Abstract
The NSABP B-30 trial addresses whether amenorrhea after adjuvant chemotherapy increases survival. Preliminary to the trial outcome analysis, we examined the incidence of amenorrhea and its relationship to symptoms and quality of life (QOL) in the standard-care arm of this adjuvant breast cancer trial. Premenopausal women treated on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm were included. Questionnaires assessing menstrual history, QOL, and symptoms were administered at baseline, day 1 of cycle 4 (or 9 weeks from start of chemotherapy for those who stopped chemotherapy early), and at 6, 12, and 24 months. Seven hundred and eight patients were evaluable for the analysis, with median potential follow-up of 57.5 months. Of these, 321 patients also participated in the QOL substudy. Of the 708 patients, 83% reported ≥1 episode of amenorrhea for ≥6 months. The estimated rate of resumption of menses at 24 months was 45.3% for women<40 years, 10.9% for women 40–50, and 3.2% for women >50 years. Those treated with tamoxifen were more likely to become amenorrheic (p = 0.003). Menstrual status was not significantly associated with QOL or symptoms. Prolonged amenorrhea is associated with a regimen that contains doxorubicin, cyclophosphamide, and docetaxel, and is age dependent and impacted by tamoxifen use. Vasomotor symptoms are common in this patient population but are not associated with menstrual status. These results can be used to inform premenopausal women about the risk and time course of amenorrhea associated with this common adjuvant therapy regimen, along with the effects on symptoms and QOL.
Keywords: Amenorrhea, Breast cancer, Docetaxel
Introduction
Survival after a diagnosis of breast cancer has been increasing for many years as a result of earlier detection and broader application of adjuvant systemic therapy. An overview analysis in 2005 reported that adjuvant chemotherapy resulted in a much larger benefit in younger women than in older women [1]; it has been postulated that part of such benefit results from ovarian ablation induced by chemotherapeutic agents. There also is evidence that either surgical or radiation-induced ovarian ablation by itself is associated with improvement in survival [1], although no additional survival benefit has been observed when ovarian ablation was added to adjuvant chemotherapy [2]. Because the majority of breast cancers in premenopausal women are hormone-receptor positive, it is imperative that we examine the extent of the effect chemotherapy has on ovarian function suppression and ovarian ablation.
The first significant report of chemotherapy-induced amenorrhea was by Fisher et al. from a National Surgical Adjuvant Breast and Bowel Project (NSABP) trial with thiotepa [3]. In this trial the incidence of such amenorrhea was much higher in women 40 years of age or older. However, the occurrence of amenorrhea was not associated with increased survival. A 20-year update of the Milan group’s cyclophosphamide, methotrexate, 5-fluorouracil (CMF) data also found no survival benefit in those patients in whom amenorrhea resulted from chemotherapy [4]. Both these reports, however, are limited by very small numbers of patients.
As a result of uncertainties about the frequency of amenorrhea with contemporary adjuvant therapy regimens and limited information about the potential benefit of ovarian suppression, we undertook a menstrual history study as part of a randomized clinical trial in node-positive breast cancer patients, NSABP B-30. The goal was to document prospectively changes in menstrual history in premenopausal women who received adjuvant chemotherapy in this protocol and to examine the patterns of amenorrhea across the three treatment arms of the study. The NSABP B-30 trial also provides a unique opportunity to assess the effect of treatment-induced amenorrhea on disease-free survival (DFS) and overall survival (S), as well as its relationship to symptoms and quality of life (QOL). No outcome results are available from the trial as yet. We report here the descriptive findings on treatment-induced amenorrhea from the control arm of the trial, which included adjuvant treatment with four cycles of doxorubicin plus cyclophosphamide followed by four cycles of docetaxel. The aims of this analysis were to describe the characteristics of patients who became amenorrheic, the patterns of amenorrhea, and the relationship between amenorrhea and patient-reported outcomes.
