Abstract
Atherosclerosis, the underlying cause of ischemic heart disease and stroke, is an inflammatory disease of arteries in a hyperlipidemic milieu. Endothelial expression of cellular adhesion molecules, such as endothelial-leukocyte adhesion molecule-1 (E-selectin) and intercellular adhesion molecule-1 (ICAM-1), plays a critical role in the initiation and progression of atherosclerosis. The dietary flavonoid, quercetin, has been reported to inhibit expression of cellular adhesion molecules, but the underlying mechanisms are incompletely understood. In this study, we found that quercetin dose-dependently (5–20 µM) inhibits lipopolysaccharide (LPS)-induced mRNA and protein expression of E-selectin and ICAM-1 in human aortic endothelial cells (HAEC). Incubation of HAEC with quercetin also significantly reduced LPS-induced oxidant production, but did not inhibit activation of the nuclear factor-kappaB (NF-κB). Furthermore, quercetin induced activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) and subsequent mRNA and protein expression of the antioxidant enzymes, heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase, quinone 1, and glutamate-cysteine ligase. The induction of Nrf2 and antioxidant enzymes was partly inhibited by the p38 mitogen-activated protein kinase (p38) inhibitor, SB203580. Our results suggest that quercetin suppresses LPS-induced oxidant production and adhesion molecule expression by inducing Nrf2 activation and antioxidant enzyme expression, which is partially mediated by p38; and the inhibitory effect of quercetin on adhesion molecule expression is not due to inhibition of NF-κB activation, but instead due to antioxidant-independent effects of HO-1.
Abbreviations: AP-1, activator protein 1; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; E-selectin, endothelial-leukocyte adhesion molecule-1; EGCG, epigallocatechin gallate; FBS, fetal bovine serum; GCL, glutamate-cysteine ligase; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; HAEC, human aortic endothelial cells; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule-1; LPS, lipopolysaccharide; NF-κB, nuclear factor-kappaB; NQO1, NAD(P)H dehydrogenase, quinone 1; Nrf2, erythroid 2-related factor 2; p38, p38 mitogen-activated protein kinase; PBS, phosphate-buffered saline; qPCR, real-time quantitative polymerase chain reaction; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha
Keywords: Atherosclerosis, Inflammation, Oxidants, Nrf2, NF-κB, Heme oxygenase-1
Graphical abstract

Highlights
-
•
Quercetin inhibits LPS-induced oxidant production and adhesion molecule expression.
-
•
Quercetin activates p38 MAP kinase and Nrf2, upregulating heme oxygenase-1 (HO-1).
-
•
HO-1 rather than NF-κB may account for quercetin’s anti-inflammatory effects.
1. Introduction
Endothelial activation with increased expression of adhesion molecules, such as endothelial-leukocyte adhesion molecule-1 (E-selectin) and intercellular adhesion molecule-1 (ICAM-1), is a critical initiating step in atherosclerosis. These cellular adhesion molecules mediate the adhesion of blood monocytes to the aortic endothelial cell surface and their transmigration into the subendothelial space of the aortic intima. The role of infection and inflammation in the initiation and progression of atherosclerosis is being increasingly recognized [17]. Evidence from animal studies suggests a significant role of lipopolysaccharide (LPS), the active constituent of endotoxin of Gram-negative bacteria, in atherosclerotic lesion development [13], [71], [22].
In addition, oxidative stress due to excessive production of oxidants and reactive oxygen species (ROS), has been implicated in the pathogenesis of atherosclerosis [47], [70], [49], [12], [51], [25], [54]. In vascular cells, ROS are generated primarily by constitutively expressed NAD(P)H oxidases [1]. While ROS play a critical role in redox-regulation of normal cellular functions, such as signal transduction, proliferation, differentiation, and apoptosis, abnormally elevated ROS levels are often associated with pathophysiological conditions [16], [5], [19], [66], [80]. Many risk factors for cardiovascular disease, such as smoking, hypercholesterolemia, hypertension, and diabetes, are associated with increased ROS production [68], [24], [63], [67], [3], and there is ample evidence that oxidative stress is an important causal factor in endothelial dysfunction, inflammation, and atherosclerosis [47], [70], [49], [12]. For example, ROS mediate endothelial dysfunction induced by obesity and body fat distribution [51], [25] and promote neutrophil adhesion to endothelial cells [46]. ROS have also been reported to attenuate the protective effect of NO [20] and to mediate the activation of endothelial cells and expression of adhesion molecules induced by LPS or pro-inflammatory cytokines [49], [54].
Certain flavonoids have been reported to suppress oxidative stress and reduce inflammatory responses in cell culture and in vivo, and to inhibit atherosclerotic lesion development in animal models [50], [40], [39]. Of the large family of flavonoids, quercetin (3, 3′, 4′, 5,7-pentahydroxyflavone) is one of the most widely distributed and is commonly found in plant-based diets. We and others have shown that quercetin inhibits adhesion molecule expression in human aortic endothelial cells (HAEC), suppresses production of pro-inflammatory cytokines in macrophages, and improves endothelial function and vessel relaxation in isolated aorta [40], [41], [60], [7], [10], [58]. Several underlying mechanisms for these effects of quercetin have been proposed, including induction of endothelial nitric oxide synthase and modulation of extracellular matrix composition [60], [28]. However, the effects of quercetin on intracellular oxidant production and activation of redox-sensitive signaling pathways in HAEC have not been reported, and, therefore, the underlying mechanisms remain incompletely understood.
The nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway plays an important role in cellular defense against oxidative and toxicological stress by upregulating expression of an array of antioxidant and detoxifying enzymes [30]. In addition, Nrf2 activation has also been reported to protect against inflammation. Nrf2 attenuates inflammatory responses by inducing the anti-inflammatory enzyme, heme oxygenase-1 (HO-1) [23], [64], [37] and by negatively regulating the expression of pro-inflammatory cytokines [32] and chemokines [36].
The goal of the present study was to gain a deeper understanding of the mechanisms whereby quercetin exerts its antioxidant and anti-inflammatory effects in endothelial cells. To this end, we investigated quercetin’s effects on cellular oxidant production and adhesion molecule expression, activation of the redox-sensitive transcription factors, nuclear factor-kappaB (NF-κB) and Nrf2, expression of antioxidant enzymes, and the involvement of specific protein kinases.
2. Materials and methods
2.1. Materials
Quercetin, LPS, and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) were purchased from Sigma-Aldrich (St. Louis, MO). All the other chemicals were of the highest grade available from Sigma-Aldrich.
