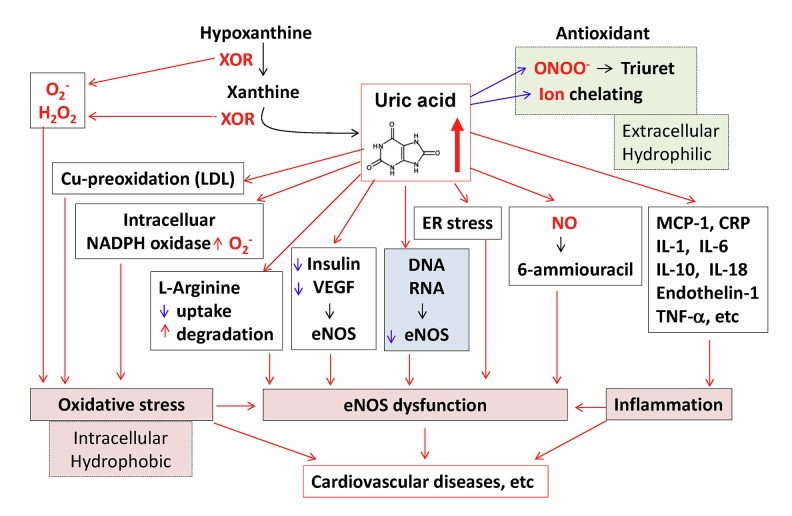
Uric acid exerts opposite effects on free radicals extracellularly or intracellularly. Circulating uric acid is believed to be a major aqueous antioxidant in humans, and it scavenges carbon centered radicals and peroxyl radicals such as peroxynitrite (ONOO−) in the hydrophilic environment and contributes to about 70% of all free radical scavenging activities in the plasma [51]. For example, uric acid protects erythrocyte membrane against lipid peroxidation and lysis induced by t-butyl hydroperoxide [52]. Uric acid can react with ONOO− to form uric acid nitration/nitrosation derivatives that can release NO and induce vasorelaxation [53]. Uric acid can also act as a chelator of iron in extracellular fluids [54]. However, uric acid loses its radical scavenging activity and becomes a strong pro-oxidant under hydrophobic conditions [55]. For example, uric acid can accelerate the copper-induced peroxidation of human LDL in the presence of pre-formed lipid hydroperoxides [56]. In addition, when uric acid enters endothelial cells, vascular smooth muscle cells, monocytes, and other types of cells via specific organic anion transporters such as URAT1 [57], it induces intracellular and mitochondrial oxidative stress through multiple mechanisms such as the stimulation of NADPH oxidase [58], and the production of pro-inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1), high-sensitivity C reactive protein, interleukin-1, interleukin-6, interleukin-10, interleukin-18, endothelin-1, and tumor necrosis factor-alpha [59]. Furthermore, uric acid blocks insulin- and vascular endothelial growth factor (VEGF)-mediated endothelial nitric oxide synthase (eNOS) activation and nitric oxide (NO) release [49,60]; induces cellular ER stress and eNOS dysfunction [61]; directly reacts with NO to form 6-aminouracil [62]; blocks the uptake of the substrate L-arginine [63]; and stimulates the degradation of L-arginine [64], thereby reducing NO bioavailability (Figure 6). In liver cells, uric acid blocks AMP-activated protein kinase and stimulates gluconeogenesis [65]. In adipocytes, uric acid induces oxidative stress and decreases adiponectin synthesis [66]. Uric acid induces oxidative stress and inhibits growth of pancreatic β-cells [67]. Uric acid stimulates vascular smooth muscle cell proliferation and induces inflammatory changes in the kidney [68]. In renal proximal tubule cells, uric acid inhibits proliferation through the PKC signaling pathway [69]. Overall, hyperuricemia contributes to the progression of cardiovascular disease and many other diseases through the oxidant property of uric acid.
Figure 6.
The oxidant-antioxidant paradox mechanisms of uric acid. Circulating uric acid is a major aqueous antioxidant in humans. It scavenges carbon centered radicals and peroxyl radicals such as peroxynitrite (ONOO−) in the hydrophilic environment. Uric acid can also act as a chelator of iron in extracellular fluids. However, uric acid becomes a strong pro-oxidant under hydrophobic conditions. Uric acid can accelerate the copper-induced peroxidation of human LDL, induces intracellular and mitochondrial oxidative stress, and stimulates expression of inflammation cytokines. Uric acid inhibits eNOS activation and NO release, induces cellular ER stress, and directly reacts with NO to form 6-aminouracil. Uric acid can block the uptake of the substrate L-arginine and stimulate the degradation of L-arginine, thereby reducing NO bioavailability. Thus, hyperuricemia-induced oxidative stress, eNOS dysfunction, and inflammation may contribute to cardiovascular diseases.