Methods
Patients and assessments
The primary aim of the NSABP B-30 trial is to determine whether four cycles of the combination of doxorubicin, docetaxel, and cyclophosphamide (TAC) will prolong DFS and S in patients with node-positive breast cancer compared to the standard arm of four cycles of doxorubicin and cyclophosphamide followed by four cycles of docetaxel (AC→T). The second primary aimis to determine whether four cycles of the combination of doxorubicin and docetaxel (AT) is at least as effective as the other two regimens in improving outcome. Patients were eligible if they had clinical T1–3, N0–1,M0 breast cancer and at least one positive axillary lymph node on axillary dissection. The AC→T regimen consisted of four cycles of doxorubicin 60 mg/m2 intravenously (IV) and cyclophosphamide 600 mg/m2 IV followed by four cycles of docetaxel 100 mg/m2 IV given every 3 weeks. No prophylactic growth factors were used. For patients who had hormone-receptor-positive tumors, tamoxifen administration began no sooner than 3 weeks and no later than 12 weeks after the last dose of chemotherapy. This protocol was a multi-institutional study approved by all local investigational review boards (IRB), and informed consent was obtained from each patient who participated in the study.
The menstrual history study included all pre- and peri-menopausal women who entered the trial. Women were defined as pre- or peri-menopausal if they had experienced a menstrual cycle within the preceding 12 months and had not undergone a hysterectomy and/or bilateral oophorectomy before randomization. A menstrual history questionnaire was adapted from previous studies of women receiving chemotherapy [5]. There were two versions: a baseline version (MHB) for all patients on B-30, submitted before randomization, and a follow-up version (MHF) for all pre- or peri-menopausal women. The MHF was completed on day 1 of cycle 4 (D1C4) or 9 weeks after start of chemotherapy, and at 6, 12, and 24 months from study entry. All patients who developed a recurrence or second primary cancer also adhered to this schedule.
The QOL sub-study was designed to include the first 2,100 patients who completed a baseline QOL questionnaire. The QOL assessment included the Functional Assessment of Cancer Therapy—Breast (FACT-B), Version 3. The FACT-B instrument is a multi-dimensional 44-item, cancer-oriented measure of QOL [6]. Five subscales provide scores for physical well-being, social/family well-being, patient/physician relationship, emotional well-being (EWB), and functional well-being. Nine additional questions refer to problems commonly experienced by women with breast cancer. The FACT-B instrument has been extensively validated and completed by thousands of cancer patients over the past several years, including patients in several NSABP studies [7]. Patient-reported symptoms were collected by means of a checklist on the QOL questionnaire, using a 7-day recall period. The symptom checklist was based on those used in the NSABP breast cancer prevention trials [8–10]. The severity of each symptom was graded from 0 (‘not at all bothered’) to 4 (‘bothered very much’). The QOL questionnaires were administered at the same time points as the MHF.
Questionnaires were administered in the office at clinic visits, although phone or mail administration was also allowed when necessary. Women were expected to complete the questionnaire on their own without help interpreting the items, although the questionnaire was to be reviewed by staff to prevent unintentional missing data. QOL Missing Data (QMD) forms were to be submitted in lieu of a QOL or MHF form for any assessment that was not obtained. The QMD form is completed by clinical staff and includes questions about the reason(s) for the missed assessment.
Data collection and analysis
The MHF questionnaire requested the date of the most recent menstrual cycle for those women who had experienced menstrual bleeding within the preceding 12 months. Patients were also asked to indicate whether they had experienced changes in menstrual cycles. Based on these data, we constructed intervals of time during which a patient was known to be amenorrheic or not amenorrheic. The cumulative duration of amenorrhea was defined as the total length of time exceeding 1 month with no menstrual period. Discontiguous intervals of amenorrhea were summed for this analysis. (For example: if a woman was amenorrheic for 6 months, then experienced bleeding but became amenorrheic again, the initial 6 months was included in the calculation.) Intervals during which menstrual status was unknown due to gaps in assessments were excluded from the duration, so that the estimated duration is a conservative under-estimate. Patients who never experienced amenorrhea were assigned a duration of 0. For those who were amenorrheic at the time of their last measurement, the duration of amenorrhea was considered censored.
The primary endpoints for this comparison of menstrual status with QOL are the FACT-B EWB scale and the trial outcome index (TOI), which is the average of the physical well-being scale, the functional well-being scale, and the breast cancer-specific module. The symptom endpoints were the vasomotor, gynecological, and dyspareunia scales of the symptom checklist.