2.2. Endothelial cells
Human aortic endothelial cells were obtained from Lonza (Walkersville, MD). Upon receipt, the cells were seeded in 75-cm2 flasks precoated with 1% (w/v) bovine gelatin (Sigma-Aldrich) at a ratio of 1:2 and were grown in EBM basal medium (Lonza) containing bovine brain extract, ascorbic acid, hydrocortisone, epidermal growth factor, 2% (v/v) fetal bovine serum (FBS; Sigma-Aldrich), and gentamicin/amphotericin-B at 37 °C under 5% CO2 in a humidified atmosphere. Medium was replaced periodically until cells reached 80–90% confluence; cells were then detached with 0.05% (w/v) trypsin-0.02% (w/v) EDTA (Sigma-Aldrich) and sub-cultured in gelatin-precoated 75 cm2 flasks at a 1:3 ratio. Cells at passage 7 were used for experiments.
2.3. Experiments
Human aortic endothelial cells were plated in 1% (w/v) gelatin-precoated, flat-bottom 96-well plates or 10-cm dishes with Medium 199 (Sigma-Aldrich) supplemented with 20% (v/v) FBS, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1 ng/ml human basic fibroblast growth factor (Roche). The cells were allowed to grow for 3–4 days until they reached confluence. Subsequently, they were incubated with or without different concentrations (5, 10, or 20 μM) of quercetin for up to 18 h, and then, in some experiments, were co-incubated for up to 5 h without or with 0.10 µg/ml LPS and the corresponding concentrations of quercetin. Previous work done in our laboratory had indicated that 18 h incubation of HAEC with quercetin was required for maximal inhibition of adhesion molecule expression [40], [41].
Stock solutions of quercetin were freshly prepared by dissolving it in DMSO. The stock solutions were subsequently diluted with Medium 199 containing 20% (v/v) FBS. The final concentrations of DMSO in the medium were ≤0.1% (v/v). Appropriate controls with the vehicle DMSO were included in all experiments. Cell viability was determined with Cell Proliferation Reagent WST-1 (Roche) according to the manufacturer’s instructions.
2.4. Gene expression of adhesion molecules and antioxidant enzymes
Confluent HAEC in 10-cm dishes were incubated with quercetin for 6–18 h and then, in some experiments, co-incubated with LPS and quercetin for 3 h. In other experiments, cells were pre-incubated with the p38 mitogen-activated protein kinase (p38) inhibitor, SB203580 (Sigma-Aldrich), for 1 h before incubation with quercetin. Total RNA was isolated from HAEC with TRIzol reagent (Life Technologies, Carlsbad, CA, USA). cDNA was synthesized using a high-capacity cDNA archive kit (Life Technologies). mRNA levels of E-selectin, ICAM-1, HO-1, NAD(P)H dehydrogenase, quinone 1 (NQO1), and the glutamate-cysteine ligase (GCL) modifier and catalytic subunits (GCLM and GCLC, respectively) were determined by real-time quantitative polymerase chain reaction (qPCR) with an ABI Prism 7500 Sequence Detection System (Life Technologies). Primers and probes used were purchased from Life Technologies as Assays on Demand, which contained a 20x mixture of PCR primers and TaqMan 6-FAM dye-labeled probes. The PCR reactions were performed with TaqMan Universal PCR Master Mix (Life Technologies). Glyceraldehyde 3-phosphate dehydrogenase or β-actin were used as internal control genes, and each gene was quantified relative to the control gene using standard curves.
2.5. Protein expression of adhesion molecules
Confluent HAEC in 96-well plates were incubated with quercetin for 18 h and then co-incubated with LPS and quercetin for 5 h. Protein levels of E-selectin and ICAM-1 on the cellular surface were measured by cell ELISA. After experimental treatments, cells were fixed in phosphate-buffered saline (PBS) containing 0.1% (v/v) glutaraldehyde. Plates were then blocked with 0.1% (v/v) Tween 20 in PBS containing 5% (w/v) skim milk at 37 °C for 1 h and subsequently incubated with a primary antibody to either E-selectin or ICAM-1 (R&D Systems, Minneapolis, MN) at 37 °C for 2 h. Thereafter, the plates were incubated with a horseradish peroxidase-linked sheep anti-mouse IgG secondary antibody (GE Healthcare, Little Chalfont Bucks, UK) at 37 °C for 1 h. Expression of E-selectin and ICAM-1 was evaluated by adding o-phenylendiamine-hydrochloride (Sigma-Aldrich). Absorbance at 492 nm was recorded in a microplate reader spectrophotometer (Spectromax 190, Molecular Devices, Sunnyvale, CA).
2.6. Intracellular oxidant production
Confluent HAEC in 96-well plates were incubated with quercetin for 18 h, washed with HBSS buffer, and then incubated with 10 µM DCFH-DA for 20 min. After washing the cells again, they were incubated with 10 µg/ml LPS in medium containing 0.1% (v/v) FBS. Fluorescence was measured immediately after adding LPS and then every hour up to 4 h using a Spectromax Gemini XS multiplate fluorometer (Molecular Devices, Sunnyvale, CA) with excitation and emission settings of 485 nm and 530 nm, respectively.
2.7. Activation of Nrf2, NF-κB, and p38 measured by ELISA
Confluent HAEC in 10-cm dishes were incubated with quercetin for 3–18 h and then, in some experiments, co-incubated with LPS and quercetin for 3 h. In other experiments, cells were pre-incubated with SB203580 for 1 h before incubation with quercetin. Nuclear extracts (Nrf2 and NF-κB) or whole-cell extracts (phosphorylated p38) of HAEC were prepared with a cell extraction kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. NF-κB and Nrf2 DNA-binding activity was measured with the corresponding TransAM transcription factor ELISA kit (Active Motif, Carlsbad, CA), following the manufacturer’s instructions. Specificity of the assays was confirmed by competition with the supplied wild-type or mutant oligonucleotide. Phosphorylated p38 was measured with a p38 MAPK alpha (pT180/Y182) SimpleStep ELISA Kit (Abcam, Eugene, OR), following the manufacturer’s instructions.
