ABSTRACT
Background:
The main reason for biomaterial related refractory infections is biofilm formation caused by bacterial adhesion on the surface of materials. Silver-hydroxyapatite (Ag/HA) nanocomposite coating can inhibit the formation of biofilm, but its mechanism is not clear.
Material and Method:
In order to clarify the mechanism, the amounts of biofilm on the Ag/HA composite coating and HA coating were determined, the release rates of silver nanoparticles in simulated body fluid (SBF) were detected by atomic absorption spectrometry, and the expression values of atlE, fbe, sap, iapB genes of Staphylococcus aureus were studied when they grew on Ag/HA composite coating and HA coating.
Results:
The amount of the biofilm on the Ag/HA composite coating was significantly less than that on the HA coating, and the bacterial adhesion was decreased. The silver nanoparticles were released continuously in SBF and the release rate decreased gradually with time. The expression values of atlE, fbe and sap were high in the initial stage of adhesion and the expression value of iapB was high in the colonies-gathering stage in the control group, but they were all significantly inhibited in the presence of Ag.
Conclusion:
These results indicated that the main antibacterial effect of Ag/HA composite coating was achieved by the release of silver nanoparticles. The addition of Ag inhibited the expression of genes related to biofilm formation, which in turn inhibited the formation of biofilms. This provided theoretical support for the clinical application of Ag/HA composite coating.
Keywords: Biofilm, Escherichia coli, joint prosthesis, silver-hydroxyapatite nanocomposite coating, Staphylococcus aureus
RESUMEN
Antecedentes:
La razón principal de las infecciones refractarias relacionadas con biomateriales es la formación de biopelículas causada por la adhesión de bacterias a la superficie de los materiales. El revestimiento con nanocompuestos de hidroxiapatita-plata (Ag/HA) puede inhibir la formación de biopelículas, pero su mecanismo no está claro.
Material y Método:
Para aclarar el mecanismo, se determinaron las cantidades de biopelículas en el revestimiento con Ag/HA y HA, se detectaron las tasas de liberación de nanopartículas de plata en el fluido corporal simulado (FCS) mediante espectrometría de absorción atómica, y se estudiaron los valores de expresión de los genes atlE, fbe, sap e iapB del Staphylococcus aureus, desarrollados en el revestimiento de compuesto Ag/HA y el revestimiento de HA.
Resultados:
La cantidad de biopelículas en el revestimiento de compuesto Ag/HA fue significativamente menor que la hallada en el revestimiento HA, y disminuyó la adhesión bacteriana. Las nanopartículas de plata fueron liberadas continuamente en el FCS y la tasa de liberación disminuyó gradualmente con el tiempo. Los valores de expresión de atlE, fbe y sap fueron altos en la etapa inicial de la adhesión, y el valor de la expresión de iapB fue alto en la etapa de formación de colonias en el grupo control, pero todos fueron significativamente inhibidos en presencia de Ag.
Conclusión:
Estos resultados indicaron que el principal efecto antibacteriano del revestimiento con compuesto Ag/HA fue logrado por la liberación de las nanopartículas de plata. La adición de Ag inhibió la expresión de los genes relacionados con la formación de biopelículas, que a su vez inhibe la formación de biopelículas. Esto proporcionó apoyo teórico a la aplicación clínica del revestimiento con compuesto Ag/HA.
INTRODUCTION
At present, the main reason for biomaterial related refractory infections is biofilm formation caused by bacterial adhesion on the surface of materials, and traditional antibiotics treat these infections poorly (1). Bacteria can cause biofilm formation in the implanted material surface and the adjacent tissues, resulting in damage to the local tissues (2). Recent studies showed that even if systematical preoperative antibiotic prophylaxis treatments were performed, total infection rates of hip and knee arthroplasty still reached 0.5% and 4%, respectively (3). It was reported that the infection rate of nail tracts was not the same, but the average incidence of serious nail tract infection was 5.8%, and it was necessary for patients to get antibiotics and remove screws or external fixator nails in hospital (4). Therefore, the postoperative infection of artificial joints and implants is still a difficult problem in orthopaedics surgery.
Due to the physical and physiological barrier function of the membrane, endophytic bacteria in the biofilms have stronger resistance to antibiotics than free bacteria (5). Therefore, it is difficult to completely remove the biofilms using antibiotics, which only kill the free bacteria on the membrane surface (6). With the increase of resistant strains, silver ions were developed and researched by scholars as an antimicrobial agent (7). Silver (Ag) has a very broad antibacterial spectrum, and can kill hundreds of pathogenic bacteria and virus (8–10). In addition, Ag has antioxidant, corrosion resistance (11), high thermal stability, low cell toxicity (12, 13) and other features. Recently, it has become a very effective method to use Ag to kill micro-organisms on biological materials and medical devices (14). Hydroxyapatite (HA) is similar to the chemical composition and crystal structure of human hard tissue, which has unique biological activity and biocompatibility (15). The HA coating on the metal material (such as titanium or titanium alloy) can enhance the excellent mechanical properties of metal materials and highlight the good biocompatibility of HA (16, 17).
