Abstract
Correlations between angiotensin-converting enzyme (ACE) genotype (I/I, I/D, D/D), disease severity at baseline and response to enzyme replacement therapy (ERT) were assessed in the Pompe disease Late-Onset Treatment Study (LOTS). No correlations were observed between ACE genotype and disease severity at baseline. However, D/D patients appeared to have a reduced response to alglucosidase alfa treatment than I/I or I/D patients, suggesting that ACE polymorphisms may influence the response to alglucosidase alfa treatment and warrants further investigation.
Abbreviations: 6MWT, 6-Minute Walk Test; ACE, angiotensin-converting enzyme; D, deletion; ERT, enzyme replacement therapy; FVC, forced vital capacity; GAA, acid-alpha glucosidase; I, insertion; IOPD, infantile-onset Pompe disease; LOPD, late-onset Pompe disease; LOTS, Late-Onset Treatment Study; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; QMT, quantitative muscle testing
Keywords: Pompe disease, Angiotensin-converting enzyme, Gene polymorphism, Enzyme replacement therapy, Alglucosidase alfa
1. Introduction
Pompe disease is characterized by a deficiency of the glycogen-degrading lysosomal enzyme, acid alpha-glucosidase (GAA) [1], [2]. In classic infantile-onset Pompe disease (IOPD), glycogen deposition in cardiac, skeletal, and respiratory muscles causes severe cardiomyopathy, hypotonia, and respiratory failure, leading to death by 1 year of age if not treated [1]. In those with onset during childhood or adulthood (late-onset Pompe disease [LOPD]), glycogen deposition is mainly in skeletal and respiratory muscles, causing progressive limb-girdle myopathy and respiratory insufficiency [3], [4].
Enzyme replacement therapy (ERT) with recombinant human GAA (alglucosidase alfa) has been shown to improve walking distance and stabilize pulmonary function in LOPD patients [4], [5], and to improve survival and motor outcomes in classic IOPD patients [6]. However, individual responsiveness to ERT varies across the patient populations [4], [5], [6]. Studies have examined the effect of individual mutations in the GAA gene and other genetic factors [7], [8] with one gene of interest encoding angiotensin converting enzyme (ACE).
ACE catalyzes the conversion of angiotensin I to the active form, angiotensin II (a potent vasoconstrictor), and degrades bradykinin (a potent vasodilator). The net effect of ACE is vasoconstriction. Half of the variation in human ACE activity is attributable to an insertion/deletion (I/D) marker allele in intron 16 of the ACE gene [9], [10], [11], [12]. An insertion (I allele) of an Alu repeat results in lower ACE activity, whereas the absence of the insertion (or deletion, D allele) results in higher activity [10]. The ACE I/D polymorphism has been linked to physical performance and exercise duration, and it has been reported that the I allele is associated with a predominance of slow-twitch muscle fibers (Type I) and the D allele with fast-twitch muscle fibers (Type II) [11], [12]. Taken together, it has been hypothesized that differences in muscle-fiber type and vasodynamics may impact the skeletal muscle response to ERT.
The D/D genotype in 14 LOPD patients was found to be associated with higher serum creatine kinase levels at diagnosis, earlier onset of disease, and the presence of muscle pain [13]. In a larger cohort of Pompe patients (N = 85), individuals with the D/D ACE genotype presented with an earlier onset of disease and muscle pain, confirming the previous observation [7]. The relationship between ACE genotype and clinical parameters in a cohort of LOPD patients who received biweekly infusions of alglucosidase alfa for > 2 years also was reported [14]. Notably, I/I genotype patients had significantly greater muscle mass measured by quantitative magnetic resonance imaging (MRI). Muscle strength and endurance variations demonstrated a similar trend, with the I/D genotype resulting in intermediate outcomes between I/I and D/D; however, these differences were not statistically significant.
We tested the hypothesis that ACE genotype was a contributing factor to disease progression and clinical response to ERT in the Pompe disease Late-Onset Treatment Study (LOTS) [5].