2.8. Activation of Nrf2, protein expression of antioxidant enzymes, and phosphorylation of p38 measured by immunoblotting
Confluent HAEC in 10-cm dishes were incubated with quercetin for 3 h (p38), 6 h (Nrf2) or 18 h (antioxidant enzymes). In some experiments, cells were pre-incubated with SB203580 for 1 h before incubation with quercetin. Nuclear and whole-cell extracts of HAEC were prepared with a cell extraction kit (Active Motif) according to the manufacturer’s instructions. Equal amounts of protein (20–25 µg) were separated on 8% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and then transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked in 5% (w/v) nonfat dry milk in PBS containing 0.1% (v/v) Tween 20 for 1 h at room temperature and were then incubated overnight at 4 °C with specific primary antibodies to Nrf2 (Santa Cruz Biotechnology, Santa Cruz, CA), HO-1, NQO1, GCLC, GCLM, p38, phosphorylated p38, β-actin (Abcam, Eugene, OR) or lamin A/C (Cell Signaling Technology, Danvers, MA), followed by incubation at room temperature for 1 h with a horseradish peroxidase-conjugated secondary antibody (Abcam). The membrane was then incubated with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Eugene, OR) and exposed to films for visualization.
2.9. Statistical analysis
Data were expressed as means±SEM of at least three independent experiments each run in triplicate and were analyzed by unpaired student’s t-test or by factorial analysis of variance (ANOVA) followed by post hoc analysis with the Holm-Sidak test. Significance was accepted at P<0.05.
3. Results
3.1. Quercetin inhibits LPS-induced adhesion molecule expression
To investigate whether quercetin can inhibit adhesion molecule expression induced by LPS, HAEC were incubated with or without 5, 10, or 20 μM quercetin for 18 h and then co-incubated with 0.10 μg/ml LPS and the corresponding concentrations of quercetin for 3 h. Transcription of E-selectin and ICAM-1 genes was evaluated by real-time qPCR. As expected, incubation of HAEC with LPS strongly upregulated mRNA levels of E-selectin and ICAM-1 compared to control cells incubated without LPS (Fig. 1a and b). Quercetin treatment dose-dependently inhibited LPS-induced increases of adhesion molecule mRNA levels: ICAM-1 message was significantly reduced by quercetin at all concentrations tested (Fig. 1b), and E-selectin mRNA levels were significantly reduced by 10 and 20 μM quercetin (Fig. 1a).
Fig. 1.
Quercetin inhibits LPS-induced adhesion molecule expression. Human aortic endothelial cells were incubated for 18 h without or with the indicated concentrations of quercetin, and then were co-incubated for 3 h (mRNA levels) or 5 h (protein levels) without or with 0.10 µg/ml LPS and the corresponding concentrations of quercetin. mRNA and protein levels of E-selectin (a, c) and ICAM-1 (b, d) were measured by real-time PCR and cell-ELISA, respectively. *Denotes significant difference from LPS-stimulated cells, P<0.05.
To investigate whether quercetin also inhibited LPS-induced upregulation of adhesion molecule protein levels, HAEC were incubated as described above, except that exposure to LPS lasted for 5 h instead of 3 h. After incubation, E-selectin and ICAM-1 protein levels on the cell surface were determined by cell-ELISA. Quercetin dose-dependently inhibited LPS-induced E-selectin and ICAM-1 protein expression (Fig. 1c and d). ICAM-1 protein levels were more effectively suppressed by quercetin than E-selectin, with >85% inhibition of ICAM-1 expression by 20 µM quercetin (Fig. 1d). These results are consistent with our previous observation that quercetin differently inhibited tumor necrosis factor alpha (TNFα)-induced expression of ICAM-1 and E-selectin, reflecting differences in the promoter regions of the genes of these two adhesion molecules [40].
During and after incubations, cells were examined with an inverted optical microscope. No noticeable morphological alterations were observed with any of the treatments. In addition, none of the concentrations of quercetin used in this study significantly reduced cell viability (data not shown).
3.2. Quercetin inhibits LPS-induced intracellular oxidant production
Oxidative stress has been reported to be an important factor in inducing adhesion molecule expression and inflammation [54], [14], [33]. Hence, we assessed the effect of quercetin on intracellular oxidant levels, using DCFH-DA as a fluorescence probe. As shown in Fig. 2, LPS significantly increased intracellular oxidant production compared to unstimulated cells. Quercetin significantly decreased LPS-induced formation of oxidants at all concentrations tested. After 4 h of incubation with LPS, 10 and 20 µM quercetin inhibited oxidant generation by about 75%, while 5 µM quercetin caused about 40% inhibition. Quercetin at 20 µM also significantly reduced basal levels of cellular oxidant production in the absence of LPS (Fig. 2). Since cells were incubated with quercetin and then washed prior to the addition of DCFH-DA, followed by incubation with LPS in the absence of quercetin, these data suggest a persistent effect of quercetin treatment on HAEC, including changes in antioxidant levels or signal transduction pathways.
Fig. 2.
Quercetin inhibits LPS-induced intracellular oxidant production. Human aortic endothelial cells were incubated for 18 h without (Control, LPS) or with the indicated concentrations of quercetin (Que). The cells were then washed and incubated for 20 min with 10 μM DCFH-DA. Subsequently, cells were washed again and 10 μg/ml LPS in medium containing 0.1% (v/v) FBS or LPS-free medium containing 0.1% (v/v) FBS was added. Fluorescence was measured immediately after adding LPS and every hour up to 4 h. *Denotes significant difference from LPS-stimulated cells, P<0.05; #denotes significant difference from control cells, P<0.05.
3.3. Quercetin does not inhibit LPS-induced NF-κB activation
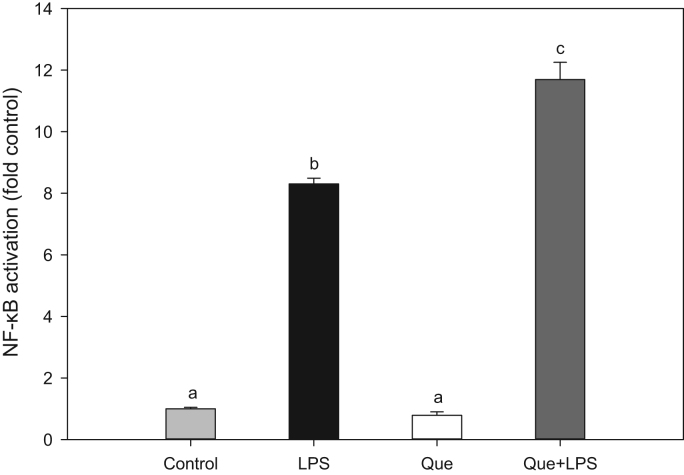
The redox-sensitive transcription factor, NF-κB, is critically involved in the regulation of many pro-inflammatory genes, such as cytokines, chemokines, and cellular adhesion molecules [34], [8]. To investigate whether the inhibitory effect of quercetin on adhesion molecule expression is mediated by NF-κB, HAEC were incubated with 20 µM quercetin for 18 h and then exposed to LPS for 3 h. Nuclear extracts were prepared and NF-κB DNA binding activity was measured by ELISA. While LPS strongly increased nuclear levels and DNA-binding activity of NF-κB, quercetin treatment did not inhibit LPS-induced NF-κB activation, and in fact appeared to further enhance it (Fig. 3). These results suggest that the inhibition of adhesion molecule expression by quercetin is not due to suppression of NF-κB activation, and that alternative pathways are involved.