With the developments in materials science, biological materials have been studied and applied, from their passive adaptation to the biological environment to purposefully designing the material component and preparing biological composite materials which have special functions (18). In recent years, a new type of composite coating, which has good biocompatibility and bioactivity, has become a research hotspot. Silver-hydroxyapatite composite coating meets the above requirements. Silver-hydroxyapatite biological composite has been shown to have superior bone conduction and bone induction by HA (19). Chen et al (20) reported in vitro antibacterial and biological properties of Ag/HA coatings prepared by application of magnetic sputtering. Research on antibacterial properties and bone compatibility or biocompatibility of silver-containing hydroxyapatite coatings prepared by application of sol-gel (21, 22) and research on in vitro antibacterial properties and cytotoxicity of silver or platinum-containing hydroxyapatite coatings prepared by application of micro arc oxidation have been reported (23). In these methods of preparation, thermal spraying, especially plasma spraying technology is mature and is currently the only widely used method in clinical practice (24). There have been scientists who successfully prepared silver-containing HA antibacterial coating using vacuum plasma spraying and detected and reported its preliminary antibacterial properties.
However, there are not many studies on the mechanism of Ag/HA nanocomposite coating on artificial joints. Staphylococcus aureus and Escherichia coli are the main bacteria in postoperative infection of artificial joints and implants (25, 26). It is worthy to research meticulously how the biofilms form and how Ag/HA nanocomposite coating inhibits biofilms, which will provide the theory support for their clinical application. In this paper, we adopted vacuum plasma spraying technology in the preparation of antibacterial HA coatings containing 0.3% and 0.6% (m/m) of silver antibacterial agents on the surface of titanium alloy (Ti-6Al-4V) substrate, and detected the sustained release rates of silver ions in simulated body fluid, the effects on the biofilm formation of bacteria (27) and the expression of genes related to biofilm formation of S aureus.
MATERIAL AND METHODS
Preparation and detection of materials
The particle size of HA powder (Sulzer Metco, Switzerland) ranged from 15 ~ 50 μm; 99.7% and 99.4% (m/m) of HA powder and 0.3% and 0.6% (m/m) of silver powder were ball milled and mixed for two hours, respectively, and then prepared for HA coating and silver containing HA composite coating (Ag/HA coating) on titanium alloy substrate (size: 30 mm × 10 mm × 2 mm and 6 mm × 2 mm) using vacuum plasma spraying equipment (Sulzer Metco, Switzerland). The spraying parameters are shown in Table 1. According to the standard of ASTM C-633, the bonding strength of the pure HA coating andAg/HA composite coating was 20.1 and 23.4 MPa, respectively (28, 29).
Table 1. Spraying parameters of vacuum plasma spraying system.
| Parameters | Values |
|---|---|
| Plasma gas Ar | 50 L·min-1 |
| Plasma gas H2 | 20 L·min-1 |
| Spraying distance | 400 mm |
| Thickness of coating layer | 300 μm |
| Pressure of vacuum chamber | 200 mbar (1 mbar = 100 Pa) |
| Powder-feeding carrier gas Ar | 1.0 L·min-1 |
| Powder-feeding rate | 30 g·min-1 |
| Current (I) | 800 A |
| Voltage (U) | 80 V |
The spectrum of X-ray diffraction (XRD) of 0.6% Ag/HA coating sample was detected by X-ray powder diffraction (PW3040/60, CuKα, 40 kV, 40 mA, scanning speed of 2°/min, scanning range of 20° ~ 120°). The spectrum of energy dispersive spectrometer (EDS), image of scanning electron microscope (SEM) and elemental analysis of 0.6% Ag/HA coating were detected using the scanning electron microscope (QUANTA 200) with EDAX energy spectrum analysis.
Inhibition zone test
Freeze-dried strains of S aureus ATCC25923 and E coli ATCC25922 were resuscitated, inoculated on the agar plate and cultivated in a 37 °C constant incubator for one day. The strains morphologically identified for pure cultures were prepared by the following steps.
Each piece of three wafer-shaped materials and one vancomycin tablet were placed on the 90 mm agar medium inoculated with S aureus (inoculation amount to 1.5 × 107 CFU ie colony forming units). Each piece of three wafer-shaped materials and one ceftazidime tablet were placed on the 90 mm agar medium inoculated with E coli (inoculation amount to 1.5 × 107 CFU). These plates were cultivated in a 37 °C constant incubator for one day. After the inhibition zones were detected, the materials and tablets were taken out and placed on the new agar medium inoculated with S aureus or E coli for continuous incubation. Then the inhibition zones were detected every three days and the media were changed. The sizes and duration of the inhibition zones were recorded. Each strain was repeated five times.
