Abstract
Because STX is a selective ligand for membrane estrogen receptors, it may be able to confer the beneficial effects of estrogen without eliciting the deleterious side effects associated with activation of the nuclear estrogen receptors. This study evaluates the neuroprotective properties of STX in the context of amyloid-β (Aβ) exposure.
MC65 and SH-SY5Y neuroblastoma cell lines, as well as primary hippocampal neurons from wild type (WT) and Tg2576 mice, were used to investigate the ability of STX to attenuate cell death, mitochondrial dysfunction, dendritic simplification, and synaptic loss induced by Aβ. STX prevented Aβ-induced cell death in both neuroblastoma cell lines; it also normalized the decrease in ATP and mitochondrial gene expression caused by Aβ in these cells. Notably, STX also increased ATP content and mitochondrial gene expression in control neuroblastoma cells (in the absence of Aβ). Likewise in primary neurons, STX increased ATP levels and mitochondrial gene expression in both genotypes. In addition, STX treatment enhanced dendritic arborization and spine densities in WT neurons and prevented the diminished outgrowth of dendrites caused by Aβ exposure in Tg2576 neurons.
These data suggest that STX can act as an effective neuroprotective agent in the context of Aβ toxicity, improving mitochondrial function as well as dendritic growth and synaptic differentiation. In addition, since STX also improved these endpoints in the absence of Aβ, this compound may have broader therapeutic value beyond Alzheimer’s disease.
Keywords: amyloid-β toxicity, selective estrogen receptor modulator, mitochondrial dysfunction, neuroprotection
INTRODUCTION
The neuroprotective and cognitive-enhancing effects of natural and synthetic estrogens have been widely demonstrated in humans [1–4], as well as in numerous experimental model systems [5–10]. However, the increased risk of thrombosis and hormone-sensitive cancers associated with treatments using the natural estrogen 17β-estradiol (E2) in post-menopausal women has limited the development of estrogen-related compounds for treating neurodegenerative diseases. The recent identification of new selective estrogen receptor modulators (SERMs) may offer a solution to this problem. The diphenylacrylamide compound STX is one example of a SERM that exhibits the neuroprotective effects of estrogen without inducing oncogenic and thrombotic side effects [11, 12]. Unlike E2 and its functional analogs, STX does not bind nuclear estrogen receptors (ER-α and ER-β), which regulate transcriptional responses to E2. Instead, STX binds the G protein-coupled estrogen receptor GqMER, which is located in the plasma membrane [9]. This uncoupling of nuclear and membrane ER activation suggests that STX could provide a novel therapeutic option for treating patients afflicted with neurodegenerative disease and age-related dementias.
Alzheimer’s disease (AD) is the most common form of dementia world-wide [13], and the risk of AD is associated with age-related loss of sex steroid hormones in both men and women [14, 15]. In AD patients, the accumulation of extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles are accompanied by neuronal loss, synaptic dysfunction, and severe cognitive impairment [16–19]. Mitochondrial dysfunction is thought to contribute to these deleterious physiological changes [20]: deficits in proteins associated with the electron transport chain (ETC) [21], decreased ATP production, and general impairment of mitochondrial activity have all been observed in the brains of AD patients, as well as in many in vitro and in vivo models of amyloid pathology [22–27].
Several estrogen-dependent signaling pathways converge on mitochondria to modulate cellular respiration and ATP production [28–30]. E2 has been shown to enhance brain mitochondrial function [31], increase mitochondrial respiratory capacity [32], and induce the expression of mitochondrial proteins [29, 32]. E2 has likewise been shown to protect against Aβ-induced cell death in vitro [33–35] and in vivo [36, 37], and to attenuate bioenergetic deficits induced by Aβ in both neuroblastoma cells [38] and mouse models of AD [39, 40].
Despite the growing literature describing the beneficial action of estradiol on brain mitochondrial function in the context of AD, comparatively little is known about the ability of SERMs (acting via extranuclear ERs) to recapitulate these neuroprotective effects. We have now addressed this issue by examining the neuroprotective effects of STX on cell viability, mitochondrial function, and neuronal morphology in several in vitro models of Aβ toxicity.
METHODS
STX preparation
STX was produced by AAPharmaSyn, LLC (Ann Arbor, MI) under contract with the authors of the synthetic protocol for STX, published in Tobias et al. [41]. Stock solutions of STX (2 mM) were prepared in 100% anhydrous dimethyl sulfoxide (DMSO), which was then diluted to working concentrations in culture medium, as described below.
MC65 Cell culture
MC65 cells were cultured in MEMα supplemented with 10% fetal bovine serum (FBS; GIBCO/Life Technologies), 2 mM L-glutamine (Sigma-Aldrich), and 0.1% tetracycline (Sigma-Aldrich). For each experiment, cells were trypsinized and resuspended in Opti-MEM without phenol red (GIBCO/Life Technologies), then treated with STX or matched DMSO concentrations in the presence and absence of tetracycline. All endpoints were compared to those obtained with tetracycline-treated cells, either with or without the addition of STX. For assays of viability, cells were plated at 10,000 cells/well in 96 well plates and assessed after 72 hr of continuous treatment. For assays of gene expression and ATP determination, cells were plated at 60,000 cells/well in 12 well plates and were harvested after 48 hr of continuous treatment.
SH-SY5Y Cell Culture
SH-SY5Y neuroblastoma cells were cultured in DMEM/F12 medium (GIBCO/Life Technologies) supplemented with 10% FBS and 1% penicillin-streptomycin (Sigma-Aldrich). For assays of gene expression and ATP production, cells were plated at 200,000 cells/well in 12-well plates. For assays of cell viability, cells were plated at 15,000 cells/well in 96 well plates. Three days after plating, cells were washed with phosphate-buffered saline (PBS) and switched to serum-free DMEM/F12 medium containing 1% N-2 growth supplement (GIBCO/Life Technologies) plus STX (100 nM). The following day, the cells were treated with 50 µM Aβ25–35 (American Peptide Company), a fragment that has been shown to recapitulate the toxic effects of full-length Aβin vitro [42, 43]. Aβ25–35 solutions were incubated at 37° C for 72 hr, prior to addition to the cell cultures. All endpoints were assessed after 48 hr of treatment.
Culture of primary hippocampal neurons
Embryonic Tg2576 mice and their wild type (WT) littermates were used to generate primary neuronal cultures. The Tg2576 line expresses the human APPswe double mutation (K670N-M671L) under the control of the hamster prion promoter [44, 45], resulting in an accumulation of Aβ1–42 in the brain and the development of age-dependent Aβ plaques. Previous studies have shown that after several weeks in culture, primary neurons isolated from these animals display metabolic alterations [46] and a dystrophic phenotype that includes impaired dendritic arborization and loss of synaptic spines [19],similar to what is observed in the brains of adult Tg2576 animals [22, 47].