Fig. 3.
Quercetin does not inhibit LPS-induced NF-κB activation. Human aortic endothelial cells were incubated for 18 h without (Control) or with 20 µM quercetin (Que) and then co-incubated for 3 h without or with 0.10 µg/ml LPS (LPS) and 20 µM quercetin (LPS+Que). Nuclear proteins were extracted and NF-κB was measured by ELISA. Results marked with different letters differ significantly, P<0.05.
3.4. Quercetin induces Nrf2 activation
Nrf2 is a transcription factor that is activated by intracellular oxidative or electrophilic stress and coordinates the expression of many genes encoding antioxidant and detoxification enzymes [30]. To investigate whether quercetin alone (i.e., in the absence of LPS), can induce Nrf2 activation, HAEC were incubated with 20 µM quercetin and nuclear Nrf2 levels were measured by ELISA and Western blotting. As shown in Fig. 4a, quercetin induced nuclear Nrf2 DNA-binding activity in a time-dependent manner. The highest level of Nrf2 activity was observed after 6 h of incubation, which was followed by a gradual return to baseline after 18 h. Quercetin treatment for 6 h also caused a significant increase in Nrf2 protein levels in nuclear extracts of HAEC measured by immunoblotting (Fig. 4b and c).
Fig. 4.
Quercetin induces Nrf2 activation. Human aortic endothelial cells were incubated without (Control) or with 20 µM quercetin (Que) for the indicated time periods; or were incubated for 18 h without or with 20 µM quercetin, respectively, and then incubated for 3 h with 0.10 µg/ml LPS (LPS and LPS+Que). Nuclear proteins were extracted and Nrf2 was measured by ELISA (a). Further, HAEC were incubated for 6 h without (Con) or with 20 µM quercetin (Que). Nuclear proteins were extracted and Nrf2 was assessed by immunoblotting (b) and quantitatively analyzed (c). *Denotes significant difference from Control (a, c), P<0.05; #denotes significant difference from LPS-stimulated cells (a), P<0.05.
To determine whether quercetin affects Nrf2 transcriptional activation in the presence of LPS, HAEC were incubated with 20 µM quercetin for 18 h and then treated with LPS for 3 h as in previous experiments. Cells treated with quercetin and LPS exhibited a two-fold increase in nuclear Nrf2 levels, as assessed by ELISA, while LPS alone had no effect (Fig. 4a). These results demonstrate that quercetin induces Nrf2 activation in both quiescent and LPS-stimulated cells, and this activation is likely the mechanism whereby quercetin exerts its antioxidant and anti-inflammatory effects in HAEC.
3.5. Quercetin induces antioxidant enzyme expression
To assess the effect of quercetin on the expression of antioxidant enzymes [65], [2], [26], HAEC were treated for up to 18 h with 5–20 µM quercetin, and mRNA levels of antioxidant enzymes were determined by real-time qPCR. We found that HO-1 gene transcription was strongly induced by quercetin in a dose- and time-dependent manner, while induction of NQO1 was less pronounced (Fig. 5a and b). mRNA levels of GCLM and GCLC were also induced by quercetin treatment (data not shown). Effects of quercetin on HO-1 and NQO1 protein expression were determined by immunoblotting, and significant induction of both enzymes was observed after incubation of HAEC for 18 h with 20 µM quercetin (Fig. 5c and d).
Fig. 5.
Quercetin induces antioxidant enzyme expression. Human aortic endothelial cells were incubated for 18 h without (Control) or with the indicated concentrations of quercetin (Que) (a); or were incubated with 20 µM quercetin for the indicated time periods (b); or were incubated for 18 h without (Control, LPS) or with the indicated concentrations of quercetin (Que) and then co-incubated for 3 h without or with 0.10 µg/ml LPS and the corresponding concentrations of quercetin (e). mRNA levels of HO-1 and NQO1 were measured by real-time PCR. Further, HAEC were incubated for 18 h without (Con) or with 20 µM quercetin (Que), and protein levels of HO-1 and NQO1 were assessed by immunoblotting (c) and quantitatively analyzed (d). *Denotes significant difference from control cells (a, d) or 0 h (b) or LPS-stimulated cells (e), P<0.05.
To determine whether LPS affects the ability of quercetin to induce antioxidant enzymes, HAEC were incubated with quercetin followed by LPS stimulation, as before. While LPS treatment alone did not affect mRNA levels of NQO1 and HO-1, quercetin significantly and dose-dependently increased mRNA levels of both HO-1 and NQO1 in LPS-treated cells (Fig. 5e), similar to the results observed in the absence of LPS (Fig. 5a). These data are further evidence that the mechanism of quercetin’s activity in HAEC is independent of LPS.
3.6. Quercetin induces p38 activation
Several mitogen-activated protein kinase signaling pathways have been reported to be involved in Nrf2 activation and the induction of antioxidant enzymes [9], [29]. p38 has been implicated as one such protein kinase, especially in the activation of HO-1 by extracts of natural products and plants [9], [48]. Hence, we assessed the effect of quercetin on the activation, i.e., phosphorylation, of p38. We found that incubation of HAEC for 3 h with 20 µM quercetin slightly but significantly increased the levels of phosphorylated p38, measured either by ELISA (Fig. 6a) or immunoblotting (Fig. 6b and c).
Fig. 6.
Quercetin induces p38 activation. HAEC were incubated for 3 h without (Con) or with 20 µM quercetin (Que). Phosphorylated p38 (P-p38) in whole cell extracts was measured by ELISA (a) or assessed by immunoblotting (b) and quantitatively analyzed (c). *Denotes significant difference from control cells (a and c).