Observation of adhesion by SEM
Three wafer-shaped materials were soaked in suspension containing 1.5 × 106 CFU/mL of S aureus or E coli and cultivated in a 37 °C constant incubator for three hours. After they were taken out and flushed with phosphate buffered saline (PBS) buffer, these materials were placed into a 2.5% glutaraldehyde solution to fix for two hours and then flushed with PBS buffer and dehydrated in graded ethanol. They were subsequently placed into a 100% isoamyl acetate solution to do replacement treatment for 25 minutes. Then the adhesion of bacteria was detected under the SEM after the materials were placed into a CO2 critical point dryer for critical point drying and sprayed by gold.
Colony number experiment
The suspension containing 3 × 108 CFU/mL of S aureus or E coli was prepared and 100 µl of the suspension was dripped on the surface of three wafer-like materials and covered with coverslips to make bacteria suspension evenly distributed with no bubbles, then cultivated in a 37 °C constant incubator for one day. The materials were placed in 4.9 mL of PBS buffer and oscillated for five minutes on the vortex oscillation apparatus to elute the bacteria. Next, 100 µl of the elution solution was used to wash the plate and the bacteria were counted after being cultivated in a 37 °C constant incubator for three days. Each strain was repeated five times. The antibacterial rate (%) was calculated by the following formula:
 |
In vitro Ag+ release test
Simulated body fluid (SBF) was formulated according to the method of Kokubo et al. The granular materials A2 and A3 were cleaned by acetone ultrasonic washing, leached with deionized water, dried, and then immersed into 50 mL of SBF for extraction at 37 °C, avoiding light. The concentration of Ag+ was detected at 1, 3, 7, 14, 21 and 49 days by the Unicam 939/959 atomic absorption spectrometer and three samples were taken at each time point.
Expression of related genes
The suspension containing 3 × 108 CFU/mL of S aureus or E coli was prepared and cultivated in a 37 °C constant incubator for two days and seven days. The materials were placed into 5 mL of PBS buffer and oscillated for five minutes on the vortex oscillation apparatus to elute the bacteria. The elution solution was centrifuged and the bacteria were collected as a pellet. RNA was extracted using the RNAprep Pure Cell/Bacteria Kit (Tiangen, Beijing, China) based on the manufacturer's protocol and then reverse transcribed to cDNA using Prime-Script™ RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China).
The atlE, fbe, sap, iapB genes of S aureus were searched in GenBank of the National Centre of Biotechnology Information (NCBI) and their polymerase chain reaction (PCR) primers were designed (Table 2). Fluorescence quantitative PCR amplification (qRT-PCR) was performed using cDNA as template and iQ™ SYBR® Green supermix (BIO-RAD, USA) as work solution on the CFX96 Touch™ Real-Time PCR Detection System (BIO-RAD, USA). Each sample was repeated four times.
Table 2. Primers of qRT-PCR for atlE, fbe, sap and iapB genes of Staphylococcus aureus.
| Gene | Protein | Forward primer (5’ to 3’) | Reverse primer (5’ to 3’) | Product size (bp) | |
|---|---|---|---|---|---|
| atlE | autolysin | TAACTGGGATAACGCAGCGG | TGGTAAGCTGGTACCGTTCTG | 204 | |
| AtlE | |||||
| fbe | fibrinogen | AGATGCGAGCGAAGGATACG | TGCATCAGTTTTCGCTGCTG | 229 | |
| binding | |||||
| protein | |||||
| sap | surface | CGTGTTCGACAATGATGGCG | GCGATGCTGTTTTCGCTTCA | 228 | |
| anchored | |||||
| protein | |||||
| iapB | intercellular | ACACATACCCACGATTTGCAT | GGAGTTCGGAGTGACTGCTT | 246 | |
| adhesion | |||||
| protein B |
Statistical analysis
SPSS 17.0 statistical software package was used for analysis. Data are shown as mean ± SD and the difference between groups was compared with t-test. When p < 0.05, the difference was significant, p < 0.01 was very significant and p < 0.001 was extremely significant.