Breeding pairs of Tg2576 mice were raised in an in-house facility at the OHSU. Mice were maintained in a climate-controlled environment with a 12-hr light/12-hr dark cycle and fed Pico Lab Rodent Diet 5LOD (LabDiets, St. Louis, MO). Diet and water were supplied ad libitum. Litters were weaned and group-housed (4–6/cage) until commencement of breeding. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional Animal Care and Use Committee of OHSU.
Hippocampal neurons were isolated from embryonic mice, based on the methods of Kaech and Banker [48]. Briefly, embryos were harvested at 18 days of gestation from anesthetized females. Hippocampi were dissected, gently minced, and trypsinized to generate suspensions of dispersed neurons, which were then plated in MEM medium (GIBCO /Life Technologies), 5% FBS (Atlanta Biologicals), and 0.6% glucose (Sigma-Aldrich). After 4 hr, the medium was removed and replaced with Neurobasal Medium supplemented with 1× GlutaMAX (GIBCO/Life Technologies) and 1× GS21 (MTI-GlobalStem). The animals were genotyped by PCR using DNA extracted from tail samples taken after dissection of the hippocampi. For analyses of gene expression and ATP levels, dissociated hippocampal cells were plated at 200,000 per well in 12-well plates that had been pre-coated with 1 mg/mL poly-L-lysine. After 7 days in vitro (DIV), cells were treated with STX (100 nM) and harvested 48 hr later for analysis.
For Sholl analyses of dendritic complexity, we used two different protocols that yielded equivalent results. In the first protocol, 150,000 hippocampal neurons were plated in 60 mm dishes in 1× MEM with 5% FBS, each dish containing four poly-L-lysine-coated glass coverslips with paraffin wax spacers [48]. After 4 hr, the coverslips were flipped into 60 mm dishes containing neural stem cell-derived glial cells (provided by Dr. Gary Banker, Jungers Center, OHSU) and maintained in 6 ml Neurobasal media with GlutaMAX and GS21 [48]. Each dish was fed every week by removing 1 ml of the culture medium and adding 1 ml fresh Neurobasal media containing GlutaMAX plus GS21, with the first feed (at 5 DIV) containing 6 µM cytosine β-D-arabinofuranoside hydrochloride (AraC; Sigma-Aldrich). The first and second feed (5 DIV and 12 DIV respectively) also contained STX (100 nM) or DMSO. At 19 DIV, each coverslip was fixed in 4% PFA in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 7.4). Coverslips were stained with Anti-MAP2B (Sigma-Aldrich #M4403; 3.3 µg/ml) and Goat anti-mouse IgG1-Cy3 (Jackson ImmunoResearch #115-165-205; 1.5µg/ml). Immunostained neurons were imaged with a Zeiss ApoTome2 microscope. In the second protocol, freshly isolated hippocampal neurons were electroporated with plasmids encoding enhanced Green Fluorescent Protein (eGFP) under the control of the CMV immediate-early enhancer and the chicken β-actin promoter [49]. 300,000 electroporated hippocampal neurons were plated with 150,000 non-electroporated neurons of the same genotype onto poly-L-lysine-coated coverslips, then cultured and processed as described above. For both protocols, blinded Sholl analyses were performed using the Fiji platform [50] with the plug-in created by Ferreira et al. [51]. At least thirty cells were analyzed per treatment condition. Statistical differences between treatment groups were calculated using Student’s unpaired t-tests.
For an analysis of dendritic spines, 150,000 hippocampal neurons were electroporated with plasmids encoding eGFP (as described above) and plated onto dishes with coverslips containing 300,000 cortical neurons of the same genotype (plated 7 days prior to the addition of the hippocampal neurons). This strategy promoted robust synapse formation while maintaining the electroporated hippocampal neurons at a density that permitted the unambiguous visualization of non-intersecting dendritic segments. After 4 hr, coverslips were flipped into 60 mm dishes containing stem cell-derived glial cells in 6 ml Neurobasal media with GlutaMAX and GS21. Each dish was fed every week with 1 ml Neurobasal media plus GlutaMAX and GS21, with the first feed (at 7 DIV) containing AraC and the second feed (at 14 DIV) containing STX (100 nM) or DMSO. Coverslips were then fixed in 4% PFA in PHEM buffer at 21 DIV and immunostained with anti-GFP (Life Technologies #A11122; 2µg/ml), detected with Alexa-488-conjugated goat anti-Rabbit secondary antibodies (Life Technologies #A11034; 2 µg/ml). The immunostained neurons were then imaged using a Zeiss ApoTome2 microscope and analyzed blind by a separate investigator not involved in the image collection. 16–20 images were collected from different neurons in each treatment group, and spines were quantified on 50–100 µm segments of dendrite length per image using FIJI software.
Quantitative real time PCR
Total RNA from neuroblastoma cells and cultured hippocampal neurons was extracted using Tri-Reagent (Molecular Research Center). RNA was reverse transcribed with the Superscript III First Strand Synthesis kit (Invitrogen) to generate cDNA, as per the manufacturer’s instructions. Relative gene expression was determined using TaqMan Gene Expression Master Mix and commercially available TaqMan primers (Life Technologies) for mitochondrially encoded NADH dehydrogenase 1 (Mt-ND1), mitochondrially encoded ATP synthase 6 (Mt-ATP6), mitochondrially encoded cytochrome c oxidase 1 (Mt-CO1), mitochondrially encoded cytochrome B (Mt-CYB), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Quantitative polymerase chain reactions (qPCR) was performed on a StepOne Plus Machine (Applied Biosystems) and analyzed using the delta-delta Ct method.
Cell number determination
Cell number was determined both by DNA quantification as well as MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4- sulfophenyl)-2H-tetrazolium) assays. For DNA quantification, media was removed from the wells and the plates were frozen at −80° C for 24 hr, prior to subsequent analysis. Cell numbers were quantified using the CyQUANT Cell Proliferation Assay kit (Thermo Fisher), as per the manufacturer’s instructions. The MTS assay was performed using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega), as per the manufacturer’s instructions.
ATP and protein quantification
ATP was quantified using the ATP determination kit (Life Technologies), as per the manufacturer’s instructions. Cells were lysed with 0.1% Triton X 100 in PBS and incubated with the reaction solution for 15 min at room temperature, prior to measurement. Values were normalized to total protein content, as determined by the BCA method (Thermo Fisher). Briefly, this colorimetric assay combines the reduction and chelation of cuprous copper (Cu1+) to peptides under alkaline conditions in the presence of sodium potassium tartrate. Reduced Cu1+ is then reacted with bicinchoninic acid (BCA) to produce a concentration-dependent reaction product detectable in a spectrophotometer at 562 nm. Protein concentrations of cell lysates were analyzed in triplicate by comparison to a standard curve generated with serial dilutions of BSA (bovine serum albumin; 0.03–2 mg/ml) in a microplate reader, as per the manufacturer’s instructions. Protein concentrations were then calculated in Excel.