Emphasis in this preliminary report is on descriptive analyses rather than hypothesis testing. Formal testing was performed to examine the association between menstrual status and QOL endpoints in a repeated measures analysis. The associations between menstrual status and tamoxifen use or vasomotor symptoms were tested with chi-squared tests. Tests were two-sided at a significance level of 0.05, with an intent-to-treat approach including all subjects with available follow-up information. We used SAS version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
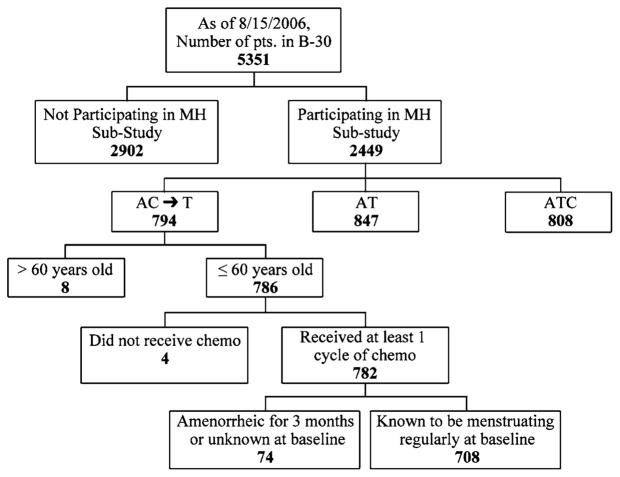
Between 1999 and 2004, 5,351 patients were randomized into the NSABP B-30 trial from 185 clinical centers throughout North America. Of these, 2,449 patients participated in the menstrual history study, and 2,170 participated in the QOL study. Of the 782 patients ≤60 years who were assigned to AC → T and who received at least one cycle of chemotherapy, 708 (91%) had menstruated within 3 months at the start of treatment (Fig. 1). These 708 patients were considered evaluable for the present report. The median duration of potential follow-up for the evaluable cohort at the time of analysis is 57.5 months. Compliance with the menstrual history assessment was high; 83.6% of the expected MHF forms were received. (Forms were expected for all patients who were still alive and had not withdrawn consent at the time of the assessment.) Of those in the evaluable cohort, 321 patients were also in the QOL sub-study; 85.3% of the expected QOL forms were received.
Fig. 1.
Patients participating in the B-30 trial and those who took part in the current study. Of the 782 patients under the age of 61 who were assigned to AC → T and who received at least one cycle of chemotherapy, 708 (91%) had menstruated within 3 months at the start of treatment. (Fig. 1)
Considering the full 24 months of assessments, 83% of patients reported at least one episode of amenorrhea lasting at least 6 months; 9% reported an episode lasting for 3–6 months; and 3% continued menstruating at least once every 3 months but reported changes in their menstrual cycles. The remaining 5% experienced neither amenorrhea nor changes in menstrual cycles.
Table 1 shows distribution of the frequency of amenorrhea for ≥6 months at the 6-month and 12-month assessments according to age and tamoxifen use. Women who were amenorrheic for >6 months at the 6-month assessment had menstruated recently enough at baseline to be considered peri-menopausal but had already begun to miss cycles before they began treatment. Women treated with tamoxifen were more likely to become amenorrheic (p = 0.003 at 6 months and p = 0.03 at 12 months). This difference was observed primarily among younger women, although this preliminary study was not powered to perform the analysis in the younger subset.
Table 1.
Frequency of amenorrhea according to age and tamoxifen use in preliminary analysis subset of NSABP B-30 trial
| Age | Amenorrhea ≥6 months at 6 months
|
Amenorrhea ≥6 months at 12 months
|
||||
|---|---|---|---|---|---|---|
| All (%) | No Tam (%) | Tam (%) | All (%) | No Tam (%) | Tam (%) | |
| ≤30 | 0/19 (0) | 0/10 (0) | 0/9 (0) | 7/17 (41) | 3/10 (30) | 4/7 (57) |
| 30.1–40 | 22/196 (11) | 6/66 (9) | 16/130 (12) | 97/151 (64) | 29/48 (60) | 68/103 (66) |
| 40.1–50 | 132/401 (33) | 18/75 (24) | 114/326 (35) | 300/332 (90) | 53/57 (93) | 247/275 (90) |
| 50.1–60 | 32/55 (58) | 6/10 (60) | 26/45 (58) | 53/54 (98) | 10/10 (100) | 43/44 (98) |
| All ages | 186/671 (28) | 30/161 (19) | 156/510 (31) | 457/554 (82) | 95/125 (76) | 362/429 (84) |
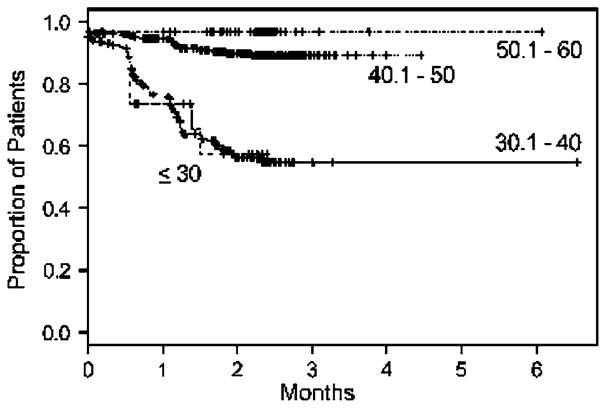
The cumulative duration of amenorrhea by age is shown in Fig. 2. By 24 months, the estimated rates of resumption of menses were 45.3% for women under age 40, 10.9% for women aged 40–50, and 3.2% for women over age 50. Seventy-four percent of women under age 30 and 69% of those 30.1–40 experienced 12 or more months of amenorrhea. Ninety-two percent of women 40.1–50 and 97% of women 50.1–60 were amenorrheic for at least 12 months. Some data were available beyond 24 months because patients continued to submit questionnaires. In some cases this might have been the result of treatment delays.