RESULTS
Composition and structure of Ag/HA
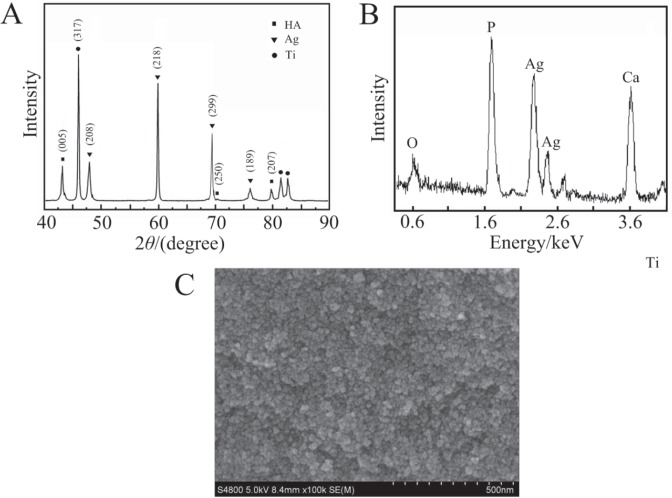
The XRD spectrum of the 0.6% Ag/HA composite coating prepared by vacuum plasma spraying is shown in Fig. 1A. The main diffraction peaks of HA: 005, 250 and 207, all appeared in the spectrum. The strongest diffraction peak of HA was 005 crystal plane at 30.5° and the next in strength was 207 crystal plane at 27.9°; these were in complete agreement with the standard spectrum (#74-0566). The strongest diffraction peaks of Ag in the Ag/HA composite coating was 218 planes at 37.3°. The second strongest peak was 299 planes at 47.1°, which was completely consistent with the standard atlas (# 04-0783). From the above analysis, the composition of the composite coating was HA and Ag, and the crystal was good.
Fig. 1. Composition and microstructure of silver-hydroxyapatite (Ag/HA) composite coating. A: the XRD spectrum; B: the EDS spectrum; C: the SEM image (the inset is the magnification of the rectangle). B and C are the EDS spectrum and SEM images of Ag/HA composite coating. The main elements of the composite coating are calcium, phosphorus, silver and oxygen (B). The coating is uniform in the nanoparticles and covers the titanium substrate, which is similar to approximate ball (C). The size of the particles is 100 ~ 300 nm from the photo of local magnification of the composite coating (C). The results show that the distribution of Ag particles in the Ag/HA composite coating is more uniform.
Inhibition zone test
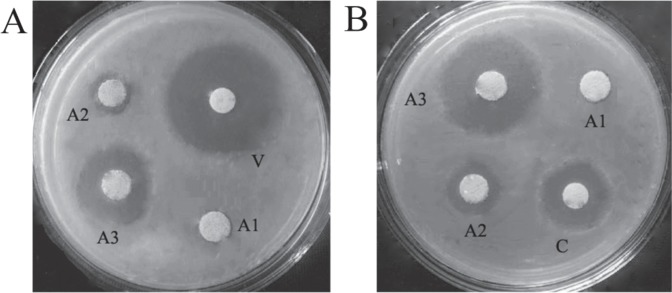
There was no antibacterial circle of material A1 in the whole process of the experiment (Fig. 2). The antibacterial circles of the two drug tablets appeared at one day. The diameter of the antibacterial circle of vancomycin tablet against S aureus was 17.76 ± 0.89 mm and that of ceftazidime tablet against E coli was 30.06 ± 3.78 mm; there was no antibacterial circle from the second day. The antibacterial circles of the materials of A2,A3 on day one were the largest diameter (Fig. 2), in which the antibacterial circles of the materials of A2 against S aureus and E coli were 14.61 ± 1.99 mm and 9.95 ± 1.28 mm, respectively, and that of A3 were 23.60 ± 1.81 mm and 18.45 ± 0.79 mm. The differences of different materials with the same bacterium and its related tablet were statistically significant (p < 0.05). The diameters of inhibition zones decreased gradually over time, the time duration of antibacterial circle appearing around material A2 against S aureus and E coli was 14 and 32 days, respectively, and that of A3 was 10 and 25 days, but there were no bacteria on the part directly in contact with materials of A2 and A3 (Fig. 2).
Fig. 2. Antibacterial effect after one day. A: Staphylococcus aureus; B: Escherichia coli. A1: hydroxyapatite (HA); A2: HA + 0.3% silver (Ag); A3: HA + 0.6% Ag; V: vancomycin; C: ceftazidime.
Bacterial adhesion
Under the microscope, there was a large number of bacteria adhering to the surface of material A1, which was distributed like a crumb or linear. There were less bacteria adhering to the surface of materials A2 and A3, whose distribution was dispersed.
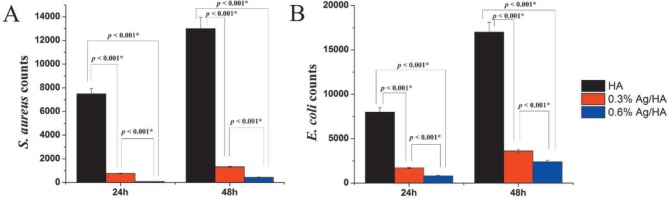
After contact with material A1, S aureus and E coli grew continuously, thus there was no antibacterial property. On the contrary, their growth slowed down significantly after contact with material A2 and A3 (Fig. 3). The antibacterial rates of A2 against S aureus and E coli were 87.75 ± 4.04% and 77.76% ± 2.39%, and that of A3 were 93.09% ± 3.99% and 84.98% ± 3.17% respectively. The difference of the same bacterium groups was statistically significant [p < 0.05] (Fig. 3).