Statistics
Statistical significance was determined using Bonferroni post-hoc tests and one-way analysis of variance with appropriate t-tests. Significance was defined as p ≤0.05. Analyses were performed using Excel or GraphPad Prism 6.
RESULTS
STX protects against Aβ-induced cytotoxicity in neuroblastoma models
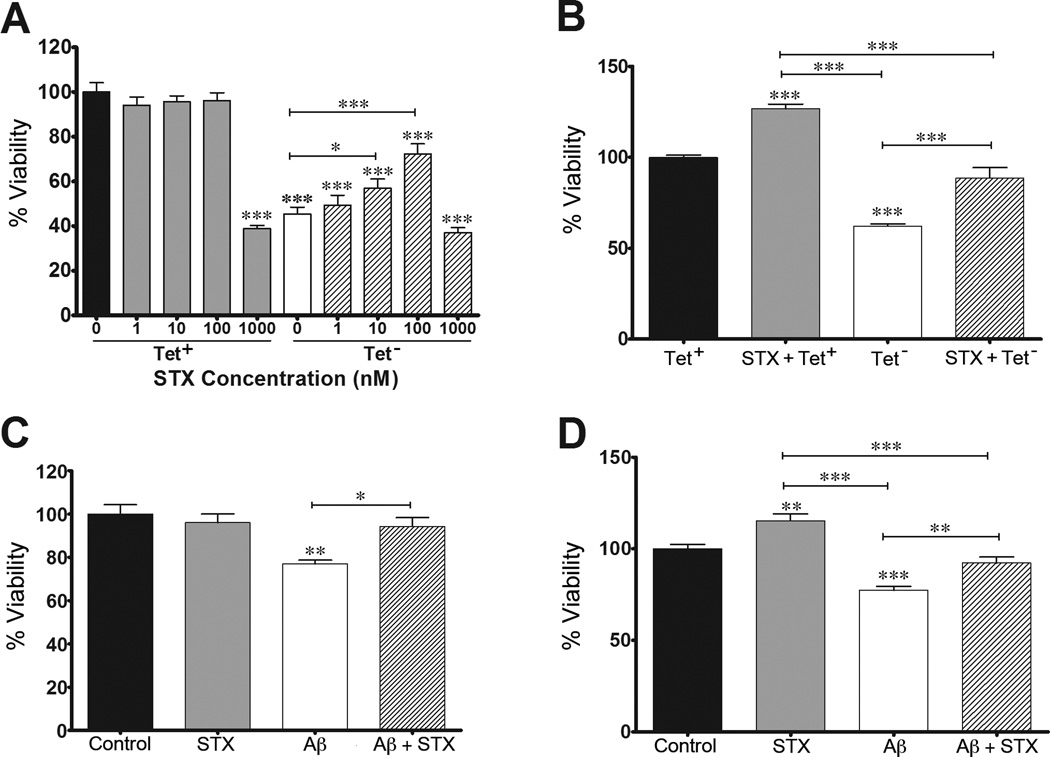
In previous work, we used two different neuroblastoma models of amyloid toxicity to investigate the protective effects of candidate therapeutic compounds. MC65 neuroblastoma cells conditionally express APP-C99 under the control of a tetracycline-responsive promoter, which in turn is cleaved by endogenous secretases to generate Aβ peptides (predominantly Aβ1–40/42) [52–54]. Following tetracycline withdrawal (Tet−), Aβ levels progressively accumulate in these cultures, resulting in extensive cell death by 72 hr [52]. Alternatively, SH-SY5Y neuroblastoma cells have been used in many studies to test the effects of exogenously introduced Aβ (including Aβ25–35 and Aβ1–42), both of which have been shown to induce progressive cell death in a concentration-dependent manner [43, 55–57]. Accordingly, we used these two cell culture models to investigate the protective effects of STX. As shown in Figure 1A, STX treatment protected MC65 cells from Aβ-induced cell death in a dose-dependent manner (between 1–100 nM). When analyzed with CyQUANT assays of cell proliferation, these dosages had no detectable effect in the absence of Aβ (Tet+ condition). The highest dose, 1000 nM, was toxic in both the Tet+ and Tet− conditions. By comparison, analyzing replicate sets of MC65 cells using the MTS assay (a measure of cellular metabolic activity) revealed that treatment with 100 nM STX significantly improved the viability of both control cultures (STX + Tet+) and cultures exposed to toxic Aβ levels (STX + Tet−). Accordingly, we used 100 nM STX for our subsequent experiments.
Figure 1. STX protects against Aβ-induced cell death in neuroblastoma cells.
A–B) MC65 neuroblastoma cells treated with STX in the presence and absence of Tetracycline (Tet). A). In control conditions (Tet+), STX at concentrations from 1–100 nM had no deleterious effect on cell viability (analyzed with the CyQUANT assay). In cultures producing toxic levels of Aβ (Tet− conditions), STX protected against Aβ-induced cell death in a dose-dependent manner (from 1–100 nM). The highest dose of STX (1000 nM) was toxic in both conditions (n = 10–12 per treatment condition). B) Replicate analysis of MC65 cell viability (using the MTS assay) showed that STX treatment (100 nM) significantly improved the percentage of viable cells in control cultures (Tet+) and in cultures producing toxic Aβ (Tet−) (n=12–14 per treatment condition). C) In SH-SY5Y cells, treatment with Aβ25–35 (50 µM) induced a significant reduction in cell viability (analyzed with the CyQUANT assay); simultaneous treatment with STX (100 nM) prevented Aβ-induced cell death (n=6–7 per treatment condition). D) Replicate analysis of SH-SY5Y cell viability (using the MTS assay) showed that STX treatment (100 nM) significantly improved the percentage of viable cells in control cultures and partially rescued the viability of cells treated with Aβ25–35 (n = 12–14 per treatment condition). *p<0.05; **p<0.01; ***p<0.001.
To test whether STX also confers beneficial effects in cells exposed to exogenous amyloid peptides, we pre-treated SH-SY5Y cells with 100 nM STX for 24 hr before the addition of 50 µM Aβ25–35, based on previous evidence that treatment with Aβ25–35 recapitulates the toxic effects of full-length Aβ [42, 58, 59]. As shown in Figure 1C, Aβ25–35 treatment resulted in a significant reduction in viability (based on CyQUANT assays of proliferation), whereas pre-treatment with STX prevented this effect. In control cells (not treated with Aβ), STX treatment alone did not alter cell viability. As in our MC65 cell assays, we found that STX pre-treatment significantly improved the metabolic activity of control SH-SY5Y cultures (as measured with MTS assays), as well as partially rescuing the viability of cells treated with Aβ25–35 (Figure 1D). In combination, these results indicate that STX can protect cells against the deleterious effects of multiple toxic forms of Aβ, whether they are generated endogenously or introduced exogenously.