Clinical XOR-Inhibitor Drugs
Since XOR is a critical enzyme to produce uric acid and ROS, it becomes an effective target of drugs for the treatment of gout and hyperuricemia-related diseases, including cardiovascular disease, hypertension, kidney disease, and many other diseases. The first XOR-inhibitor drug was allopurinol [4-hydroxypyrazolo (3,4-d) pyrimidine], which was discovered by American scientists, Nobel laureates Gertrude Elion and George Hitchings, in 1940s [70]. It was approved by the US Food and Drug Administration (FDA) in 1966 for treating recurrent acute gouty arthritis, tophi, urate nephropathy, uric acid kidney stones, and chemotherapy-induced hyperuricemia [10,70]. Allopurinol, a hypoxanthine analog, is hydrolyzed by XOR to produce oxypurinol, which binds tightly to the reduced molybdenum ion, Mo (IV), in the enzyme, and thus inhibits uric acid synthesis [71]. Oxypurinol has long persistence in tissues and is responsible for much of the pharmacological activity of allopurinol. Besides gout, allopurinol treatment significantly reduces the risk of myocardial infarction, reduces all-cause and cardiovascular mortality in high-risk patients [39,72], and improves endothelial functions [49]. These encouraging clinical data have led to the increased use of allopurinol for these diseases [73]. However, due to its adverse effects, allopurinol is currently not indicated for broad use in asymptomatic hyperuricemia and its related cardiovascular disease, or in diseases other than gout [73]. Asymptomatic hyperuricemia is a term traditionally applied to the condition in which the SUA level is elevated without symptoms or signs of uric acid crystal deposition disease, such as gout or uric acid renal disease. Asymptomatic hyperuricemia is a risk factor of cardiovascular disease and the other diseases mentioned above.
It is estimated that allopurinol is used by more than 1.2 million patients in the USA and UK [73]. Allopurinol is generally well tolerated, but 2–5% of treated patients experience adverse effects [74,75]. Importantly, it is one of the most common drugs associated with life-threatening hypersensitivity reactions, including bone marrow depression, hepatotoxicity, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis, and drug rash with eosinophilia and systemic manifestations [76–78]. These severe reactions with allopurinol occur in 0.1 to 0.4% patients, with a high mortality (27–32%) and a high morbidity, including renal failure and eye sequelae [77,79]. The hypersensitivity reaction may occur even after months or years of medication. The recently increased clinical use of allopurinol for cardiovascular and chronic kidney diseases showed a proportional increase in its induced-hypersensitivity reactions [78]. Other adverse effects of allopurinol include peripheral neuritis, interstitial nephritis, renal toxicity due to impairment of pyrimidine metabolism, and congenital malformations when used during pregnancy [76,79,80]. Although the mechanism of allopurinol toxicity is not fully understood, it is hypothesized that the accumulation of its metabolite, oxypurinol, along with immunologic and genetic factors may modify the cellular proteins and trigger an autoimmune response against skin or liver cells [81].
Febuxostat [2-(3-cyano-4-isobutoxy-phenyl)-4-methyl-1,3-thiazole-5 carboxylic acid] is a non-purine XOR-inhibitor drug approved by the European Medicines Agency in 2008 and US FDA in 2009 for use in patients intolerant to allopurinol [81]. It was discovered by scientists at the Japanese pharmaceutical company Teijin in 1998. Febuxostat is structurally distinct from allopurinol and is able to inhibit both the oxidized Mo (VI) and reduced Mo (IV) forms of XOR, thus resulting in a more effective blockade of uric acid and ROS production [82]. Clinically, febuxostat provides greater hypouricemic activity and less toxicity than allopurinol [83,84]. However, initial clinical studies showed that febuxostat can also lead to cutaneous adverse effects in about 2% of patients [84–86]. Cases of severe febuxostat hypersensitivity reactions such as SJS and anaphylactic shock are reported [85,87]. These serious adverse effects with febuxostat are potentially associated with a history of skin reaction to allopurinol, particularly in patients with renal failure [85,88,89]. Moreover, febuxostat was associated with a higher incidence of hepatotoxicity in clinical patients [90]. A case report also showed that febuxostat induced rhabdomyolysis [91]. Thus, febuxostat is currently not recommended for the treatment of asymptomatic hyperuricemia [10].
Topiroxostat [4-[5-(4-Pyridinyl)-1H-1,2,4-triazol-3-yl]-2-pyridinecarbonitrile] is another XOR-inhibitor drug, approved in Japan in 2013 for the treatment of patients with hyperuricemia, including gout [92]. Topiroxostat (formerly known as FYX-051) was discovered by Fujiyahujin Co. in Japan, and it is a non-purine, hybrid-type XOR-inhibitor, which not only forms a covalent linkage to molybdenum via oxygen in the hydroxylation reaction intermediate, but also interacts with amino acid residues of the solvent channel [93]. Topiroxostat has high bioavailability and safety in animals. However, the information on its adverse effects in humans is not available due to its short duration of clinical use in Japan.