Furthermore, we assessed the role of p38 in quercetin-induced Nrf2 activation and subsequent antioxidant enzyme induction in HAEC by incubating cells with SB203580, a specific p38 inhibitor. Cells were treated with 15 µM SB203580 for 1 h, followed by incubation for up to 12 h with 20 µM quercetin. We found that SB203580 partially inhibited quercetin-induced Nrf2 activation, measured by ELISA (Fig. 7a) and immunoblotting (Fig. 7b and c). In parallel, SB203580 also attenuated quercetin-induced gene transcription of HO-1 and NQO1 (Fig. 7d). The inhibition of quercetin-induced antioxidant gene expression by SB203580 was stronger than the inhibition of Nrf2 activation, suggesting an amplifying effect. Taken together, these results suggest that quercetin induces Nrf2 activation and antioxidant enzyme gene transcription via activation of p38, although other protein kinases may be involved in the response.
Fig. 7.
Quercetin-induced Nrf2 activation and antioxidant gene transcription are inhibited by the p38 inhibitor, SB203580. Human aortic endothelial cells were incubated for 1 h without (Con) or with 15 µM of the p38 inhibitor, SB203580 (SB), and then were incubated without or with 20 µM quercetin (Que) for 6 h (a–c) or 12 h (d). Nuclear proteins were extracted and Nrf2 was measured by ELISA (a) or assessed by immunoblotting (b) and quantitatively analyzed (c); or mRNA levels of HO-1 and NQO1 were measured by real-time PCR (d). Results marked with different letters differ significantly, P<0.05 (a, c, d).
4. Discussion
In the present study, we found that quercetin exerts antioxidant and anti-inflammatory effects in HAEC that may be relevant to the inhibition of atherogenesis in humans. In our experiments, we used the aglycone (or “free”) form of quercetin and showed dose-dependent effects in the concentration range of 5–20 µM. Naturally occurring quercetin in plants exists in the form of glycosides, which are ubiquitously contained in fruits and vegetables, particularly onions, apples, kale, and broccoli (Michael 1992). In the small intestine, quercetin glycosides are absorbed into intestinal epithelial cells, where they are hydrolyzed and the remaining quercetin aglycone is glucuronidated or sulfated. Those metabolites of quercetin are released into the blood stream and then may be further metabolized in the liver by sulfate transferase and catechol O-methyl transferase.
Therefore, quercetin detected in plasma is mostly in conjugated forms under normal physiological conditions, and quercetin aglycone is only found in the sub-micromolar concentration range [56], [62], [45]. In tissues, however, quercetin aglycone can be the predominant form [6], [79]. Both monocytes and macrophages can release β-glucuronidase [18], [77], which catalyzes the deglucuronidation of quercetin glucuronides, producing quercetin aglycone [4]. In the initial stages of atherosclerosis, monocytes are recruited to the arterial wall and subsequently mature into resident macrophages. It has been reported that β-glucuronidase activity increases in human atherosclerotic aortas [43] and in the aorta of rabbits in the early stages of experimental atherosclerosis [44]. LPS-induced inflammation also has been shown to significantly increase deconjugation of quercetin-3-O-glucuronide by macrophages, which is essential for quercetin’s anti-inflammatory effects [27]. In LPS-challenged rats, the aglycone: monoglucoronide ratio of luteolin, which has a structure similar to quercetin, increases from 0.23 to 0.78 [61].
We observed antioxidant and anti-inflammatory effects of quercetin at concentrations as low as 5 µM. Total quercetin (conjugated and aglycone) concentration in human plasma can reach 3.95 µM after consuming shallot dry skin [72]. Dietary supplementation with quercetin may result in even higher plasma levels. If the majority of the quercetin is converted to its aglycone form at the site of inflammation and endothelial dysfunction, i.e., in the subendothelial space of the arterial intima, the local tissue concentration of quercetin aglycone may well reach levels comparable to the concentrations used in this study.
To better understand the mechanism(s) by which quercetin exerts its antioxidant and anti-inflammatory effects in HAEC, we investigated whether the strong inhibitory effect of quercetin on LPS-induced adhesion molecule expression, in particular ICAM-1, was associated with decreased oxidant production and NF-κB activation. While we found that oxidant production was strongly inhibited by quercetin, NF-κB activation was not inhibited, as we reported previously using TNFα [40]. Transcription of ICAM-1 is under the control not only of NF-κB, but also activator protein 1 (AP-1) [34], [8], with both transcription factors being involved in ICAM-1 induction by LPS [59]. However, NF-κB and AP-1 are differentially induced [34], [42], [52]. For example, the matrix metalloproteinase-9 gene has binding sites for both NF-κB and AP-1 in its promoter; however, its induction by 12-O-tetradecanoylphorbol-13-acetate is only dependent on AP-1 but not NF-κB [38]. Furthermore, the flavan-3-ol, epigallocatechin gallate (EGCG), inhibits AP-1 induction by TNFα but does not affect TNFα-induced DNA binding of NF-κB [81]. In human umbilical vein endothelial cells, induction of ICAM-1 expression by H2O2, a critical oxidant in cell signaling, occurs through the AP-1/Ets elements in the promoter region of the ICAM-1 gene, not through the activity of NF-κB, which mediates induction of ICAM-1 by TNFα [57]. In fact, activation of AP-1 by ROS is well established [38], [57]. In addition, some flavonoids, including quercitrin and quercetin, have been reported to inhibit the activity of AP-1 [38], [15].
In the present study, we further observed that quercetin induced transcription of HO-1 and NQO1, two antioxidant enzymes known to reduce ROS production and attenuate inflammation in vitro and in vivo. For example, induction of HO-1 reduces ROS generation and inhibits adhesion molecule expression and monocyte adhesion in cultured cells [76], [55], [69], and NQO1 protects against oxidative stress in animal models and tissue culture [21], [35]. Interestingly, HO-1 also inhibits AP-1 nuclear translocation and DNA-binding activity [75], [74]. As mentioned above, TNFα-induced AP-1 DNA-binding activity is inhibited by EGCG; this effect of EGCG appears to be mediated by HO-1, and bilirubin is involved in the action of HO-1 on AP-1 [81]. In addition, HO-1 and bilirubin attenuate endothelial activation induced by TNFα or oxidized low-density lipoprotein [31]. In our study, HO-1 was strongly induced by quercetin, which suggests a possible mechanism for the inhibitory effect of quercetin on ICAM-1 expression involving AP-1 (Fig. 8).