Fig. 3. Colony forming units (CFU) of Staphylococcus aureus and Escherichia coli in direct contact with composite materials. A: S aureus; B: E coli.
*the difference was significant; HA: hydroxyapatite; Ag: silver
In vitro Ag+ release
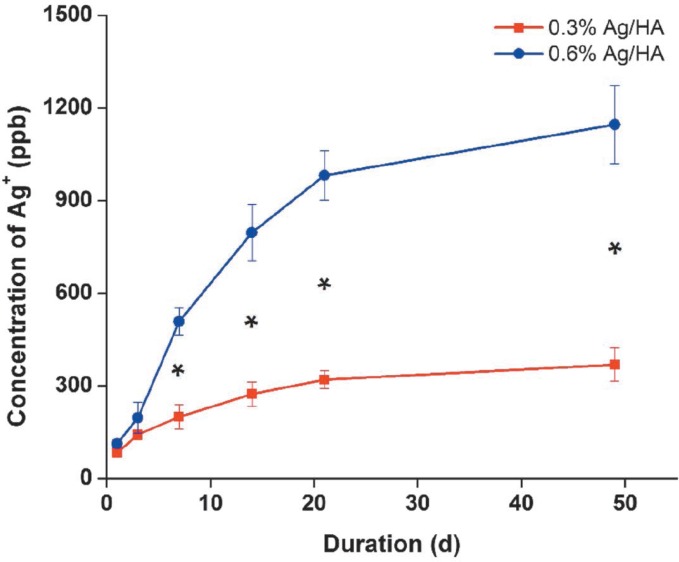
The concentrations of Ag+ in SBF increased gradually at 1, 3, 7, 14, 49 and 21 days. At the beginning, the release rate of Ag+ was very fast, but decreased gradually with the extension of time (Fig. 4). There was no significant difference in the concentrations two and three days after immersion (p > 0.05) and there were significant differences in the concentrations after seven days [p < 0.05] (Fig. 4). The concentrations of Ag+ in the A3 group were higher than those in the A2 group at each point [p < 0.05] (Fig. 4).
Fig. 4. Release amount of Ag+ at different durations in simulated body fluid (SBF). *the difference was significant; Ag: silver; HA: hydroxyapatite.
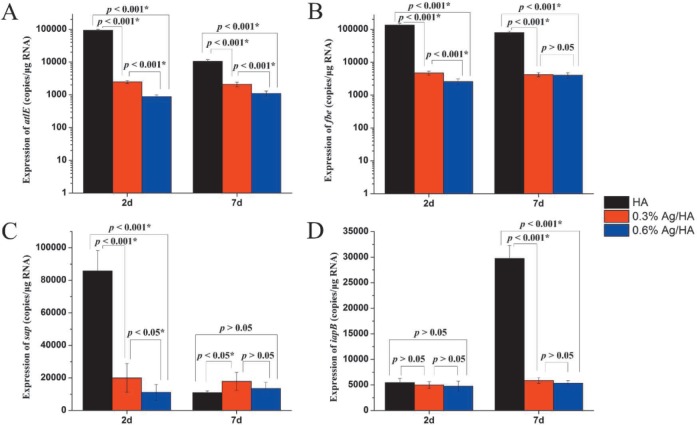
Expression of related genes
From the qRT-PCR results (Figs. 5 A and B), we found that the expressions of atlE and fbe genes were similar. On both day two and day seven, the expressions of atlE and fbe genes in the 0.3% Ag/HA and 0.6% Ag/HA groups were all much lower than those in the HA group (p < 0.001) and the expressions of atlE and fbe genes in the 0.3% Ag/HA group were much lower than in the 0.6% Ag/HA group (p < 0.001). This showed that Ag+ had obvious inhibition effect on atlE and fbe genes, and the inhibition effect was enhanced with the increase of Ag+ concentration.
Fig. 5. The expression of four genes of Staphylococcus aureus in direct contact with composite materials after two and seven days.
*the difference was significant; HA: hydroxyapatite; Ag: silver
On day two, the expression of sap gene in the HA group was much higher than that in the 0.3% Ag/HA and 0.6% Ag/HA groups (p < 0.001), but there was no difference on day seven [p > 0.05] (Fig. 5C). When comparing day two and day seven, the expression of sap gene in the HA group decreased significantly but there was no difference in the 0.3% Ag/HA and 0.6% Ag/HA groups, indicating that sap gene expressed abundantly in the initial bacterial attachment stage but decreased in the biofilm accumulation phase. Due to the effect of Ag+, the expression of sap gene was always inhibited in the 0.3% Ag/HA and 0.6% Ag/HA groups (Fig. 5C).