2. Materials and methods
LOTS, a randomized, double-blind, placebo-controlled trial involving 90 LOPD patients, evaluated the safety and efficacy of biweekly infusions (20 mg/kg) of alglucosidase alfa compared to placebo for 78 weeks in juvenile and adult LOPD patients. All the patients enrolled in the study had biallelic mutations in the GAA gene. Detailed methods and results of LOTS have been reported [5]. Only patients who completed ≥ 26 weeks of the study were included in this analysis (n = 58 alglucosidase alfa-treated; n = 30 placebo-treated).
The I/D allele status of a 287 base-pair Alu repeat in intron 16 of the ACE gene was determined by polymerase chain reaction (PCR) analysis using methods described previously [15] with slight modifications (2 U Platinum™ Taq polymerase, 1 × Platinum™ Taq PCR buffer-Mg, 3 mM MgCl2, 0.25 mM dNTP mix, and 0.5 mM of the forward and reverse primers). Results were verified by repeat analysis. One-way ANOVA analysis was used to evaluate correlations between ACE genotype (I/I, I/D, and D/D) and baseline Pompe disease characteristics. Student's t-test was conducted to compare alglucosidase alfa treatment and placebo within each ACE genotype.
3. Results
At baseline, patients with the D/D genotype did not present with earlier onset of disease (mean age at first symptoms: 27.4 ± 12.7 years vs. 28.5 ± 11.5 [I/D] and 28.6 ± 14.4 [I/I]), longer duration of disease at trial entry (mean duration since symptom onset: 18.3 ± 10.2 years vs. 17.6 ± 11.6 [I/D] and 20.4 ± 9.9 [I/I]), or more severe disease as measured by 6-Minute Walk Test (6MWT) (mean: 343.0 ± 144.4 m vs. 339.2 ± 125.6 [I/D] and 293.1 ± 147.4 [I/I]), or percent predicted forced vital capacity (FVC) (mean: 53.3 ± 15.4% vs. 56.2 ± 15.6 [I/D] and 52.4 ± 12.5 [I/I]). Differences between ACE genotypes were not statistically significant.
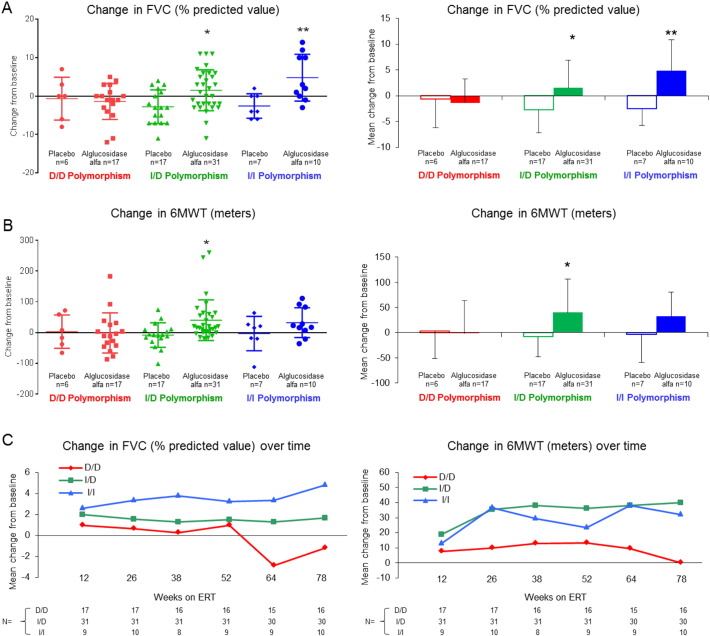
Following ERT, LOTS patients with the D/D genotype appeared less responsive to alglucosidase alfa treatment than patients with the I/I or I/D genotypes (Fig. 1). Patients with the I/D and I/I genotype who received ERT showed significantly greater improvements in FVC (mean change: 1.516 [P = 0.028] and 4.8 [P = 0.007], respectively) compared to D/D patients (mean change: − 1.412) receiving treatment (Fig. 1A). Similarly, the mean increase in the 6MWT was significantly higher in I/D patients on ERT (mean change: 40.16 [P = 0.022]) compared to the D/D group (mean change: − 1.412), with a trend for an improved response in patients with the I/I genotype (mean change: 32) (Fig. 1B). Fig. 1C presents the change from baseline in patients treated with alglucosidase alfa and also illustrates a trend toward lower responsiveness to alglucosidase alfa in D/D genotype patients. Importantly, while the mean changes in FVC and 6MWT do not appear to indicate an overall response to alglucosidase alfa within the D/D genotype group, it is important to note that a variable response to ERT was observed, and several patients demonstrated gains in the 6MWT and FVC with this genotype (Fig. 1A, B).