Development of New XOR Inhibitors
Besides the 3 approved XOR-inhibitor drugs (allopurinol, febuxostat, and topiroxostat), there is a continuous and intensive effort to develop new XOR-inhibitor drugs because of the following reasons: current drugs (allopurinol and febuxostat) are associated with certain adverse effects and are not indicated for broad use in patients with asymptomatic hyperuricemia; hyperuricemia has been demonstrated to be an independent risk factor for cardiovascular disease, renal diseases, and many other diseases; and long-term control of asymptomatic hyperuricemia may be an effective strategy to prevent/treat these hyperuricemia-related diseases. Thus, there is an urgent need to develop new XOR inhibitors with no or milder adverse effects. In recent years, several synthesized purine analogs were reported to have XOR inhibitory effects: 9-benzoyl 9-deazaguanines [94], N-(1,3-diaryl-3-oxopropyl)amides [95] and 5,6-dihydropyrazolo/pyrazolo[1,5-c]quinazoline derivatives [96] and naphthopyrans [97]; non-purine analogs such as thiadiazolopyrimidin-5-ones [98], aryl-2H-pyrazole derivatives [99], 2-amino-5-alkylidene-thiazol-4-ones [100], and others [101,102]; and natural or natural derivatives such as riparsaponin [103], genistein [104], morin [105], curcumin analogs [106], and others [107–109] (Table 1). Recently, we have focused on the natural derivatives for the development of novel XOR inhibitors [109], which could be possible alternatives for allopurinol and febuxostat, or at least in combination therapy to minimize the adverse effects of current drugs, in particular in long-term applications for symptomatic and asymptomatic hyperuricemia-related diseases. We found that 3,4-dihydroxy-5-nitrobenzaldehyde (DHNB), a derivative of natural protocatechuic aldehyde, is a strong XOR-inhibitor in a cell-free system and in a mouse model. DHNB displays potent mixed-type inhibition of XOR and shows an additive effect with allopurinol at low concentrations. In addition, DHNB, but not allopurinol, directly scavenged ROS, including ONOO− and HOCl. DHNB has a different chemical structure from the current clinical XOR-inhibitor drugs (Figure 7), and showed much less toxicity in the mouse model as compared with allopurinol. In a mouse model, a large dose (500 mg/kg) of allopurinol caused high mortality and fur loss of survivors and their offspring; while DHNB did not show any adverse effects at this dose [109]. In fact, natural protocatechuic aldehyde (3,4-Dihydroxybenzyl aldehyde, DHB-CHO) only showed a weak inhibitory effect on XOR activity [109]. DHB-CHO is a phenolic aldehyde, which is found in cork [110] and the mushroom Phellinus linteus [111]. Phellinus linteus is a Chinese traditional medicine and has been widely used in China, Japan, and Korea for centuries to treat a broad range of diseases, including gout. Phellinus linteus extract showed an XOR inhibitory effect in vitro [112]. DHB-CHO can be used as a precursor in the vanillin synthesis [113]. As a derivative of DHC-CHO, DHNB showed a much stronger XOR inhibitory effect than DHC-CHO in vitro, and has much less toxicity than allopurinol in mice. Thus, DHNB is considered as a prime candidate for use as an XOR-inhibitor drug. Further preclinical and clinical studies of DHNB are warranted.
Table 1.
Recent development of new XOR inhibitors reported in the literature.
| Compound | Mechanisms | References |
|---|---|---|
| 9-Benzoyl 9-deazaguanines | Purine analogs | Rodrigues MV et al., 2016 [94] |
| N-(1,3-Diaryl-3-oxopropyl)amides | Purine analogs | Nepali K et al., 2011 [95] |
| 5,6-Dihydropyrazolo/pyrazolo[1,5-c]quinazoline derivatives | Purine analogs | Kumar D et al., 2014 [96] |
| Naphthopyrans | Non-purine analogs | Sharma S et al., 2014 [97] |
| Thiadiazolopyrimidin-5-ones | Non-purine analogs | Sathisha KR et al., 2016 [98] |
| Aryl-2H-pyrazole derivatives | Non-purine analogs | Sun ZG et al., 2015 [99] |
| 2-Amino-5-alkylidene-thiazol-4-ones | Non-purine analogs | Smelcerovic Z et al., 2015 [100] |
| 2-(Indol-5-yl)thiazoles | Non-purine analogs | Song JU et al., 2015 [101] |
| 1-Hydroxy/methoxy-4-methyl-2-phenyl-1H-imidazole-5-carboxylic acid derivatives | Non-purine analogs | Chen S et al., 2015 [102] |
| Riparsaponin | Natural substance | Xu F et al., 2014 [103] |
| Genistein (4′,5,7-Trihydroxyisoflavone) | Natural substance | Lin S et al., 2015 [104] |
| Morin | Natural substance | Zhang J et al., 2016 [105] |
| Curcumin analogs | Natural derivatives | Shen L et al., 2009 [106] |
| Oxidation product of caffeic acid | Natural derivatives | Masuda T et al., 2014 [107] |
| Aloe-emodin derivatives | Natural derivatives | Shi DH et al., 2014 [108] |
| DHNB (3,4-Dihydroxy-5-nitrobenzaldehyde) | Natural derivatives | Lü JM et al., 2013 [109] |
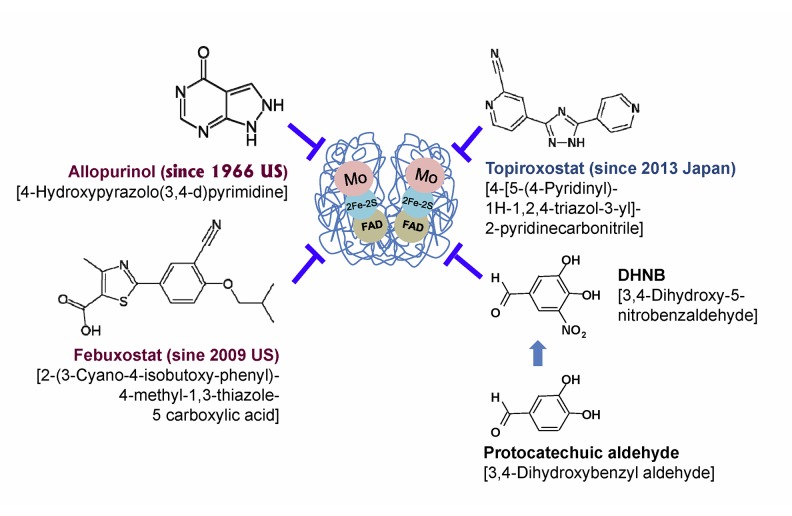
Figure 7.