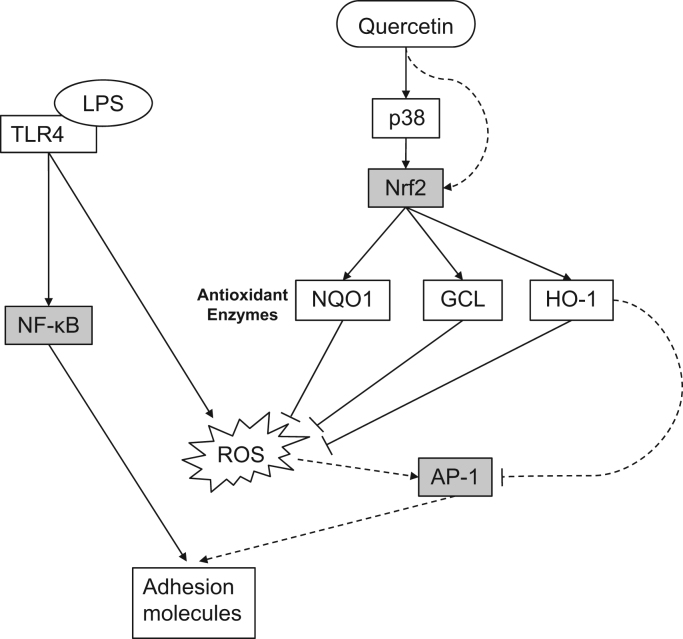
Fig. 8.
Scheme summarizing the antioxidant and anti-inflammatory effects of quercetin in human aortic endothelial cells. Quercetin induces Nrf2 nuclear translocation and DNA binding activity via activation (phosphorylation) of p38. Since the p38 inhibitor, SB203580, did not completely suppress Nrf2 activation by quercetin, (an) alternative pathway(s) may be involved. Activation of Nrf2 results in gene transcription and protein expression of the antioxidant enzymes, HO-1, NQO1, and GCL, which in turn inhibit LPS-induced formation of oxidants (ROS) via the TLR4 pathway. Induction of HO-1 may inhibit AP-1 activity and adhesion molecule expression by (an) antioxidant-independent mechanism(s). In contrast, quercetin did not inhibit LPS-induced NF-κB activation. Solid lines: based on our data presented in this paper; dashed lines: based on previously reported data [73], [34], [8], [75], [74], [53], [49], [37], [57].
Numerous studies have shown that protein kinases are involved in the induction of Nrf2 and antioxidant enzymes. Depending on the models and inhibitors used, results of those studies are sometimes inconsistent or even contradictory. The extracellular regulatory kinase inhibitor, PD98059, inhibits quercetin-induced HO-1 protein expression in RAW264.7 cells, but the c-Jun N-terminal kinases inhibitor, SP600125, or the p38 inhibitor, SB203580, does not show any effect [11]. In a different study, SB203580, rather than SP600125, PD98059, or the phosphoinositide 3-kinase inhibitor, LY294002, represses HO-1 accumulation induced by Ginkgo biloba extract [9]. Protein kinase C also has been reported to mediate the induction of Nrf2 and HO-1 by genistein in Caco-2 cells [78]. In the present study, the p38 inhibitor, SB203580, inhibited the induction of Nrf2 and antioxidant enzymes significantly but not completely, which suggests that other protein kinases or protein kinase-independent pathways may be involved in quercetin’s effects on Nrf2 activation and antioxidant enzyme induction.
Our findings are summarized in Fig. 8. Quercetin induces Nrf2 via activation of p38. The partial inhibition of quercetin-induced Nrf2 activation by SB203580 suggests alternative mechanisms for Nrf2 activation by quercetin. Activation of Nrf2 results in gene transcription and protein expression of antioxidant enzymes, which reduce excess oxidant levels stimulated by LPS through the Toll-like receptor 4 pathway. Induction of HO-1 can inhibit AP-1 activity, which may be responsible for the inhibition of adhesion molecule expression by quercetin, rather than inhibition of NF-kB activation.
5. Conclusions
In the present study, we found that LPS-stimulated adhesion molecule expression and oxidant formation in HAEC are strongly inhibited by quercetin. Quercetin induces expression of HO-1, NQO1, and GCL by activating Nrf2, which is partially dependent on p38. The antioxidant effect of quercetin against basal and LPS-induced oxidant formation in HAEC is readily explained by the induction of antioxidant enzymes, whereas the anti-inflammatory effect of quercetin on LPS-induced adhesion molecule expression is likely due to increased expression of HO-1 and may occur through antioxidant-independent mechanisms.
Acknowledgments
We thank Dr. Alexander Michels, Dr. Kate Shay, and Stephen Lawson from the Linus Pauling Institute for their careful review and helpful critique of this work. This research was supported by the donor-funded Research Innovation Fund of the Linus Pauling Institute at Oregon State University; it did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ago T., Kuroda J., Kamouchi M., Sadoshima J., Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system. Circ. J. 2011;75(8):1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 2.Anwar A.A., Li F.Y., Leake D.S., Ishii T., Mann G.E., Siow R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005;39(2):227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Asano H., Horinouchi T., Mai Y., Sawada O., Fujii S., Nishiya T., Minami M., Katayama T., Iwanaga T., Terada K., Miwa S. Nicotine- and tar-free cigarette smoke induces cell damage through reactive oxygen species newly generated by PKC-dependent activation of NADPH oxidase. J. Pharmacol. Sci. 2012;118(2):275–287. doi: 10.1254/jphs.11166fp. [DOI] [PubMed] [Google Scholar]
- 4.Bartholomé R., Haenen G., Hollman C.H., Bast A., Dagnelie P.C., Roos D., Keijer J., Kroon P.A., Needs P.W., Arts I.C. Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by beta-glucuronidase from neutrophils. Drug Metab. Pharmacokinet. 2010;25(4):379–387. doi: 10.2133/dmpk.dmpk-10-rg-002. [DOI] [PubMed] [Google Scholar]
- 5.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bieger J., Cermak R., Blank R., de Boer V.C., Hollman P.C., Kamphues J., Wolffram S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008;138(8):1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- 7.Chao P.Y., Huang Y.P., Hsieh W.B. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adhes. Migr. 2013;7(2):237–245. doi: 10.4161/cam.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.C., Chow M.P., Huang W.C., Lin Y.C., Chang Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol. Pharmacol. 2004;66(3):683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 9.Chen J.S., Huang P.H., Wang C.H., Lin F.Y., Tsai H.Y., Wu T.C., Lin S.J., Chen J.W. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214(2):301–309. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Cho S.Y., Park S.J., Kwon M.J., Jeong T.S., Bok S.H., Choi W.Y., Jeong W.I., Ryu S.Y., Do S.H., Lee C.S., Song J.C., Jeong K.S. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell Biochem. 2003;243(1–2):153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 11.Chow J.M., Shen S.C., Huan S.K., Lin H.Y., Chen Y.C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharmacol. 2005;69(12):1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo A., Moretti F., Burgio V.L., Bravi C., Guido F., Levrero M., Puri P.L. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J. Cell Physiol. 2003;195(3):402–410. doi: 10.1002/jcp.10191. [DOI] [PubMed] [Google Scholar]
- 13.Cuaz-Pérolin C., Billiet L., Baugé E., Copin C., Scott-Algara D., Genze F., Büchele B., Syrovets T., Simmet T., Rouis M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 2008;28(2):272–277. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 14.De Mattia G., Bravi M.C., Laurenti O., Cassone-Faldetta M., Proietti A., De Luca O., Armiento A., Ferri C. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia. 1998;41(11):1392–1396. doi: 10.1007/s001250051082. [DOI] [PubMed] [Google Scholar]