Fig. 2.
Cumulative duration of amenorrhea (no menstrual bleeding for more than 1 month) by age in preliminary analysis subset of NSABP B-30 trial. 697 patients (11 patients removed due to inadequate data)
Mean scores for the QOL endpoints are shown in Table 2. QOL varied significantly over time (p<0.0001 for both the TOI and EWB). The mean TOI declined initially during treatment and then rose to levels higher than baseline. The EWB increased during treatment and increased again after the completion of treatment. The mean and variance of the TOI and EWB at baseline were comparable to published norms for breast cancer patients [11]. Menstrual status was not significantly associated with either of the sub-scales in longitudinal analysis (p = 0.42 for TOI and p = 0.76 for EWB).
Table 2.
Mean scores for quality of life endpoints: FACT-B trial outcome index (TOI) and emotional well-being (EWB) scale (mean, SD) in preliminary analysis subset of NSABP B-30 trial
| Baseline | Day 1, cycle 4 | Six months | Eighteen months | Twenty-four months | |
|---|---|---|---|---|---|
| TOI | 66.9 (12.7) | 60.7 (15.0) | 60.8 (15.9) | 71.8 (13.4) | 72.2 (13.8) |
| EWB | 17.1 (4.4) | 18.3 (4.4) | 18.9 (4.2) | 19.7 (4.0) | 19.6 (4.1) |
The rates of menopausal symptoms (vasomotor symptoms, gynecological symptoms, and dyspareunia reported at least ‘a little bit’ bothersome) were similar between women who were amenorrheic and those who were not at each time point (Table 3). Vasomotor symptoms were especially common at 6 months, regardless of menstrual status. At 12 months women with amenorrhea had higher rates of vasomotor symptoms (90% versus 55%), but the difference was not statistically significant (p = 0.12). Rates of all menopausal symptoms remained much higher compared to baseline at 12 months.
Table 3.
Menopausal symptoms in preliminary analysis subset of NSABP B-30 trial
| Amenorrheic (%) | Not amenorrheic (%) | |
|---|---|---|
| Vasomotor (hot flashes, night sweats, cold sweats) | ||
| Baseline | 0/0 | 110/319 (34) |
| Day 1, cycle 4 | 0/0 | 200/276 (72) |
| Six months | 70/82 (85) | 163/184 (89) |
| Twelve months | 169/188 (90) | 21/38 (55) |
| Gynecological (genital itching or irritation, vaginal discharge, vaginal bleeding or spotting) | ||
| Baseline | 0/0 | 97/319 (30) |
| Day 1, cycle 4 | 0/0 | 176/276 (64) |
| Six months | 48/82 (59) | 103/184 (56) |
| Twelve months | 81/188 (43) | 21/38 (55) |
| Dyspareunia (vaginal dryness, pain with intercourse) | ||
| Baseline | 0/0 | 32/319 (10) |
| Day 1, cycle 4 | 0/0 | 91/273 (33) |
| Six months | 35/82 (43) | 75/184 (41) |
| Twelve months | 83/188 (44) | 15/37 (41) |
Counts and percentages reporting any symptom at least “a little bit”
Discussion
This study is the first to evaluate amenorrhea in detail after treatment with a sequential chemotherapy regimen containing anthracycline/cyclophosphamide followed by docetaxel. Eighty-three percent of the premenopausal patients in our study experienced amenorrhea for at least 6 months and an additional 9% for at least 3 months. The incidence was higher and the duration of amenorrhea was longer in women over 40. The incidence of amenorrhea was increased with tamoxifen use. However, there were no significant associations between amenorrhea and QOL indices or symptoms in this small subset of patients.