Chemical structure of XOR-inhibitor drugs and DHNB. Allopurinol [4-hydroxypyrazolo(3,4-d) pyrimidine] is a synthetic hypoxanthine analog. It is hydrolyzed by XOR to produce oxypurinol, which binds tightly to the reduced molybdenum ion, Mo (IV), in the enzyme and thus inhibits uric acid synthesis. Febuxostat [2-(3-cyano-4-isobutoxy-phenyl)-4-methyl-1,3-thiazole-5 carboxylic acid] and topiroxostat [4-[5-(4-pyridinyl)-1H-1,2,4-triazol-3-yl]-2-pyridinecarbonitrile] are synthetic non-purine analogs. DHNB [3,4-Dihydroxy-5-nitrobenzaldehyde] is a derivative of natural protocatechuic aldehyde (3,4-Dihydroxybenzyl aldehyde, DHB-CHO).
Self-Nanoemulsifying Drug Delivery Systems (SNEDDS)
In order to develop new and effective XOR-inhibitor drugs, the oral delivery system is a critical aspect of this effort. Many approved drugs and candidate drugs exhibit low solubility in water, which leads to limited oral bioavailability [114]. Various formulations have been developed to improve the bioavailability and dissolution rate of poorly water-soluble drugs. Among them, self-nanoemulsifying drug delivery systems (SNEDDS) are the most promising technologies currently used for this purpose. SNEDDS are isotropic mixtures of drug, surfactant, and co-surfactant that can rapidly form fine oil-in-water emulsions, which form nano-sized droplets (50–200 nm) in an aqueous media with mild agitation [114,115]. The physicochemical properties, drug solubilization capacity, and physiological fate are dependent on the selection of the SNEDDS components. SNEDDS may offer numerous advantages, including spontaneous nanoparticle formation, ease of manufacture, thermodynamic stability, and improved solubilization of candidate drugs. These lipophilic drug-containing nano-droplets with small size and larger surface area may result in a higher loading capability and improved bioavailability of the drugs. Interestingly, SNEDDS may have unique biopharmaceutical mechanisms such as reduced intra-enterocyte metabolism of the drug by CYP P450 enzymes, reduced P-glycoprotein (P-gp) efflux activity, and hepatic first-pass metabolism bypass via lymphatic absorption. Greater bioavailability means that less drug need be used for the therapy; therefore, SNEDDS formulation may lower costs of drugs and reduce the stomach irritation and toxicity of oral drugs. Recently, SNEDDS have been used to deliver a natural substance called morin, a XOR-inhibitor [105]. Oral delivery of morin by SNEDDS significantly enhanced its urate-lowering effect in a hyperuricemic rat model. Also, SNEDDS enhanced morin concentrations in the liver and kidneys, and inhibited activity of hepatic XOR. Thus, SNEDDS has great potential to contribute to the development of new XOR-inhibitor drugs. It could also be used for improving the therapeutic efficacy of clinical XOR-inhibitor drugs (allopurinol, febuxostat, and topiroxostat). Currently, we are studying the application of SNEDDS technology to DHNB to improve its efficacy in the hyperuricemia mouse models.
Conclusions
Uric acid is the final oxidation product of purine metabolism in humans. Xanthine oxidoreductase (XOR) is a critical enzyme, catalyzing the oxidation of hypoxanthine to xanthine to uric acid with ROS production. Hyperuricemia is caused by overproduction or under-excretion of uric acid and is the underlying cause of gout. In recent years, the prevalence of hyperuricemia has increased worldwide and more clinical studies have confirmed that hyperuricemia is a significant and independent risk factor for cardiovascular disease, hypertension, diabetes, and many other diseases. Pathophysiological roles of hyperuricemia may be involved in oxidative stress, endothelial dysfunction, and systemic inflammation. Accordingly, urate-lowering therapy may play an important role in the management of hyperuricemia-related cardiovascular disease and other diseases. Currently available XOR-inhibitor drugs, including allopurinol and febuxostat, may have significant adverse effects such as hypersensitivity drug reactions and they are currently not indicated for broad use in many hyperuricemia-related diseases. Thus, there is an urgent need for the development of new XOR inhibitors with no or much milder adverse effects. New XOR inhibitors may have different chemical structures with current clinical XOR-inhibitor drugs, and could be used in the combination therapy at a small dose to reduce toxicity and potential drug resistance. New formulations for improving the bioavailability of drugs are also a critical aspect of the development of new XOR inhibitors. Self-nanoemulsifying drug delivery systems (SNEDDS) are the most promising technologies currently used for this purpose.
Footnotes
Source of support: This work was supported by a research grant from the National Institutes of Health (R21 AR-063017 to C. Chen) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas, U.S.A.