- 15.Ding M., Zhao J., Bowman L., Lu Y., Shi X. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin. Int. J. Oncol. 2010;36(1):59–67. [PubMed] [Google Scholar]
- 16.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 17.Epstein S.E., Zhu J., Najafi A.H., Burnett M.S. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119(24):3133–3141. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 18.Ferreri N.R., Beck L., Spiegelberg H.L. Beta-Glucuronidase release from human monocytes induced with aggregated immunoglobulins of different classes. Cell Immunol. 1986;98(1):57–67. doi: 10.1016/0008-8749(86)90267-4. [DOI] [PubMed] [Google Scholar]
- 19.Forman H.J., Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002;166(12 Pt 2):S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 20.Förstermann U., Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 21.Gaikwad A., Long D.J., Stringer J.L., Jaiswal A.K. In vivo role of NAD(P)H: quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J. Biol. Chem. 2001;276(25):22559–22564. doi: 10.1074/jbc.M101053200. [DOI] [PubMed] [Google Scholar]
- 22.Gitlin J.M., Loftin C.D. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice. Cardiovasc. Res. 2009;81(2):400–407. doi: 10.1093/cvr/cvn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He C.H., Gong P., Hu B., Stewart D., Choi M.E., Choi A.M., Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J. Biol. Chem. 2001;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 24.Heitzer T., Just H., Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94(1):6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Huang C.J., McAllister M.J., Slusher A.L., Webb H.E., Mock J.T., Acevedo E.O. Obesity-related oxidative Stress: the impact of physical activity and diet manipulation. Sports Med. Open. 2015;1(1):32. doi: 10.1186/s40798-015-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C.S., Lin A.H., Liu C.T., Tsai C.W., Chang I.S., Chen H.W., Lii C.K. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activation. Mol. Nutr. Food Res. 2013;57(11):1918–1930. doi: 10.1002/mnfr.201300063. [DOI] [PubMed] [Google Scholar]
- 27.Ishisaka A., Kawabata K., Miki S., Shiba Y., Minekawa S., Nishikawa T., Mukai R., Terao J., Kawai Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS One. 2013;8(11):e80843. doi: 10.1371/journal.pone.0080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov V., Ivanova S., Kalinovsky T., Niedzwiecki A., Rath M. Plant-derived micronutrients suppress monocyte adhesion to cultured human aortic endothelial cell layer by modulating its extracellular matrix composition. J. Cardiovasc. Pharmacol. 2008;52(1):55–65. doi: 10.1097/FJC.0b013e31817e692f. [DOI] [PubMed] [Google Scholar]
- 29.Kang K.W., Lee S.J., Kim S.G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005;7(11–12):1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 30.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura K., Ishikawa K., Wada Y., Kimura S., Matsumoto H., Kohro T., Itabe H., Kodama T., Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler. Thromb. Vasc. Biol. 2005;25(1):155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 32.Khor T.O., Huang M.T., Kwon K.H., Chan J.Y., Reddy B.S., Kong A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580–11584. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 33.Kunsch C., Medford R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999;85(8):753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 34.Lakshminarayanan V., Beno D.W., Costa R.H., Roebuck K.A. Differential regulation of interleukin-8 and intercellular adhesion molecule-1 by H2O2 and tumor necrosis factor-alpha in endothelial and epithelial cells. J. Biol. Chem. 1997;272(52):32910–32918. doi: 10.1074/jbc.272.52.32910. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278(14):12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 36.Levonen A.L., Inkala M., Heikura T., Jauhiainen S., Jyrkkänen H.K., Kansanen E., Määttä K., Romppanen E., Turunen P., Rutanen J., Ylä-Herttuala S. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007;27(4):741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.C., Liu X.M., Peyton K., Wang H., Yang W.C., Lin S.J., Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008;28(4):739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin C.W., Hou W.C., Shen S.C., Juan S.H., Ko C.H., Wang L.M., Chen Y.C. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008;29(9):1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- 39.Loke W.M., Proudfoot J.M., Hodgson J.M., McKinley A.J., Hime N., Magat M., Stocker R., Croft K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010;30(4):749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- 40.Lotito S.B., Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J. Biol. Chem. 2006;281(48):37102–37110. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 41.Lotito S.B., Zhang W.J., Yang C.S., Crozier A., Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic. Biol. Med. 2011;51(2):454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer M., Schreck R., Baeuerle P.A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller B.F., Kothari H.V. Increased activity of lysosomal enzymes in human atherosclerotic aortas. Exp. Mol. Pathol. 1969;10(3):288–294. doi: 10.1016/0014-4800(69)90058-6. [DOI] [PubMed] [Google Scholar]
- 44.Mrhova O., Zemplenyi T., Lojda Z. Beta-glucuronidase activity of the aorta in early stages of experimental rabbit atherosclerosis. J. Atheroscler. Res. 1963;3:44–49. doi: 10.1016/s0368-1319(63)80094-0. [DOI] [PubMed] [Google Scholar]
- 45.Mullen W., Edwards C.A., Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006;96(1):107–116. doi: 10.1079/bjn20061809. [DOI] [PubMed] [Google Scholar]