Assessment tools for menstrual history are not well established. The instrument used in this study asked whether or not the patient had experienced menstruation in the previous 12 months and, if so, the date of that menstrual cycle. The use of patient calendars or diaries has been reported in several large clinical studies [12–14], and in this study women were given calendars for their own use, but calendar data were not collected. This approach allows uncertainty. For example, a woman with a recent menstrual cycle might have been amenorrheic in the previous interval. For the present analysis, we used all available information and were most often able to reconstruct the sequence of menstrual cycles. However, an approach requiring full calendars would yield more complete data.
There have been many reports confirming that chemotherapy-induced amenorrhea is more frequent in women as they get older and is more often irreversible. This is most certainly due to the fact that there are fewer active ovarian follicles with increasing age. Chemotherapy-induced amenorrhea has long been thought to be associated with an increase in chemotherapy efficacy due to its effect on ovarian suppression. However, the literature is not clear on this point because most studies are small, do not define or collect amenorrhea data consistently and prospectively, do not distinguish estrogen receptor status, and do not report tamoxifen interactions. This issue was recently reviewed [15].
The International Breast Cancer Study Group carefully addressed amenorrhea in its Trial 13–93 [16]. Premenopausal women with positive lymph nodes were randomized to an anthracycline-containing regimen followed by immediate or delayed CMF and to tamoxifen or no further treatment. In 735 premenopausal women, amenorrhea was defined as at least one report of no menses during the first 15 months after randomization. Ninety-one percent of women ≥35 years reported amenorrhea compared to 42% of those<35 years. The study found that patients<40 years of age had a higher incidence of amenorrhea when tamoxifen was also given. Achievement of amenorrhea was correlated with improved DFS in women with ER-positive tumors (HR for amenorrhea versus not = 0.61; 95% CI, 0.44–0.86) and was similar in those patients who did and those who did not receive tamoxifen. That study supports the role of ovarian suppression as adjuvant treatment for premenopausal breast cancer patients.
Since most adjuvant regimens currently include anthracyclines, alkylating agents, and a taxane, either in sequence or combination, it is difficult to determine the contribution of the taxane to the induction of amenorrhea. Most of the existing data have been collected in very small studies, and the results are conflicting. The largest study compared 420 patients who received docetaxel, doxorubicin, cyclophosphamide (TAC) to 403 patients receiving a standard 5-fluorouracil, doxorubicin, cyclophosphamide (FAC) regimen [17]. The definition of amenorrhea in this study was ≥3 months without a menstrual cycle. A higher incidence of amenorrhea was reported with TAC (61.7% versus 52.4% p = 0.007). However, the method of data collection and the time period during which the data were collected were not stated. These factors are critical to our ability to assess the true impact of chemotherapy-induced amenorrhea. It would also be interesting to know whether the development of amenorrhea was associated with an increase in DFS, since this study did report a significant benefit with TAC versus FAC in patients who had ER-positive tumors.
Another study evaluated menstrual bleeding in 595 women ≤45 years of age with stage I–III breast cancer who received either neoadjuvant or adjuvant chemotherapy [14]. This was a prospective study that evaluated monthly bleeding calendars and clinical, demographic, QOL, and treatment data. Again, older age was associated with less bleeding and less recovery of menstruation. Patients receiving AC with or without the addition of paclitaxel or docetaxel had a significant decrease in menstruation after chemotherapy, which gradually returned in 45–55% of patients at 3 years. This was contrary to what was seen with CMF chemotherapy, where bleeding continued initially but steadily decreased over 3 years. This study also reported that women who received tamoxifen had less bleeding at 1 year but no difference at 3 years.
The addition of tamoxifen to chemotherapy has been reported to increase amenorrhea in other studies [18–20]. There is an increase in circulating estrogen in premenopausal women given tamoxifen, which may be due to the direct stimulation by tamoxifen of ovarian estrogen production. Another possibility is that because tamoxifen increases gonadotropins, this could result in a failure of negative feedback. How this increase in circulating estrogens results in amenorrhea is unclear.