References
- 1.Galassi FM, Borghi C. A brief history of uric acid: from gout to cardiovascular risk factor. Eur J Intern Med. 2015;26:373. doi: 10.1016/j.ejim.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Hu X, Fan Y, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. 2016;6:19520. doi: 10.1038/srep19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: A systematic review and meta-analysis. Clin Chem Lab Med. 2016;54(1):7–15. doi: 10.1515/cclm-2015-0523. [DOI] [PubMed] [Google Scholar]
- 4.Borghi C, Desideri G. Urate-lowering drugs and prevention of cardiovascular disease: The emerging role of xanthine oxidase inhibition. Hypertension. 2016;67(3):496–98. doi: 10.1161/HYPERTENSIONAHA.115.06531. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan M, Kumar R. A comprehensive review on bioactive fused heterocycles as purine-utilizing enzymes inhibitors. Med Chem Res. 2015;24:2259–82. [Google Scholar]
- 6.Scheele C. Examen chemicum calculi urinari. Opuscula. 1776;2:73. [Google Scholar]
- 7.Kippen I, Klinenberg JR, Weinberger A, Wilcox WR. Factors affecting urate solubility in vitro. Ann Rheum Dis. 1974;33:313–17. doi: 10.1136/ard.33.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipkowitz MS. Regulation of uric acid excretion by the kidney. Curr Rheumatol Rep. 2012;14(2):179–88. doi: 10.1007/s11926-012-0240-z. [DOI] [PubMed] [Google Scholar]
- 9.Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18(9):1295–306. [PubMed] [Google Scholar]
- 10.Khanna D, FitzGerald JD, Khanna PP, et al. American College of Rheumatology Guidelines for Management of Gout Part I: Systematic non-pharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64(10):1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 1995;9:995–1003. [PubMed] [Google Scholar]
- 12.Hille R, Nishino T, Bittner F. Molybdenum enzymes in higher organisms. Coord Chem Rev. 2011;255:1179–205. doi: 10.1016/j.ccr.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellsten-Westing Y. Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry. 1993;100:215–22. doi: 10.1007/BF00269094. [DOI] [PubMed] [Google Scholar]
- 14.Cejková J, Ardan T, Filipec M, Midelfart A. Xanthine oxidoreductase and xanthine oxidase in human cornea. Histol Histopathol. 2002;17(3):755–60. doi: 10.14670/HH-17.755. [DOI] [PubMed] [Google Scholar]
- 15.Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Investig. 1999;79:967–74. [PubMed] [Google Scholar]
- 16.Enroth C, Eger BT, Okamoto K, et al. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc Natl Acad Sci USA. 2000;97:10723–28. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim Biophys Acta. 2014;1842(9):1502–17. doi: 10.1016/j.bbadis.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 19.Kratzer JT, Lanaspa MA, Murphy MN, et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci USA. 2014;111(10):3763–68. doi: 10.1073/pnas.1320393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proctor P. Similar functions of uric acid and ascorbate in man? Nature. 1970;228:868. doi: 10.1038/228868a0. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe S, Kang Dh, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of saltsensitivity. Hypertension. 2002;40:355–60. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 22.Inouye E, Park KS, Asaka A. Blood uric acid level and IQ: A study in twin families. Acta Genet Med Gemellol (Roma) 1984;33:237–42. doi: 10.1017/s0001566000007273. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Evans JE, Rock K. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 24.Grassi D, Ferri L, Desideri G, et al. Chronic hyperuricemia, uric acid deposit and cardiovascular risk. Curr Pharm Des. 2013;19:2432–38. doi: 10.2174/1381612811319130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: A systematic review and meta-analysis. Biomed Res Int. 2015;2015:762820. doi: 10.1155/2015/762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelis MF, Warms PC, Fusco RD, Davis BB. Hypouricemia and hyperuricosuria in Laennec cirrhosis. Arch Intern Med. 1974;134:681–83. [PubMed] [Google Scholar]
- 27.Levartovsky D, Lagziel A, Sperling O, et al. XDH gene mutation is the underlying cause of classical xanthinuria: A second report. Kidney Int. 2000;57:2215–20. doi: 10.1046/j.1523-1755.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 28.Windpessl M, Ritelli M, Wallner M, Colombi M. A novel homozygous SLC2A9 mutation associated with renal-induced hypouricemia. Am J Nephrol. 2016;43(4):245–50. doi: 10.1159/000445845. [DOI] [PubMed] [Google Scholar]
- 29.Kim HO, Ihm CG, Jeong KH, et al. A case report of familial renal hypouricemia confirmed by genotyping of SLC22A12, and a literature review. Electrolyte Blood Press. 2015;13(2):52–57. doi: 10.5049/EBP.2015.13.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundy JS, Becker MA, Baraf HS, et al. Pegloticase Phase 2 Study Investigators. Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum. 2008;58:2882. doi: 10.1002/art.23810. [DOI] [PubMed] [Google Scholar]
- 31.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: Prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 32.Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol. 2007;3:443–49. doi: 10.1038/ncprheum0556. [DOI] [PubMed] [Google Scholar]
- 33.Chou CT, Lai JS. The epidemiology of hyperuricaemia and gout in Taiwan aborigines. Br J Rheumatol. 1998;37(3):258–62. doi: 10.1093/rheumatology/37.3.258. [DOI] [PubMed] [Google Scholar]
- 34.Katsiki N, Karagiannis A, Athyros VG, Mikhailidis DP. Hyperuricaemia: More than just a cause of gout? J Cardiovasc Med. 2013;14:397–402. doi: 10.2459/JCM.0b013e3283595adc. [DOI] [PubMed] [Google Scholar]