STX increases ATP production and induces mitochondrial gene expression in neuroblastoma cells
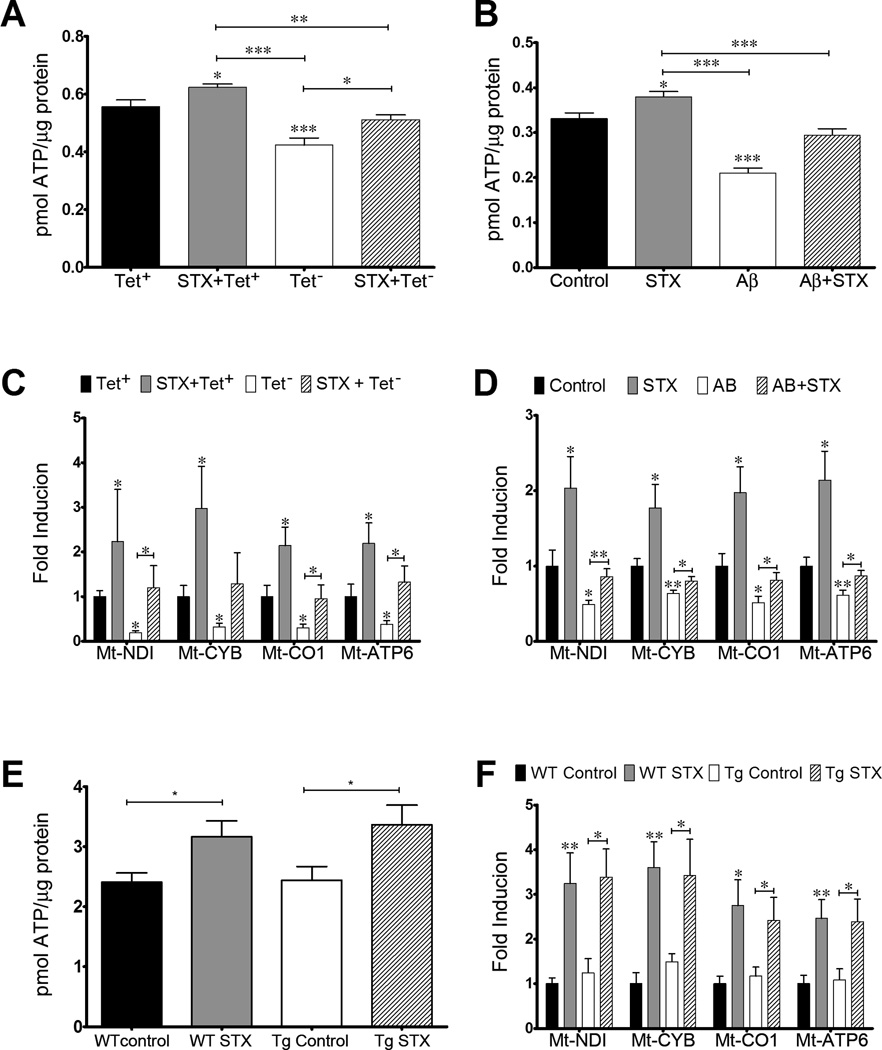
We also used our neuroblastoma cell assays to examine whether STX could protect against the reduction in ATP levels caused by Aβ exposure, as an assay of mitochondrial function. As shown in Figure 2A, ATP levels were significantly reduced in MC65 cells by 48 hr after tetracycline removal (Tet−), coincident with the accumulation of Aβ in these cultures (similar to our previous results; [60]). In contrast, STX treatment attenuated this decrease (Figure 2A), while STX treatment also significantly increased ATP production in MC65 cells that were maintained in the presence of tetracycline (and therefore not expressing Aβ). Similarly, treating SH-SY5Y cells with exogenous Aβ caused a decrease in ATP levels that was prevented by STX pre-treatment (Figure 2B), while treating control SH-SY5Y cells with STX increased intracellular ATP levels (in the absence of Aβ).
Figure 2. STX protects against Aβ-induced loss of mitochondrial function in neuroblastoma cells and hippocampal neurons.
A–B): STX improves mitochondrial function in neuroblastoma cells. A). 100 nM STX increased ATP production in control MC65 cells (Tet+condition) and attenuated the decrease in ATP production caused by the induction of Aβ expression (Tet− condition). B) 100 nM STX increased ATP production in control SH-SY5Y cells and attenuated the decrease in ATP production caused by treatment with Aβ peptides (n=10–12 per treatment condition). C-D): STX (100 nM) also increased ETC gene expression in control cells and attenuated the decrease in ETC gene expression caused by Aβ exposure in both MC65 cells (C) and SH-SY5Y cells (D) (n = 12–15 per treatment condition). E) STX (100 nM) increased ATP production in hippocampal neurons from both WT and Tg2576 animals. F) STX also increased the expression of ETC genes in neurons from both genotypes (n = 6 per treatment condition). *p<0.05; **p<0.01; ***p<0.001
We also evaluated whether STX prevented the down-regulation of mitochondrial DNA-derived genes by Aβ; specifically, we examined the expression of Mt-ND1, Mt-CYB, Mt-CO1 and Mt-ATP, which encode proteins in complexes I, III, IV and V of the ETC, respectively. All four ETC genes were repressed in MC65 cells expressing Aβ (under Tet− conditions), whereas STX treatment attenuated this effect (Figure 2C). Consistent with our analysis of ATP levels, control MC65 treated with STX (under Tet+ conditions) also coordinately increased the expression of all the mitochondrial genes, independent of Aβ expression. Likewise in SH-SY5Y cells, Aβ treatment significantly repressed the expression of the mitochondrial genes, while STX treatment prevented this decrease (Figure 2D), restoring their expression to control levels. Once again, treating control SH-SY5Y cells with STX (in the absence of Aβ) also enhanced the basal expression of all four ETC genes to a similar degree. In combination, these results provide additional evidence that STX bolsters mitochondrial function while protecting against the deleterious effects of Aβ exposure, suggesting that this compound might also have beneficial effects in the context of other disease-associated insults that affect cellular metabolism and viability.
STX increases ATP production and mitochondrial gene expression in primary hippocampal neurons
Because the metabolism of neuroblastoma cells differs substantially from that of neurons [61], we also evaluated the effects of STX on the mitochondrial parameters of primary neurons, to confirm that similar mechanistic pathways could be regulated by this compound in a more physiologically relevant cellular context. As shown in Figure 2E, STX treatment significantly increased ATP content in cultured hippocampal neurons isolated either from WT littermate control mice or from Tg2576 mice, which overexpress the human APPswe double mutation and accumulate Aβ1–42 [44, 45]. Of note is there was no difference in ATP content between the control-treated WT and Tg2576 neurons in this experiment (analyzed using neurons at 9 DIV). STX treatment also significantly increased the expression of ETC genes in hippocampal neurons from both WT and Tg2576 animals to a similar degree (Figure 2F). As observed with our analysis of ATP levels, no differences between the two genotypes were detected in the basal expression of any these genes.