Fig. 1.
Changes in forced vital capacity (FVC) in the sitting position and 6-Minute Walk Test (6MWT) from baseline (BL) to 78 weeks or patient's last study timepoint by ACE genotype. (A) Change in FVC (individual patient dot plot [left] and mean changes [right]). (B) Change in 6MWT (individual patient dot plot [left] and mean changes [right]). (C) Mean changes over time in patients treated with alglucosidase alfa. *P < 0.01 and **P < 0.05 compared to D/D alglucosidase alfa group according to ANOVA. Error bars represent standard deviation (SD).
The D/D genotype was not associated with increases in peak IgG titers, sustained antibody titers, or inhibitory antibodies (data not shown), indicating that the correlation between ACE genotype and response to ERT is not associated with an immune response to alglucosidase alfa.
4. Discussion
Our finding that LOPD patients with the ACE D/D genotype demonstrate a reduced response to alglucosidase alfa relative to those with I/I or I/D genotypes is consistent with earlier findings [14]. We also demonstrated the presence of this association regardless of baseline disease severity and anti-GAA IgG antibody levels. However, unlike patients in earlier studies [7], [13], LOTS patients with the D/D genotype did not present with an earlier onset of disease or a longer duration of disease at study entry.
Our results suggest that the ACE I/D polymorphism may influence the response to alglucosidase alfa treatment independent of the patient's disease severity at treatment initiation. These findings raise pertinent questions about the mechanisms by which the ACE genotype influences the muscle response to alglucosidase alfa treatment. Does the D/D genotype result in increased vasoconstriction, and therefore less enzyme delivery to skeletal muscle? Do patients with the D/D genotype have an increase in Type II muscles fibers (fast-twitch, anaerobic, glycogen-rich) that decreases their response to treatment? Type I muscles fibers (slow-twitch, aerobic, mitochondria-rich) have an increased capillary density, which has been correlated with increased glycogen clearance after ERT in a mouse model of Pompe disease [16]. On the other hand, Type II muscle fibers exhibit increased autophagic pathology in Pompe mice, which may be responsible for the mis-targeting of ERT within the muscle fiber [17]. Finally, it is important to note that individual patient responses to alglucosidase alfa were variable across the three ACE genotypes, and many patients with the D/D genotype demonstrated improvements in the functional assessments evaluated. This supports the concept that an individualized treatment approach and careful follow-up are warranted for Pompe disease patients receiving ERT.
In conclusion, Pompe disease patients with the ACE D/D genotype appeared to show a decreased response to alglucosidase alfa, although the mechanism underlying this observation is not yet known. Continued genotyping of Pompe disease patients for the ACE polymorphism and further investigation of therapeutic response are warranted.
Conflict of interest
All authors are employees of Sanofi, and the study was funded by Sanofi Genzyme.
Acknowledgments
The authors wish to thank all of the investigators and their patients who participated in the Late-Onset Treatment Study (LOTS); Laurie LaRusso of Chestnut Communications, Walpole, MA, for medical writing support paid for by Sanofi Genzyme; and Adrienne Aiello and Marianne B. Zajdel of Sanofi Genzyme for medical writing and editorial assistance.