- 35.Storhaug HM, Norvik JV, Toft I, et al. Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: A gender specific analysis from The Tromsø Study. BMC Cardiovasc Disord. 2013;13:115. doi: 10.1186/1471-2261-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prev Cardiol. 2015;22(4):513–18. doi: 10.1177/2047487313519346. [DOI] [PubMed] [Google Scholar]
- 37.Yuan H, Yu C, Li X, et al. Serum uric acid levels and risk of metabolic syndrome: A dose-response meta-snalysis of prospective studies. J Clin Endocrinol Metab. 2015;100(11):4198–207. doi: 10.1210/jc.2015-2527. [DOI] [PubMed] [Google Scholar]
- 38.Shih MH, Lazo M, Liu SH, et al. Association between serum uric acid and nonalcoholic fatty liver disease in the US population. J Formos Med Assoc. 2015;114(4):314–20. doi: 10.1016/j.jfma.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubreuil M, Zhu Y, Zhang Y, et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis. 2015;74:1368–72. doi: 10.1136/annrheumdis-2014-205269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacIsaac RL, Salatski J, Higgins P, et al. Allopurinoil and cardiovascular outcomes in adultes with hypertension. Hypertension. 2016;67:535–40. doi: 10.1161/HYPERTENSIONAHA.115.06344. [DOI] [PubMed] [Google Scholar]
- 41.Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur J Intern Med. 2016;29:3–8. doi: 10.1016/j.ejim.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen AY, Lü JM, Yao Q, Chen C. Entacapone is an antioxidant more potent than vitamin C and vitamin E for scavenging of hypochlorous acid and peroxynitrite, and the inhibition of oxidative stress-induced cell death. Med Sci Monit. 2016;22:687–96. doi: 10.12659/MSM.896462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panasenko OM, Evgina SA, Driomina ES, et al. Hypochlorite induces lipoproteins and lipid peroxidation in blood phospholipid liposomes. Free Radic Biol Med. 1995;19:133–40. doi: 10.1016/0891-5849(94)00211-2. [DOI] [PubMed] [Google Scholar]
- 44.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288(2):481–87. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 45.Ogino K, Kato M, Furuse Y, et al. Uric acid-lowering treatment with benzbromarone in patients with heart failure: A double-blind placebo-controlled crossover preliminary study. Circ Heart Fail. 2010;3(1):73–81. doi: 10.1161/CIRCHEARTFAILURE.109.868604. [DOI] [PubMed] [Google Scholar]
- 46.Minhas KM, Saraiva RM, Schuleri KH, et al. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98(2):271–79. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 47.Pritsos CA. Cellular distribution, metabolism and regulation of the xanthine oxidoreductase enzyme system. Chem Biol Interact. 2000;129(1–2):195–208. doi: 10.1016/s0009-2797(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 48.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glycosaminoglycans: Kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys. 1997;339:125–35. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 49.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–42. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 50.George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114(23):2508–16. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 51.Warning WS. Uric acid: An important antioxidant in acute ischaemic stroke. QJM. 2002;95:691–93. doi: 10.1093/qjmed/95.10.691. [DOI] [PubMed] [Google Scholar]
- 52.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skinner KA, White CR, Patel R, et al. Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem. 1998;273(38):24491–97. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- 54.Ghio AJ, Kennedy TP, Stonehuerner J, et al. Iron regulates xanthine oxidase activity in the lung. Am J Physiol. 2002;283:L563–72. doi: 10.1152/ajplung.00413.2000. [DOI] [PubMed] [Google Scholar]
- 55.Muraoka S, Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–89. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 56.Bagnati M, Perugini C, Cau C, et al. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: A study using uric acid. Biochem J. 1999;340:143–52. [PMC free article] [PubMed] [Google Scholar]
- 57.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417( 6887):447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 58.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34:1951–56. doi: 10.1161/01.STR.0000081983.34771.D2. [DOI] [PubMed] [Google Scholar]
- 59.Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart J. 2006;27(10):1174–81. doi: 10.1093/eurheartj/ehi879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YJ, Yoon Y, Lee KY, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28(7):3197–204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 61.Li P, Zhang L, Zhang M, et al. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: A mechanism for uric acid-induced endothelial dysfunction. Int J Mol Med. 2016;37(4):989–97. doi: 10.3892/ijmm.2016.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skinner KA, White CR, Patel R, et al. Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem. 1998;273:24491–97. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- 63.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu RevCell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 64.Martyn KD, Frederick LM, von Loehneysen K, et al. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Cicerchi C, Li N, Kratzer J, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: Evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28(8):3339–50. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293(2):C584–96. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Yamamoto T, Hisatome I, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375:89–96. doi: 10.1016/j.mce.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 68.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266(13):8604–8. [PubMed] [Google Scholar]