There was no difference in the expression of iapB gene on day two (p > 0.05). However, it increased significantly in the HA group on day seven (p < 0.001), but it remained as on day two in the 0.3% Ag/HA and 0.6% Ag/HA groups [p > 0.05] (Fig. 5D). There was no difference of expression of iapB gene between day two and day seven (p > 0.05). This indicated that the sap gene expressed rarely in the initial bacterial attachment stage but increased sharply in the biofilm accumulation phase. However, the expression of the sap gene was always inhibited in the 0.3% Ag/HA and 0.6% Ag/HA groups (Fig. 5D).
DISCUSSION
In the department of orthopaedics, more and more studies have focussed on the performance of implants to prevent bacterial colonization and biofilm formation. In addition to the use of antibiotics for bone cement prevention of infection, the study of local antimicrobial methods mainly focussed on the surface of the implant and the use of antibacterial materials. Although the incidence of systemic antibiotics can prevent postoperative infection, for implant-related infection, using systemic antibiotics only is not enough to prevent bacterial colonization on the surface of the implant and the surrounding tissue. If bacteria are planted on the surface of the implant, using antibiotics does little to reduce the formation of infection. Gristina et al (30, 31) studied the interaction between the complex environment of the body and the implant. The authors suggested that the polluted bacteria will compete with immune cells of the body for planting on the surface of the implant. In this case, the bacteria have a better advantage to adapt to the environment because of the rapid and extreme variability. The body's immune system is weakened by the presence of foreign bodies, and loses the competition to the bacteria. It is a reasonable method to prevent bacterial colonization from the surface of the implant if the implant is released immediately after implantation (32).
The bacterial biofilm is the growth process formed in order to adapt to changes in the environment and attach to a body tissue or biological material surface, which is composed of bacteria and extracellular matrix corresponding with planktonic cells. After the formation of the biofilm, bacteria are encapsulated in the extracellular matrix and increase their resistance on clearance of the immune system and effect of antibiotics, which make the two mechanisms difficult to effectively remove the focus of infection. Bacteria in the biofilm can also grow continuously and be released into the blood, which causes chronic infection and recurrence of infection after discontinued treatment of antibiotics and leads to a protracted illness (33). In recent years, basic research and clinical research on bacterial biofilm has become a hot spot in the field of anti-infection.
Bacterial biofilm refers to the highly organized multicellular group structure, in which bacteria are attached to the living or non-living surface and are covered by its own secretory extracellular slime substance. The formation of S aureus biofilm is divided into two stages: the first is initial attachment on biomaterial by bacterial protein of surface hydrophobicity or polysaccharide intercellular adhesion, and the second is biofilm formation mainly mediated through the polysaccharide intercellular adhesion [PIA] (34, 35). Bacterial initial attachment is a process in which bacteria directly attach to the surface of biological material via the factor of the bacteria. Biological material is soon covered by all kinds of attachment proteins after being implanted in the body. Once combined in biological materials or the receptor protein absorption on the material surface layer, bacteria ligandis firmly stick on the surface of biological materials.
Plasma proteins (fibrinogen, fibrin and albumin) affect the surface absorption of S aureus on the surface of the material; this is also an important factor to determine the composition of the biofilm. Fibrinogen and fibrin are beneficial to the bacterial adhesion and the formation of platelet complex, which forms a thrombus containing bacteria on the surface of the material, while the absorption of albumin on the formation of the complex with the platelet is not conducive to bacterial adhesion. There are many factors involved in the initial attachment phase of S aureus, such as capsular polysaccharide adhesion (PSPA), autolysin AtlE, fibrinogen binding protein (FBE) and staphylococcal surface protein [SSP] (36). Following the initial attachment, the biofilm is formed by cell proliferation and mutual adhesion with formation of multilayer cell clumps and is coated with mucus on the external surface. After adhesion to the surface, the cell adjusts its gene expression to produce a large number of exopolysaccharide (EPS) as well as to grow. Bacteria can be glued by EPS to form bacterial clumps, ie microcolony. A large number of microcolonies makes the biofilm thick, so the development of the EPS molecule is very important for the development of the biofilm structure. Mature biofilm is a highly organized structure and is composed of microcolonies. Between these microcolonies, there are many pipelines which can transport nutrients, enzymes, metabolites and the discharge of wastes. Biofilm formation is a dynamic process and its structure is inhomogeneous. Inheterogeneity is another important feature of bacterial biofilm involved in the differentiation of functional bacteria and bacterial resistance. Some factors are associated with the aggregation stage, such as PIA, haemagglutinin and accumulation associated protein (AAP). Polysaccharide intercellular adhesion is essential to biofilm formation (37).
atlE gene expresses to secrete autolysin and mediates bacterial adhesion in the adhesion stage. The adhesion ability of strain deleted atlE gene decreases significantly. After being implanted into the human body, biological material will soon be covered by blood components including fibronectin, fibrinogen, laminin, platelet and others. The expression of fbe and sap genes results in synthesis of fibre protein binding protein mediated attachment of S aureus and fibrin. During the accumulation phase, iapABD gene expresses to synthesize extracellular matrix and finally forms pileus-like multilayer structure in mature biofilms. In addition to genetic regulation, the biofilm formation of S aureus is also involved with environmental factors such as glucose, ethanol and blood components. The infection of S aureus is associated with the implantation of biological material and not with the genetic factors of strains. In vitro experiments prove that under the condition of serum components, adhesion phenomenon is not an important virulence factor (38, 39). Genes are just some of the factors that affect the biofilm formation, but not the determining factor. Therefore, it is not enough to prove that the formation of these genes and the formation of the biofilm are necessary. This study only confirmed that the Ag/HA composite coating is also involved in the change of the surface activity, and the specific mechanism needs to be further explored.