Clinicians must inform premenopausal women about the potential risk of temporary and/or permanent amenorrhea at the start of adjuvant chemotherapy. Few prospective datasets using self-report information are available in the literature, and only a limited amount of information comes from studies of contemporary taxane-containing regimens. The additional effect of adjuvant tamoxifen on amenorrhea after chemotherapy has undergone little study. NSABP B-30, when completed and analyzed, will provide one of the largest databases on treatment-induced amenorrhea and its QOL and survival outcomes. In the meantime, the data in this report provide important information for physicians and their patients about this medical complication of adjuvant therapy and should be useful in helping patients anticipate and manage these effects of treatment.
Acknowledgments
Public Health Service grants U10CA-12027, U10CA-37377, U10CA-69651, and U10CA-69974 from the National Cancer Institute, Department of Health and Human Services provided support for this article. The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Cancer Institute or the U.S. federal government. We wish to thank Barbara C. Good, PhD, Director of Scientific Publications for the NSABP, for editorial assistance.
Footnotes
This work represents original research by the authors. Previously presented at ASCO 2005 as a poster (abstr # 537).
Contributor Information
Sandra M. Swain, Email: Sandra.M.Swain@Medstar.net, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Washington Cancer Institute, Washington Hospital Center, 110 Irving Street NW, Washington, DC 20010, USA
Stephanie R. Land, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Marcie W. Ritter, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Joseph P. Costantino, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Reena S. Cecchini, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Eleftherios P. Mamounas, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Altman Health Foundation, Canton, OH, USA
Norman Wolmark, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Allegheny General Hospital, Pittsburgh, PA, USA.
Patricia A. Ganz, National Surgical Adjuvant Breast and Bowel Project, Pittsburgh, PA, USA. Schools of Medicine and Public Health, Jonsson Comprehensive Cancer Center, Los Angeles, CA, USA
References
- 1.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Ovarian ablation in early breast cancer: overview of the randomised trials. Lancet. 1996;348:1189–1196. [PubMed] [Google Scholar]
- 3.Fisher B, Ravdin RG, Ausman RK, Slack NH, Moore GE, Noer RJ. Surgical adjuvant chemotherapy in cancer of the breast: results of a decade of cooperative investigation. Ann Surg. 1968;168:337–356. doi: 10.1097/00000658-196809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonadonna G, Valagussa P, Moliterni A, Zametti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up. N Engl J Med. 1995;332:901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast Cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 6.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy—breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 7.Land SR, Kopec JA, Yothers G, Anderson S, Day R, Tang G, Ganz PA, Fisher B, Wolmark N. Health-related quality of life in anxillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004;86:153–164. doi: 10.1023/B:BREA.0000032983.87966.4e. [DOI] [PubMed] [Google Scholar]
- 8.Day R National Surgical Adjuvant Breast and Bowel Project P-1 Study (NSABP P-1) Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. Ann NY Acad Sci. 2001;949:143–150. [PubMed] [Google Scholar]
- 9.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Baseline quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 10.Land SR, Wickerham DL, Costantino JP, Ritter MW, Vogel VG, Lee M, Pajon ER, Wade JL, III, Dakhil S, Lockhart JB, Jr, Wolmark N, Ganz PA. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 11.Cella D. Manual of the Functional Assessment of Cancer Therapy (FACT) Scales and the Functional Assessment of HIV Infection (FAHI) Scale, version 3. Lippincott-Raven; Philadelphia: 1996. [Google Scholar]
- 12.Andolsek KM. Cycle control with triphasic norgestimate and ethinyl estradiol, a new oral contraceptive agent. Acta Obstet Gynecol Scand Suppl. 1992;156:22–26. doi: 10.3109/00016349209156511. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, Yazlle ME, deSouza RN, Pinho Neto JS, de Leal WB, Leal C, Hippolito SB, Abranches AD. Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg di-hydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate. Contraception. 1997;55:175–181. doi: 10.1016/s0010-7824(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 14.Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 15.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 16.Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, Price KN, Coates AS, Gelber RD International Breast Cancer Study Group. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13–93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 18.Boccardo F, Rubagotti A, Bruzzi P, Cappellini M, Isola G, Nenci I, Piffanelli A, Scanni A, Sismondi P, Santi L, et al. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients: results of a multicentric Italian study. Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative Group. J Clin Oncol. 1990;8:1310–1320. doi: 10.1200/JCO.1990.8.8.1310. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 20.Rose DP. Effect of cytotoxic agents and antihormonal therapy on endocrine function. In: Bulbrook RD, Taylor DJ, editors. Commentaries on Research in Breast Disease. Alan R. Liss; New York: 1979. [Google Scholar]