- 69.Han HJ, Lim MJ, Lee YJ, et al. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007;292:F373–81. doi: 10.1152/ajprenal.00104.2006. [DOI] [PubMed] [Google Scholar]
- 70.Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamoto K, Eger BT, Nishino T, et al. Mechanism of inhibition of xanthine oxidoreductase by allopurinol: crystal structure of reduced bovine milk xanthine oxidoreductase bound with oxipurinol. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):888–93. doi: 10.1080/15257770802146577. [DOI] [PubMed] [Google Scholar]
- 72.Grimaldi-Bensouda L, Alpérovitch A, Aubrun E, et al. PGRx MI Group: Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. 2014;103:51–57. doi: 10.1136/annrheumdis-2012-202972. [DOI] [PubMed] [Google Scholar]
- 73.Struthers A, Shearer F. Allopurinol: novel indications in cardiovascular disease. Heart. 2012;98(21):1543–45. doi: 10.1136/heartjnl-2012-302249. [DOI] [PubMed] [Google Scholar]
- 74.McInnes GT, Lawson DH, Jick H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann Rheum Dis. 1981;40:245–49. doi: 10.1136/ard.40.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aubock J, Fritsch P. Asymptomatic hyperuricaemia and allopurinol induced toxic epidermal necrolysis. Br Med J. 1985;290:1969–70. doi: 10.1136/bmj.290.6486.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin MS, Dai YS, Pwu RF, et al. Risk estimates for drugs suspected of being associated with Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Intern Med J. 2005;35(3):188–90. doi: 10.1111/j.1445-5994.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 77.Yaylacı S, Demir MV, Temiz T, et al. Allopurinol-induced DRESS syndrome. Indian J Pharmacol. 2012;44(3):412–14. doi: 10.4103/0253-7613.96351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs: The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 79.Ramasamy SN, Korb-Wells CS, Kannangara DR, et al. Allopurinol hypersensitivity: A systematic review of all published cases, 1950–2012. Drug Saf. 2013;36:953–80. doi: 10.1007/s40264-013-0084-0. [DOI] [PubMed] [Google Scholar]
- 80.Lee MH, Stocker SL, Anderson J, et al. Initiating allopurinol therapy: Do we need to know the patient’s human leucocyte antigen status? Intern Med J. 2012;42(4):411–16. doi: 10.1111/j.1445-5994.2011.02567.x. [DOI] [PubMed] [Google Scholar]
- 81.Hassan S, Wetz R, Zouein E. Allopurinol causing drug rash with eosinophilia and sysyrmic symptoms syndrome: A challenging diagnosis. Int J Gen Med. 2011;4:789–92. doi: 10.2147/IJGM.S24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011;377(9760):165–77. doi: 10.1016/S0140-6736(10)60665-4. [DOI] [PubMed] [Google Scholar]
- 82.Love BL, Barrons R, Veverka A, Snider KM. Urate-lowering therapy for gout: Focus on febuxostat. Pharmacotherapy. 2010;30:594–608. doi: 10.1592/phco.30.6.594. [DOI] [PubMed] [Google Scholar]
- 83.Calogiuri G, Nettis E, Di Leo E, et al. Allopurinol hypersensitivity reactions: desensitization strategies and new therapeutic alternative molecules. Inflamm Allergy Drug Targets. 2013;12:19–28. doi: 10.2174/1871528111312010004. [DOI] [PubMed] [Google Scholar]
- 84.Chohan S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol. 2011;38:1957–59. doi: 10.3899/jrheum.110092. [DOI] [PubMed] [Google Scholar]
- 85.Abeles AM. Febuxostat hypersensitivity. J Rheumatol. 2012;39:659. doi: 10.3899/jrheum.111161. [DOI] [PubMed] [Google Scholar]
- 86.Schumacher HR, Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59(11):1540–48. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 87. [Accessed April 27, 2016]. www.accessdata.fda.gov.
- 88.Mauck M, Taintor A, Jha P. Cross-sensitivity of allopurinol and febuxostat-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. J Gen Intern Med. 2010;25:S504–5. [Google Scholar]
- 89.Bardin T, Chalès G, Pascart T, et al. Risk of cutaneous adverse events with febuxostat treatment in patients with skin reaction to allopurinol. A retrospective, hospital-based study of 101 patients with consecutive allopurinol and febuxostat treatment. Joint Bone Spine. 2016;83(3):314–17. doi: 10.1016/j.jbspin.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Bohm M, Vuppalanchi R, Chalasani N Drug-Induced Liver Injury Network (DILIN) Febuxostat-induced acute liver injury. Hepatology. 2016;63(3):1047–49. doi: 10.1002/hep.28403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang Y, Kim MJ, Jang HN, et al. Rhabdomyolysis associated with initiation of febuxostat therapy for hyperuricaemia in a patient with chronic kidney disease. J Clin Pharm Ther. 2014;39(3):328–30. doi: 10.1111/jcpt.12144. [DOI] [PubMed] [Google Scholar]
- 92.Hosoya T, Sasaki T, Hashimoto H, et al. Clinical efficacy and safety of topiroxostat in Japanese male hyperuricemic patients with or without gout: An exploratory, phase 2a, multicentre, randomized, double-blind, placebo-controlled study. J Clin Pharm Ther. 2016;41(3):298–305. doi: 10.1111/jcpt.12392. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto K, Okamoto K, Ashizawa N, Nishino T. FYX-051: A novel and potent hybrid-type inhibitor of xanthine oxidoreductase. J Pharmacol Exp Ther. 2011;336(1):95–103. doi: 10.1124/jpet.110.174540. [DOI] [PubMed] [Google Scholar]