STX attenuates Aβ-induced impairments in dendritic morphology in hippocampal neurons
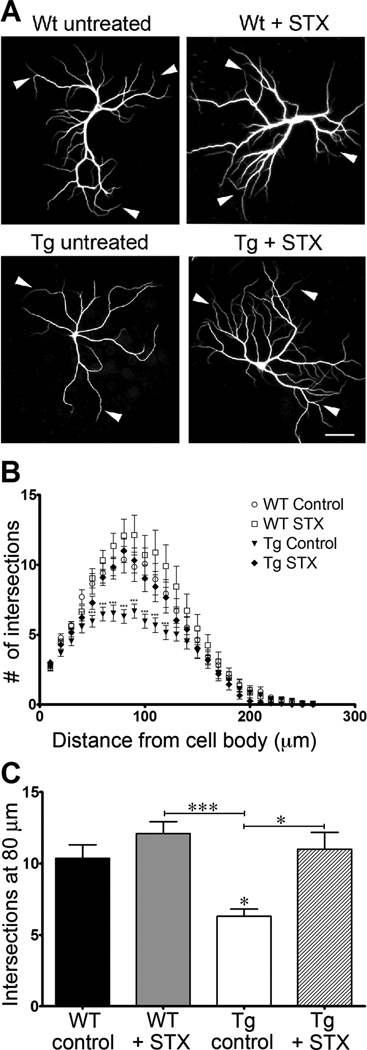
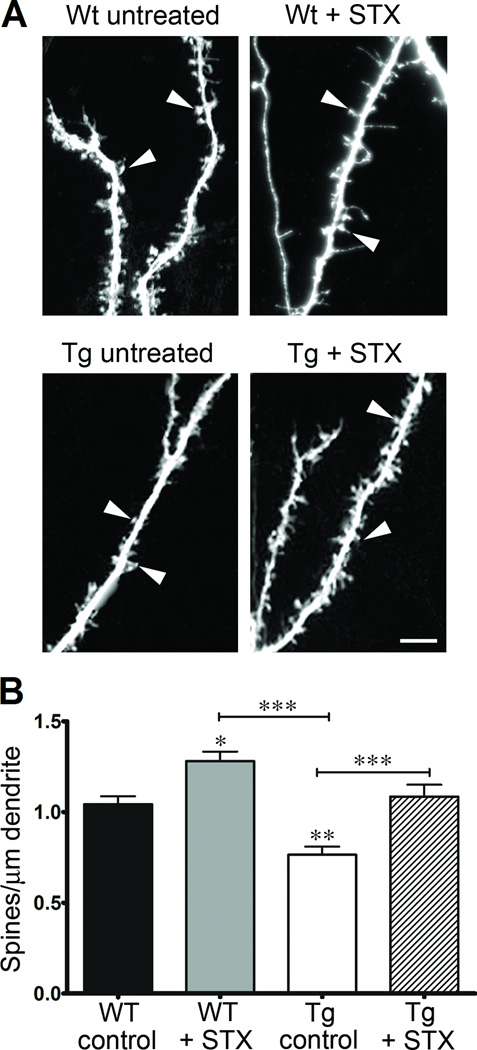
As previously reported, cultured neurons isolated from the brains of Tg2576 mice progressively develop neurodegenerative phenotypes (compared to WT control neurons), including substantially reduced dendritic complexity and lower spine densities caused by their chronic production of Aβ1–42 [19, 62]. Consistent with these reports, we observed that Tg2576 hippocampal neurons exhibited substantially reduced patterns of dendritic arborization after 19 days in culture, compared to neurons from WT littermate controls (Figure 3A). Sholl analyses revealed that STX treatment restored the dendritic complexity of Tg576 neurons to WT levels (Figures 3B–C). We obtained equivalent results when we used cultures containing non-electroporated neurons or cultures containing neurons expressing eGFP. These results are consistent with previous evidence that inducing the expression of eGFP in cultured hippocampal neurons does not significantly alter their overall development or survival rates [48]. Using this protocol, we also observed a similar protective effect of STX on the elaboration of dendritic spines in neurons expressing eGFP. As shown in Figure 4A–B, spine densities in Tg2576 neurons were significantly reduced compared to WT neurons after 3 weeks in culture, recapitulating previous findings by other investigators [19]. In contrast, treatment with STX treatment rescued this deficit (Figure 4B), restoring Tg2576 spine densities to control levels. In addition, STX also resulted in a significant increase in the spine densities of WT neurons, compared to vehicle-control treated neurons. These results demonstrate that STX treatments prevented the deleterious effects of Aβ on both neuronal metabolism and dendritic morphology.
Figure 3. STX increases dendritic arborization in hippocampal neurons from WT and Tg2576 mice.
A) Representative images of neurons from each treatment group. B) Sholl analysis of the total number of dendritic branches of cultured hippocampal neurons from Tg2576 and WT littermate control mice (n = 30 neurons per treatment condition). STX treatment (100 nM) induced an increase in dendritic complexity in WT control neurons, and restored the extent of dendritic arborization of Tg2576 neurons to control levels. C) STX increased the maximal extent of arborization (measured at 80 µm from the cell body) in WT neurons and restored the maximal arborization of Tg2576 neurons to control levels. *p<0.05; ***p<0.001. Scale bar in 3A = 25 µm
Figure 4. STX increases dendritic spine densities in hippocampal neurons from WT and Tg2576 mice.
A) Representative images of dendritic spines from each treatment condition; arrowheads indicate representative spines included in this analysis. B) STX (100 nM) increased the number of dendritic spines in WT hippocampal neurons and restored spine densities of Tg2576 neurons to control levels (n = 16–20 dendritic segments per treatment condition). *p<0.05; **p<0.01; ***p<0.001. Scale bar in 4A = 5 µm
DISCUSSION
SERMs are promising therapeutic compounds because of their ability to provide the neuroprotective effects of natural estrogen without its negative side effects. STX is one such compound that has been shown to confer beneficial neurological effects by binding selectively to a G protein-coupled membrane estrogen receptor (GqMER), rather than the classical nuclear estrogen receptors [9, 63]. Notably, STX does not induce the deleterious oncogenic and thrombotic effects seen with E2 treatments, indicating that it might provide an improved strategy for hormone replacement therapy [11, 12]. In this study, we report that STX is also an effective neuroprotective compound, mitigating the toxic effects of several different forms of Aβ in a variety of different assays.