CONCLUSION
Silver-hydroxyapatite nanocomposite coating can obviously inhibit the formation of biofilm. The amount of biofilm on the Ag/HA composite coating was significantly less than that on the HA coating, and the bacterial adhesion was decreased. The expression values of atlE, fbe and sap were high in the initial stage of adhesion and that of iapB was high in the coloniesgathering stage in the control group, but they were all significantly inhibited in the presence of Ag. These results indicate that the main antibacterial effect of Ag/HA composite coating was achieved by the release of silver nanoparticles. This provided theoretical support for the clinical application of Ag/HA composite coating, which has a very broad medical application prospect.
REFERENCES
- 1.Chen W, Oh S, Ong AP, Oh N, Liu Y, Courtney HS, et al. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process. J Biomed Mater Res A. 2007;82:899–906. doi: 10.1002/jbm.a.31197. [DOI] [PubMed] [Google Scholar]
- 2.Stoodley P, Kathju S, Hu FZ, Erdos G, Levenson JE, Mehta N, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clin Orthop Relat Res. 2007;437:31–40. doi: 10.1097/01.blo.0000175129.83084.d5. [DOI] [PubMed] [Google Scholar]
- 3.Stigter M, Bezemer J, de Groot K, Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Rel. 2004;99:127–137. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Green SA. Complications of external skeletal fixation. Clin Orthop Relat Res. 2007;180:109–116. [PubMed] [Google Scholar]
- 5.Lindsay D, von Holey A. Bacterial biofilms within the clinical setting: what healthcare professionals should know. J Hosp Infect. 2006;64:313–325. doi: 10.1016/j.jhin.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2007;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stobie N, Duffy B, McCormack DE, Colreavy J, Hidalgo M, McHale P, et al. Prevention of staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials. 2008;29:963–969. doi: 10.1016/j.biomaterials.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 8.Kawashita M, Tsuneyama S, Miyaji F, Kokubo T, Kozuka H, Yamamoto K, et al. Antibacterial silver-containing silica glass prepared by sol-gel method. Biomaterials. 2000;21:393–398. doi: 10.1016/s0142-9612(99)00201-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Q, Liu Y, Wang C. Development and evaluation of electroless Ag-PTFE composite coatings with anti-microbial and anti-corrosion properties. Appl Surf Sci. 2005;252:1620–1627. [Google Scholar]
- 10.Betts AJ, Dowling DP, McConnell ML, Pope C. The influence of platinum on the performance of silver-platinum anti-bacterial coatings. Mater Design. 2005;26:217–222. [Google Scholar]
- 11.Kim TN, Feng QL, Kim JO, Wu J, Wang H, Chen GC, et al. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J Mater Sci Mater Med. 1998;9:129–134. doi: 10.1023/a:1008811501734. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Stevens SE., Jr. Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals. 1998;11:27–32. doi: 10.1023/a:1009253223055. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL, Doherty PJ, Vince DG, Grashoff GJ, Williams DF. The biocompatibility of silver. Crit Rev Biocomp. 1989;5:221–243. [Google Scholar]
- 14.Bellantone M, Williams HD, Hench LL. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob Agents Chemother. 2002;46:1940–1945. doi: 10.1128/AAC.46.6.1940-1945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanovska AA, Stanislavov AS, Sukhodub LB, Kuznetsov VN, Illiashenko VY, Danilchenko SN, et al. Silver-doped hydroxyapatite coatings formed on Ti-6Al-4V substrates and their characterization. Mater Sci Eng C Mater Biol Appl. 2014;36:215–220. doi: 10.1016/j.msec.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Kenney EB, Lekovic V, Carranza FA, Jr, Dimitrijeric B, Han T, Takei H. A comparative clinical study of solid and granular porous hydroxylapatite implants in human periodontal osseous defects. J Biomed Mater Res. 1988;22:1233–1243. doi: 10.1002/jbm.820221211. [DOI] [PubMed] [Google Scholar]
- 17.Daculsi G, Passuti N, Martin S, Deudon C, Daculsi G, Passuti N, et al. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990;24:379–396. doi: 10.1002/jbm.820240309. [DOI] [PubMed] [Google Scholar]
- 18.Shirkhanzadeh M. Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes. J Mater Sci Mater Med. 1998;9:67–72. doi: 10.1023/a:1008838813120. [DOI] [PubMed] [Google Scholar]