- 94.Rodrigues MV, Barbosa AF, da Silva JF, et al. 9-Benzoyl 9-deazaguanines as potent xanthine oxidase inhibitors. Bioorg Med Chem. 2016;24(2):226–31. doi: 10.1016/j.bmc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Nepali K, Agarwal A, Sapra S, et al. N-(1,3-Diaryl-3-oxopropyl)amides as a new template for xanthine oxidase inhibitors. Bioorg Med Chem. 2011;19(18):5569–76. doi: 10.1016/j.bmc.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 96.Kumar D, Kaur G, Negi A, et al. Synthesis and xanthine oxidase inhibitory activity of 5,6-dihydropyrazolo/pyrazolo[1,5-c]quinazoline derivatives. Bioorg Chem. 2014;57:57–64. doi: 10.1016/j.bioorg.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Sharma S, Sharma K, Ojha R, et al. Microwave assisted synthesis of naphthopyrans catalysed by silica supported fluoroboric acid as a new class of non-purine xanthine oxidase inhibitors. Bioorg Med Chem Lett. 2014;24(2):495–500. doi: 10.1016/j.bmcl.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 98.Sathisha KR, Gopal S, Rangappa KS. Antihyperuricemic effects of thiadiazolopyrimidin-5-one analogues in oxonate treated rats. Eur J Pharmacol. 2016;776:99–105. doi: 10.1016/j.ejphar.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 99.Sun ZG, Zhou XJ, Zhu ML, et al. Synthesis and biological evaluation of novel aryl-2H-pyrazole derivatives as potent non-purine xanthine oxidase inhibitors. Chem Pharm Bull (Tokyo) 2015;63(8):603–7. doi: 10.1248/cpb.c15-00282. [DOI] [PubMed] [Google Scholar]
- 100.Smelcerovic Z, Veljkovic A, Kocic G, et al. Xanthine oxidase inhibitory properties and anti-inflammatory activity of 2-amino-5-alkylidene-thiazol-4-ones. Chem Biol Interact. 2015;229:73–81. doi: 10.1016/j.cbi.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Song JU, Choi SP, Kim TH, et al. Design and synthesis of novel 2-(indol-5-yl)thiazole derivatives as xanthine oxidase inhibitors. Bioorg Med Chem Lett. 2015;25(6):1254–58. doi: 10.1016/j.bmcl.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 102.Chen S, Zhang T, Wang J, et al. Synthesis and evaluation of 1-hydroxy/methoxy-4-methyl-2-phenyl-1H-imidazole-5-carboxylic acid derivatives as non-purine xanthine oxidase inhibitors. Eur J Med Chem. 2015;103:343–53. doi: 10.1016/j.ejmech.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 103.Xu F, Zhao X, Yang L, et al. A new cycloartane-type triterpenoid saponin xanthine oxidase inhibitor from Homonoia riparia Lour. Molecules. 2014;19(9):13422–31. doi: 10.3390/molecules190913422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin S, Zhang G, Pan J, Gong D. Deciphering the inhibitory mechanism of genistein on xanthine oxidase in vitro. J Photochem Photobiol B. 2015;153:463–72. doi: 10.1016/j.jphotobiol.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Shuai X, Li J, et al. Biodistribution, hypouricemic efficacy and therapeutic mechanism of morin phospholipid complex loaded self-nanoemulsifying drug delivery systems in an experimental hyperuricemic model in rats. J Pharm Pharmacol. 2016;68(1):14–25. doi: 10.1111/jphp.12492. [DOI] [PubMed] [Google Scholar]
- 106.Shen L, Ji HF. Insights into the inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett. 2009;19(21):5990–93. doi: 10.1016/j.bmcl.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 107.Masuda T, Shingai Y, Takahashi C, et al. Identification of a potent xanthine oxidase inhibitor from oxidation of caffeic acid. Free Radic Biol Med. 2014;69:300–7. doi: 10.1016/j.freeradbiomed.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 108.Shi DH, Huang W, Li C, et al. Design, synthesis and molecular modeling of aloe-emodin derivatives as potent xanthine oxidase inhibitors. Eur J Med Chem. 2014;75:289–96. doi: 10.1016/j.ejmech.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 109.Lü JM, Yao Q, Chen C. 3,4-Dihydroxy-5-nitrobenzaldehyde (DHNB) is a potent inhibitor of xanthine oxidase: A potential therapeutic agent for treatment of hyperuricemia and gout. Biochem Pharmacol. 2013;86(9):1328–37. doi: 10.1016/j.bcp.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Conde E, Cadahía E, García-Vallejo MC, de Simón BF. Polyphenolic composition of quercus suber cork from different spanish provenances. J Agric Food Chem. 1998;46:3166–71. [Google Scholar]
- 111.Lee YS, Kang YH, Jung JY, et al. Protein glycation inhibitors from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2008;31(10):1968–72. doi: 10.1248/bpb.31.1968. [DOI] [PubMed] [Google Scholar]
- 112.Song YS, Kim SH, Sa JH, et al. Antiangiogenic, angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J Ethnopharmacol. 2003;88:113–16. doi: 10.1016/s0378-8741(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 113.Rao SR, Ravishankar GA. Biotransformation of protocatechuic aldehyde and caffeic acid to vanillin and capsaicin in freely suspended and immobilized cell cultures of Capsicum frutescens. J Biotechnol. 2000;76(2–3):137–46. doi: 10.1016/s0168-1656(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 114.Cherniakov I, Domb AJ, Hoffman A. Self-nano-emulsifying drug delivery systems: An update of the biopharmaceutical aspects. Expert Opin Drug Deliv. 2015;12(7):1121–33. doi: 10.1517/17425247.2015.999038. [DOI] [PubMed] [Google Scholar]
- 115.Mazzaferro S, Bouchemal K, Ponchel G. Oral delivery of anticancer drugs III: Formulation using drug delivery systems. Drug Discov Today. 2013;18:99–104. doi: 10.1016/j.drudis.2012.08.007. [DOI] [PubMed] [Google Scholar]