STX treatment protected against Aβ-induced cell death and mitochondrial dysfunction in neuroblastoma cells, and it attenuated Aβ-induced dendritic simplification and spine loss in primary hippocampal neurons. In both our MC65 and SH-SY5Y models of Aβ toxicity, STX treatment significantly reduced Aβ-induced cell death. It is notable that we obtained similar results in the MC65 cells exposed to full-length Aβ peptides (Aβ1–40/42) and SH-SY5Y cells exposed to the shorter peptides (Aβ25–35) that have been shown to recapitulate the cytotoxic effects of Aβ oligomers [42, 58, 59]. Likewise, the coherence of our observations in both neuroblastoma lines and cultured neurons suggests that the protective effects of STX are not limited to a particular set of in vitro conditions [64]. In addition, our results are consistent with previous reports that STX is neuroprotective in whole-animal models of ischemic stroke [9, 63], supporting the hypothesis that this compound might be beneficial in the context of a variety of neurodegenerative conditions. However the molecular mechanisms underlying this protective effect have yet to be determined. Both STX and E2 can rapidly activate multiple signaling pathways independent of altered gene expression, demonstrating the involvement of non-nuclear estrogen receptors [65, 66]. In rats, STX-dependent stimulation of GqMER results in the activation of both the ERK/MAPK [67] and PKCδ/PKA signaling pathways [67, 68]. Alternatively, experiments using neuroblastoma cells haveshown that activation of GqMER can also initiate PI3K signaling [69]. Each of these pathways has been shown to contribute to neuroprotective responses in a variety of model systems [70–72] and have been linked with E2-dependent neuronal growth responses, including dendritic branching and spine elaboration [73–77]. These observations suggest that one or more of these signaling pathways might also mediate the protective effects of STX in our neuroblastoma and neuronal culture assays (a topic for future investigations).
Reduced dendritic arborization, diminished spine numbers, synaptic loss, and neuronal death have been widely reported in AD patients and in animal models of this disease [17, 18, 47, 78–82]. Loss of dendritic complexity and reduced spine densities are also prominent features of AD and correlate significantly with cognitive decline [16]. These same morphological changes occur in neurons isolated from Tg2576 mice that overexpress human APPswe, a mutant form of APP that causes the accumulation of Aβ1–42 and early-onset AD in human patients [19]. In this study, we found that STX treatment reversed the deleterious effects of Aβ on dendritic arborization and spine density in hippocampal neurons isolated from Tg2576 mice. Notably, STX also improved spine densities in cultures of WT neurons. These results are consistent with the well-documented beneficial effects of E2 treatment on dendritic arborization and spine density [73, 74, 83–85], supporting other evidence that STX can confer the neuroprotective responses of natural estrogen in a variety of contexts [9, 63]. Whether these effects of STX on neuronal morphology involve the same mechanisms underlying its cytoprotective actions remain to be defined.
We also found that STX improved mitochondrial function in two different neuroblastoma models of Aβtoxicity. STX treatment attenuated both the decrease in ATP content and reduced ETC gene expression caused by Aβ exposure, while increasing these parameters in control cultures (not treated with Aβ). In contrast, we found that STX did not significantly reduce the more general effects of H2O2 on viability (unpublished data), supporting the hypothesis that STX mediates its protective actions on cellular metabolism via one or more specific signaling pathways (as summarized above).
We were able to recapitulate many of the same effects on mitochondrial endpoints in primary neurons: STX increased ATP content and ETC gene expression in both WT and Tg2576 neurons. In contrast to our analyses of neuronal morphology, however, we detected no significant differences in baseline ATP levels or mitochondrial function between the genotypes. This result is likely due to the fact that the neurons used these assays were only grown for 9 days in culture before mitochondrial gene expression and ATP levels were measured, whereas other investigators reported that phenotypic changes between the genotypes were only detectable after 19 DIV [19]. Previous studies have also shown that E2 can increase the expression of ETC genes in cultured spinal cord neurons [86] and can modulate mitochondrial dynamics and bioenergetics in the brain [87]. Likewise, estrogen treatments can reverse deficits in ATP production caused by either Aβ or tau accumulation in SH-SY5Y cells [38]. To our knowledge, however, this is the first report that the protective effects of STX include enhanced mitochondrial activity, providing additional evidence that this compound provides the beneficial actions of natural estrogens without their adverse side effects.
Despite the fact that many of the mitochondrial effects of estrogens appear to be modulated by non-nuclear ERs [88, 89], research to date has focused on hormone analogs and other compounds that act primarily via the classical nuclear ERs. Future experiments will be needed to investigate the specific role of membrane estrogen receptors (including GqMER) in transducing the protective effects of STX on mitochondrial function. Although the mechanisms underlying these effects have not been fully elucidated, manipulations that target PKA signaling have been reported to increase ETC activity in rat hippocampal neurons and to reverse the bioenergetic impairment caused by Aβ treatment [90, 91], while ERK and PI3K activation have been shown to restore ATP production in a neuronal model of mitochondrial injury [92]. As noted above, these same signaling pathways have been implicated in regulating STX-responses in other contexts [67–69]. In light of recent evidence that mitochondrial abnormalities accompany impairments in dendritic arborization and spine density [16], our studies will provide a framework for investigating whether the protective effects of STX on neuronal morphology are functionally linked to improved mitochondrial activity.
The experiments described in this study demonstrate the neuroprotective action of STX in several models of Aβ toxicity and support the potential value of this compound as a novel therapeutic agent for treating AD. Interestingly, many of the beneficial effects of STX were also observed in control cultures and thus appear to be independent of Aβ exposure. These observations suggest that STX might be suitable for treating a variety of conditions that have been linked with mitochondrial dysfunction, including other neurodegenerative diseases and age-related deficits in cognitive function.
Acknowledgments
This work was funded by a Department of Veterans Affairs Merit Review grant awarded to J. Quinn, and by NIH grants NS078363 and AG025525 awarded to P. Copenhaver. The authors thank Dr. Martin Kelly for providing his expertise and the STX used in this study, and Dr. Gary Banker and Ms. Barbara Smoody for their support of our experiments with primary hippocampal neurons. We also wish to thank Ms. Jenna Fisk for her assistance with image acquisition in some of our studies. Dr. Doris Kretzschmar provided critical input on this manuscript. We gratefully acknowledge Dr. Stefanie Kaech and Ms. Aurelie Snyder for their invaluable assistance with the confocal imaging analysis that was performed in the Advanced Light Microscopy Core, Jungers Center, OHSU. Confocal imaging was supported in part by NIH P30 NS061800, and by the OHSU School of Medicine Faculty Innovation Fund (to P. Copenhaver). MC65 cells were generously provided by Randy Woltjer, MD, PhD.