- 19.Chaki TK, Wang PE. Densification and strengthening of silver reinforced hydroxyapatite-matrix composite prepared by sintering. J Mater Sci Mater Med. 1994;5:533–542. [Google Scholar]
- 20.Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, et al. In vitro anti-bacterial and biological properties of magnetron cosputtered silver-containing hydroxyapatite coating. Biomaterials. 2006;27:5512–5517. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Zheng X, Xie Y, Ding C, Ruan H, Fan C, et al. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing HA coatings. J Mater Sci Mater Med. 2008;19:3603–3609. doi: 10.1007/s10856-008-3529-8. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH, et al. Antibiofilm dentin primer with quaternary ammonium and silver nanoparticles. J Dent Res. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song WH, Ryu HS, Hong SH. Antibacterial properties of Ag (or Pt)-containing calcium phosphate coatings formed by micro-arc oxidation. J Biomed Mater Res A. 2009;88:246–254. doi: 10.1002/jbm.a.31877. [DOI] [PubMed] [Google Scholar]
- 24.Ersek RA, Denton DR. Silver impregnated porcine xenografts for treatment of meshed autografts. Ann Plast Surg. 1984;13:482–487. doi: 10.1097/00000637-198412000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Barth E, Myrvik QM, Wagner W, Gristina AG. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials. 1989;10:325–328. doi: 10.1016/0142-9612(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 26.Gristina AG, Shibata Y, Giridhar G, Kreger A, Myrvik QN. The glycocalyx, biofilm, microbes, and resistant infection. Semin Arthroplasty. 1994;5:160–170. [PubMed] [Google Scholar]
- 27.Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res. 2001;80:363–370. doi: 10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]
- 28.Yang YC, Chang E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V substrate. Biomaterials. 2001;22:1827–1836. doi: 10.1016/s0142-9612(00)00364-1. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X, Huang M, Ding C. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials. 2000;21:841–849. doi: 10.1016/s0142-9612(99)00255-0. [DOI] [PubMed] [Google Scholar]
- 30.Gristina AG, Giridhar G, Gabriel BL, Naylor PT, Myrvik QN. Cell biology and molecular mechanisms in artificial device infections. Int J Artif Organs. 1993;16:755–763. [PubMed] [Google Scholar]
- 31.Gristina AG, Rovere GD, Shoji H, Nicastro JF. An in vitro study of bacterial response to inert and reactive metals and to methyl methacrylate. J Biomed Mater Res. 1976;10:273–281. doi: 10.1002/jbm.820100208. [DOI] [PubMed] [Google Scholar]
- 32.Qu J, Lu X, Li D, Ding Y, Leng Y, Weng J, et al. Silver/hydroxyapatite composite coatings on porous titanium surfaces by sol-gel method. J Biomed Mater Res B Appl Biomater. 2011;97:40–48. doi: 10.1002/jbm.b.31784. [DOI] [PubMed] [Google Scholar]
- 33.Rudkin JK, Laabei M, Edwards AM, Joo HS, Otto M, Lennon KL, et al. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58:1100–1107. doi: 10.1128/AAC.01618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herkendell K, Shukla VR, Patel AK, Balani K. Domination of volumetric toughening by silver nanoparticles over interfacial strengthening of carbon nanotubes in bactericidal hydroxyapatite biocomposite. Mater Sci Eng C Mater Biol Appl. 2014;34:455–467. doi: 10.1016/j.msec.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Li J, Wang L, Huang D, Zuo Y, Li Y. The release properties of silver ions from Ag-nHA/TiO2/PA66 antimicrobial composite scaffolds. Biomed Mater. 2010;5:044105–044105. doi: 10.1088/1748-6041/5/4/044105. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J Dent. 2013;41:504–513. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim PN, Shi Z, Neoh KG, Ho B, Tay BY, Thian ES. The effects of silver, silicon-containing apatite towards bacteria and cell responses. Biomed Mater. 2014;9:015010–015010. doi: 10.1088/1748-6041/9/1/015010. [DOI] [PubMed] [Google Scholar]
- 38.Eto S, Miyamoto H, Shobuike T, Noda I, Akiyama T, Tsukamoto M, et al. Silver oxide-containing hydroxyapatite coating supports osteoblast function and enhances implant anchorage strength in rat femur. J Orthop. 2015;33:1391–1397. doi: 10.1002/jor.22903. [DOI] [PubMed] [Google Scholar]
- 39.Chung RJ, Hsieh MF, Huang CW, Perng LH, Wen HW, Chin TS. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J Biomed Mater Res B Appl Biomater. 2006;76:169–178. doi: 10.1002/jbm.b.30365. [DOI] [PubMed] [Google Scholar]