References
- 1.van der Ploeg A.T., Reuser A.J. Pompe's disease. Lancet. 2008;372:1342–1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- 2.Hirschhorn R., Reuser A.J.J. Glycogen storage disease type II: acid α-glucosidase (acid maltase deficiency) In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Kinzler K.W., Vogelstein B., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. [Google Scholar]
- 3.Cupler E.J., Berger K.I., Leshner R.T., Wolfe G.I., Han J.J., Barohn R.J., Kissel J.T., AANEM Consensus Committee on Late-onset Pompe Disease Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012;45:319–333. doi: 10.1002/mus.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toscano A., Schoser B. Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J. Neurol. 2013;260:951–959. doi: 10.1007/s00415-012-6636-x. [DOI] [PubMed] [Google Scholar]
- 5.van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J., Herson S., Kishnani P.S., Laforet P., Lake S.L., Lange D.J., Leshner R.T., Mayhew J.E., Morgan C., Nozaki K., Park D.J., Pestronk A., Rosenbloom B., Skrinar A., van Capelle C.I., van der Beek N.A., Wasserstein M., Zivkovic S.A. A randomized study of alglucosidase alfa in late-onset Pompe's disease. New. Engl. J. Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 6.Kishnani P.S., Corzo D., Nicolino M., Byrne B., Mandel H., Hwu W.L., Leslie N., Levine J., Spencer C., McDonald M., Li J., Dumontier J., Halberthal M., Chien Y.H., Hopkin R., Vijayaraghavan S., Gruskin D., Bartholomew D., van der Ploeg A., Clancy J.P., Parini R., Morin G., Beck M., De la Gastine G.S., Jokic M., Thurberg B., Richards S., Bali D., Davison M., Worden M.A., Chen Y.T., Wraith J.E. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 7.De Filippi P., Saeidi K., Ravaglia S., Dardis A., Angelini C., Mongini T., Morandi L., Moggio M., Di Muzio A., Filosto M., Bembi B., Giannini F., Marrosu G., Rigoldi M., Tonin P., Servidei S., Siciliano G., Carlucci A., Scotti C., Comelli M., Toscano A., Danesino C. Genotype-phenotype correlation in Pompe disease, a step forward. Orphanet. J. Rare. Dis. 2014;9:102. doi: 10.1186/s13023-014-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroos M., Hoogeveen-Westerveld M., van der Ploeg A., Reuser A.J. The genotype-phenotype correlation in Pompe disease. Am. J. Med. Genet. Part C. 2012;160C:59–68. doi: 10.1002/ajmg.c.31318. [DOI] [PubMed] [Google Scholar]
- 9.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danser A.H., Schalekamp M.A., Bax W.A., van den Brink A.M., Saxena P.R., Riegger G.A., Schunkert H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 11.Puthucheary Z., Skipworth J.R., Rawal J., Loosemore M., Van Someren K., Montgomery H.E. The ACE gene and human performance: 12 years on. Sports Med. 2011;41:433–448. doi: 10.2165/11588720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B., Tanaka H., Shono N., Miura S., Kiyonaga A., Shindo M., Saku K. The I allele of the angiotensin-converting enzyme gene is associated with an increased percentage of slow-twitch type I fibers in human skeletal muscle. Clin. Genet. 2003;63:139–144. doi: 10.1034/j.1399-0004.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 13.de Filippi P., Ravaglia S., Bembi B., Costa A., Moglia A., Piccolo G., Repetto A., Dardis A., Greco G., Ciana G., Canevari F., Danesino C. The angiotensin-converting enzyme insertion/deletion polymorphism modifies the clinical outcome in patients with Pompe disease. Genet. Med. 2010;12:206–211. doi: 10.1097/GIM.0b013e3181d2900e. [DOI] [PubMed] [Google Scholar]
- 14.Ravaglia S., De Filippi P., Pichiecchio A., Ponzio M., Saeidi Garaghani K., Poloni G.U., Bini P., Danesino C. Can genes influencing muscle function affect the therapeutic response to enzyme replacement therapy (ERT) in late-onset type II glycogenosis? Mol. Genet. Metab. 2012;107:104–110. doi: 10.1016/j.ymgme.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Rigat B., Hubert C., Corvol P., Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1) Nucleic Acids Res. 1992;20:1433. doi: 10.1093/nar/20.6.1433-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawes M.L., Kennedy W., O'Callaghan M.W., Thurberg B.L. Differential muscular glycogen clearance after enzyme replacement therapy in a mouse model of Pompe disease. Mol. Genet. Metab. 2007;91:343–351. doi: 10.1016/j.ymgme.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda T., Ahearn M., Roberts A., Mattaliano R.J., Zaal K., Ralston E., Plotz P.H., Raben N. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol. Ther. 2006;14:831–839. doi: 10.1016/j.ymthe.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]