REFERENCES
- 1.Henderson V, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Karim R, Shoupe D, Kono N, Dustin L, Allayee H, Mack WJ. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci. 2013;110:20290–20295. doi: 10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorenstein C, Rennó J, Jr, Vieira Filho AH, Gianfaldoni A, Gonçalves MA, Halbe HW, Fernandes CE, Demétrio FN. Estrogen replacement therapy and cognitive functions in healthy postmenopausal women: a randomized trial. Arch Womens Ment Health. 2011;14:367–373. doi: 10.1007/s00737-011-0230-6. [DOI] [PubMed] [Google Scholar]
- 3.Ryan J, Scali J, Carriere I, Ritchie K, Ancelin ML. Hormonal treatment, mild cognitive impairment and Alzheimer's disease. Int Psychogeriatr. 2008;20:47–56. doi: 10.1017/S1041610207006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acosta J, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown C, Suzuki S, Jelks KA, Wise PM. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27:240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DonCarlos L, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34:S113–S122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gröger M, Plesnila N. The neuroprotective effect of 17β-estradiol is independent of its antioxidative properties. Brain Res. 2014;1589C:61–67. doi: 10.1016/j.brainres.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Han D, Scott EL, Dong Y, Raz L, Wang R, Zhang Q. Attenuation of mitochondrial and nuclear p38α signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia. Mol Cell Endocrinol. 2015;400:21–31. doi: 10.1016/j.mce.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Song XJ, Gao PH, Zhou XY, Zhou SN. Estradiol prevents Aβ 25–35-inhibited long-term potentiation induction through enhancing survival of newborn neurons in the dentate gyrus. Int J Neurosci. 2015;22:1–9. doi: 10.3109/00207454.2014.995267. [DOI] [PubMed] [Google Scholar]
- 11.Qiu JRO, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;783:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 14.Barron A, Pike CJ. Sex hormones, aging, and Alzheimer's disease. Front Bioscie (Elite Ed) 2012;4:976–997. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pike C, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baloyannis S. Dendritic pathology in Alzheimer's disease. J Neurol Sci. 2009;283:153–157. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- 17.Dumanis S, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavroudis I, Fotiou DF, Manani MG, Njaou SN, Frangou D, Costa VG, Baloyannis SJ. Dendritic pathology and spinal loss in the visual cortex in Alzheimer's disease: a Golgi study in pathology. Int J Neurosci. 2011;121:347–354. doi: 10.3109/00207454.2011.553753. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leuner K, Müller WE, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer's disease. Mol Neurobiol. 2012;46:186–193. doi: 10.1007/s12035-012-8307-4. [DOI] [PubMed] [Google Scholar]
- 21.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 22.Cuadrado-Tejedor M, Cabodevilla JF, Zamarbide M, Gómez-Isla T, Franco R, Perez-Mediavilla A. Age-related mitochondrial alterations without neuronal loss in the hippocampus of a transgenic model of Alzheimer's disease. Curr Alzheimer Res. 2013;10:390–405. doi: 10.2174/1567205011310040005. [DOI] [PubMed] [Google Scholar]
- 23.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McManus M, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhein V, Baysang G, Rao S, Meier F, Bonert A, Müller-Spahn F, Eckert A. Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol. 2009;29:1063–1071. doi: 10.1007/s10571-009-9398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saraiva A, Borges MM, Madeira MD, Tavares MA, Paula-Barbosa MM. Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer's disease. J Submicrosc Cytol. 1985;17:459–464. [PubMed] [Google Scholar]
- 28.Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci. 2006;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen R, Johnson LA, Zuloaga DG, Limoli CL, Raber J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem. 2013;25:303–313. doi: 10.1111/jnc.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinton R. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in Neurosciences. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin R, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. 2012;24:236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosoda T, Nakajima H, Honjo H. Estrogen protects neuronal cells from amyloid beta-induced apoptotic cell death. Neuroreport. 2001;12:1965–1970. doi: 10.1097/00001756-200107030-00038. [DOI] [PubMed] [Google Scholar]
- 34.Napolitano M, Costa L, Piacentini R, Grassi C, Lanzone A, Gulino A. 17β-estradiol protects cerebellar granule cells against β-amyloid-induced toxicity via the apoptoticmitochondrial pathway. Neurosci Lett. 2014;21:134–139. doi: 10.1016/j.neulet.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27:1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szego E, Csorba A, Janáky T, Kékesi KA, Abrahám IM, Mórotz GM, Penke B, Palkovits M, Murvai U, Kellermayer MS, Kardos J, Juhász GD. Effects of estrogen on beta-amyloidinduced cholinergic cell death in the nucleus basalis magnocellularis. Neuroendocrinology. 2011;93:90–105. doi: 10.1159/000321119. [DOI] [PubMed] [Google Scholar]
- 37.Yenki P, Khodagholi F, Shaerzadeh F. Inhibition of phosphorylation of JNK suppresses Aβ-induced ER stress and upregulates prosurvival mitochondrial proteins in rat hippocampus. J Mol Neurosci. 2013;49:262–269. doi: 10.1007/s12031-012-9837-y. [DOI] [PubMed] [Google Scholar]
- 38.Grimm A, Biliouris EE, Lang UE, Götz J, Mensah-Nyagan AG, Eckert A. Sex hormone-related neurosteroids differentially rescue bioenergetic deficits induced by amyloid-β or hyperphosphorylated tau protein. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-1988-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging. 2012;33:1507–1521. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobias S, Qiu J, Kelly MJ, Scanlan TS. Synthesis and biological evaluation of SERMs with potent nongenomic estrogenic activity. Chem Med Chem. 2006;1 doi: 10.1002/cmdc.200500098. [DOI] [PubMed] [Google Scholar]
- 42.Yankner B, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 43.Lee C, Park GH, Lee SR, Jang JH. Attenuation of beta-amyloid-induced oxidative cell death by sulforaphane via activation of NF-E2-related factor 2. Oxidative Medicine and Cellular Longevity. 2013;2013:313510. doi: 10.1155/2013/313510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 45.King D, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behav Brain Res. 1999;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 46.Calkins M, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F b-amyloid precursor protein and Alzheimer’s disease. J Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaech S, Banker G. Culturing hippocampal neurons. Nat. Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 49.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 50.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira T, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjostrom PJ, van Meyel DJ. Neuronal morphometry directly from bitmap images. Nature Methods. 2014;11:982–984. doi: 10.1038/nmeth.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sopher B, F K, Kavanagh TJ, Furlong CE, Martin GM. Neurodegenerative mechanisms in Alzheimer disease. A role for oxidative damage in amyloid beta protein precursor-mediated cell death. Mol Chem Neuropathol. 1996;29:153–168. doi: 10.1007/BF02814999. [DOI] [PubMed] [Google Scholar]
- 53.Maezawa I, Hong HS, Wu HC, Battina SK, Rana S, Iwamoto T, Radke GA, Pettersson E, Martin GM, Hua DH, Jin LW. A novel tricyclic pyrone compound ameliorates cell death associated with intracellular amyloid-beta oligomeric complexes. J Neurochem. 2006;98:57–67. doi: 10.1111/j.1471-4159.2006.03862.x. [DOI] [PubMed] [Google Scholar]