- 46.Niu X.F., Smith C.W., Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ. Res. 1994;74(6):1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- 47.Pamukcu B., Lip G.Y., Shantsila E. The nuclear factor--kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb. Res. 2011;128(2):117–123. doi: 10.1016/j.thromres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 48.Park E.J., Kim Y.M., Park S.W., Kim H.J., Lee J.H., Lee D.U., Chang K.C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxicol. 2013;55:386–395. doi: 10.1016/j.fct.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Park H.S., Chun J.N., Jung H.Y., Choi C., Bae Y.S. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006;72(3):447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Pavlica S., Gebhardt R. Protective effects of flavonoids and two metabolites against oxidative stress in neuronal PC12 cells. Life Sci. 2010;86(3–4):79–86. doi: 10.1016/j.lfs.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Perticone F., Ceravolo R., Candigliota M., Ventura G., Iacopino S., Sinopoli F., Mattioli P.L. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50(1):159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 52.Pinkus R., Weiner L.M., Daniel V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996;271(23):13422–13429. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- 53.Pocock J., Gómez-Guerrero C., Harendza S., Ayoub M., Hernández-Vargas P., Zahner G., Stahl R.A., Thaiss F. Differential activation of NF-kappa B, AP-1, and C/EBP in endotoxin-tolerant rats: mechanisms for in vivo regulation of glomerular RANTES/CCL5 expression. J. Immunol. 2003;170(12):6280–6291. doi: 10.4049/jimmunol.170.12.6280. [DOI] [PubMed] [Google Scholar]
- 54.Pueyo M.E., Gonzalez W., Nicoletti A., Savoie F., Arnal J.F., Michel J.B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2000;20(3):645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 55.Pullikotil P., Chen H., Muniyappa R., Greenberg C.C., Yang S., Reiter C.E., Lee J.W., Chung J.H., Quon M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 2012;23(9):1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rechner A.R., Kuhnle G., Hu H., Roedig-Penman A., van den Braak M.H., Moore K.P., Rice-Evans C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002;36(11):1229–1241. doi: 10.1080/246-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 57.Roebuck K.A., Rahman A., Lakshminarayanan V., Janakidevi K., Malik A.B. H2O2 and tumor necrosis factor-alpha activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J. Biol. Chem. 1995;270(32):18966–18974. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez M., Lodi F., Vera R., Villar I.C., Cogolludo A., Jimenez R., Moreno L., Romero M., Tamargo J., Perez-Vizcaino F., Duarte J. Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J. Nutr. 2007;137(4):910–915. doi: 10.1093/jn/137.4.910. [DOI] [PubMed] [Google Scholar]
- 59.Sawa Y., Ueki T., Hata M., Iwasawa K., Tsuruga E., Kojima H., Ishikawa H., Yoshida S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J. Histochem. Cytochem. 2008;56(2):97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen Y., Croft K.D., Hodgson J.M., Kyle R., Lee I.L., Wang Y., Stocker R., Ward N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012;84(8):1036–1044. doi: 10.1016/j.bcp.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Shimoi K., Saka N., Nozawa R., Sato M., Amano I., Nakayama T., Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001;29(12):1521–1524. [PubMed] [Google Scholar]
- 62.Soleas G.J., Yan J., Goldberg D.M. Measurement of trans-resveratrol, (+)-catechin, and quercetin in rat and human blood and urine by gas chromatography with mass selective detection. Methods Enzymol. 2001;335:130–145. doi: 10.1016/s0076-6879(01)35238-2. [DOI] [PubMed] [Google Scholar]
- 63.Taddei S., Virdis A., Ghiadoni L., Magagna A., Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97(22):2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 64.Tamion F., Richard V., Renet S., Thuillez C. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(6):G1308–G1314. doi: 10.1152/ajpgi.00154.2007. [DOI] [PubMed] [Google Scholar]
- 65.Tanigawa S., Fujii M., Hou D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 67.Timimi F.K., Ting H.H., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998;31(3):552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 68.Ting H.H., Timimi F.K., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95(12):2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 69.Tsai H.Y., Huang P.H., Lin F.Y., Chen J.S., Lin S.J., Chen J.W. Ginkgo biloba extract reduces high-glucose-induced endothelial reactive oxygen species generation and cell adhesion molecule expression by enhancing HO-1 expression via Akt/eNOS and p38 MAP kinase pathways. Eur. J. Pharm. Sci. 2013;48(4–5):803–811. doi: 10.1016/j.ejps.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Tsukimori K., Fukushima K., Tsushima A., Nakano H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension. 2005;46(4):696–700. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- 71.Westerterp M., Berbée J.F., Pires N.M., van Mierlo G.J., Kleemann R., Romijn J.A., Havekes L.M., Rensen P.C. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116(19):2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 72.Wiczkowski W., Romaszko J., Bucinski A., Szawara-Nowak D., Honke J., Zielinski H., Piskula M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008;138(5):885–888. doi: 10.1093/jn/138.5.885. [DOI] [PubMed] [Google Scholar]
- 73.Yao P., Nussler A., Liu L., Hao L., Song F., Schirmeier A., Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007;47(2):253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Yasui Y., Nakamura M., Onda T., Uehara T., Murata S., Matsui N., Fukuishi N., Akagi R., Suematsu M., Akagi M. Heme oxygenase-1 inhibits cytokine production by activated mast cells. Biochem. Biophys. Res. Commun. 2007;354(2):485–490. doi: 10.1016/j.bbrc.2006.12.228. [DOI] [PubMed] [Google Scholar]
- 75.Yeh C.H., Chen T.P., Wang Y.C., Lin Y.M., Lin P.J. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J. Surg. Res. 2009;155(1):147–156. doi: 10.1016/j.jss.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 76.Youn G.S., Kwon D.J., Ju S.M., Choi S.Y., Park J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. 2013;46(8):410–415. doi: 10.5483/BMBRep.2013.46.8.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeya H.I., Keku E., Richards F., Spurr C.L. Monocyte and granulocyte defect in chronic lymphocytic leukemia. Am. J. Pathol. 1979;95(1):43–54. [PMC free article] [PubMed] [Google Scholar]
- 78.Zhai X., Lin M., Zhang F., Hu Y., Xu X., Li Y., Liu K., Ma X., Tian X., Yao J. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 2013;57(2):249–259. doi: 10.1002/mnfr.201200536. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L., Angst E., Park J.L., Moro A., Dawson D.W., Reber H.A., Eibl G., Hines O.J., Go V.L., Lu Q.Y. Quercetin aglycone is bioavailable in murine pancreas and pancreatic xenografts. J. Agric. Food Chem. 2010;58(12):7252–7257. doi: 10.1021/jf101192k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y., Toborek M., Hennig B. Epigallocatechin gallate-mediated protection against tumor necrosis factor-α-induced monocyte chemoattractant protein-1 expression is heme oxygenase-1 dependent. Metabolism. 2010;59(10):1528–1535. doi: 10.1016/j.metabol.2010.01.018. [DOI] [PubMed] [Google Scholar]