- 54.Woltjer R, McMahan W, Milatovic D, Kjerulf JD, Shie FS, Rung LG, Montine KS, Montine TJ. Effects of chemical chaperones on oxidative stress and detergent-insoluble species formation following conditional expression of amyloid precursor protein carboxy-terminal fragment. Neurobiol Dis. 2007;25:427–437. doi: 10.1016/j.nbd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- 56.Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH. The beta-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain. 2008;131:90–108. doi: 10.1093/brain/awm260. [DOI] [PubMed] [Google Scholar]
- 57.Gray N, Morre J, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A. Caffeoylquinic acids in Centella asiatica protect against amyloid-beta toxicity. J Alzheimers Dis. 2014;40:359–373. doi: 10.3233/JAD-131913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pike C, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 59.Murvai U, Somkuti J, Smeller L, Penke B, Kellermayer MS. Structural and nanomechanical comparison of epitaxially and solution-grown amyloid beta25–35 fibrils. Biochim Biophys Acta. 2015;1854:327–332. doi: 10.1016/j.bbapap.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Gray N, Sampath H, Zweig JA, Quinn JF, Soumyanath A. Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. J Alzheimers Dis. 2015;45:933–946. doi: 10.3233/JAD-142217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider L, Giordano S, Zelickson BR, S Johnson M, A Benavides G, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic Biol Med. 2011;51:2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.An K, Jung JH, Jeong AY, Kim HG, Jung SY, Lee K, Kim HJ, Kim SJ, Jeong TY, Son Y, Kim HS, Kim JH. Neuritin can normalize neural deficits of Alzheimer's disease. Cell Death & Disease. 2014;5:e1523. doi: 10.1038/cddis.2014.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Etgen AM, Jover-Mengual T, Zukin RS. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications. Frontiers in Neuroendocrinology. 2011;32:336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alasia S, Aimar P, Merighi A, Lossi L. Context-dependent toxicity of amyloid-beta; peptides on mouse cerebellar cells. J Alzheimers Dis. 2012;30:41–51. doi: 10.3233/JAD-2012-120043. [DOI] [PubMed] [Google Scholar]
- 65.Kelly M, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 66.Rønnekleiv O, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25:165–177. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 67.Nag S, Mokha SS. Activation of a Gq-coupled membrane estrogen receptor rapidly attenuates α2-adrenoceptor-induced antinociception via an ERK I/II-dependent, non-genomic mechanism in the female rat. Neuroscience. 2014;16:122–134. doi: 10.1016/j.neuroscience.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark S, Rainville J, Zhao X, Katzenellenbogen BS, Pfaff D, Vasudevan N. Estrogen receptor-mediated transcription involves the activation of multiple kinase pathways in neuroblastoma cells. J Steroid Biochem Mol Biol. 2014;139:45–53. doi: 10.1016/j.jsbmb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Li L, Xu B, Zhu Y, Chen L, Sokabe M, Chen L. DHEA prevents Aβ25–35-impaired survival of newborn neurons in the dentate gyrus through a modulation of PI3K-Akt-mTOR signaling. Neuropharmacology. 2010;59:323–333. doi: 10.1016/j.neuropharm.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 by acteoside through ERK and PI3 K/Akt pathway confer neuroprotection against beta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21:368–378. doi: 10.1007/s12640-011-9292-5. [DOI] [PubMed] [Google Scholar]
- 72.Xing G, Dong M, Li X, Zou Y, Fan L, Wang X, Cai D, Li C, Zhou L, Liu J, Niu Y. Neuroprotective effects of puerarin against beta-amyloid-induced neurotoxicity in PC12 cells via aPI3K-dependent signaling pathway. Brain Res Bull. 2011;85:212–218. doi: 10.1016/j.brainresbull.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015 doi: 10.1016/j.brainres.2014.12.056. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, Kawato S. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–558. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 75.Beyer C, Raab H. Nongenomic effects of oestrogen: embryonic mouse midbrain neurones respond with a rapid release of calcium from intracellular stores. Eur J Neurosci. 1998;10:255–262. doi: 10.1046/j.1460-9568.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 76.Beyer C, Karolczak M. Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signalling. J Neurosci Res. 2000;59:107–116. [PubMed] [Google Scholar]
- 77.Ishii H, Tsurugizawa T, Ogiue-Ikeda M, Asashima M, Mukai H, Murakami G, Hojo Y, Kimoto T, Kawato S. Local production of sex hormones and their modulation of hippocampal synaptic plasticity. Neuroscientist. 2007;13:323–334. doi: 10.1177/10738584070130040601. [DOI] [PubMed] [Google Scholar]
- 78.Dickstein D, Brautigam H, Stockton SD, Jr, Schmeidler J, Hof PR. Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau. Brain Struct Funct. 2010;214:161–179. doi: 10.1007/s00429-010-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemes J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F b-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 80.Kazim S, Blanchard J, Dai CL, Tung YC, LaFerla FM, Iqbal IG, Iqbal K. Disease modifying effect of chronic oral treatment with a neurotrophic peptidergic compound in a triple transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2014;71C:110–130. doi: 10.1016/j.nbd.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Mavroudis I, Manani MG, Petrides F, Petsoglou K, Njau SD, Costa VG, Baloyannis SJ. Dendritic and spinal pathology of the Purkinje cells from the human cerebellar vermis in Alzheimer's disease. Psychiatr Danub. 2013;25:221–226. [PubMed] [Google Scholar]
- 82.Mavroudis I, Manani M, Petrides F, Petsoglou C, Njau S, Costa V, Baloyannis S. Dendritic and spinal alterations of neurons from Edinger-Westphal nucleus in Alzheimer's disease. Folia Neuropathol. 2014;52:197–204. doi: 10.5114/fn.2014.43791. [DOI] [PubMed] [Google Scholar]
- 83.Brocca M, Pietranera L, Beauquis J, De Nicola AF. Estradiol increases dendritic length and spine density in CA1 neurons of the hippocampus of spontaneously hypertensive rats: a Golgi impregnation study. Exp Neurol. 2013;247:158–164. doi: 10.1016/j.expneurol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Dominguez R, Jalali C, de Lacalle S. Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J Neurosci. 2004;24:982–990. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Ex Ther. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800:1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Felty Q, Roy D. Estrogen, mitochondria, and growth of cancer and non-cancer cells. J Carcinog. 2005;4:1. doi: 10.1186/1477-3163-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arnold S, Victor MB, Beyer C. Estrogen and the regulation of mitochondrial structure and function in the brain. J Steroid Biochem Mol Biol. 2012;131:2–9. doi: 10.1016/j.jsbmb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Sarkar S, Jun S1, Simpkins JW2. Estrogen amelioration of Aβ-induced defects in mitochondria is mediated by mitochondrial signaling pathway involving ERβ, AKAP and Drp1. Brain Res. 2015;1616:101–111. doi: 10.1016/j.brainres.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao L, Yang YF, Gao YB, Wang SM, Wang LF, Zuo HY, Dong J, Xu XP, Su ZT, Zhou HM, Zhu LL, Peng RY. Upregulation of HIF-1α via activation of ERK and PI3K pathway mediated protective response to microwave-induced mitochondrial injury in neuron-like cells. Mol Neurobiol. 2014;50:1024–1034. doi: 10.1007/s12035-014-8667-z. [DOI] [PubMed] [Google Scholar]